Montmorillonite and Composite Amino Acid Overcome the Challenges of Straw Return in Cold-Region Soil: Synergistic Mechanisms of Rapid Straw Humification and Carbon Sequestration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Materials
2.3. Litterbag Experiments
2.4. Corn Planting Experiments
2.5. Measurement Method of Indicators
2.6. Data Statistics and Analysis
3. Results and Discussion
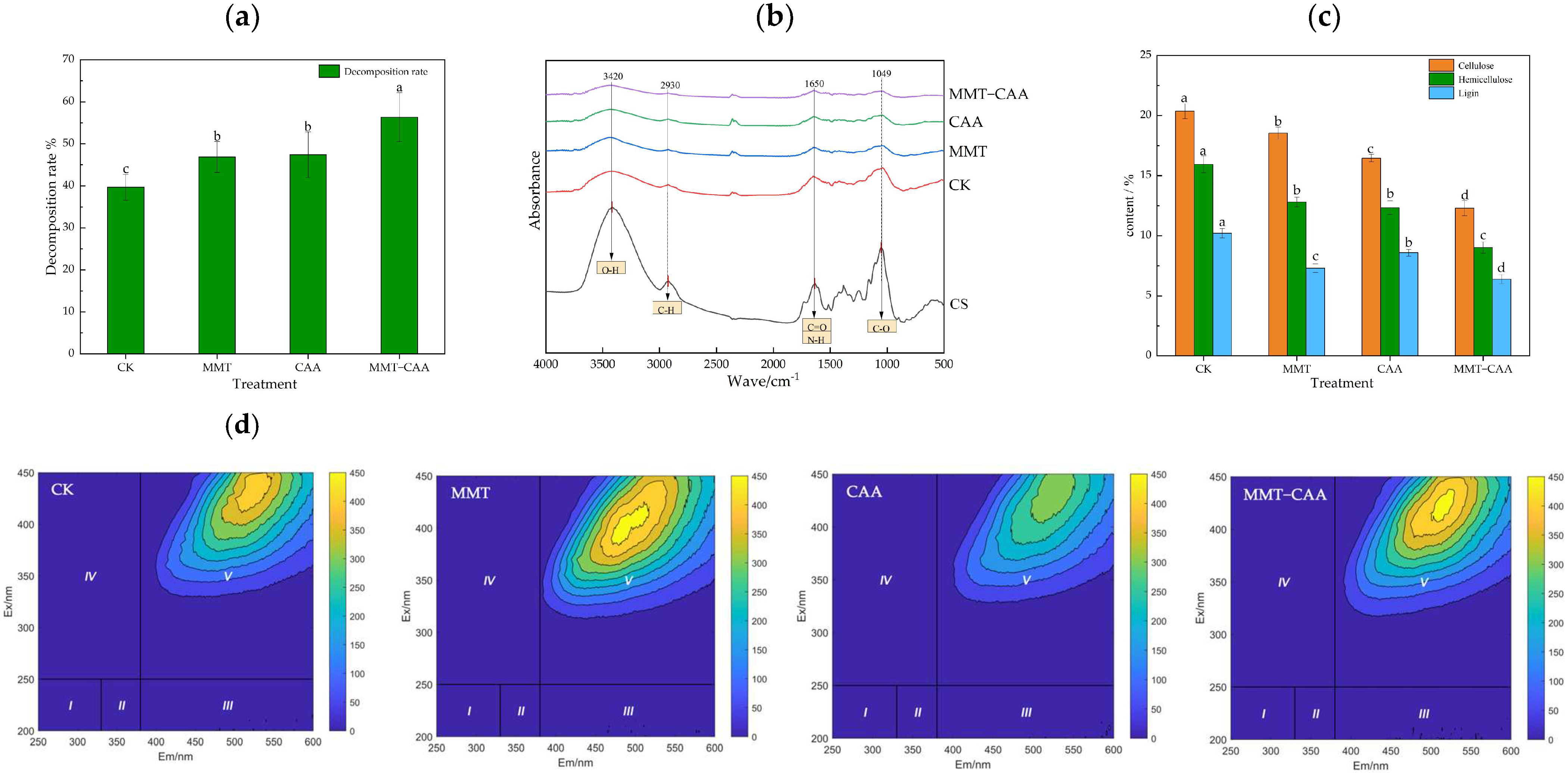
3.1. Direct Evidence of Montmorillonite and Composite Amino Acid Promoting Straw Decomposition
3.2. Montmorillonite and Composite Amino Acid Promote Humification of Corn Straw and Make Humic Acid Structure More Stable
3.3. Microbial Communities and Co-Degradation Systems Revealed the Important Roles of Montmorillonite and Composite Amino Acid in Straw Degradation
3.4. Montmorillonite and Composite Amino Acid Promote the Growth of Corn Seedlings and Reduce Greenhouse Gas Emissions
3.5. Possible Mechanisms of Humification and Carbon Sequestration Promoted by Montmorillonite and Composite Amino Acid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.H.; Gao, Q.; Chen, X.P.; Zhang, W.S. Temporal and spatial characteristics of resources input and environmental effects for maize production in the three provinces of northeast China. Sci. Agric. Sin. 2022, 55, 3170–3184. [Google Scholar] [CrossRef]
- Yan, C.; Yan, S.S.; Jia, Y.T.; Dong, K.S.; Ma, C.M.; Gong, Z.P. Decomposition characteristics of rice straw returned to the soil in northeast China. Nutr. Cycl. Agroecosyst. 2019, 114, 211–224. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, J.; Sun, L.; Wu, J.G. The combined application of swine manure and straw strips to the field can promote the decomposition of corn straw in “broken skin yellow” of black soil. BMC Plant Biol. 2025, 25, 218. [Google Scholar] [CrossRef]
- Yang, Y.; Long, Y.; Li, S.W.; Liu, X.H. Straw Return Decomposition Characteristics and Effects on Soil Nutrients and Maize Yield. Agriculture 2023, 13, 1570. [Google Scholar] [CrossRef]
- Mohammad, G.; Elnaz, A.; Petr, K.; Jan, M.; Marek, K.; Trong, N.H. Carbon pool dynamic and soil microbial respiration affected by land use alteration: A case study in humid subtropical area. Land 2023, 12, 459. [Google Scholar] [CrossRef]
- Zhou, J.C.; Luo, Y.Q.; Chen, J. Dilemmas in linking microbial carbon use efficiency with soil organic carbon dynamics. Glob. Change Biol. 2025, 31, e70047. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Ren, J.N.; Manzoni, S.; Fatichi, S. Temperature controls the relation between soil organic carbon and microbial carbon use efficiency. Glob. Change Biol. 2024, 30, e17492. [Google Scholar] [CrossRef]
