Abstract
This study investigates the impact of environmental changes induced by systematic manipulation of flooding depth and breeding density on greenhouse gas emissions in the field-based giant rice–fish hybrid farming model. Compared with traditional agricultural practices, increasing cultured density in giant rice–fish co-cultivation significantly alleviated the adverse consequences of flooding on soil nutrient dynamics, microbial activity community structure, and greenhouse gas emissions. Relative to the traditional alternating wet and dry irrigation, the soil concentrations of ammonium, total nitrogen, and phosphate significantly increased. Cultured fish had significantly increased soil microbial biomass carbon, nitrogen, and phosphorus contents and improved soil β-glucosidase and aryl-sulfatase activates relative to flooding alone. Cultured fish increased the relative abundances of Actinobacteria, Nitrospirae, Planctomycetes, Verrucomicrobia, and Aminicenantes. An increasing cultured fish density reduced cumulative methane and nitrous oxide emissions and GWP (global warming potential). Relative to the continuous flooding throughout the growing period, cumulative methane emissions and GWP in the flooding with high-density cultured fish were reduced by 5.32% and 1.48%, respectively. Notably, this co-cultivation strategy has the potential to transform traditional practices for sustainable agriculture. Nevertheless, it is imperative to remain vigilant about the potential consequences of greenhouse gas emissions associated with these innovative practices. Continuous monitoring and refinement are essential to ensure the long-term sustainability and viability of this agricultural approach.
1. Introduction
Intensive agriculture has significantly degraded farmland environments, such as via soil compaction and water eutrophication, and increased greenhouse gas emissions, while substantially enhancing food security [1,2,3,4]. Compared to traditional biological and non-biological measures, this study focuses on strategies to maintain ecological stability in rice paddies [5,6]. In recent years, co-cultivation practices in rice paddies have garnered significant attention for their dual ability to stabilize environments and generate both social and economic benefits, offering a promising alternative to alleviate conventional farming approaches’ adverse environment effect [7,8].
Paddy field co-cultivation, a practice with a 2000-year history in China, has long been prevalent in the south and southeast rice-growing regions [9,10]. Co-cultivation can significantly reduce the need for fertilizers and pesticides in rice fields [11]. The management of cultured fish substantially reduced weed density, dipterous insect populations, and pesticide usage compared to traditional paddy cultivation [12]. Additionally, rice–fish co-cultivation has increased rice nitrogen use efficiency, resulting in a 24% reduction in fertilizer application [13]. Consequently, paddy field co-cultivation ensures economic viability and reduces the environmental impact of unreasonable agricultural activities [14].
In agricultural production, rice paddies have been the main source of greenhouse gas emissions. In the pursuit of higher rice yields and economic returns, excessive amounts of fertilizers and pesticides have been applied, resulting in soil nutrient levels exceeding the requirements for rice growth in traditional paddy fields [15]. A previous research study found that fertilizer application significantly increased the nutrient cycling and stimulated microbial activity in paddy soil [16], but excessive chemical fertilizer application in rice fields is a major contributor to greenhouse gas emissions and exacerbates the greenhouse effect in traditional rice fields [17,18]. In addition, inappropriate irrigation practices have led to water wastage and adverse effects on soil greenhouse gas emissions [19]. Current research has indicated that the anaerobic conditions in traditional flooded paddy fields stimulated the decomposition of organic molecules and the methanogenic and denitrifying bacterial activity, ultimately resulting in elevated methane and nitrous oxide emissions [20,21]. Co-cultivation practices in rice paddies can affect greenhouse gas emissions by changing soil aeration, nutrient cycling, and microbial activity and communities [22,23]. However, the conclusion remains controversial.
The impact of paddy field co-cultivation on greenhouse gas emissions remains controversial. Previous research has shown that co-cultured catfish reduced nitrous oxide (N2O) and ammonia (NH3) emissions by 85.6% and 26.0%, respectively, compared to monoculture rice, while cultured shrimp reduced them by 108.3% and 22.6%, respectively [24]. Nevertheless, current studies have reported an increase in methane emissions from co-cultured fish [11]. We hypothesized that the paddy field co-cultivation greenhouse gas emissions will correlate with the soil environmental and soil microbial community.
Hence, it is imperative to comprehensively assess the impact of giant rice co-cultivation on greenhouse gas emissions. The purpose of this study is to (1) quantify the effects of giant rice co-cultivation on greenhouse gas emissions, (2) elucidate the response of the soil microbial activity and community to giant rice co-cultivation, and (3) investigate the relationship between greenhouse gas emissions and soil microbial activity in the giant rice co-cultivation mode. Our study will provide references and suggestions for improving the paddy co-cultured production modes and mitigate the negative impact on the paddy environment.
2. Materials and Methods
2.1. Field Description and Experimental Design
The experiments were conducted from May 2022 to October 2022 at the Ecological Planting and Cultivation Rice Field Experimental and Demonstration Park, located at the Changsha Station of Agricultural Environment Observation and Research, Chinese Academy of Sciences in Changsha, Hunan, China (112°52′ E, 27°59′ N). The site’s altitude ranged between 45 and 50 m, with surface pressure levels of 1001.17~1003.08 hPa. The climate typifies a subtropical humid monsoon. The soil type in the experimental area was paddy soil, characterized by an organic matter (OM) at 16.61 g kg−1, total nitrogen (TN) at 2.02 g kg−1, total phosphorus (TP) at 0.31 g kg−1, and pH level of 5.5. The rice variety used in this study was giant rice, resulting from remote hybridization between indica and wild rice and subsequent directed breeding by the Institute of Subtropical Agriculture, Chinese Academy of Sciences. The giant rice plants selected for this experiment reached an impressive average height of 1.80–2.0 m and offer a solution by allowing simultaneous rice cultivation and aquaculture through deep flooding, reducing extensive fish pond excavation and paddy destruction. The chosen fish species for the study was carp (Cyprinus carpio). Field plots, each covering an area of 300 m2, were employed for the experiment. These plots were demarcated by ridges measuring 45 cm in height and 30 cm in width. Rice plants were spaced 50 cm apart in rows. Each field plot consisted of three replicates. The experimental treatments encompassed traditional alternating wet and dry irrigation (CK); continuous flooding throughout the growing period (WGPF); flooding in combination with low-density cultured fish (FFD1); and flooding with high-density cultured fish (FFD2). The cultured fish densities employed were 2 (low-density) and 4 (high-density) fish per square meter, respectively. Flooding depth was maintained at 30 cm, initiated at the jointing stage and concluded at the mature stage.
