White Lupin and Hairy Vetch as Green Manures: Impacts on Yield and Nutrient Cycling in an Organic Almond Orchard
Abstract
1. Introduction
2. Materials and Methods
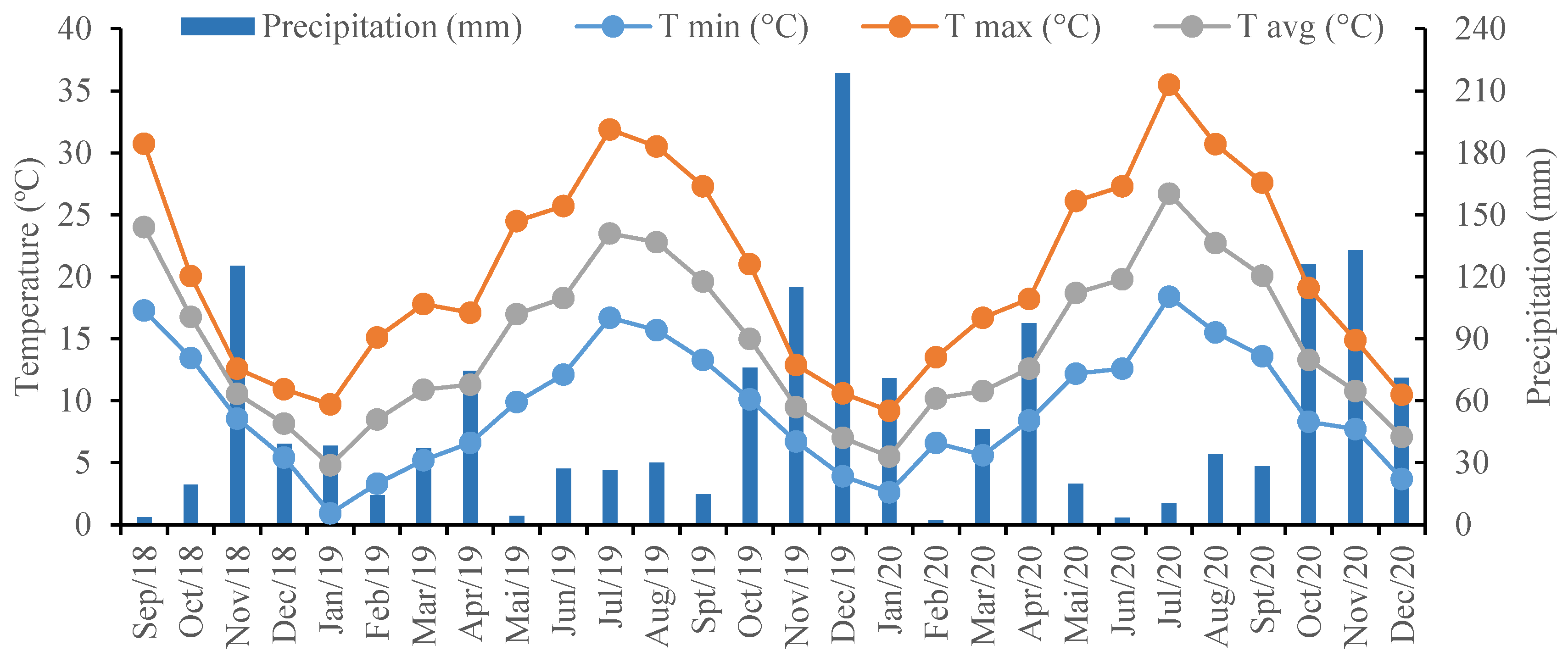
2.1. Site Characterization
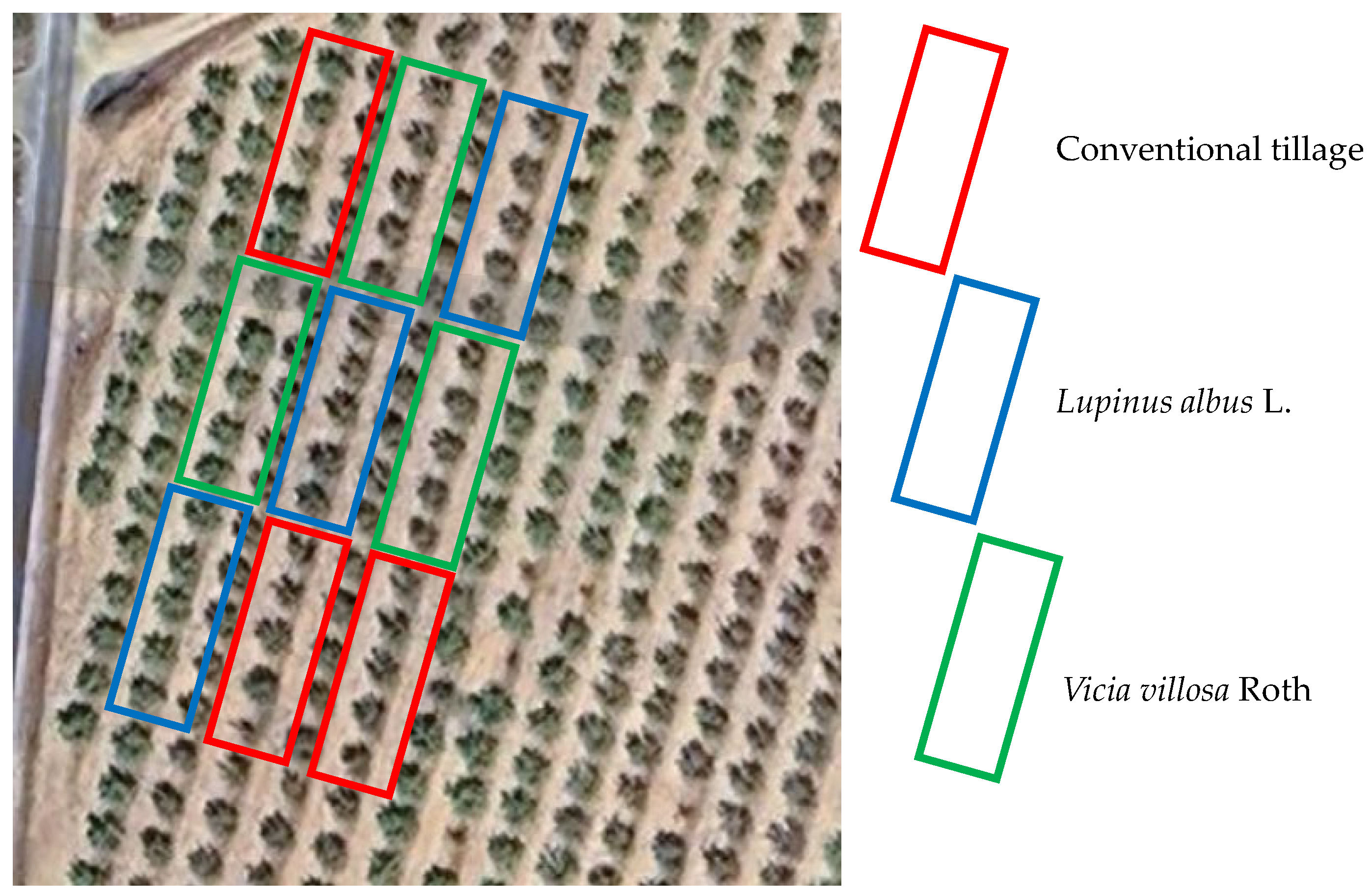
2.2. Experimental Design and Treatment Characterization
2.3. Plant Tissue and Soil Sampling
2.4. Laboratory Analyses
2.5. Data Analysis
3. Results