- Zhu, C.C.; Zhong, W.; Han, C.; Deng, H.; Jiang, Y.B. Driving factors of soil organic carbon sequestration under straw returning across China’s uplands. J. Environ. Manag. 2023, 335, 117590. [Google Scholar] [CrossRef]
- Wang, K.K.; Hu, W.S.; Xu, Z.Y.; Xue, Y.H.; Zhang, Z.; Liao, S.; Zhang, Y.; Li, X.; Ren, T.; Cong, R.; et al. Seasonal temporal characteristics of in situ straw decomposition in different types and returning methods. J. Soil Sci. Plant Nutr. 2022, 22, 4228–4240. [Google Scholar] [CrossRef]
- Jing, X.D.; Chai, X.H.; Long, S.Q.; Tian, L.; Si, M.R.; Zheng, X.; Cai, X.Y. Urea/sodium hydroxide pretreatments enhance decomposition of maize straw in soils and sorption of straw residues toward herbicides. J. Hazard. Mater. 2022, 431, 128467. [Google Scholar] [CrossRef]
- Jia, P.Y.; Wang, X.; Liu, S.M.; Hua, Y.T.; Zhou, S.X.; Jiang, Z.X. Combined use of biochar and microbial agent can promote lignocellulose degradation and humic acid formation during sewage sludge-reed straw composting. Bioresour. Technol. 2022, 370, 128525. [Google Scholar] [CrossRef]
- Ma, D.G.; Chen, H.Y.; Liu, D.X.; Feng, C.W.; Hua, Y.H.; Gu, T.; Guo, X.; Zhou, Y.; Wang, H.; Tong, G.; et al. Soil-derived cellulose-degrading bacteria: Screening, identification, the optimization of fermentation conditions, and their whole genome sequencing. Front. Microbiol. 2024, 15, 1409697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Dou, S.; Ndzelu, B.S.; Guan, X.W.; Zhang, B.H.; Bai, Y. Effects of different corn straw amendments on humus composition and structural characteristics of humic acid in black soil. Commun. Soil Sci. Plant Anal. 2020, 51, 107–117. [Google Scholar] [CrossRef]
- Liu, X.; Dou, S.; Zheng, S. Effects of corn straw and biochar returning to fields every other year on the structure of soil humic acid. Sustainability 2022, 14, 15946. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Dou, S.; Dong, S.S.; Tan, C.; Li, L.B.; Lin, C.M. Effect of deep incorporation of corn stover in addition to chemical fertilization on composition of soil humus and structure of humic acid in soil. Acta Pedol. Sin. 2016, 53, 694–702. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Z.X.; Li, W.Q.; Zhao, Y.D.; Xia, J.; Dong, W.Y.; Chen, B.Q. Effects of different straw return modes on soil carbon, nitrogen, and greenhouse gas emissions in the semiarid maize field. Plants 2024, 13, 2503. [Google Scholar] [CrossRef]
- Song, C.C.; Zhang, J.B. Effects of soil moisture, temperature, and nitrogen fertilization on soil respiration and nitrous oxide emission during maize growth period in northeast China. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2009, 59, 97–106. [Google Scholar] [CrossRef]