2.2. Methane and Nitrous Oxide Gas Collection and Determination
Gas was collected using the static chamber method at seven-day intervals from the jointing stage until maturity. Gas (30 mL) was collected into glass vials at 0, 10, 20, and 30 min, and the temperature was recorded at the same time. Methane and nitrous oxide concentrations were determined by gas chromatography. The nitrous oxide monitor was an ECD (electron capture detector), the filling material in the separation column was 80/100-mesh PorpakQ, the detection temperature was 330 °C, the column temperature was 65 °C, the carrier gas was nitrogen, and the flow rate was 40 mL min−1. The methane detector was an FID (hydrogen flame ionization detector), the carrier gas was ammonia gas, the flow rate was 40 mL min−1, the temperature of the detector was 200 °C, and the temperature of the separation column was 55 °C. The emission rate was obtained by linear regression through the four gas concentrations. The methane and nitrous oxide flux was calculated according to the following equation:
where F is the methane or nitrous oxide flux (mg m−2 h−1), P is the methane or nitrous oxide density in the standard state (kg m−3), h is the height of the chamber (m), dc/dt is the rate of change in the concentrations of methane or nitrous oxide in the static chamber, and T is the average temperature in the static chamber (°C).
F = P × h × dc/dt × (273/(273 + T))
Cumulative methane and nitrous oxide emissions were calculated using the following equation:
where i is the ith measurement, F is the methane or nitrous oxide flux (mg m−2 h−1), (Fi +Fi + 1) is the flux measured on two consecutive sampling days, d represents the days between two adjacent days of the measurements, and 24 is the number of hours in a day.
CE = Σ((Fi + Fi + 1)/2 × 10−3 × d × 24 × 10)
The global warming potential (GWP) was used to evaluate the total radiative forcing of these greenhouse gases. GWP was calculated as follows:
where GWP is the cumulative CO2 equivalent emission (kg CO2-eq ha−1), CECH4 and CEN2O are cumulative CH4 and N2O emissions (kg ha−1), respectively; and 28 and 265 are the CO2 equivalent mass per unit mass of the CH4 and N2O gas emitted integrated over a 100-year time horizon, respectively.
GWP = (CECH4 × 28) + (CEN2O × 265)
2.3. Analyses of Soil Properties
The soil samples were air-dried at room temperature, ground, and sieved to measure the physicochemical properties of the soil, including pH, Eh, organic matter (OM), and total nitrogen and phosphorus (TN and TP) contents. Detailed analytical methods were used according to the methods described by Lu [25]. The soil organic matter was determined using the potassium dichromate–sulfuric acid external heating method. The soil total nitrogen and total phosphorus were pretreated using mixed sulfuric acid and perchloric acid digested and measured using a continuous flow analyzer. The soil pH and Eh were measured using a multi-parameter analyzer. Fresh soil was used for the analyses of ammonium (NH4+-N) and nitrate (NO3−-N) with the methods described by Lu [25] and Wang et al. [26]. Soil ammonium and nitrate were extracted using potassium chloride and measured using a flow analyzer.
2.4. Analyses of Soil Microbial Activities
Urease activity in soil was determined by phenol-sodium hypochlorite colorimetry [26]. The activities of β-glucosidase, chitinase, acid phosphatase, and arylsulfonase were determined by Carine et al. [27]. The activities of these enzymes were determined by colorimetry of p-nitrophenol release. The substrates used for each enzyme assay are listed in Table S1. The soil microbial biomass was extracted using chloroform fumigation and potassium sulfate leaching [28]. The soil microbial biomass was extracted using a combination of chloroform fumigation and potassium sulfate leaching techniques. Microbial biomass carbon content is determined through titration analysis, while microbial biomass nitrogen and phosphorus levels are quantified via flow analyzer-based methods.
2.5. Soil DNA Extraction, Illumina-MiSeq Sequencing, and Data Analysis
Whole genomic DNA was extracted from soil samples using a commercial soil DNA kit (Omega Bio-tek, Norcross, GA, USA). After testing the quality, concentration, and purity of the DNA samples, DNA splitting was performed using a Covaris M220 (China Genetics Ltd., Shenzhen, China) to construct a double-end library. Paired-end libraries were constructed using NEXTFLEX Rapid DNA-Seq (Bioo Scientific, Austin, TX, USA). Double-end sequencing was performed on Majorbio Biomedical Technologies’ Illumina Hiseq Xten (Illumina Inc., San Diego, CA, USA). Raw sequencing data from our current study has been submitted to NCBI (PRJNA873258). Paired Illumina reads were fastp removed in adapters and low-quality reads (length < 50 bp or quality value < 20 or N bases). Macrogenomic data were assembled using MEGAHIT, 1.1.2 (https://github.com/voutcn/MEGAHIT, accessed on 12 August 2025, version 1.1.2), open reading frames (orf) were predicted for each assembled overlap group using MetaGene, and non-redundant gene catalogues were constructed using CD-HIT. Representative sequences from the non-redundant gene catalogue were aligned with the NCBI NR database [29]. Taxonomy annotation was performed using Diamond (https://bbuchfink.github.io/diamond/, accessed on 12 August 2025, version 0.8.35). Functional genes annotated in the NR database (http://ardb.cbcb.umd.edu/, accessed on 12 August 2025, version 0.8.35) [30] were compared using Diamond. Shannon and Simpson indices for microbial communities were quantified based on previous studies.
2.6. Statistical Analysis
Significant differences between treatments were analyzed using Duncan’s multiple tests. The relationship between soil microbial communities, greenhouse gas emissions, and soil physicochemical properties was quantitatively analyzed using Pearson correlation analysis. Duncan’s multiple test and Pearson’s correlation analysis were carried out using IBM SPSS Statistics 24.0 software. Using Shannon and Simpson indexes, principal component analysis (PCoA) of soil microorganisms was performed using R 3.1.0 (https://www.r-project.org/). Redundancy analysis (RDA) tests were conducted using CANOCO (version 4.5). The IBM SPSS Amos 26 was used to develop a partial least squares path model (PLS-PM) of the indirect and direct effects of co-culture related to greenhouse gas emissions. The quality of the PLS-PM was evaluated using the chi-square test normalized by degrees of freedom (χ2/df) (which should not exceed 3), the goodness-of-fit index (GFI) (which should exceed 0.9), and the root mean square error of approximation (RMSEA) (which should be approximately less than 0.1).
3. Results
3.1. Soil Properties
Table 1 presents the results of soil properties in our study. In comparison to the CK treatment, the flooding alone or combination of flooding and cultured fish in rice fields significantly increases soil pH (p < 0.05). The highest soil pH (5.39 ± 0.18) was found in the WGPF treatment, with no significant differences (p < 0.05) between the FFD1 and FFD2 treatments. Soil Eh levels significantly decrease in the CK treatment (p < 0.05), and soil Eh in the CK treatment decreases by 6.12% compared to flooding alone. However, relative to the CK treatment, soil Eh significantly increases in the cultured fish treatment, with no significant difference (p < 0.05) between the FFD1 and FFD2 treatments. While flooding alone did not significantly affect soil NH4+ and TN concentrations compared to the CK treatment (p < 0.05), cultured fish significantly increased these concentrations (p < 0.05), with no significant difference related to breeding density. Soil concentrations of OM, TP, and NO3− significantly decrease with flooding alone compared to the CK treatment (p < 0.05). Conversely, cultured fish significantly increase these soil parameters (p < 0.05). Relative to flooding alone, in low-density and highly cultured treatment, soil OM, TP, and NO3− concentration significantly increased by 45.22%, 44%, and 23.37% and 50.22%, 50%, and 36.36%, respectively. The highest soil PO3− concentration (20.53 ± 0.14 mg kg−1) was found in the FFD2 treatment, with no significant difference (p < 0.05) observed among the other three treatments.