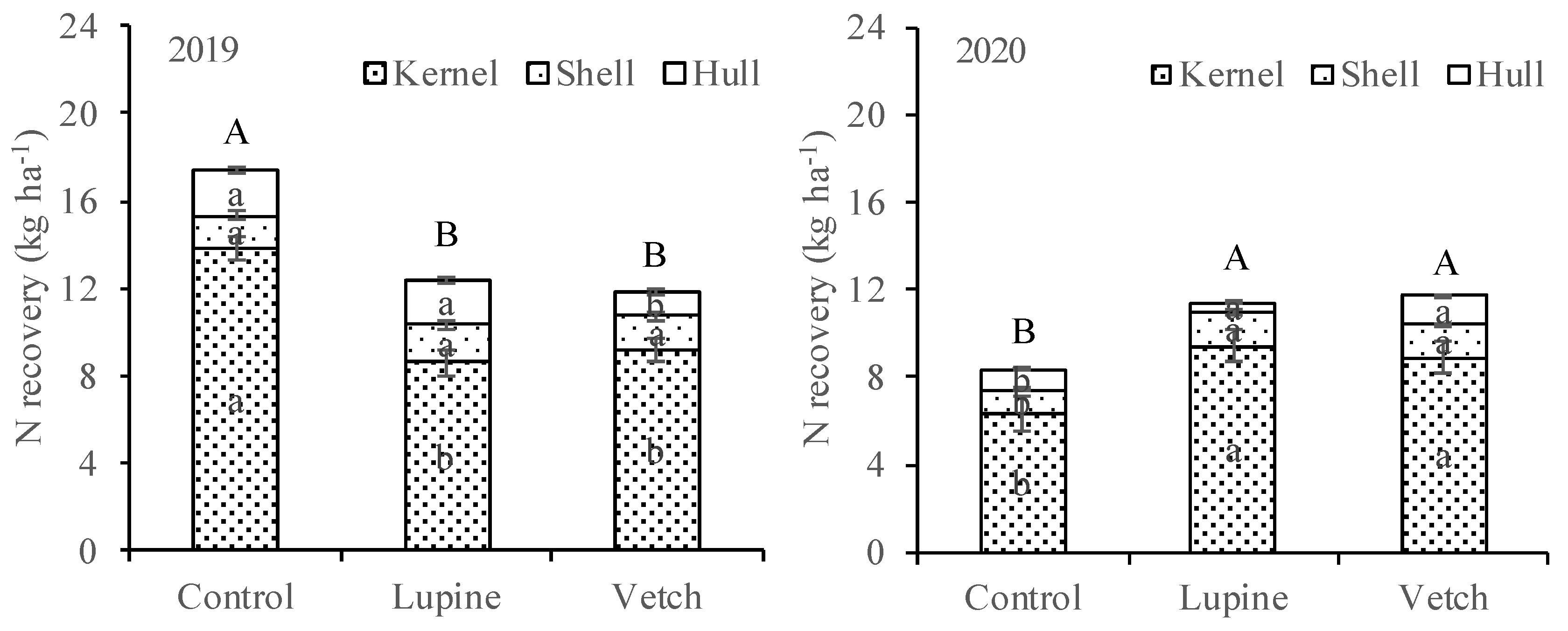
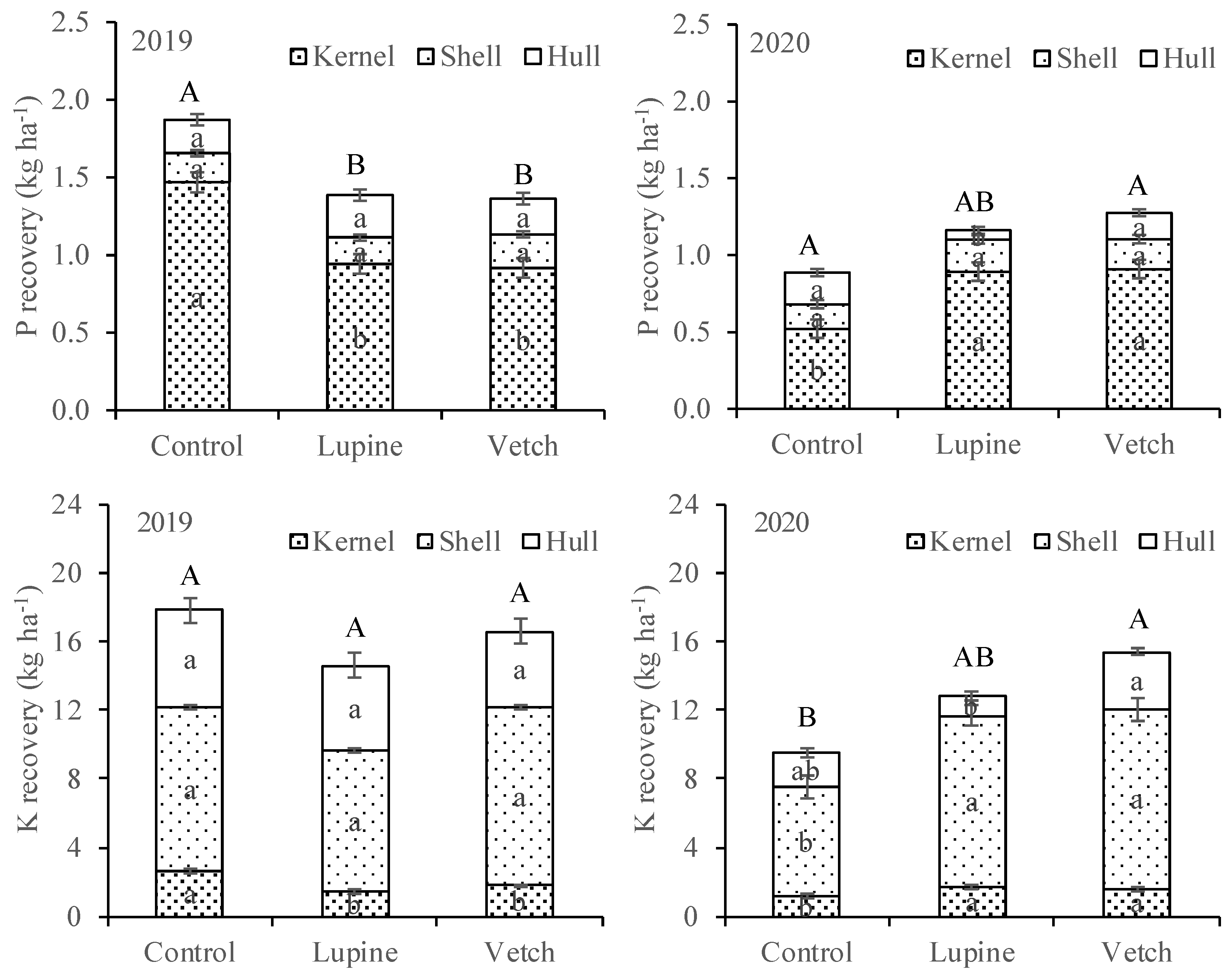
3.1. Nutrient Concentration and Recovery in Cover Crop Tissues
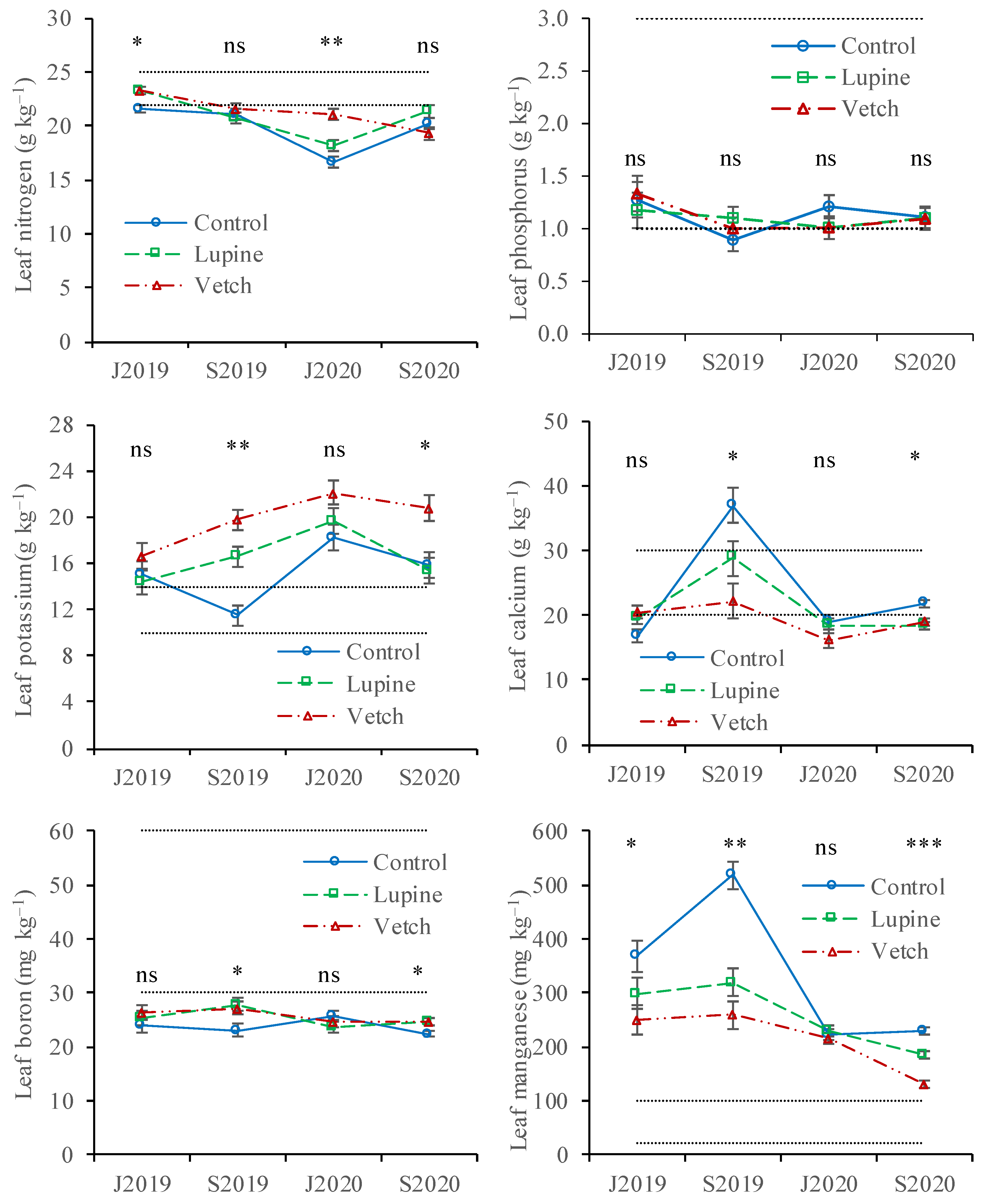
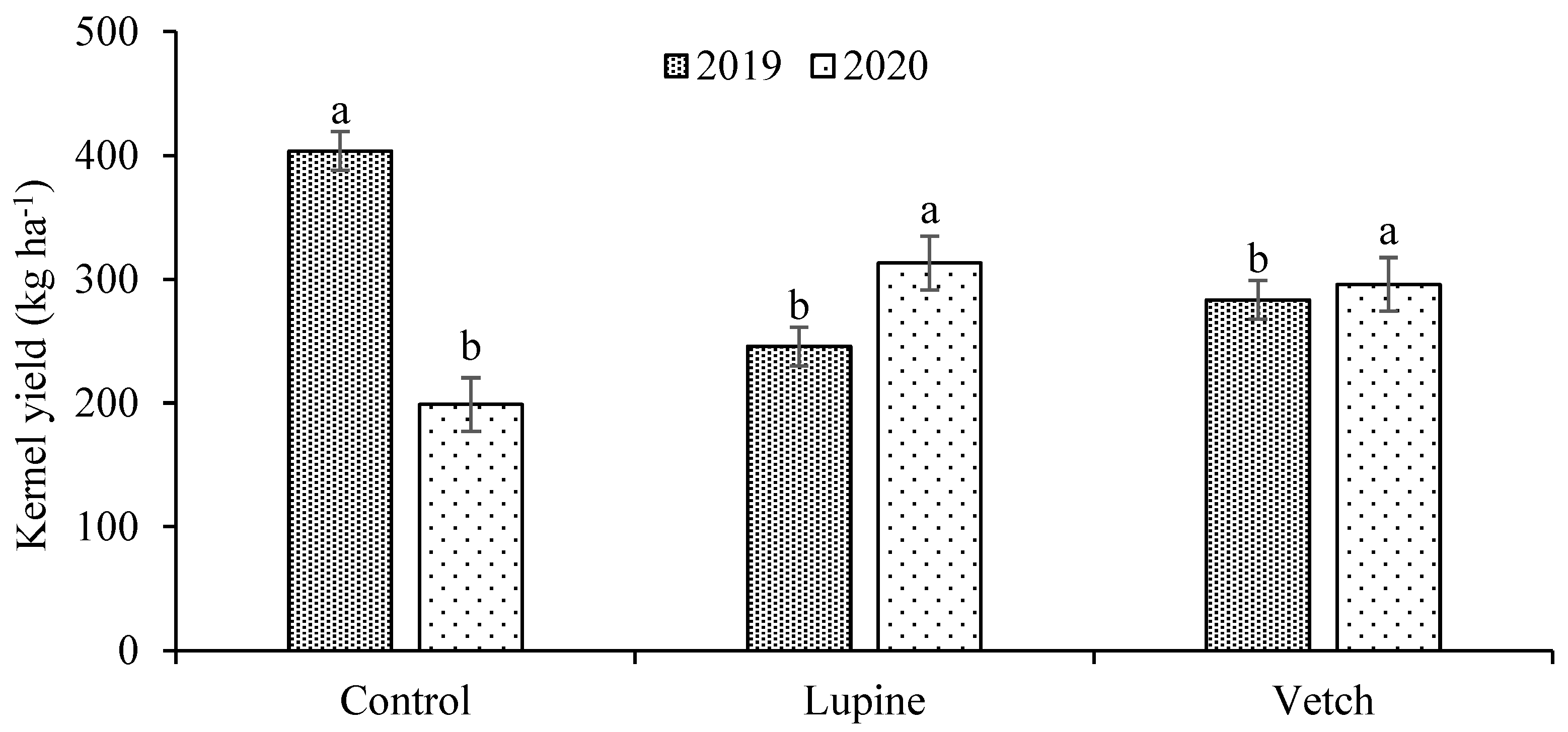
3.2. Nutrient Concentration in Leaves and Almonds, and Almond Yield
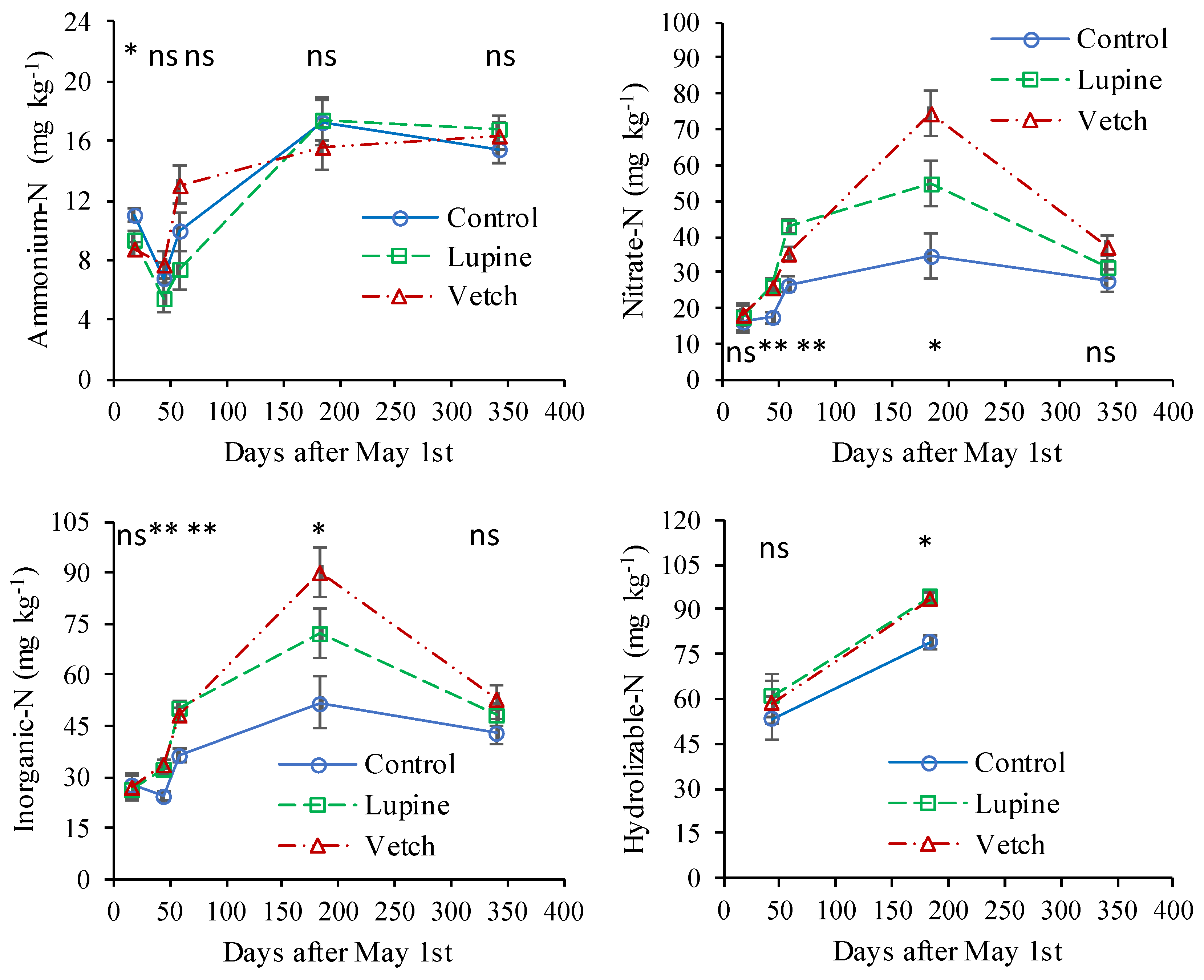
3.3. Indicators of Soil Nitrogen Availability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arquero, O.; Parra, M.A. Exigencias