- Filip, Z.; Haider, K.; Martin, J.P. Influence of clay minerals on the formation of humic substances by Epicoccum nigrum and Stachybotrys chartarum. Soil Biol. Biochem. 1972, 4, 147–154. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Song, Q.L.; Liang, Q.X.; Sun, Y.; Song, F.Q. Effects of different nitrogen fertilizer application rates on soil microbial structure in paddy soil when combined with rice straw return. Microorganisms 2025, 13, 79. [Google Scholar] [CrossRef]
- Qiu, L.F. Effects of Clay Minerals on Straw Degradation and Agglomeration Formation. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar] [CrossRef]
- Sarkar, B. Clay minerals—Organic matter interactions in relation to carbon stabilization in soils. In The Future of Soil Carbon; Academic Press: Cambridge, MA, USA, 2018; pp. 71–86. [Google Scholar] [CrossRef]
- Singh, M.; Sarkar, B.; Sarkar, S.; Churchman, J.; Beerling, D.J. Stabilization of soil organic carbon as influenced by clay mineralogy. Adv. Agron. 2017, 148, 33–84. [Google Scholar] [CrossRef]
- Li, R.H.; Li, X.Y.; Li, H.R.; Zhao, K.P.; Peng, K. Structural characteristics of clay minerals and their progress in CO2 adsorption. Bulletin. Ceram. Soc. 2022, 41, 141–152. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.B.; Song, X.M.; Nian, X.J.; Zhang, H. Effect on microbial growth and activity of amino acid nutrition. Heilongjiang Agric. Sci. 2010, 5, 13–15. [Google Scholar]
- Feng, Z.Y.; Wu, P.D.; Xie, X.L.; Zhou, Y.; Zhu, H.H.; Yao, Q. Feather-Based compost drastically regulates soil microbial community and lettuce growth in a subtropical soil: The possible role of amino acids. J. Soil Sci. Plant Nutr. 2021, 21, 709–721. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhang, Y.F.; Zhang, Q.G.; Xu, L.X.; Li, R.; Luo, X.P.; Zhang, X.; Tong, J. Earthworms modify microbial community structure and accelerate maize stover decomposition during vermicomposting. Environ. Sci. Pollut. Res. 2015, 22, 17161–17170. [Google Scholar] [CrossRef]
- Wang, Y.N. The Utility Model Relates to an Organic Polypeptidase Activity Regulator. Chinese Patent CN104961544A, 5 June 2015. [Google Scholar]
- Li, D.D.; Li, Z.Q.; Zhao, B.Z.; Zhang, J.B. Relationship between the chemical structure of straw and composition of main microbial groups during the decomposition of wheat and maize straws as affected by soil texture. Biol. Fertil. Soils 2020, 56, 11–24. [Google Scholar] [CrossRef]
- Wang, Y.M.; Sha, Y.; Ren, Z.Z.; Huang, Y.W.; Gao, Q.; Wang, S.J.; Li, X.Y.; Feng, G.Z. Conservative strip tillage system in maize maintains high yield and mitigates GHG emissions but promotes N2O emissions. Sci. Total Environ. 2024, 932, 173067. [Google Scholar] [CrossRef]
- NYT3494-2019; Agricultural biomass raw materials-Determination of cellulose, hemicellulose, and lignin. Ministry of Agriculture of the PRC: Beijing, China, 2019.
