Ball-Milling-Assisted Fe3O4 Loadings of Rice Straw Biochar for Enhanced Tetracycline Adsorption in Aquatic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Straw Biochar
2.3. Response Surface Optimization of Ball-Milling-Assisted Fe3O4 Loading Conditions
2.4. Characterization of Biochar Materials
2.5. Adsorption Studies of Tetracycline onto Modified Biochar
2.5.1. Adsorption Kinetics Experiments
2.5.2. Adsorption Isotherm Experiment
2.5.3. pH Effect Experiments
2.6. Determination of TC Concentration
2.7. Calculation of Adsorption Capacity
3. Results and Discussion
3.1. Optimization of Ball-Milling-Assisted Fe3O4 Loading Conditions
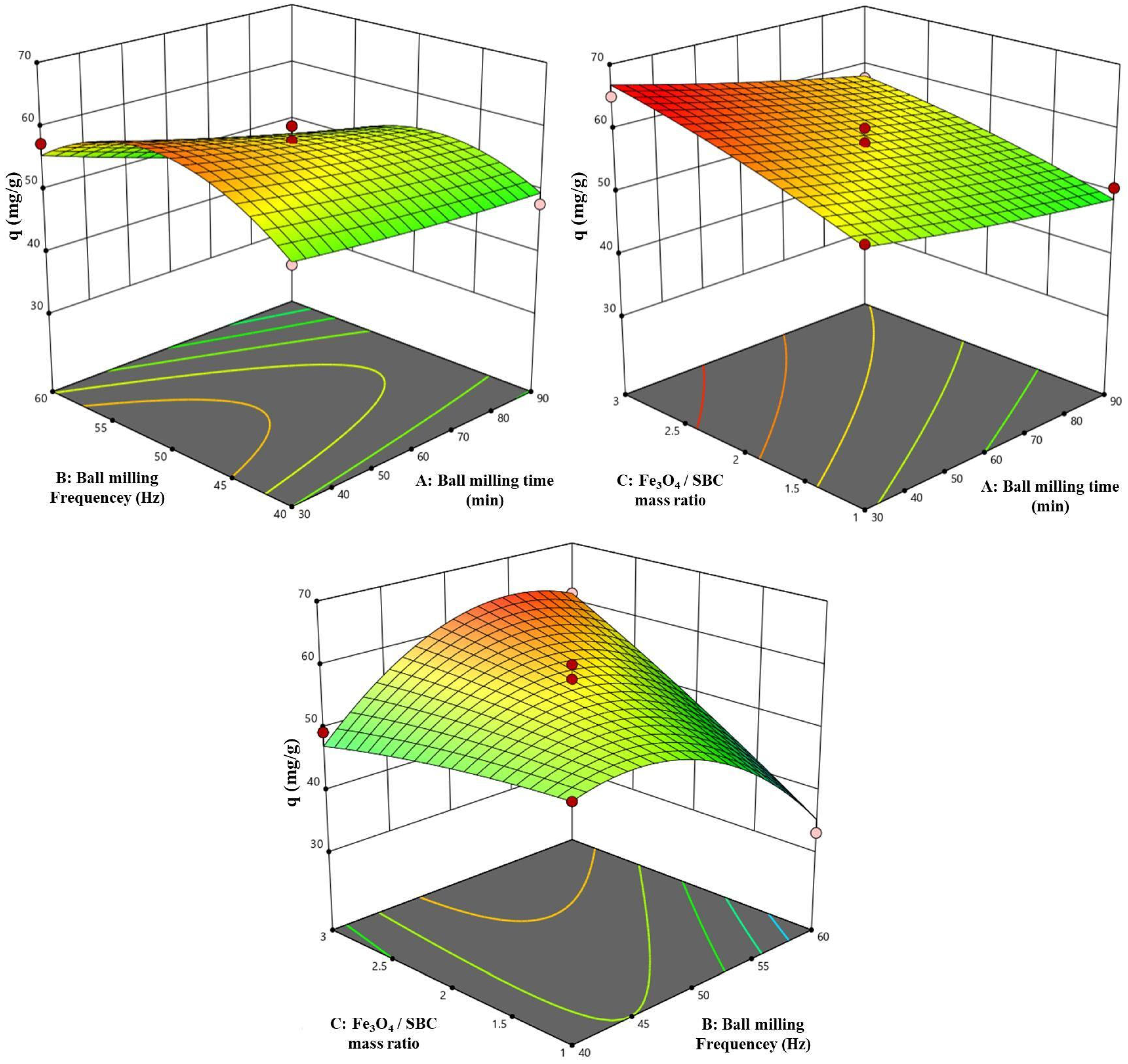
3.1.1. Results of Box–Behnken Response Surface Experiments
3.1.2. Influence of Ball-Milling-Assisted Fe3O4 Modification Conditions
3.2. Characterization of Biochar
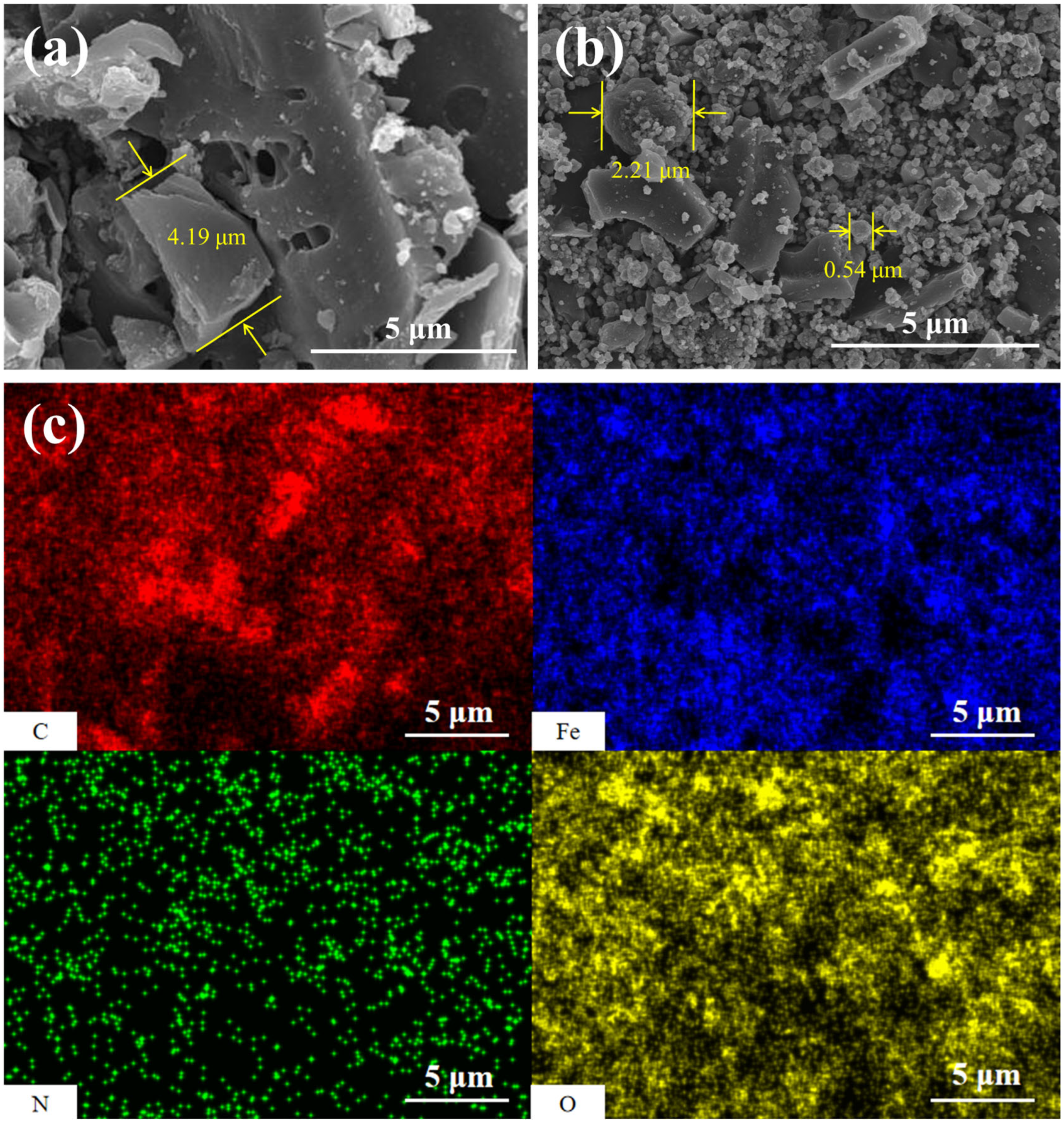
3.2.1. SEM
3.2.2. FTIR
3.2.3. XRD
3.2.4. BET
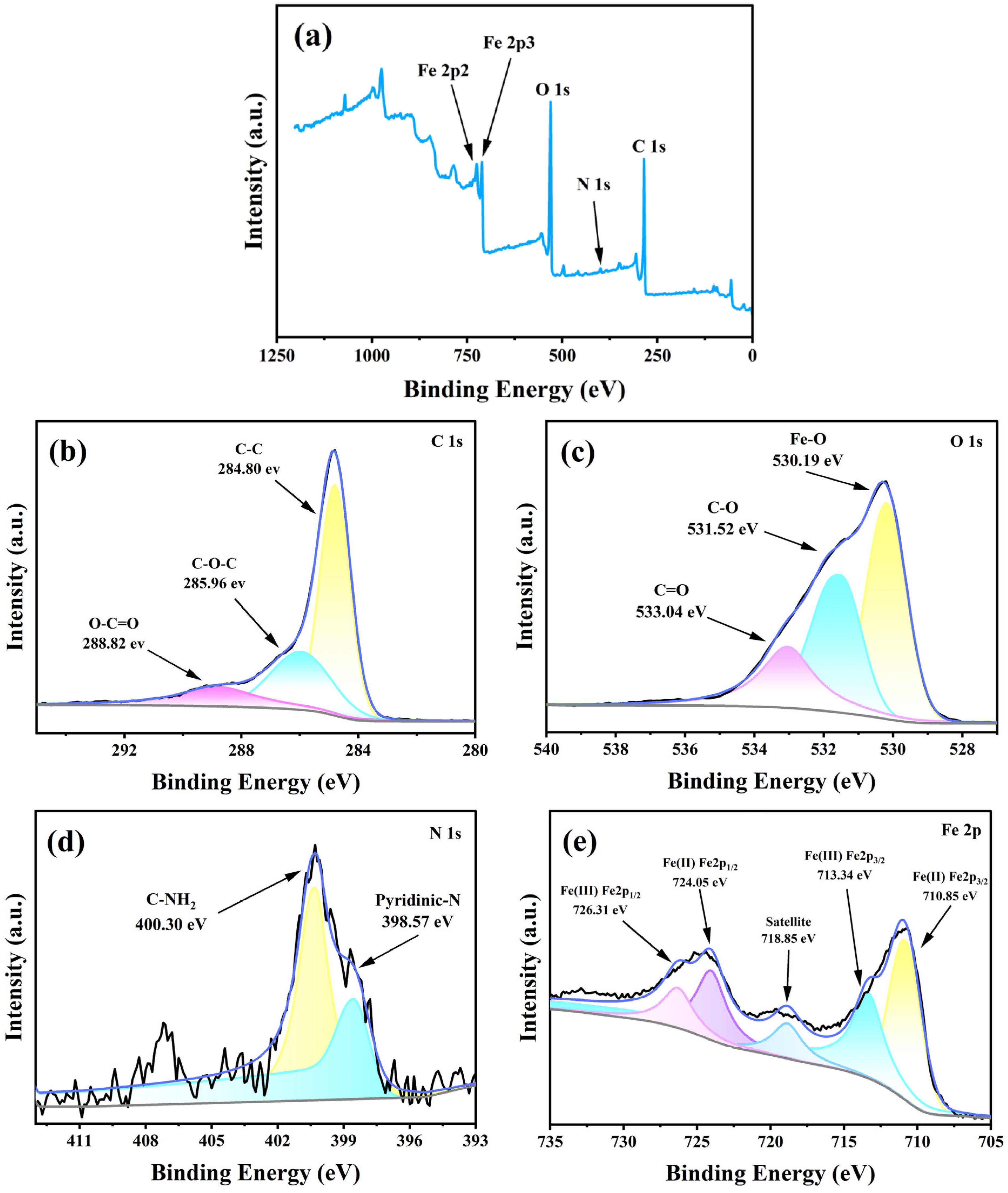
3.2.5. XPS
3.3. Adsorption Experiments of TC by Fe3O4@BM-SBC
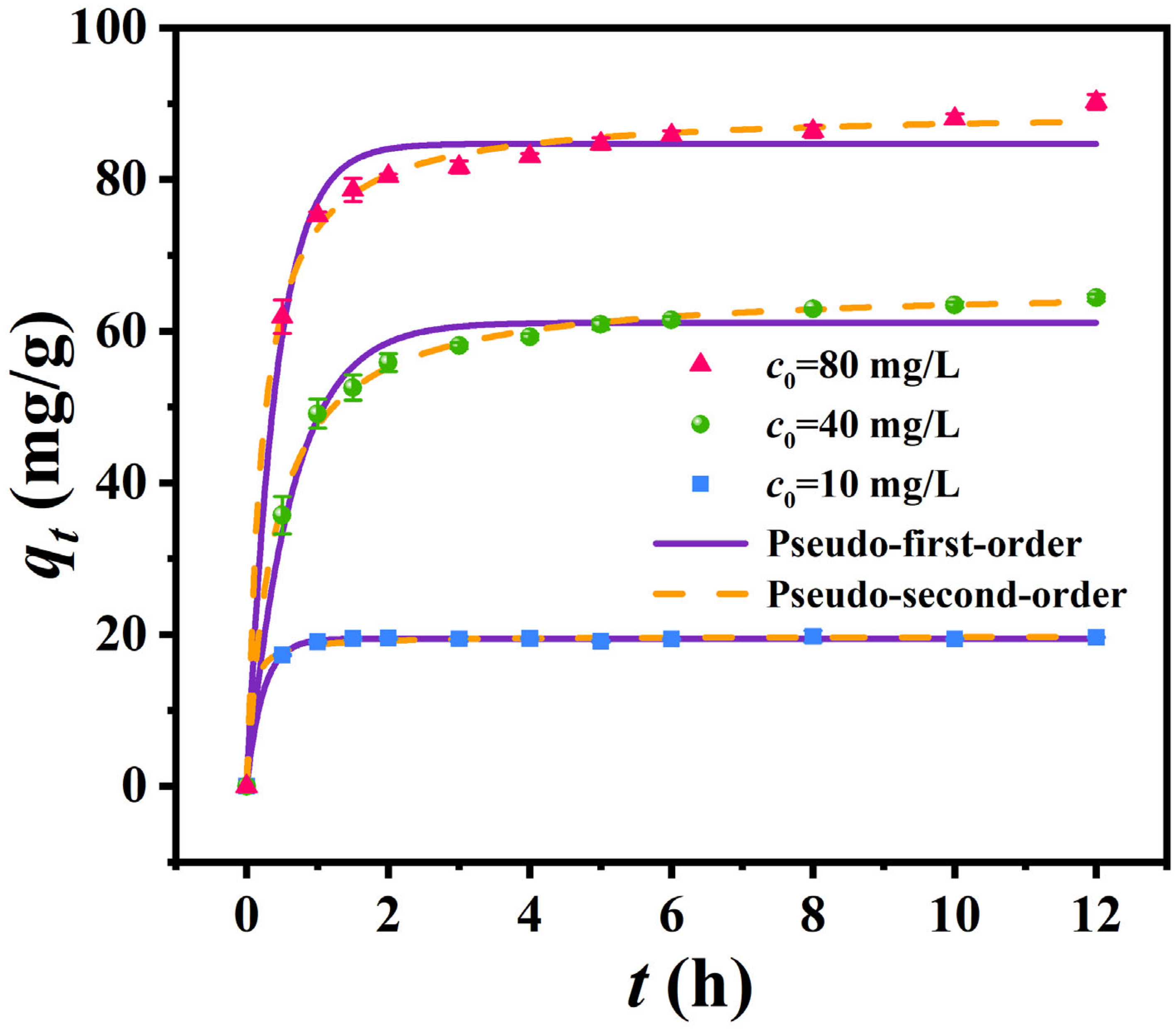
3.3.1. Adsorption Kinetics
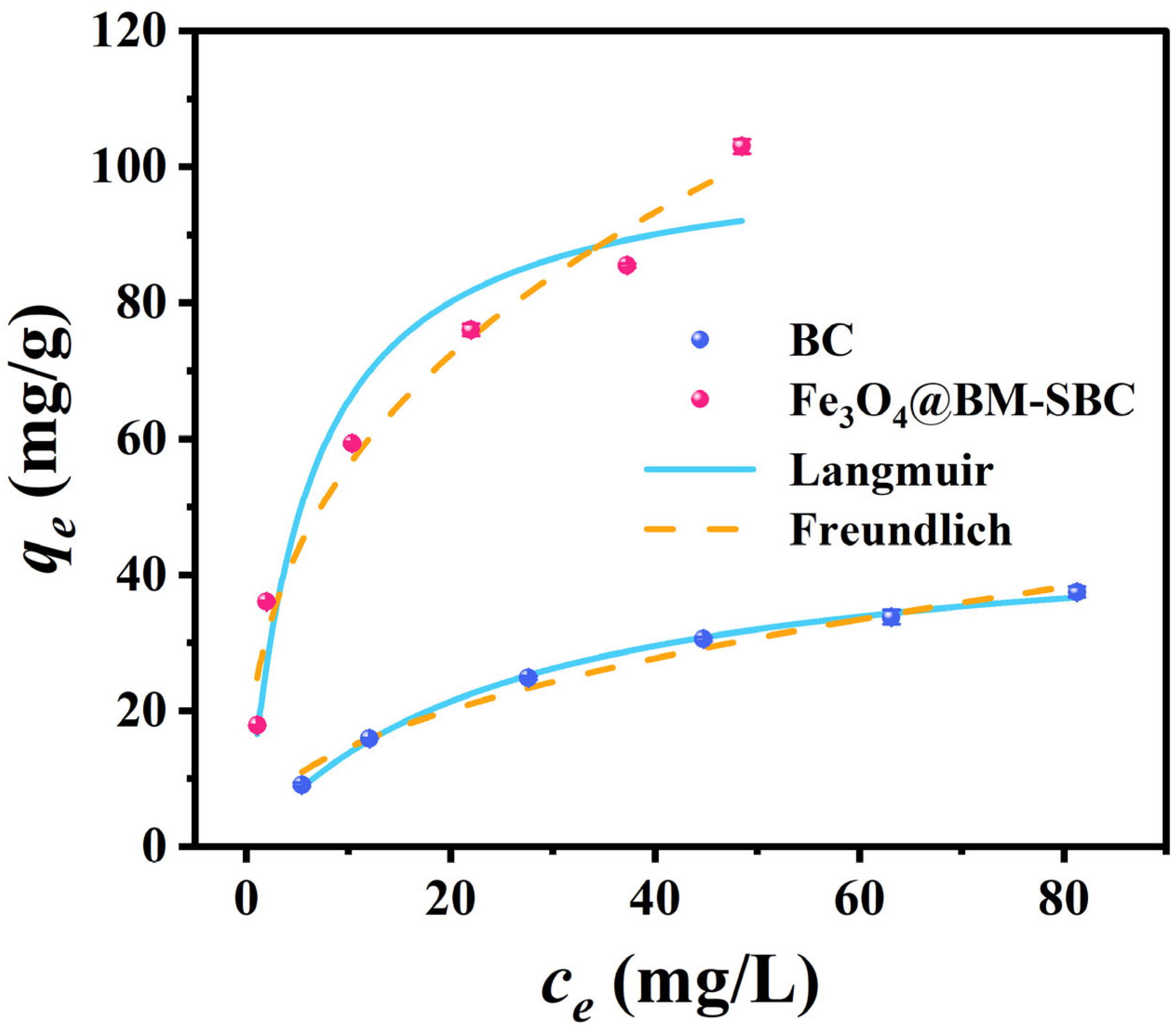
3.3.2. Adsorption Isotherms
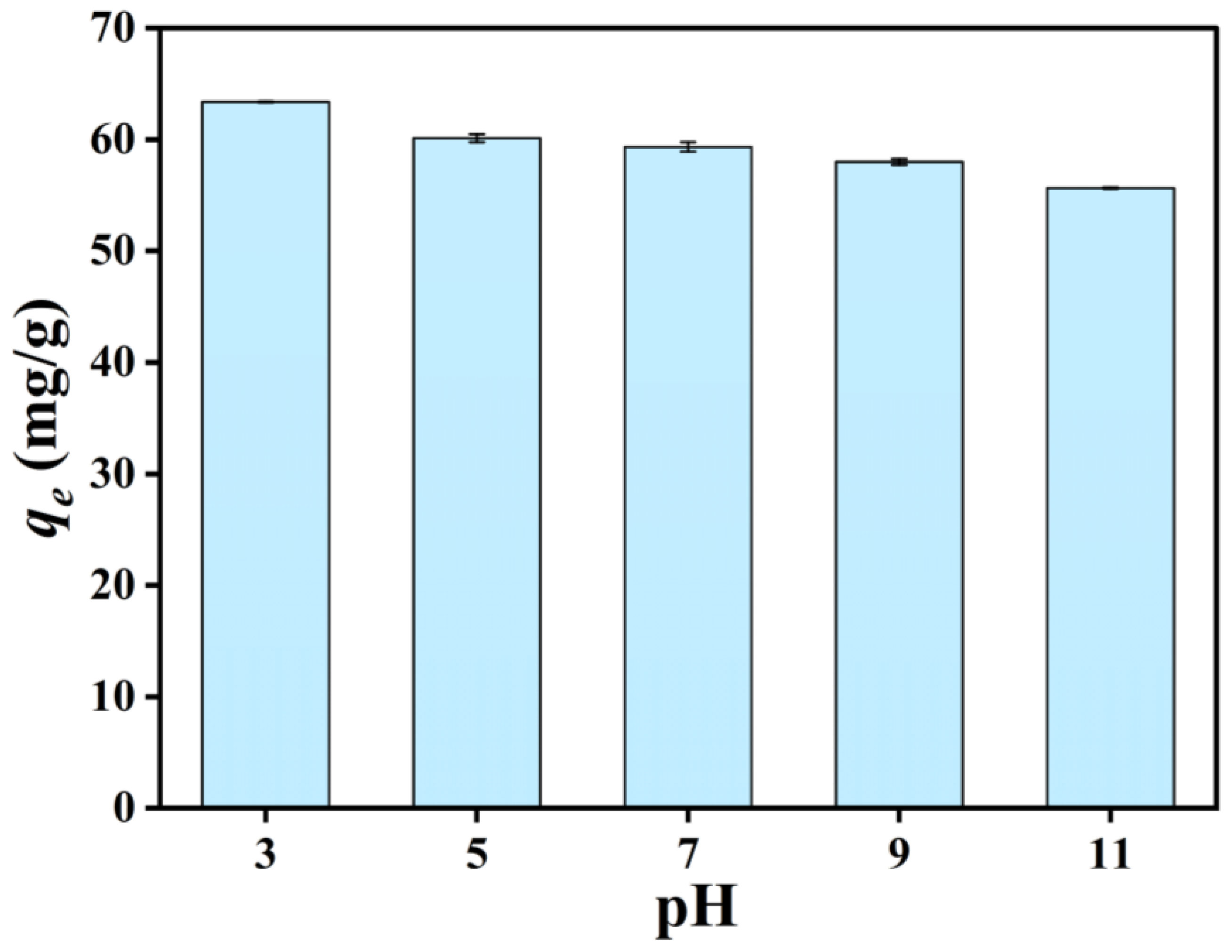
3.3.3. Effect of pH
3.4. Adsorption Mechanisms
3.5. Future Considerations on Feedstock and Pyrolysis Influence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barasarathi, J.; Abdullah, P.S.; Uche, E.C. Application of magnetic carbon nanocomposite from agro-waste for the removal of pollutants from water and wastewater. Chemosphere 2022, 305, 135384. [Google Scholar] [CrossRef]
- Sibhatu, A.K.; Weldegebrieal, G.K.; Sagadevan, S.; Tran, N.N.; Hessel, V. Photocatalytic activity of CuO nanoparticles for organic and inorganic pollutants removal in wastewater remediation. Chemosphere 2022, 300, 134623. [Google Scholar] [CrossRef]
- Bonetto, L.R.; Ferrarini, F.; de Marco, C.; Crespo, J.S.; Guégan, R.; Giovanela, M. Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J. Water Process Eng. 2015, 6, 11–20. [Google Scholar] [CrossRef]
- Chaba, M.J.M.; Nomngongo, P.N. Effective adsorptive removal of amoxicillin from aqueous solutions and wastewater samples using zinc oxide coated carbon nanofiber composite. Emerg. Contam. 2019, 5, 143–149. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tao, H.; Wang, Y.J.; Ma, Z.Y.; Zhou, Z.Y. Pollution characteristics and risk assessment of tetracycline antibiotics in farmland soil in Yinchuan. Environ. Sci. 2021, 42, 4933–4941. [Google Scholar]