medioambientales. In Manual del Cultivo del Almendro; Junta de Andalucía: Sevilha, Spain, 2013; pp. 15–19. [Google Scholar]
- Aguiar, C. Fenologia, dormência e biologia da reprodução. In Amendoeira: Estado da Produção; Centro Nacional de Competências dos Frutos Secos (CNCFS): Bragança, Portugal, 2017; pp. 42–97. [Google Scholar]
- Prgomet, I.; Pascual-Seva, N.; Morais, M.C.; Aires, A.; Barreales, D.; Ribeiro, A.C.; Silva, A.P.; Barros, A.I.; Gonçalves, B. Physiological and biochemical performance of almond trees under deficit irrigation. Sci. Hortic. 2020, 261, 108990. [Google Scholar] [CrossRef]
- Arrobas, M.; Ribeiro, A.; Barreales, D.; Pereira, E.L.; Rodrigues, M.Â. Soil and foliar nitrogen and boron fertilization of almond trees grown under rainfed conditions. Eur. J. Agron. 2019, 106, 39–48. [Google Scholar] [CrossRef]
- Almagro, M.; de Vente, J.; Boix-Fayos, C.; García-Franco, N.; Melgares de Aguilar, J.; González, D.; Solé-Benet, A.; Mar-tínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strat. Glob. Change 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- FAOSTAT. Production: Crops and Livestock Products. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 February 2025).