- Li, Y.; Zhang, J.J.; Zhao, X.M.; Li, M.T.; Gao, Q.; Zhao, L.P. Effect of long-term application of corn stalks and inorganic fertilizers on fluorescence characteristics of water extractable organic matter in a meadow black soil of northeast China. Toxicol. Environ. Chem. 2016, 98, 541–550. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.N.; Wang, X.D. Changes of the components and energy of corn stalk during decomposition process in cropland with different fertility. Afr. J. Agric. Res. 2013, 8, 1411–1417. [Google Scholar] [CrossRef]
- Pimentel, G.L.; Barreto, C.S.M.; Oliveira, S.D.M.D.; Cherubin, M.R.; Demattê, J.A.M.; Cerri, C.E.P.; Cerri, C.C. Diffuse reflectance infrared fourier transform (DRIFT) spectroscopy to assess decomposition dynamics of sugarcane straw. BioEnergy Res. 2019, 12, 909–919. [Google Scholar] [CrossRef]
- Duan, Z.X.; Kang, Z.C.; Kong, X.F.; Qiu, G.K.; Wang, Q.Y. Development of a novel low-temperature-tolerant microbial consortium for efficient degradation and recycling of farmland straw. J. Environ. Chem. Eng. 2025, 13, 115884. [Google Scholar] [CrossRef]
- Flaig, W.; Küster, E.; Haider, K.; Beutelspacher, G.; Filip, Z.; Martin, J. Influence of clay minerals on the formation of humic substances by some soil fungi. Pochvovedenie. 1971, 6, 51–59. [Google Scholar]
- Wang, S.; Xu, J.P.; Chen, D.Y.; Jiang, S.; Li, X.J.; Sheng, B.H.; Schaeffer, S. Structure characteristics of mineral-microbial residues formed by microbial utilization of lignin based on the participations of different clay mineral types. Spectrosc. Spectr. Anal. 2018, 38, 2903–2909. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.Y.; Xu, J.P.; Lei, W.Y.; Zhou, C.Y.; Chen, C.; Li, F.H.; Wang, N. Humus composition of mineral–microbial residue from microbial utilization of lignin involving different mineral types. Can. J. Soil Sci. 2019, 99, 208–216. [Google Scholar] [CrossRef]
- Xu, Y.; Dou, S.; Zhang, Y.F.; Tian, Y.X.; Duan, H.M.; Bai, Y. Does amendment of montmorillonite promote humification of white alfalfa. Acta Pedol. Sin. 2020, 57, 1230–1239. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Yang, Y.J.; Du, W.; Cui, Z.Y.; Zhao, T.W.; Wang, X.H.; Lv, J.L. Spectroscopic characteristics of dissolved organic matter during pig manure composting with bean dregs and biochar amendments. Microchem. J. 2020, 158, 105226. [Google Scholar] [CrossRef]
- Gao, P.; Shen, H.B.; Wang, Y.X.; Cai, S.S.; Xu, Y.Y.; Yang, H.Y.; Wang, C.; Zhang, G.L. Effects of corn straw returning methods on content and components of soil water soluble organic carbon in the semiarid black soil region of northeast China. Agric. Res. Arid Areas 2024, 42, 127–135. [Google Scholar] [CrossRef]
- Hassouna, M.; Massiani, C.; Dudal, Y.; Pech, N.; Theraulaz, F. Changes in water extractable organic matter (WEOM) in a calcareous soil under field conditions with time and soil depth. Geoderma 2010, 155, 75–85. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wang, S. Review of different microorganisms effect on humus formation. J. Jilin Agric. Univ. 2011, 33, 119–125. [Google Scholar] [CrossRef]