Table 1.
Soil physical–chemical properties and greenhouse gas emissions of the three treatments.
3.2. Greenhouse Gas Emissions
Table 1 shows the cumulative greenhouse gas emission results. Flooding water alone and low-density cultured fish did not alleviate the adverse effects of greenhouse gas emissions relative to CK treatment. However, increasing culture density reduced greenhouse gas emissions in our research. Cumulative methane and nitrous oxide emissions and GWP were all reduced in the FFD2 treatment compared to the FFD1 treatment, decreasing by 9.79%, 9.18%, and 9.68%, respectively. Additionally, relative to the WGPF treatment, cumulative methane emissions and GWP in the FFD2 treatment were reduced by 5.32% and 1.48%, respectively.
3.3. Soil Microbial Activity
The results of soil microbial activity are presented in Figure 1a. Cultured fish showed significant increases in soil β-glucosidase activities compared to the CK treatment, with even higher activities observed in the FFD2 treatment compared to FFD1. There were no significant differences in β-glucosidase activities found between the CK and WGPF treatments. Flooding alone produced significant increases in soil chitinase and phosphatase activities compared to the CK treatment. However, it led to decreased soil urease and aryl-sulfatase activities. There was no significant difference in soil chitinase activities observed between the WGPF and FFD1 treatments; however, soil chitinase activities in FFD2 treatment showed a significant decrease. Cultured fish showed reductions in soil urease and phosphatase activities compared with flooding alone. However, cultured fish showed significant increases in soil aryl-sulfatase activities compared with flooding alone. Soil contents of microbial biomass carbon (MBC) (Figure 1b), microbial biomass nitrogen (MBN) (Figure 1c), and microbial biomass phosphorus (MBP) (Figure 1d) were significantly increased by cultured fish relative to the CK and flooding alone treatment. The MBC, MBN, and MBP contents in high-density cultured fish treatment were 1.33, 1.14, and 1.78 and 1.13, 2.44, and 1.77 times higher than that of the CK and flooding alone treatment, respectively, and those in low-density cultured fish treatment were 1.35, 1.52, and 2.04 and 1.14, 2.55, and 2.02 times higher than that of the CK and flooding alone treatment, respectively.
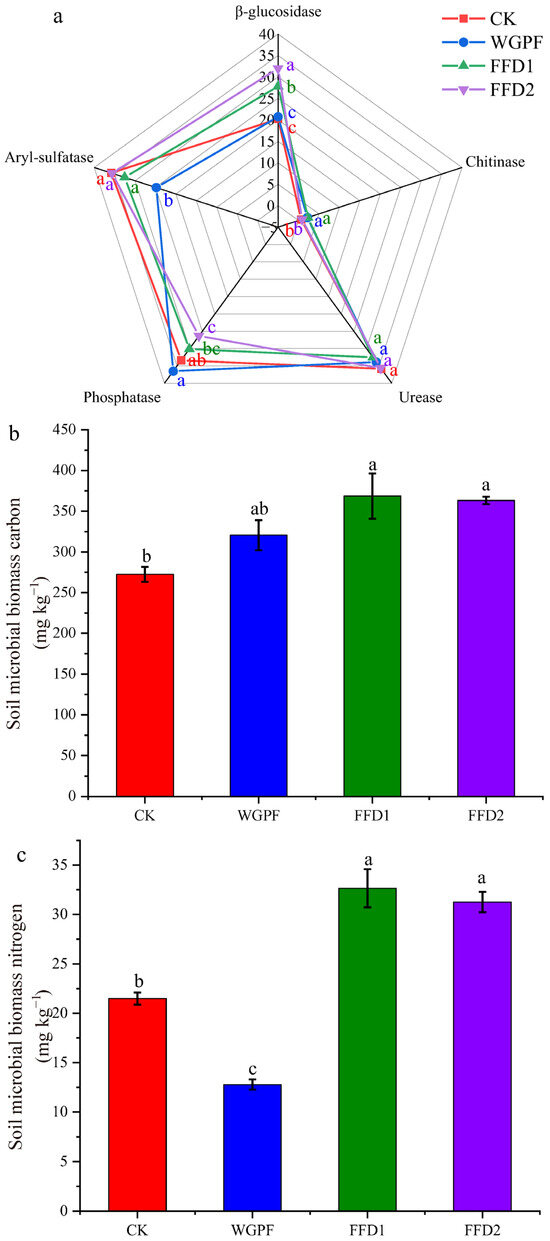
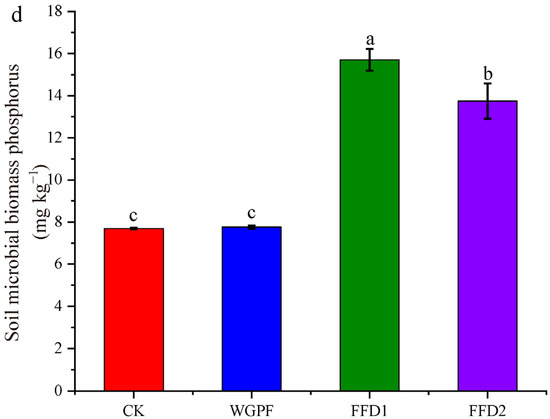
Figure 1.
Soil enzyme activity and soil microbial activity in each treatment. (a) Soil enzyme activity; (b) soil microbial biomass carbon; (c) soil microbial biomass nitrogen; (d) soil microbial biomass phosphorus. CK, control; WGPF, whole growing period flooding alone; FFD1, flooding and low-density cultured fish; FFD2, flooding and high-density cultured fish. Different letters indicate significant difference (p < 0.05).