- Almeida, A. Instalação da cultura. In Amendoeira: Estado da Produção; Centro Nacional de Competências dos Frutos Secos (CNCFS): Bragança, Portugal, 2017; pp. 127–139. [Google Scholar]
- Iglesias, I.; Foles, P.; Oliveira, C.M. A Amendoeira em Portugal e Espanha: Situação, inovação tecnológica, custos, rentabilidade e perspetivas. Agriterra 2021, 3, 36–46. [Google Scholar]
- Instituto Nacional de Estatística (INE). Estatísticas Agrícolas 2023; INE: Lisboa, Portugal, 2024. [Google Scholar]
- European Union. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, L150, 1–92. Available online: http://data.europa.eu/eli/reg/2018/848/oj (accessed on 1 August 2025).
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education Limited: Edinburgh, UK, 2017. [Google Scholar]
- Arrobas, M.; Raimundo, S.; Conceição, N.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.Â. On sandy, boron-poor soils, liming induced severe boron deficiency and drastically reduced the dry matter yield of young olive trees. Plants 2023, 12, 4161. [Google Scholar] [CrossRef] [PubMed]
- Arrobas, M.; Thais Nepomuceno Carvalho, J.; Raimundo, S.; Poggere, G.; Rodrigues, M.Â. The safe use of compost derived from municipal solid waste depends on its composition and conditions of application. Soil Use Manag. 2022, 38, 917–928. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A. Nitrogen fixation. In Marschner’s Mineral Nutrition of Plants; Elsevier: Chennai, India, 2023; pp. 615–650. ISBN 978-0-12-819773-8. [Google Scholar]
- De Vries, S.; de Vries, J. Azolla: A model system for symbiotic nitrogen fixation and evolutionary developmental biology. In Current Advances in Fern Research; Fernández, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–46. ISBN 978-3-319-75103-0. [Google Scholar]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 8539. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.P.; Swanepoel, C.M.; Nelson, W.C.D.; Beukes, D.J.; Van Der Laan, M.; Hargreaves, J.N.G.; Rötter, R.P. Simulating medium-term effects of cropping system diversification on soil fertility and crop productivity in southern Africa. Eur. J. Agron. 2020, 119, 126089. [Google Scholar] [CrossRef]
- Ton, A. Advantages of grain legume-cereal intercropping in sustainable agriculture. Turk. JAF Sci. Technol. 2021, 9, 1560–1566. [Google Scholar] [CrossRef]
- Dimande, P.; Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Ground management through grazing in rainfed olive orchards provides high olive yields and has other potential benefits for both the soil and the farmer. Agriculture 2024, 14, 897. [Google Scholar] [CrossRef]
- Buoso, S.; Zamboni, A.; Franco, A.; Commisso, M.; Guzzo, F.; Varanini, Z.; Pinton, R.; Tomasi, N.; Zanin, L. Nodulating white lupins take advantage of the reciprocal interplay between N and P nutritional responses. Physiol. Plant. 2022, 174, e13607. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Arrobas, M.; Claro, A.M.; Ferreira, I.Q.; Rodrigues, M.Â. The effect of legume species grown as cover crops in olive orchards on soil phosphorus bioavailability. J. Plant Nutr. 2015, 38, 2294–2311. [Google Scholar] [CrossRef]
- Giese, G.; Velasco-Cruz, C.; Roberts, L.; Heitman, J.; Wolf, T.K. Complete vineyard floor cover crops favorably limit grapevine vegetative growth. Sci. Hortic. 2014, 170, 256–266. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Bertolla, C.; Urbani, S.; Taticchi, A.; Esposto, S.; Servili, M.; Sifola, M.I.; Pellegrini, S.; Pagliai, M.; et al. Changes of soil properties and tree performance induced by soil management in a high-density olive orchard. Eur. J. Agron. 2012, 41, 18–27. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Arrobas, M. Cover cropping for increasing fruit production and farming sustainability. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–295. ISBN 978-0-12-818732-6. [Google Scholar]
- Keesstra, S.; Pereira, P.; Novara, A.; Brevik, E.C.; Azorin-Molina, C.; Parras-Alcántara, L.; Jordán, A.; Cerdà, A. Effects of Soil Management techniques on soil water erosion in apricot orchards. Sci. Total Environ. 2016, 551–552, 357–366. [Google Scholar] [CrossRef]