- Ao, M.M.; Wei, J.; Chen, Z.L.; Liu, L.; Song, Y.H. Research progress on environmental behaviors and ecotoxicity of tetracycline antibiotics. J. Environ. Eng. Technol. 2021, 11, 314–324. [Google Scholar]
- Xu, L.Y.; Zhang, H.; Xiong, P.; Zhu, Q.Q.; Liao, C.Y.; Jiang, G.B. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ghaemi, A.; Khanizadeh, A.; Yazdian, F.; Mollajavadi, Y.; Arshad, R.; Rahdar, A. Breast cancer detection based on cancer antigen 15-3; emphasis on optical and electrochemical methods: A review. Biosens. Bioelectron. 2024, 260, 116425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; He, Z.P.; Zhou, M.; Xie, M.L.; He, T.P.; Zhao, Y.L.; Chen, X.Y.; Wu, Y.H.; Xu, Z.G. In-situ synthesis of biochar modified PbMoO4: An efficient visible light-driven photocatalyst for tetracycline removal. Chemosphere 2021, 284, 131260. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Cao, M.B.; Liu, X.; Yang, H.B.; Li, Y.S. A series of novel carbohydrate-based carbon adsorbents were synthesized by self-propagating combustion for tetracycline removal. Bioresour. Technol. 2021, 332, 125059. [Google Scholar] [CrossRef] [PubMed]
- Kirova, G.; Velkova, Z.; Stoytcheva, M.; Gochev, V. Tetracycline removal from model aqueous solutions by pretreated waste Streptomyces fradiae biomass. Biotechnol. Biotechnol. Equip. 2021, 35, 953–963. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T.; Meyer, A.S.; Pulimi, M. Removal of tetracycline in enzymatic membrane reactor: Enzymatic conversion as the predominant mechanism over adsorption and membrane rejection. J. Environ. Chem. Eng. 2022, 10, 106973. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Sudakaran, S.V.; Ravichandran, K.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Green synthesis of NiFe nanoparticles using Punica granatum peel extract for tetracycline removal. J. Clean. Prod. 2019, 210, 767–776. [Google Scholar] [CrossRef]
- Tahira, M.; Batool, F.; Noreen, S.; Mustaqeem, M.; Munawar, K.S.; Kanwal, S.; Shahbaz, K.; Arshad, A.; Ali, H.M. Unlocking the potential of de-oiled seeds of Citrus sinensis loaded with metal nanoparticles for Congo red degradation and removal: A green water treatment strategy with bibliometric analysis. Front. Sustain. Food Syst. 2024, 8, 1430624. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.P.; Zhang, T.; Zhu, Y.L.; Qiu, F.X. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.T.; Bao, Q.H.; Zheng, J.; Deng, D.; Duan, Y.Q.; Shen, L.Q. The physicochemical characterization, equilibrium, and kinetics of heavy metal ions adsorption from aqueous solution by arrowhead plant (Sagittaria trifolia L.) stalk. J. Food Biochem. 2018, 42, e12448. [Google Scholar] [CrossRef]