3.4. Soil Microbial Community Structure
Figure 2 illustrates the dominant phyla in the soil microbial composition. Flooding alone produced significant increases in the relative abundances of Chloroflexi and Eisenbacteria compared with the CK treatment, with increases by 31.47% and 141.86%, respectively. In contrast, the relative abundances of Actinobacteria, Nitrospirae, Gemmatimonadetes, Verrucomicrobia, and Aminicenantes decreased by 29.13%, 28.60%, 44.47%, 46.92%, and 62.85%, respectively. There were no significant differences in relative abundances of Acidobacteria and Planctomycetes found between the CK and WGPF treatments. Cultured fish increased the relative abundances of Actinobacteria, Nitrospirae, Planctomycetes, Verrucomicrobia, and Aminicenantes, while decreasing the relative abundances of Chloroflexi, Acidobacteria, and Eisenbacteria, relative to flooding alone. The Shannon indices of soil microbial activity were significantly improved by flooding with or without cultured fish, and they were 2.13 ± 0.01, 2.24 ± 0.02, 2.25 ± 0.02, and 2.35 ± 0.14 for the CK, WGPF, FFD1, and FFD2 treatments, respectively (Figure 2b). Simpson indices of the soil microbial activity were also affected by flooding with or without cultured fish. The Simpson indices of the soil microbial activity were 0.20 ± 0.01, 0.17 ± 0.01, 0.19 ± 0.01, and 0.17 ± 0.03 for the CK, WGPF, FFD1, and FFD2 treatments, respectively (Figure 2c). The results of PCoA are shown in Figure 3. The PCoA analysis explained 88.87% of the total variation in microbial communities, and there was significant change in microbial communities in the WGPF and FFD1 treatments relative to the CK treatment.
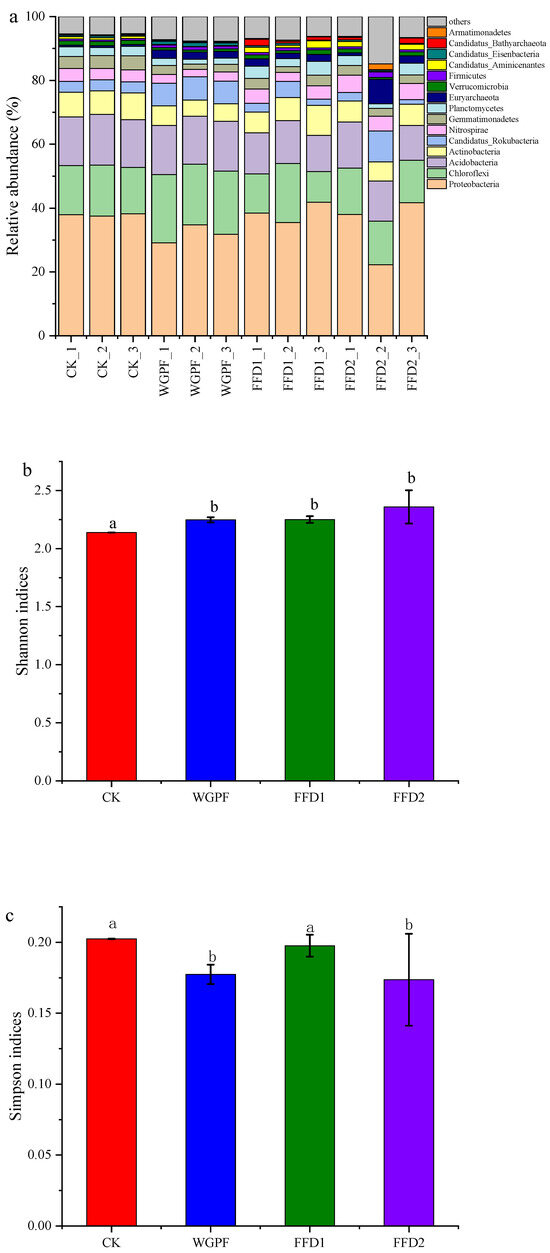
Figure 2.
Soil microbial community structure and soil microbial diversity index in each treatment. (a) Soil microbial community structure; (b) Shannon indices; (c) Simpson indices; CK, control; WGPF, whole growing period flooding alone; FFD1, flooding and low-density cultured fish; FFD2, flooding and high-density cultured fish. Different letters indicate significant difference (p < 0.05).
Figure 3.
Two-dimensional PCoA plots revealing the community structures. CK, control; WGPF, whole growing period flooding alone; FFD1, flooding and low-density cultured fish; FFD2, flooding and high-density cultured fish.
3.5. Comprehensive Relationships Among Soil Physicochemical Properties, Greenhouse Gas Emissions, Microbial Activities, and Communities
The linear correlations among soil physicochemical properties, greenhouse gas emissions, and microbial activities are illustrated in Figure 4. Cumulative nitrous oxide emissions were positively correlated with soil TN, NH4+, OM, β-glucosidase activities, MBC, and MBP, while negatively correlated with soil phosphatase activities. Cumulative methane emissions and GWP were positively correlated with soil pH, NH4+, MBC, and MBP, but negatively correlated with soil NO3−. Redundancy analysis (RDA) provided insights into the relationships between soil microbes and greenhouse gas emissions. The results of RDA shown that CEN2O was significantly and positively correlated with Bathyarchaeota, Nitrospirae, Aminicenantes, and Planctomycetes. CECH4 was significantly and positively correlated with Euryarchaeota, Firmicutes, Armatimonadetes, and Eisenbacteria (Figure 5). The Partial Least Squares Path Model (PLS-PM) revealed direct effects of the OM concentration and absolute abundances of methanogenic genes on cumulative methane emissions, while the NO3− concentration had a direct effect on cumulative nitrous oxide emissions (Figure 6).
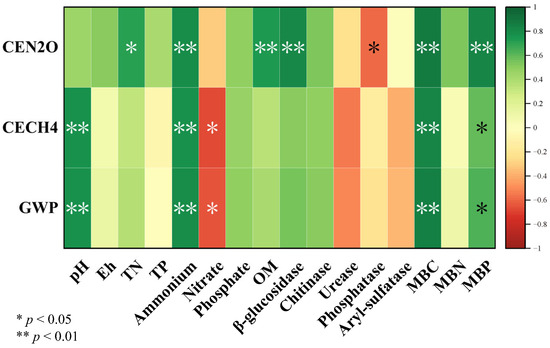
Figure 4.
Pearson’s correlation analyses of the relationships among the soil physicochemical properties, enzyme activities, and microbial activity. TN, total nitrogen; TP, total phosphorus; NH4+, ammonium; NO3−, nitrate; PO3−, phosphate; OM, organic matter; MBC, soil microbial biomass carbon; MBN, soil microbial biomass nitrogen; MBP, soil microbial biomass phosphorus; CEN2O, cumulative nitrous oxide emissions; CECH4, cumulative methane emissions; GWP, global warming potential.
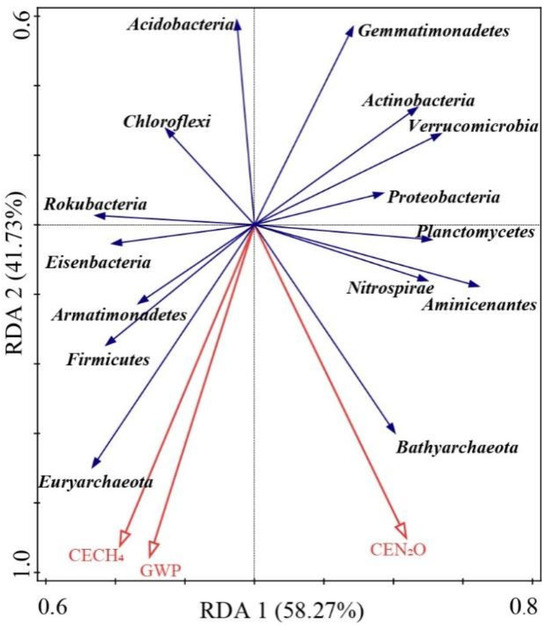
Figure 5.