- Torres, M.A.R.R.; Ordóñez-Fernández, R.; Giráldez, J.V.; Márquez-García, J.; Laguna, A.; Carbonell-Bojollo, R. Efficiency of four different seeded plants and native vegetation as cover crops in the control of soil and carbon losses by water erosion in olive orchards. Land Degrad. Dev. 2018, 29, 2278–2290. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; López-Romano, H.; Carral, P.; Álvarez-González, A.M.; Herranz-Luque, J.-E.; Sastre-Rodríguez, B.E.; García-Díaz, A.; Muñoz-Organero, G.; Marques, M.J. Ten-year impact of cover crops on soil organic matter quantity and quality in semi-arid vineyards. Land 2023, 12, 2143. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Correia, C.M.; Claro, A.M.; Ferreira, I.Q.; Barbosa, J.C.; Moutinho-Pereira, J.M.; Bacelar, E.A.; Fernandes-Silva, A.A.; Arrobas, M. Soil Nitrogen Availability in Olive Orchards after Mulching Legume Cover Crop Residues. Sci. Hortic. 2013, 158, 45–51. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Dimande, P.; Pereira, E.L.; Ferreira, I.Q.; Freitas, S.; Correia, C.M.; Moutinho-Pereira, J.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Driouech, N.; Abou Fayad, F.; Ghanem, A.; Al-Bitar, L. Agronomic performance of annual self-reseeding legumes and their self-establishment potential in the Apulia region of Italy. In Cultivating the Future Based on Science, Proceeding of the Second Conference of the International Society of Organic Agriculture Research (ISOFAR); Neuhoff, D., Halberg, N., Alfodi, T., Lockeretz, W., Thommen, A., Rasmussen, I.A., Hermansen, J., Vaarst, M., Lueck, L., Caporali, F., et al., Eds.; ISOFAR: Modena, Italy, 2008; Volume 1, pp. 396–399. [Google Scholar]
- Alcántara, C.; Soriano, M.A.; Saavedra, M.; Gómez, J.A. Sistemas de manejo Del Suelo. In El Cultivo del Olivo; Mundi-Prensa: Madrid, Spain, 2017; pp. 335–417. [Google Scholar]
- Ferreira, I.Q.; Rodrigues, M.Â.; Claro, A.M.; Arrobas, M. Management of nitrogen-rich legume cover cops as mulch in traditional olive orchards. Commun. Soil Sci. Plant Anal. 2015, 46, 1881–1894. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative agriculture—A literature review on the practices and mechanisms used to improve soil health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Instituto Português do Mar e da Atmosfera (IPMA). Normais Climatológicas. 2025. Available online: https://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 15 June 2025).
- Working Group WRB (WRB). World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; IUSS: Vienna, Austria, 2022; p. 38. [Google Scholar]
- Bryson, G.; Mills, H.A.; Sasseville, D.N.; Jones, J.B., Jr.; Barker, A.V. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops, 3rd ed.; Micro-Macro Publishing: Athens, GA, USA, 2014; ISBN 978-1-878148-01-8. [Google Scholar]
- Temminghoff, E.E.J.M.; Houba, V.J.G. Plant Analysis Procedures; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper, 9; ISRIC: Wageningen, The Netherlands; FAO: Rome, Italy, 2002; ISBN 90-6672-044-1. [Google Scholar]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Nitrate by ultraviolet spectrophotometric method. In Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Aguiar, P.; Arrobas, M.; Nharreluga, E.A.; Rodrigues, M.Â. Different species and cultivars of broad beans, lupins, and clovers demonstrated varying environmental adaptability and nitrogen fixation potential when cultivated as green ma-nures in northeastern Portugal. Sustainability 2024, 16, 10725. [Google Scholar] [CrossRef]
- Haim, D.; Shalom, L.; Simhon, Y.; Shlizerman, L.; Kamara, I.; Morozov, M.; Albacete, A.; Rivero, R.M.; Sadka, A. Alternate bearing in fruit trees: Fruit presence induces polar auxin transport in citrus and olive stem and represses IAA release from the bud. J. Exp. Bot. 2021, 72, 2450–2462. [Google Scholar] [CrossRef]
- Jangid, R.; Kumar, A.; Masu, M.M.; Kanade, N.; Pant, D. Alternate bearing in fruit crops: Causes and control measures. Asian J. Agric. Hortic. Res. 2023, 1, 10–19. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Benlloch, M.; Navarro, C.; Martin, G.C. The Time of Floral Induction in the Olive. J. Am. Soc. Hortic. Sci. 1992, 117, 304–307. [Google Scholar] [CrossRef]
- Martin, G.C.; Ferguson, L.; Sibbett, G.S. Flowering, pollination, fruiting, alternate bearing, and abscission. In Olive Production Manual; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2005; Volume 3353, pp. 49–54. [Google Scholar]
- Rosati, A.; Paoletti, A.; Al Hariri, R.; Famiani, F. Fruit production and branching density affect shoot and whole-tree wood to leaf biomass ratio in olive. Tree Physiol. 2018, 38, 1278–1285. [Google Scholar] [CrossRef]