- Ren, L.; Lin, H.X.; Meng, F.C.; Zhang, F. One-step solvothermal synthesis of Fe3O4@Carbon composites and their application in removing of Cr(VI) and Congo red. Ceram. Int. 2019, 45, 9646–9652. [Google Scholar] [CrossRef]
- Rong, J.; Qiu, F.X.; Zhang, T.; Zhang, X.Y.; Zhu, Y.; Xu, J.C.; Yang, D.Y.; Dai, Y.T. A strategy toward 3D hydrophobic composite resin network decorated with biological ellipsoidal structure rapeseed flower carbon for enhanced oils and organic solvents selective absorption. Chem. Eng. J. 2017, 322, 397–407. [Google Scholar] [CrossRef]
- Jing, Y.L.; Zhang, Y.H.; Han, I.; Wang, P.; Mei, Q.W.; Huang, Y.J. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Clare, A.; Shackley, S.; Joseph, S.; Hammond, J.; Pan, G.X.; Bloom, A. Competing uses for China’s straw: The economic and carbon abatement potential of biochar. GCB Bioenergy 2015, 7, 1272–1282. [Google Scholar] [CrossRef]
- Yu, P.P.; Yu, H.Z.; Cheng, J.H.; Nie, J.R.; Liu, Y.X.; Niu, Q.J.; Yang, Q.Z.; Liu, Y.C.; Ji, G.Y. Enhancing enzymatic hydrolysis of rice straw by acid-assisted mechanocatalytic depolymerization pretreatment. Agronomy 2024, 14, 2550. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.G.; Wang, Q.D.; Li, X.G.; Guo, Y.X.; Song, W.; Li, Y.F. Ball milling boosted the activation of peroxymonosulfate by biochar for tetracycline removal. J. Environ. Chem. Eng. 2021, 9, 106870. [Google Scholar] [CrossRef]
- Shao, S.S.; Sun, T.R.; Li, X.H.; Wang, Y.F.; Ma, L.X.; Liu, Z.F.; Wu, S.L. Preparation of heavy bio-oil-based porous carbon by pyrolysis gas activation and its performance in the aldol condensation for aviation fuel as catalyst carrier. Ind. Crops Prod. 2024, 218, 118963. [Google Scholar] [CrossRef]
- Ding, K.L.; Lin, H.; Liu, L.Y.; Jia, X.W.; Zhang, H.; Tan, Y.F.; Liang, X.Y.; He, Y.H.; Liu, D.; Han, L.J.; et al. Effect of ball milling on enzymatic sugar production from fractionated corn stover. Ind. Crops Prod. 2023, 196, 116502. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Morgan, H.M.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal behavior and kinetic study of the effects of zinc-modified biochar catalyst on lignin and low-density polyethylene (LDPE) co-pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