Redundancy analyses of relationships between soil microbial and greenhouse gas emission. CEN2O, cumulative nitrous oxide emissions; CECH4, cumulative methane emissions; GWP, global warming potential.
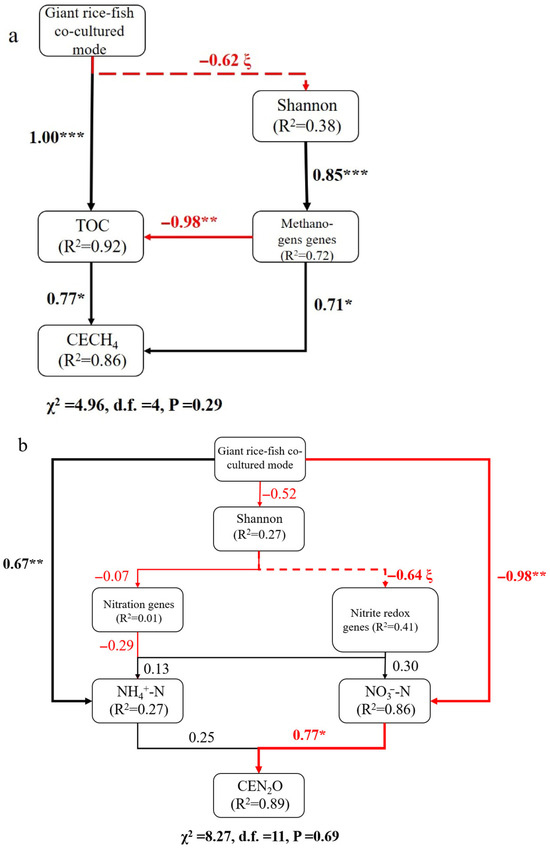
Figure 6.
Structural equation model (SEM). The effect of planting and breeding modes, nutrients, and microbial factors on (a) methane cumulative emissions and (b) cumulative emission of nitrous oxide. Arrows represent the flow of causality among parameters. Arrow width is proportional to the standardized path coefficients and can be interpreted as the relative importance of each factor. Dashed arrows represent marginally significant relationships, black arrows represent positive linear relationships, and red arrows represent negative linear relationships. * p < 0.05; ** p < 0.01; *** p < 0.001; ξ p < 0.1.
4. Discussion
4.1. Effects of Flooding and Cultured Fish on Soil Nutrients
Soil nutrients can be profoundly influenced by soil moisture and aeration levels [31]. Prolonged flooding increases soil moisture significantly, leading to anaerobic soil conditions [32]. Consequently, flooding raises the soil pH in paddy soil, while reduced aeration induces a shift from oxidizing to reducing soil conditions, resulting in decreased soil Eh [33]. However, it is worth noting that fish disturbance can alleviate the impact of anaerobic conditions on soil oxidation–reduction processes [34], explaining the increased soil Eh observed with cultured fish in our study. Reduced soil Eh enhances the soil denitrification capacity [35], which can explain the decrease in soil NO3−-N concentration due to flooding. Fish excreta are rich in nutrients and contribute to soil nutrient enrichment upon decomposition [36]. Accordingly, our study found that cultured fish significantly increased soil concentrations of OM, TN, NH4+-N, TP, and AP. Soil microbes play a crucial role in enhancing soil nutrient availability [37], and we observed an increase in the relative abundances of Actinobacteria, Nitrospirae, Verrucomicrobia, and Planctomycetes with cultured fish. Actinobacteria and Verrucomicrobia are known to degrade various organic substances, including cellulose, polysaccharides, proteins, fats, organic acids, and humus through chitinase production [38,39]. Nitrospirae and Planctomycetes contribute to enhanced soil nitrification [40,41], consistent with the observed increases in soil TN and NO3−-N by cultured fish.
4.2. Effect of Flooding and Cultured Fish on Greenhouse Gas Emissions
Greenhouse gas emissions are influenced by soil aeration [15,42]. Prolonged flooding reduces soil aeration and oxygen levels [43], creating anaerobic conditions that promote the growth of methanogenic bacteria [44]. Additionally, anaerobic soils stimulate denitrifying bacteria and soil denitrification potential [45,46]. As a result, our study found that flooding alone produced significant increases in cumulative methane and nitrous oxide emissions compared to the CK treatment. The decomposition of fish excreta is a substantial source of greenhouse gas emissions [47]. Therefore, cultured fish produced significant increases in cumulative methane and nitrous oxide emissions relative to the CK and flooding alone treatment. Previous research found that the soil structure and nutrients can influence the soil microbial community structure and greenhouse gas emissions [48]. Actinobacteria, Verrucomicrobia, and Gemmatimonadetes are involved in soil nutrient conversion and carbon and nitrogen fixation [49,50]. However, we observed that decreased relative abundances of Actinobacteria, Verrucomicrobia, and Gemmatimonadetes with flooding alone, which result in inhibited microbial carbon and nitrogen fixation function, led to increased methane and nitrous oxide emissions in our research. Furthermore, cultured fish decreased the relative abundances of Chloroflexi, which contributed to reduced microbial carbon fixation and nitrous oxide reduction [4], resulting in increased greenhouse gas emissions in our research. Nitrospirae enhance soil nitrification rates and increase NO3−-N concentrations [51]. Soil nitrogen conversion rates are accelerated by chitinase secreted by Planctomycetes [52]. Aminicenantes contain genes related to nitrate and nitric oxide reduction and contribute to increased nitrous oxide emissions [53]. Verrucomicrobia, which contain denitrifying genes, can also increase nitrous oxide emissions [54]. Consequently, cultured fish significantly increased the relative abundances of Nitrospirae, Aminicenantes, Planctomycetes, and Verrucomicrobia, leading to elevated methane and nitrous oxide emissions.
4.3. Effect of Flooding and Cultured Fish on Soil Microbial Activity and Community Structure
Cultured fish showed significant increases in soil microbial biomass in our research. Previous research has demonstrated that fish manure and fish disturbances improve soil nutrients and structure, resulting in enhanced soil microbial activities and biomass [55]. We observed significant increases in soil β-glucosidase and aryl-sulfatase activities with cultured fish compared to the CK treatment, likely due to increased soil nutrients and microbial biomass [56]. Conversely, cultured fish led to reduced soil urease and phosphatase activities but increased soil aryl-sulfatase activities compared to flooding alone. The relative abundances of Planctomycetes significantly increased, possibly due to their ability to produce substantial aryl-sulfatases [57]. However, in our research, increasing Verrucomicrobia accelerated phosphorus dissolution, leading to decreased phosphatase activities [58]. Furthermore, a previous study found that increased nitrogen and OM contents in the soil resulted in decreased soil urease and chitinase activities [59].