- Klopp, H.W.; Arriaga, F.; Bleam, W. Influence of exchangeable sodium and clay mineralogy on soil water retention and hydraulic conductivity. J. Soil Water Conserv. 2020, 75, 755–764. [Google Scholar] [CrossRef]
- Wu, Y.D.; Li, F.B.; Liu, T.X. Mechanism of extracellular electron transfer among microbe–humus–mineral in Soil: A review. Acta Pedol. Sin. 2016, 53, 277–291. [Google Scholar] [CrossRef]
- Dan, U.; Johnson, C.E. Characterization of organic matter in a northern hardwood forest soil by 13C-NMR spectroscopy and chemical methods. Geoderma 2003, 111, 123–149. [Google Scholar] [CrossRef]
- Dou, S.; Zhang, J.J.; Li, K. Effect of organic matter applications on 13C-NMR spectra of humic acids of soil. Eur. J. Soil Sci. 2008, 59, 532–539. [Google Scholar] [CrossRef]
- Sinovuyo, B.N.; Dou, S.; Zhang, X.W.; Zhang, Y.F.; Ma, R.; Liu, X. Tillage effects on humus composition and humic acid structural characteristics in soil aggregate-size fractions. Soil Tillage Res. 2021, 213, 105090. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, J.H.; Rong, J.L.; Hu, J.; He, L. Effects of different conditioners on humus composition and humic acid structural characteristics in black soil under the combined application of pig manure and straw. J. Soil Sci. Plant Nutr. 2023, 23, 6246–6256. [Google Scholar] [CrossRef]
- Yu, H.; Li, P.; Bo, G.; Shen, G. Studies on the humic acid structure and microbial nutrient restriction mechanism during organic-inorganic co-composting. J. Environ. Manag. 2024, 353, 120186. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, S.H.; Fan, M. Lignin in straw and its applications as an adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Dolfing, J.; Wang, B.Z.; Chen, R.R.; Huang, M.S.; Li, Z.P.; Lin, X.G.; Feng, Y.Z. Bacterial communities involved directly or indirectly in the anaerobic degradation of cellulose. Biol. Fertil. Soils 2019, 55, 11–22. [Google Scholar] [CrossRef]
- Meng, J.Y.; Li, H.; Fan, Z.Y.; Feng, F.Y. Isolation and identifi cation of cellulose-degrading bacteria under low Isolation and identification of cellulose-degrading bacteria under low temperature. Chin. J. Appl. Environ. 2014, 20, 152–156. [Google Scholar] [CrossRef]
- Wang, N.; Xu, J.P.; Chen, D.Y.; Wang, S. Mechanism of humification process of lignin catalyzed by two kinds of clay minerals. J. Jilin Univ. 2020, 58, 441–448. [Google Scholar] [CrossRef]
- Jiao, M.N.; Yue, F.X.; Ren, X.N.; Zhan, X.Z.; Xu, W.Y.; Tang, D.K.H.; Xiao, R.; Li, R.H. Enhanced humification attributed by the integration of Fenton reagent and oxalic acid during a co-composting of swine manure and corn straw: Impacts and the possible mechanisms. Chem. Eng. J. 2024, 498, 155579. [Google Scholar] [CrossRef]
- Li, Y.X.; Kuramae, E.E.; Fahad, N.; Enze, W.; Zhang, Z.G.; Li, J.; Yao, Z.M.; Tian, L.; Sun, Y.; Luo, S.Y.; et al. Addition of cellulose degrading bacterial agents promoting keystone fungal-mediated cellulose degradation during aerobic composting: Construction the complex co-degradation system. Bioresour. Technol. 2023, 381, 129132. [Google Scholar] [CrossRef]