- Iqbal, J.; Kiran, S.; Hussain, S.; Iqbal, R.K.; Ghafoor, U.; Younis, U.; Zarei, T.; Naz, M.; Germi, S.G.; Danish, S.; et al. Acidified biochar confers improvement in quality and yield attributes of Sufaid Chaunsa mango in saline soil. Horticulturae 2021, 7, 418. [Google Scholar] [CrossRef]
- Li, R.N.; Wang, Z.W.; Guo, J.L.; Li, Y.; Zhang, H.Y.; Zhu, J.M.; Xie, X.Y. Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci. Technol. 2018, 77, 1127–1136. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S.; Chen, M.F. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, X.Y.; Teng, H.W.; Xu, J.L.; Sheng, L.X. Purification mechanism of city tail water by constructed wetland substrate with NaOH-modified corn straw biochar. Ecotoxicol. Environ. Saf. 2022, 238, 113597. [Google Scholar] [CrossRef]
- Hu, X.L.; Xue, Y.W.; Long, L.; Zhang, K.J. Characteristics and batch experiments of acid- and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 19932–19940. [Google Scholar] [CrossRef]
- Sun, M.C.; Ma, Y.K.; Yang, Y.J.; Zhu, X.F. Effect of iron impregnation ratio on the properties and adsorption of KOH activated biochar for removal of tetracycline and heavy metals. Bioresour. Technol. 2023, 380, 129081. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhang, M.; Lv, Z.N.; Sun, Y.K.; Li, P.H.; Zhou, R.J. Research on efficient removal of ciprofloxacin through sequential rice straw biochar modification via alkali activation and manganese oxides. Environ. Technol. Innov. 2024, 34, 103611. [Google Scholar] [CrossRef]
- Heo, Y.; Lee, E.H.; Lee, S.W. Adsorptive removal of micron-sized polystyrene particles using magnetic iron oxide nanoparticles. Chemosphere 2022, 307, 135672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Wang, B.; Theng, B.K.G.; Wu, P.; Liu, F.; Wang, S.S.; Lee, X.Q.; Chen, M.; Li, L.; Zhang, X.Y. Formation and mechanisms of nano-metal oxide-biochar composites for pollutant removal: A review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar] [CrossRef]
- Jiang, M.Z.; Luo, J.H.; Qiu, M.T.; Peng, K.; Wang, G.J.; Wang, Y.H.; Chen, X.Y.; Wu, Y.H.; Liu, W.S. Enhanced tetracycline removal via Z-scheme activation by a novel magnetic ZnO/Fe3O4-modified biochar. Biomass Bioenergy 2025, 200, 108042. [Google Scholar] [CrossRef]