The soil microbial community structure is influenced by microbial growth strategies and environmental properties [59]. Flooding reduces soil aeration, which inhibits soil microbial activity [60]. In contrast, cultured fish improve the soil structure, increasing aeration and soil nutrient levels [61]. Therefore, we found that cultured fish mitigated the adverse effects of flooding and enhanced soil microbial diversity. Flooding decreases soil aeration and increases soil moisture, degrading soil texture and slowing nutrient decomposition [62]. Dry soil conditions favor Gemmatimonadetes [63], which explains the decreased relative abundances of Gemmatimonadetes with flooding. Increases in OM and NO3−-N content in the soil promoted the relative abundance of Actinobacteria, Verrucomicrobia, and Nitrospirae. Conversely, flooding’s adverse effects decreased the relative abundance of Actinobacteria, Verrucomicrobia, and Nitrospirae, while promoting Chloroflexi, which are commonly found in nutrient-poor soils [64]. Cultured fish improved soil aeration, alleviating the decline in Nitrospirae [65]. Additionally, cultured fish significantly increased soil nitrogen and the OM content, leading to increased relative abundances of Actinobacteria and Verrucomicrobia [66]. Acidobacteria thrive in acidic conditions, and the alkaline conditions induced by flooding and fish manure decomposition inhibited Acidobacteria relative abundances [67]. While flooding negatively impacted soil conditions and microbial activity, the disturbances caused by cultured fish ameliorated these effects, ultimately affecting the soil microbial community structure.
5. Conclusions
In conclusion, the microbial community structure affected by these innovative practices significantly influenced the greenhouse gas emissions. Our study revealed that the increased relative abundances of Actinobacteria, Nitrospirae, Planctomycetes, Verrucomicrobia, and Aminicenantes due to flooding with or without cultured fish contributed to higher cumulative methane and nitrous oxide emissions. However, increasing the culture density reduced cumulative nitrous oxide and methane emissions. Compared to FFD1 treatment, an increased culture density decreased nitrous oxide emissions by 9.18%. Cumulative methane emissions and GWP in the FFD2 treatment were reduced by 5.32% and 1.48%, respectively. Flooding alone reduced soil concentrations of OM, TP, and NO3−-N, while cultured fish alleviated soil nutrient deficiency and significantly increased soil concentrations of OM, NH4+-N, NO3−-N, TN, and TP. Cultured fish also enhanced soil microbial activity, including MBC, MBN, MBP, β-glucosidase, and aryl-sulfatase activities. Therefore, our study suggests that cultured fish can mitigate the adverse effects of flooding on soil nutrient cycling, microbial activity, and community structure. Nevertheless, it is essential to consider the impact of greenhouse gas emissions, and our future research will continue to investigate strategies for mitigating these emissions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15081982/s1, Table S1: Substrates used for each enzyme determination. Table S2: The basic information of metagenomic sequencing after quality control, sequence assembly, and gene prediction.
Author Contributions
Conceptualization, A.W.; methodology, A.W., D.Z. and J.Z.; software, S.L.; validation, A.W., M.Z., Y.L., X.Z. and B.C.; investigation, S.L.; data curation, S.L.; writing—original draft preparation, A.W.; writing—review and editing, A.W., M.Z. and B.C.; supervision, A.W.; funding acquisition, D.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Double First-class Construction Project of Hunan Agricultural University (kxk201801007 and SYL2019025); National Natural Science Foundation of China (32360306); and Academic Discipline (Professional Degree) Project Construction—Biological and Pharmaceutical Engineering (01050156007).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GWP | Global Warming Potential |
References
- Viana, C.M.; Freire, D.; Abrantes, P.; Rocha, J.; Pereira, P. Agricultural land systems importance for supporting food security and sustainable development goals: A systematic review. Sci. Total Environ. 2022, 806, 150718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Shen, C.; Yang, F.; Wang, J.; Ge, Y. Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes in a long-term paddy field fertilization experiment. Land Degrad. Dev. 2020, 32, 420–429. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Rezanezhad, F.; Zak, D.; Lennartz, B. The influence of microtopography on soil carbon accumulation and nutrient release from a rewetted coastal peatland. Geoderma 2023, 438, 116637. [Google Scholar] [CrossRef]
- Ma, C.; Zeng, W.; Li, J.; Li, S.; Peng, Y. Metabolomics uncovers adaptation discrepancy among anammox granular sludge with different granule size: Metabolic pathway regulation by consortia cooperation. Sci. Total Environ. 2023, 864, 161086. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Huang, S.; Wu, L.; Cai, Z.; Xu, M. Long-term combined application of organic and inorganic fertilizers increases crop yield sustainability by improving soil fertility in maize–wheat cropping systems. J. Integr. Agric. 2025, 24, 290–305. [Google Scholar] [CrossRef]
- Cropping, P. Crop residue management for nutrient cycling and improving soil productivity in rice-based cropping systems in the tropics. Adv. Agron. 2005, 85, 269. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Cao, Y.; Shi, W.; Xie, E.; Mu, N.; Du, G.; Shen, Y.; Tang, D.; Cheng, Z. Nitrogen nutrition contributes to plant fertility by affecting meiosis initiation. Nat. Commun. 2022, 13, 485. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.; Zhang, S.; Hu, W.; Yi, G.; Chen, W.; Cheng, L.; Zhang, Q. Preparation of a new biochar-based microbial fertilizer: Nutrient release patterns and synergistic mechanisms to improve soil fertility. Sci. Total Environ. 2023, 860, 160478. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Chen, Y.; Chen, Z. Spatiotemporal dynamics of rice–crayfish field in Mid-China and its socioeconomic benefits on rural revitalisation. Appl. Geogr. 2022, 139, 102636. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Zhang, J. Ratoon rice production in central China: Environmental sustainability and food production. Sci. Total Environ. 2021, 764, 142850. [Google Scholar] [CrossRef]
- Yifan, L.; Tiaoyan, W.; Shaodong, W.; Xucan, K.; Zhaoman, Z.; Hongyan, L.; Jiaolong, L. Developing integrated rice-animal farming based on climate and farmers choices. Agr. Syst. 2023, 204, 103554. [Google Scholar] [CrossRef]