- Wang, X.G.; Tian, L.; Li, Y.X.; Zhong, C.; Tian, C.J. Effects of exogenous cellulose-degrading bacteria on humus formation and bacterial community stability during composting. Bioresour. Technol. 2022, 359, 127458. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Bai, Y.L.; Lu, Y.L.; Ni, L.; Wang, Y.H.; Xu, M.Z. Quantitative relationship between effective accumulated temperature and plant height and leaf area index of summer maize under different nitrogen, phosphorus and potassium levels. Sci. Agric. Sin. 2021, 54, 4761–4777. [Google Scholar] [CrossRef]
- Luo, S.Z.; Liu, W.W.; Ren, Q.; Wei, H.Q.; Wamg, C.; Xi, X.H.; Nie, S.; Dong, L.; Ma, D.; Zhou, G.Q. Leaf area index estimation in maize and soybean using UAV LiDAR data. Precis. Agric. 2024, 25, 1915–1932. [Google Scholar] [CrossRef]
- Duan, W.X.; Zhang, H.Y.; Xie, B.T.; Wang, Q.M.; Wang, B.Q.; Zhang, L.M. Influence of application methods of polypeptidase activity promoter on the dry matter accumulation and distribution, root yield, and quality of raw-edible sweet potato. J. Nucl. Agric. Sci. 2019, 33, 1016–1023. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhen, Q.; Cui, Y.X.; Zhang, P.P.; Zhang, X.C. Use of montmorillonite-enriched siltstone for improving water condition and plant growth in sandy soil. Ecol. Eng. 2020, 145, 105740. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wan, P.; Li, J.; Lai, X.; Zhang, G.L.; Chang, H. Effects of straw returning combined with nitrogen fertilizer on paddy soil carbon sequestration and green-house-gas emission in central south region of china. J. Agric. Resour. Environ. 2016, 33, 508–517. [Google Scholar] [CrossRef]
- Li, J.H.; Li, H.; Zhang, Q.; Shao, H.B.; Gao, C.H.; Zhang, X.Z. Effects of fertilization and straw return methods on the soil carbon pool and CO2 emission in a reclaimed mine spoil in Shanxi Province, China. Soil Tillage Res. 2019, 195, 104361. [Google Scholar] [CrossRef]
- Daly, E.J.; Hernandez-Ramirez, G. Sources and priming of soil N2O and CO2 production: Nitrogen and simulated exudate additions. Soil Biol. Biochem. 2020, 149, 107942. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.B.; Zhao, B.Z.; Xin, X.L.; Zhou, G.X.; Tan, J.F.; Zhao, J.H. Carbon mineralization and microbial attributes in straw-amended soils as affected by moisture levels. Pedosphere 2014, 24, 167–177. [Google Scholar] [CrossRef]
- Hurkuck, M.; Althoff, F.; Jungkunst, H.F.; Jugold, A.; Keppler, F. Release of methane from aerobic soil: An indication of a novel chemical natural process. Chemosphere 2011, 86, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lang, M.; Han, X.Z.; Miao, S.J.; Qiao, Y.F. Gross nitrogen transformations in black soil under different land uses and management systems. Biol. Fertil. Soils 2016, 52, 233–241. [Google Scholar] [CrossRef]