- Lyu, H.H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J.C. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Qu, J.H.; Wu, Z.H.; Liu, Y.; Li, R.L.; Wang, D.; Wang, S.Q.; Wei, S.Q.; Zhang, J.R.; Tao, Y.; Jiang, Z.; et al. Ball milling potassium ferrate activated biochar for efficient chromium and tetracycline decontamination: Insights into activation and adsorption mechanisms. Bioresour. Technol. 2022, 360, 127407. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhao, M.Y.; Zhou, M.; Li, Y.C.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.Q.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Huang, B.Y.; Huang, D.; Zheng, Q.; Yan, C.H.; Feng, J.P.; Gao, H.J.; Fu, H.Q.; Liao, Y.W. Enhanced adsorption capacity of tetracycline on porous graphitic biochar with an ultra-large surface area. RSC Adv. 2023, 13, 10397–10407. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.H.; Dabbour, M.; Golly, M.K.; Agyekum, A.A.; Ma, H.L. Effect of sonication pretreatment parameters and their optimization on the antioxidant activity of Hermitia illucens larvae meal protein hydrolysates. J. Food Process. Preserv. 2019, 43, e14068. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Process optimization of intermediate-wave infrared drying: Screening by Plackett–Burman; comparison of Box-Behnken and central composite design and evaluation: A case study. Ind. Crops Prod. 2021, 162, 113287. [Google Scholar] [CrossRef]
- Yang, Z.K.; Li, M.R.; Li, Y.X.; Li, Z.H.; Huang, X.W.; Wang, X.; Shi, J.Y.; Zou, X.B.; Zhai, X.D.; Povey, M. Improving properties of Litsea cubeba oil Pickering emulsion-loaded gelatin-based bio-nanocomposite film via optimizing blending ratio: Application for mango preservation. Food Hydrocoll. 2023, 145, 109052. [Google Scholar] [CrossRef]
- Mintah, K.B.; He, R.H.; He, R.H.; Agyekum, A.A.; Dabbour, M.; Golly, M.K.; Ma, H.L. Edible insect protein for food applications: Extraction, composition, and functional properties. J. Food Process. Eng. 2020, 43, e13362. [Google Scholar] [CrossRef]
- Zheng, J.W.; Zhang, X.X.; Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Beta, T.; Huang, W.Y.; Li, Y. Extraction optimization of Arctium lappa L. polysaccharides by Box–Behnken response surface design and their antioxidant capacity. Starch-Stärke 2022, 74, 2100305. [Google Scholar] [CrossRef]
- Naseem, Z.; Iqbal, J.; Zahid, M.; Shaheen, A.; Hussain, S.; Yaseen, W. Use of hydrogen-bonded supramolecular eutectic solvents for eco-friendly extraction of bioactive molecules from Cymbopogon citratus using Box–Behnken design. J. Food Meas. Charact. 2020, 15, 1487–1498. [Google Scholar] [CrossRef]