- Mortillaro, J.-M.; Dabbadie, L.; Raminoharisoa, A.E.; Paradis, A.; Martel, P.; Andriamarolaza, R.; Raliniaina, M.; Mikolasek, O.; Aubin, J. Trophic functioning of integrated rice-fish farming in Madagascar: Insights from stable isotopes (δ13C & δ15N). Aquaculture 2022, 555, 738240. [Google Scholar] [CrossRef]
- Yuan, P.; Li, X.; Ni, M.; Cao, C.; Jiang, L.; Iqbal, A.; Wang, J. Effects of straw return and feed addition on the environment and nitrogen use efficiency under different nitrogen application rates in the rice–crayfish system. Plant Soil 2022, 475, 411–426. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Chen, G.; Cheng, W.; Shen, Y. Effects of long-term rice–crayfish coculture systems on soil nutrients, carbon pools, and rice yields in Northern Zhejiang province, China. Agronomy 2024, 14, 1014. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Fu, Q.; Li, T.; Hou, R.; Li, Q.; Li, M.; Meng, F. Characteristics of greenhouse gas emissions from farmland soils based on a structural equation model: Regulation mechanism of biochar. Environ. Res. 2022, 206, 112303. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, R.; Chen, Y.; Jiang, L.; Lei, L.; Xu, H.; Feng, S. Effects of secondary release of chromium and vanadium on soil properties, nutrient cycling and bacterial communities in contaminated acidic paddy soil. J. Environ. Manag. 2023, 326, 116725. [Google Scholar] [CrossRef]
- Belenguer-Manzanedo, M.; Alcaraz, C.; Camacho, A.; Ibáñez, C.; Català-Forner, M.; Martínez-Eixarch, M. Effect of post-harvest practices on greenhouse gas emissions in rice paddies: Flooding regime and straw management. Plant Soil 2022, 474, 77–98. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Shen, J.; Chen, D.; Zheng, L.; Ge, T.; Li, Y.; Wu, J. Reduction in net greenhouse gas emissions through a combination of pig manure and reduced inorganic fertilizer application in a double-rice cropping system: Three-year results. Agr. Ecosyst. Environ. 2022, 326, 107799. [Google Scholar] [CrossRef]
- Sun, J.; Chen, L.; Ogle, S.; Cheng, K.; Xu, X.; Li, Y.; Pan, G. Future climate change may pose pressures on greenhouse gas emission reduction in China’s rice production. Geoderma 2023, 440, 116732. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.M.; Yuan, Z.; Jia, Z.; Zhang, Y. Nitrous oxide act as an alternative electron acceptor for microbial methane oxidation in oxygen-deficient microcosms. Geoderma 2025, 455, 117213. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Jia, Z.; Zhu, L.; Zheng, P.; Kartal, B.; Hu, B. Nitrogen input promotes denitrifying methanotrophs’ abundance and contribution to methane emission reduction in coastal wetland and paddy soil. Environ. Pollut. 2022, 302, 119090. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Chen, X.; Shen, L.; Chu, Y.; Qiu, L.; Hu, G.; Song, C.; Li, D.; Meng, S. Co-culture of red swamp crayfish Procambarus clarkia influenced glycoside hydrolase families and fungal communities in the rice-paddy soils. Appl. Soil. Ecol. 2023, 186, 104816. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Shen, W.; Yao, H.; Meng, X.; Zeng, J.; Zhang, G.; Zamanien, K. A meta-analysis of ecological functions and economic benefits of co-culture models in paddy fields. Agr. Ecosyst. Environ. 2023, 341, 108195. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Lu, R.K. Analysis Methods of Soil and Agricultural Chemistry; Chinese Agricultural Science and Technology Press: Beijing, China, 2000; pp. 146–315. (In Chinese) [Google Scholar]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Carine, F.; Yvan, C.; Steven, C. Enzyme activities in apple orchard agroecosystems: How are they affected by management strategy and soil properties. Soil Biol. Biochem. 2009, 41, 61–68. [Google Scholar] [CrossRef]
- Li, F.; Feng, J.; Zhou, X.; Xu, C.; Jijakli, M.H.; Zhang, W.; Fang, F. Impact of rice-fish/shrimp co-culture on the N2O emission and NH3 volatilization in intensive aquaculture ponds. Sci. Total Environ. 2019, 655, 284–291. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Connor, R.; Feldgarden, M.; Fine, A.M.; Funk, K.; Hoffman, J.; et al. Database resources of the National Center for Biotechnology Information in 2025. Nucleic Acids Res. 2025, 53, D20–D29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Pop, M. ARDB—Antibiotic resistance genes database. Nucleic Acids Res. 2009, 37 (Suppl. S1), D443–D447. [Google Scholar] [CrossRef] [PubMed]
- Khoshru, B.; Khoshmanzar, E.; Lajayer, B.A.; Ghorbanpour, M. Soil Moisture–Mediated Changes in Microorganism Biomass and Bioavailability of Nutrients in Paddy Soil, Plant Stress Mitigators; Elsevier: Amsterdam, The Netherlands, 2023; pp. 479–494. [Google Scholar]
- Wang, H.; Wu, Q.; Han, Y. Effects of Drying-Flooding Alternation on Sediment–Water Nitrogen Fluxes in Hydro-fluctuation Belt of the Danjiangkou Reservoir. Water Air Soil Pollut. 2022, 233, 71. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Shi, H.; Liu, Y. Redox dependence of manganese controls cadmium isotope fractionation in a paddy soil-rice system under unsteady pe+ pH conditions. Sci. Total Environ. 2022, 806, 150675. [Google Scholar] [CrossRef]
- Uwimana, A.; van Dam, A.; Irvine, K. Effects of conversion of wetlands to rice and fish farming on water quality in valley bottoms of the Migina catchment, southern Rwanda. Ecol. Eng. 2018, 125, 76–86. [Google Scholar] [CrossRef]
- Smith, M.S.; Tiedje, J.M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 1979, 11, 261–267. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, J.; Dong, S.; Kitazawa, D. Sustainability assessment of marine aquaculture considering nutrients inflow from the land in Kyushu Area. Water 2022, 14, 943. [Google Scholar] [CrossRef]
- Wang, A.; Zou, D.; Xu, Z.; Chen, B.; Zhang, X.; Chen, F.; Zhang, M. Combined effects of spent mushroom substrate and dicyandiamide on carbendazim dissipation in soils: Double-edged sword effects and potential risk controls. Environ. Pollut. 2023, 319, 120992. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Chen, M.; Zhao, X.; Liu, H.; Ge, M.; Li, N.; Ning, Z.; Gao, W.; Fan, C. Molecular insights into effects of PBAT microplastics on latosol microbial diversity and DOM chemodiversity. J. Hazard. Mater. 2023, 450, 131076. [Google Scholar] [CrossRef]
- Wu, D.; Ren, C.; Ren, D.; Tian, Y.; Li, Y.; Wu, C.; Li, Q. New insights into carbon mineralization in tropical paddy soil under land use conversion: Coupled roles of soil microbial community, metabolism, and dissolved organic matter chemodiversity. Geoderma 2023, 432, 116393. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Liu, Y.; Wei, Y.; Li, J.; Ding, G.-C. Carbon amendment rather than nitrate fertilization dominated the reassembly of the total, denitrifying, and DNRA bacterial community in the anaerobic subsoil. J. Soils Sediments 2023, 23, 1–14. [Google Scholar] [CrossRef]