- Joseph, C.B.; Emma, P.M.; Matthew, W.M.; Stephen, C.H. Quantifying the legacy of snowmelt timing on soil greenhouse gas emissions in a seasonally dry montane forest. Glob. Change Biol. 2018, 24, 5933–5947. [Google Scholar] [CrossRef]
- Pi, K.F.; Bieroza, M.; Brouchkov, A.; Chen, W.T.; Dufour, L.J.P. The cold region critical zone in transition: Responses to climate warming and land use change. Annu. Rev. Environ. Resour. 2021, 46, 111–134. [Google Scholar] [CrossRef]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- Masyagina, V.O.; Menyailo, V.O. The impact of permafrost on carbon dioxide and methane fluxes in Siberia: A meta-analysis. Environ. Res. 2020, 182, 109096. [Google Scholar] [CrossRef] [PubMed]





| pH | Organic Matter (g·kg−1) | Total N (g·kg−1) | Alkaline N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) | |
|---|---|---|---|---|---|---|
| Primary soil | 6.13 | 12.87 | 1.27 | 103.74 | 23.90 | 180.79 |
| Name | Asp | Thr | Ser | Glu | Gly | Cys | Val | Met | Ile | Leu | Phe | His | Lys | Arg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/mL) | 0.439 | 0.968 | 1.121 | 2.11 | 1.292 | 0.692 | 3.144 | 2.644 | 1.747 | 3.716 | 2.82 | 0.395 | 4.173 | 3.575 |
| Treatment | TOC (g·kg−1) | WEOC (g·kg−1) | UV254 (cm−1) | SUV254 (L·mg−1·m−1) | f 450/f 500 |
|---|---|---|---|---|---|
| CK | 488.393 ± 10.400 a | 19.503 ± 0.438 d | 0.366 | 0.938 c | 1.21278 |
| MMT | 436.243 ± 14.984 bc | 21.549 ± 0.436 c | 0.462 | 1.072 a | 1.39466 |
| CAA | 455.157 ± 9.989 b | 23.343 ± 0.319 b | 0.390 | 0.835 d | 1.17967 |
| MMT-CAA | 425.747 ± 10.137 c | 24.375 ± 0.477 a | 0.501 | 1.028 b | 1.20813 |
| Alkyl C (0–50 ppm) | O-Alkyl C (50–110 ppm) | Aromatic C (110–160 ppm) | Carbonyl C (160–200 ppm) | Aliphatic C (0–110 ppm) | Aliphatic C /Aromatic C | Alkyl C /O-Alkyl C | Aromaticity | |
|---|---|---|---|---|---|---|---|---|
| CK | 0.226 | 0.477 | 0.256 | 0.041 | 0.703 | 2.742 | 0.472 | 26.726 |
| MMT | 0.221 | 0.406 | 0.326 | 0.047 | 0.627 | 1.921 | 0.545 | 34.237 |
| CAA | 0.224 | 0.397 | 0.304 | 0.075 | 0.621 | 2.043 | 0.565 | 32.860 |
| MMT-CAA | 0.210 | 0.363 | 0.374 | 0.054 | 0.573 | 1.532 | 0.579 | 39.487 |
| Treatment | Chao1 | Shannon | Simpson | |
|---|---|---|---|---|
| Bacteria | CK | 246.31 ± 6.16 a | 2.68 ± 0.06 b | 0.86 b |
| MMT | 255.42 ± 47.01 a | 3.10 ± 0.10 a | 0.90 a | |
| CAA | 290.38 ± 12.77 a | 3.23 ± 0.25 a | 0.91 a | |
| MMT-CAA | 259.87 ± 33.58 a | 2.96 ± 0.086 a | 0.91 a | |
| Fungi | CK | 149.81 ± 4.99 ab | 2.14 ± 0.174 b | 0.77 b |
| MMT | 158.26 ± 4.52 ab | 2.67 ± 0.34 a | 0.86 a | |
| CAA | 134.77 ± 12.01 b | 2.02 ± 0.10 b | 0.73 b | |
| MMT-CAA | 187.30 ± 4.84 a | 2.81 ± 0.06 a | 0.88 a |
| Species Abundance | Treatment | ||||
|---|---|---|---|---|---|
| CK | MMT | CAA | MMT-CAA | ||
| Bacteria | Proteobacteria | 0.819 a | 0.784 ab | 0.755 b | 0.663 c |
| Bacteroidetes | 0.173 c | 0.204 bc | 0.227 b | 0.307 a | |
| Actinobacteria | 0.006 b | 0.012 ab | 0.012 ab | 0.024 a | |
| Fungi | Ascomycota | 0.796 a | 0.652 b | 0.857 a | 0.800 a |
| Basidiomycota | 0.057 b | 0.108 a | 0.028 b | 0.126 a | |
| Treatment | Nodes | Edges | Positive/% | MD | GD | AD | APL | |