- Cai, T.; Du, H.H.; Liu, X.L.; Tie, B.Q.; Zeng, Z.X. Insights into the removal of Cd and Pb from aqueous solutions by NaOH–EtOH-modified biochar. Environ. Technol. Innov. 2021, 24, 101007. [Google Scholar] [CrossRef]
- Che, N.J.; Qu, J.; Wang, J.Q.; Liu, N.; Li, C.L.; Liu, Y.L. Adsorption of phosphate onto agricultural waste biochars with ferrite/manganese modified-ball-milled treatment and its reuse in saline soil. Sci. Total Environ. 2024, 915, 169841. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.Y.; Xing, Y.C.; You, T.Y. Biochar as adsorbents for environmental microplastics and nanoplastics removal. J. Environ. Chem. Eng. 2024, 12, 113377. [Google Scholar] [CrossRef]
- Derbe, T.; Gindose, T.G.; Sani, T.; Zereffa, E.A. Synthesis of zeolite-A/Fe3O4/biochar/MOF-5 composite for the defluoridation of drinking water. Appl. Water Sci. 2025, 15, 161. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Galletti, C.; Chiaramonti, D.; Bensaid, S.; Antunes, E.; Fino, D. Enhancing biochar production: A technical analysis of the combined influence of chemical activation (KOH and NaOH) and pyrolysis atmospheres (N2/CO2) on yields and properties of rice husk-derived biochar. J. Environ. Manag. 2024, 370, 123034. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.H.; Su, Y.L.; Wu, D.; Zhao, Y.P.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Ashouri, A.; Pourian, S.; Nasiri, B.; Moradi, A. Pd@dppe@Fe3O4 as a magnetically recyclable catalyst for C–C bond formation: Efficient phenylation of aldimines under mild conditions. Appl. Surf. Sci. Adv. 2025, 28, 100796. [Google Scholar] [CrossRef]
- Shan, D.N.; Deng, S.B.; Zhao, T.N.; Wang, B.; Wang, Y.J.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef]
- Fan, X.B.; Peng, L.L.; Wang, X.H.; Han, S.Q.; Yang, L.Z.; Wang, H.L.; Hao, C. Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Ind. Crops Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Xing, Y.C.; Shen, X.L.; Niu, Q.J.; Duan, H.W.; Tang, C.S.; Tao, B.; Chen, S.Y.; Shangguan, Q.Y.; Feng, B.; Yu, H.Z.; et al. Thermally and chemically stable Fe/Mg-layered double oxides-biochar for enhanced polystyrene nanoplastic adsorption and sustainable recycling. Chem. Eng. J. 2025, 508, 160918. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.P.; Tang, J.C.; Sun, H.W. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Cui, Z.W.; Ren, Y.F.; Wang, W.; Zhang, L.M.; Zhang, L.Y.; Wang, X.Y.; He, J.Y. Adsorption characteristics and mechanism of cadmium in water by alkali and magnetic composite modified wheat straw biochar. Huan Jing Ke Xue 2020, 41, 3315–3325. [Google Scholar]