- Shan, A.; Huang, L.; Chen, D.; Lin, Q.; Liu, R.; Wang, M.; Kang, K.J.; Pan, M.; Wang, G.; He, Z. Trade-offs between fertilizer-N availability and Cd pollution potential under crop straw incorporation by 15 N stable isotopes in rice. Environ. Sci. Pollut. Res. 2023, 30, 51075–51088. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, M.; Liang, X.; Zhang, H.; Xiang, J.; Zhao, L.; Fan, X. Rice Yield and Nitrogen Use Efficiency Under Climate Change: Unraveling Key Drivers with Least Absolute Shrinkage and Selection Operator Regression. Agronomy 2025, 15, 677. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Yang, Y.; Fu, G.; Tao, L.; Xiong, J. Effects and oxygen-regulated mechanisms of water management on cadmium (Cd) accumulation in rice (Oryza sativa). Sci. Total Environ. 2022, 846, 157484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, L.; Xu, J.; He, Y. Improved understanding on biochar effect in electron supplied anaerobic soil as evidenced by dechlorination and methanogenesis processes. Sci. Total Environ. 2023, 857, 159346. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Liu, R.; Yang, F.; He, G.; Wei, C. Denitrifying bacteria agent together with composite materials enhanced soil chemical properties and denitrifying functions in rare earth tailings: A field study. J. Hazard. Mater. 2023, 448, 130913. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, S.; Ni, K.; Shi, Y.; Yi, X.; Ma, Q.; Cai, Y.; Ma, L.; Ruan, J. Long-term nitrogen addition increases denitrification potential and functional gene abundance and changes denitrifying communities in acidic tea plantation soil. Environ. Res. 2023, 216, 114679. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, S.; Hua, K.; Yin, Y.; Chu, H.; Wang, D.; Guo, X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Su, J.; Ji, W.; Sun, X.; Wang, H.; Kang, Y.; Yao, B. Effects of different management practices on soil microbial community structure and function in alpine grassland. J. Environ. Manag. 2023, 327, 116859. [Google Scholar] [CrossRef]
- Lu, C.; Hou, K.; Zhou, T.; Wang, X.; Zhang, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J. Characterization of the responses of soil micro-organisms to azoxystrobin and the residue dynamics of azoxystrobin in wheat–corn rotation fields over two years. Chemosphere 2023, 318, 137918. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhuang, S.; Gao, J.; Tang, L.; Harindintwali, J.D.; Wang, F. Aeration increases soil bacterial diversity and nutrient transformation under mulching-induced hypoxic conditions. Sci. Total Environ. 2022, 817, 153017. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Li, Y.; Zhao, P.; Nie, Z.; Liu, H. Comammox Nitrospira and AOB communities are more sensitive than AOA community to different fertilization strategies in a fluvo-aquic soil. Agr. Ecosyst. Environ. 2023, 342, 108224. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Wei, S.; Jiang, Y.; Zhang, Y.; Cai, Y.; Ma, Q. Metagenomics reveals N-induced changes in carbon-degrading genes and microbial communities of tea (Camellia sinensis L.) plantation soil under long-term fertilization. Sci. Total Environ. 2023, 856, 159231. [Google Scholar] [CrossRef]
- Deng, L.; Meile, C.; Fiskal, A.; Bölsterli, D.; Han, X.; Gajendra, N.; Dubois, N.; Bernasconi, S.M.; Lever, M.A. Deposit-feeding worms control subsurface ecosystem functioning in intertidal sediment with strong physical forcing. PNAS Nexus 2022, 1, pgac146. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, C.; Liu, G.; Luo, X.; Rauan, A.; Zhang, C.; Li, T.; Yu, H.; Dong, S.; Gao, Q. A hydroponic plants and biofilm combined treatment system efficiently purified wastewater from cold flowing water aquaculture. Sci. Total Environ. 2022, 821, 153534. [Google Scholar] [CrossRef]
- Bhanwaria, R.; Singh, B.; Musarella, C.M. Effect of organic manure and moisture regimes on soil physiochemical properties, microbial biomass Cmic: Nmic: Pmic turnover and yield of mustard grains in arid climate. Plants 2022, 11, 722. [Google Scholar] [CrossRef]
- Vuyyuru, M.; Sandhu, H.S.; Erickson, J.E.; Ogram, A.V. Soil chemical and biological fertility, microbial community structure and dynamics in successive and fallow sugarcane planting systems. Agroecol. Sust. Food 2020, 44, 768–794. [Google Scholar] [CrossRef]
- Gadler, P.; Faber, K. New enzymes for biotransformations: Microbial alkyl sulfatases displaying stereo-and enantioselectivity. Trends Biotechnol. 2007, 25, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Carlos, F.S.; Schaffer, N.; Marcolin, E.; Fernandes, R.S.; Mariot, R.; Mazzurana, M.; Roesch, L.F.W.; Levandoski, B.; de Oliveira Camargo, F.A. A long-term no-tillage system can increase enzymatic activity and maintain bacterial richness in paddy fields. Land Degrad. Dev. 2021, 32, 2257–2268. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Adams, J.; Liu, J.; Liu, J.; Zhang, S.; et al. Co–elevation of CO2 and temperature enhances nitrogen mineralization in the rhizosphere of rice. Biol. Fertil. Soils 2024, 60, 729–741. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Gao, P.; Dou, Z. The environmental, nutritional, and economic benefits of rice-aquaculture animal coculture in China. Energy 2022, 249, 123723. [Google Scholar] [CrossRef]
- Mhlanga, B.; Pellegrino, E.; Thierfelder, C.; Ercoli, L. Conservation agriculture practices drive maize yield by regulating soil nutrient availability, arbuscular mycorrhizas, and plant nutrient uptake. Field Crop Res. 2022, 277, 108403. [Google Scholar] [CrossRef]
- Guo, X.; Du, S.; Guo, H.; Min, W. Long-term saline water drip irrigation alters soil physicochemical properties, bacterial community structure, and nitrogen transformations in cotton. Appl. Soil Ecol. 2023, 182, 104719. [Google Scholar] [CrossRef]
- Zhang, R.; Rong, L.; Zhang, L. Soil nutrient variability mediates the effects of erosion on soil microbial communities: Results from a modified topsoil removal method in an agricultural field in Yunnan plateau, China. Environ. Sci. Pollut. R 2022, 29, 3659–3671. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Bing, X.; HUANG, D.; Mao, Y.; Hao, Q.; Hongfeng, C.; Keqiang, Z.; Rongliang, Q.; Rongrong, Y. Effects of rhamnolipids on bacterial communities in contaminated soil and earthworm guts. Pedosphere 2022, 33, 927–937. [Google Scholar] [CrossRef]
- Ramotowski, D.; Shi, W. Nitrapyrin-based nitrification inhibitors shaped the soil microbial community via controls on soil pH and inorganic N composition. Appl. Soil Ecol. 2022, 170, 104295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).