|---|---|---|---|---|---|---|---|---|
| Bacteria | CK | 53 | 199 | 56.78 | 0.2392 | 0.1444 | 7.5094 | 1.9289 |
| MMT | 65 | 276 | 63.32 | 0.2620 | 0.1327 | 8.4923 | 1.9519 | |
| CAA | 67 | 213 | 64.79 | 0.3053 | 0.0963 | 6.3582 | 2.1746 | |
| MMT-CAA | 71 | 348 | 61.78 | 0.2226 | 0.1400 | 9.8028 | 1.8938 | |
| Fungi | CK | 42 | 79 | 50.53 | 0.3388 | 0.1218 | 4.7500 | 2.1462 |
| MMT | 43 | 68 | 55.88 | 0.4753 | 0.0753 | 3.1628 | 2.8671 | |
| CAA | 35 | 57 | 71.93 | 0.3476 | 0.0958 | 3.2571 | 2.2505 | |
| MMT-CAA | 40 | 95 | 55.70 | 0.4030 | 0.0918 | 3.7619 | 2.2285 |
| Treatment | Plant Height (cm) | Stem Diameter (cm) | Leaf Area (cm2) | Leaf SPAD |
|---|---|---|---|---|
| CK | 44.28 ± 3.21 d | 1.09 ± 0.15 d | 90.64 ± 15.91 d | 31.86 ± 0.53 c |
| MMT | 55.31 ± 2.98 c | 1.25 ± 0.16 c | 113.45 ± 18.64 c | 40.80 ± 0.93 b |
| CAA | 61.22 ± 3.02 b | 1.53 ± 0.11 b | 147.32 ± 10.93 b | 41.38 ± 2.39 b |
| MMT-CAA | 68.67 ± 1.98 a | 1.73 ± 0.08 a | 154.30 ± 14.79 a | 44.96 ± 0.78 a |
| Treatment | Average Greenhouse Gas Emission Fluxes | Cumulative Greenhouse Gas Emissions | ||||
|---|---|---|---|---|---|---|
| CO2 (mg m−2 h−1) | N2O (μg m−2 h−1) | CH4 (μg m−2h−1) | CO2 (kg ha−1) | N2O (kg ha−1) | CH4 (kg ha−1) | |
| CK | 215.24 ± 7.14 b | 68.48 ± 2.62 b | −36.59 ± 1.71 a | 518.195 ± 16.69 b | 0.164 ± 0.013 b | −0.090 ± 0.005 a |
| MMT | 138.69 ± 6.08 c | 57.66 ± 1.49 c | −34.84 ± 1.91 a | 319.064 ± 14.35 c | 0.137 ± 0.006 c | −0.086 ± 0.002 a |
| CAA | 287.43 ± 6.75 a | 81.23 ± 2.73 a | −47.85 ± 1.5 c | 695.057 ± 26.41 a | 0.185 ± 0.017 a | −0.117 ± 0.004 c |
| MMT-CAA | 223.759 ± 5.77 b | 65.41 ± 1.72 b | −41.48 ± 1.64 b | 542.533 ± 29.02 b | 0.152 ± 0.009 b | −0.101 ± 0.003 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Galliane, T.F.J.; Zhao, C.; Feng, Y.; Li, M. Montmorillonite and Composite Amino Acid Overcome the Challenges of Straw Return in Cold-Region Soil: Synergistic Mechanisms of Rapid Straw Humification and Carbon Sequestration. Agronomy 2025, 15, 1979. https://doi.org/10.3390/agronomy15081979
Chen X, Galliane TFJ, Zhao C, Feng Y, Li M. Montmorillonite and Composite Amino Acid Overcome the Challenges of Straw Return in Cold-Region Soil: Synergistic Mechanisms of Rapid Straw Humification and Carbon Sequestration. Agronomy. 2025; 15(8):1979. https://doi.org/10.3390/agronomy15081979
Chicago/Turabian StyleChen, Xingyan, Tchoumtchoua Foka Joseline Galliane, Chongyang Zhao, Yanhui Feng, and Mingtang Li. 2025. "Montmorillonite and Composite Amino Acid Overcome the Challenges of Straw Return in Cold-Region Soil: Synergistic Mechanisms of Rapid Straw Humification and Carbon Sequestration" Agronomy 15, no. 8: 1979. https://doi.org/10.3390/agronomy15081979
APA StyleChen, X., Galliane, T. F. J., Zhao, C., Feng, Y., & Li, M. (2025). Montmorillonite and Composite Amino Acid Overcome the Challenges of Straw Return in Cold-Region Soil: Synergistic Mechanisms of Rapid Straw Humification and Carbon Sequestration. Agronomy, 15(8), 1979. https://doi.org/10.3390/agronomy15081979