- Zhao, S.Y.; Li, M.X.; Ding, J.; Yang, S.S.; Zang, Y.N.; Zhao, Y.; Gao, X.L.; Ren, N.Q. Fabrication of rGO/Fe3O4 magnetic composite for the adsorption of anthraquinone-2-sulfonate in water phase. Water 2021, 13, 2315. [Google Scholar] [CrossRef]
- Tong, F.; Huang, Q.; Liu, L.Z.; Fan, G.P.; Shi, G.L.; Lu, X.; Gao, Y. Interactive effects of inorganic–organic compounds on passivation of cadmium in weakly alkaline soil. Agronomy 2023, 13, 2647. [Google Scholar] [CrossRef]
- Zhao, L.Y.; He, P.X.; Li, Q.; Pan, H.H.; Xie, T.; Huang, S.Y.; Cao, S.H.; Liu, X.X. Efficiently removal of tetracycline from water by Fe3O4-sludge biochar. Water Air Soil Pollut. 2023, 235, 39. [Google Scholar]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Benis, K.Z. Understanding the physicochemical structure of biochar affected by feedstock, pyrolysis conditions, and post-pyrolysis modification methods—A meta-analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]

| Influencing Factors | Coded | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ball-milling time/min | A | 30 | 60 | 90 |
| Ball-milling frequency/Hz | B | 40 | 50 | 60 |
| Fe3O4 to SBC mass ratio | C | 1:1 | 2:1 | 3:1 |
| No. | Ball-Milling Time/min | Ball-Milling Frequency/Hz | Fe3O4-to-Biochar Mass Ratio | Adsorption Capacity/(mg/g) |
|---|---|---|---|---|
| 1 | 30 | 40 | 2 | 52.496 |
| 2 | 90 | 40 | 2 | 47.856 |
| 3 | 30 | 60 | 2 | 57.440 |
| 4 | 90 | 60 | 2 | 44.081 |
| 5 | 30 | 50 | 1 | 55.740 |
| 6 | 90 | 50 | 1 | 50.805 |
| 7 | 30 | 50 | 3 | 65.058 |
| 8 | 90 | 50 | 3 | 57.666 |
| 9 | 60 | 40 | 1 | 52.751 |
| 10 | 60 | 60 | 1 | 33.111 |
| 11 | 60 | 40 | 3 | 49.507 |
| 12 | 60 | 60 | 3 | 61.382 |
| 13 | 60 | 50 | 2 | 57.234 |
| 14 | 60 | 50 | 2 | 57.352 |
| 15 | 60 | 50 | 2 | 60.222 |
| 16 | 60 | 50 | 2 | 56.329 |
| 17 | 60 | 50 | 2 | 57.922 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F | p | Significance |
|---|---|---|---|---|---|---|
| Model | 860.58 | 9 | 95.62 | 20.69 | 0.0003 | ** |
| A—Ball-milling time | 114.96 | 1 | 114.96 | 24.88 | 0.0016 | ** |
| B—Ball-milling frequency | 5.44 | 1 | 5.44 | 1.18 | 0.3140 | - |
| Fe3O4-to-biochar mass ratio | 212.24 | 1 | 212.24 | 45.93 | 0.0003 | ** |
| AB | 19.01 | 1 | 19.01 | 4.11 | 0.0822 | - |
| AC | 1.51 | 1 | 1.51 | 0.3266 | 0.5856 | - |
| BC | 248.30 | 1 | 248.30 | 53.73 | 0.0002 | ** |
| A2 | 0.6502 | 1 | 0.6502 | 0.1407 | 0.7187 | - |
| B2 | 252.02 | 1 | 252.02 | 54.53 | 0.0002 | ** |
| C2 | 3.32 | 1 | 3.32 | 0.7177 | 0.4249 | - |
| Residual | 32.35 | 7 | 4.62 | |||
| Lack of Fit | 23.78 | 3 | 7.93 | 3.70 | 0.1192 | - |
| Pure Error | 8.57 | 4 | 2.14 | |||
| Total | 892.93 | 16 |
| Sample | C | N | O | Fe | O/C | Total |
|---|---|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (wt%) | (%) | (wt%) | |
| BC | 64.21 | 5.96 | 29.59 | 0.24 | 46.08 | 100 |
| Fe3O4@BM-SBC | 45.55 | 2.52 | 28.98 | 22.95 | 63.62 | 100 |
| Sample | BET Surface Area/(m2/g) | Total Pore Volume/(cm3/g) | Average Pore Diameter/nm |
|---|---|---|---|
| BC | 74.12 | 0.37 | 19.98 |
| Fe3O4@BM-SBC | 76.37 | 0.10 | 5.37 |
| Model | Parameter | c0 (TC)/(mg/L) | ||
|---|---|---|---|---|
| 10 | 40 | 80 | ||
| Pseudo-first-order | qe/(mg/g) | 19.450 | 61.154 | 84.707 |
| k1/h−1 | 4.359 | 1.569 | 2.413 | |
| R2 | 0.999 | 0.986 | 0.985 | |
| Pseudo-second-order | qe/(mg/g) | 19.765 | 65.946 | 89.207 |
| k2/g·(mg·h)−1 | 0.858 | 0.039 | 0.053 | |
| R2 | 0.997 | 0.998 | 0.997 | |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL | qmax | R2 | KF | n−1 | R2 | |
| L/mg | mg/g | mg(1-1/n)·L1/n·g−1 | ||||
| BC | 0.040 | 47.882 | 0.998 | 5.027 | 0.463 | 0.985 |
| Fe3O4@BM-SBC | 0.176 | 102.875 | 0.939 | 24.047 | 0.368 | 0.977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yu, H.; Xing, Y.; Zhao, Q.; Ashan, R.; Feng, B.; Tao, B.; Shangguan, Q.; Liu, Y.; Zhang, H.; et al. Ball-Milling-Assisted Fe3O4 Loadings of Rice Straw Biochar for Enhanced Tetracycline Adsorption in Aquatic Systems. Agronomy 2025, 15, 1987. https://doi.org/10.3390/agronomy15081987
Liu Y, Yu H, Xing Y, Zhao Q, Ashan R, Feng B, Tao B, Shangguan Q, Liu Y, Zhang H, et al. Ball-Milling-Assisted Fe3O4 Loadings of Rice Straw Biochar for Enhanced Tetracycline Adsorption in Aquatic Systems. Agronomy. 2025; 15(8):1987. https://doi.org/10.3390/agronomy15081987
Chicago/Turabian StyleLiu, Yuxin, Haizhang Yu, Yuchen Xing, Qi Zhao, Rukeya Ashan, Bo Feng, Bo Tao, Qianyi Shangguan, Yucheng Liu, Haiyan Zhang, and et al. 2025. "Ball-Milling-Assisted Fe3O4 Loadings of Rice Straw Biochar for Enhanced Tetracycline Adsorption in Aquatic Systems" Agronomy 15, no. 8: 1987. https://doi.org/10.3390/agronomy15081987
APA StyleLiu, Y., Yu, H., Xing, Y., Zhao, Q., Ashan, R., Feng, B., Tao, B., Shangguan, Q., Liu, Y., Zhang, H., & Ji, G. (2025). Ball-Milling-Assisted Fe3O4 Loadings of Rice Straw Biochar for Enhanced Tetracycline Adsorption in Aquatic Systems. Agronomy, 15(8), 1987. https://doi.org/10.3390/agronomy15081987