- Ryan, M.G.; Oren, R.; Waring, R.H. Fruiting and sink competition. Tree Physiol. 2018, 38, 1261–1266. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, J.P. Functions of macronu-trients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier Ltd.: Chennai, India, 2023; pp. 201–281. ISBN 978-0-12-384905-2. [Google Scholar]
- Morais, M.C.; Aires, A.; Barreales, D.; Rodrigues, M.Â.; Ribeiro, A.C.; Gonçalves, B.; Silva, A.P. Combined soil and foliar nitrogen fertilization effects on rainfed almond tree performance. J. Soil. Sci. Plant Nutr. 2020, 20, 2552–2565. [Google Scholar] [CrossRef]
- Barbieri, P.; Starck, T.; Voisin, A.-S.; Nesme, T. Biological nitrogen fixation of legumes crops under organic farming as driven by cropping management: A review. Agric. Syst. 2023, 205, 103579. [Google Scholar] [CrossRef]
- Ojiem, J.O.; Franke, A.C.; Vanlauwe, B.; de Ridder, N.; Giller, K.E. Benefits of legume–maize rotations: Assessing the impact of diversity on the productivity of smallholders in Western Kenya. Field Crop. Res. 2014, 168, 75–85. [Google Scholar] [CrossRef]
- Mesfin, S.; Gebresamuel, G.; Haile, M.; Zenebe, A. Potentials of legumes rotation on yield and nitrogen uptake of sub-sequent wheat crop in northern Ethiopia. Heliyon 2023, 9, e16126. [Google Scholar] [CrossRef] [PubMed]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners—the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F.-J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: Chennai, India, 2023; pp. 283–385. ISBN 978-0-12-819773-8. [Google Scholar]
- Bell, R. Diagnosis and prediction of deficiency and toxicity of nutrients. In Marschner’s Mineral Nutrition of Plants; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: Chennai, India, 2023; pp. 477–495. ISBN 978-0-12-819773-8. [Google Scholar]
- Portela, E.; Ferreira-Cardoso, J.; Louzada, J.; Gomes-Laranjo, J. Assessment of boron application in chestnuts: Nut yield and quality. J. Plant Nutr. 2015, 38, 973–987. [Google Scholar] [CrossRef]
- Arrobas, M.; Raimundo, S.; Correia, C.M.; Rodrigues, M.Â. Omitting the application of nitrogen or potassium reduced the growth of young chestnut (Castanea sativa) trees, while a lack of boron decreased fruit yield. Soil Syst. 2024, 8, 104. [Google Scholar] [CrossRef]
- Silva, A.P.; Aires, A.; Barreales, D.; Rodrigues, M.Â.; Ribeiro, A.C.; Gonçalves, B.; Morais, M.C. Effects of foliar and soil boron fertilization on yield, leaf physiological traits and fruit attributes in rainfed almond orchards. Agronomy 2022, 12, 2005. [Google Scholar] [CrossRef]
- Arrobas, M.; Silva, J.; Busato, M.R.; Ferreira, A.C.; Raimundo, S.; Pereira, A.; Finatto, T.; de Mello, N.A.; Correia, C.M.; Rodrigues, M.Â. Large chestnut trees did not respond to annual fertiliser applications, requiring a long-term approach to establishing effective fertilisation plans. Soil Syst. 2023, 7, 2. [Google Scholar] [CrossRef]
- Phillips, I.R.; Greenway, M. Changes in water-soluble and exchangeable ions, cation exchange capacity, and phosphorusmax in soils under alternating waterlogged and drying conditions. Commun. Soil Sci. Plant Anal. 1998, 29, 51–65. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil Res. 2014, 52, 483. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 517877. [Google Scholar] [CrossRef]
| Soil Properties | Soil Properties (Cont.) | ||
|---|---|---|---|
| 1 Organic C (g kg−1) | 12.7 ± 4.15 | 6 Exchang. Ca (cmolc kg−1) | 6.7 ± 0.57 |
| 2 pH (H2O) | 6.1 ± 0.32 | 6 Exchang. Mg (cmolc kg−1) | 1.5 ± 0.17 |
| 3 Extract. P (mg kg−1, P2O5) | 38.5 ± 4.84 | 6 Exchang. K (cmolc kg−1) | 0.4 ± 0.01 |
| 3 Extract. K (mg kg−1, K2O) | 168.8 ± 50.15 | 6 Exchang. Na (cmolc kg−1) | 0.2 ± 0.02 |
| 4 Extract. B (mg kg−1) | 0.3 ± 0.09 | 7 CEC (cmolc kg−1) | 8.8 ± 0.66 |
| 5 Extract. Fe (mg kg−1) | 34.0 ± 0.89 | 8 Clay (g kg−1) | 90.6 ± 6.35 |
| 5 Extract. Mn (mg kg−1) | 16.9 ± 0.12 | 8 Silt (g kg−1) | 163.2 ± 11.73 |
| 5 Extract. Zn (mg kg−1) | 0.7 ± 0.02 | 8 Sand (g kg−1) | 746.20 ± 5.88 |
| 5 Extract. Cu (mg kg−1) | 1.7 ± 0.20 | Texture (USDA) | Sandy loam |
| Lupine | Vetch | Lupine | Vetch | |
|---|---|---|---|---|
| Nutrient Concentration | Nutrient Recovery | |||
| g kg−1 | kg ha−1 | |||
| Carbon | 530.5 ± 3.5 b | 543.2 ± 7.8 a | 3118.4 ± 214.3 a | 3415.5 ± 356.5 a |
| Nitrogen | 29.5 ± 3.3 a | 23.9 ± 2.9 b | 172.5 ± 12.0 a | 150.7 ± 25.5 a |
| Phosphorus | 2.1 ± 0.7 a | 1.6 ± 0.4 a | 12.4 ± 3.2 a | 9.6 ± 2.1 a |
| Potassium | 23.1 ± 6.7 a | 9.3 ± 4.8 b | 134.1 ± 32.6 a | 56.4 ± 22.6 b |
| Calcium | 7.7 ± 3.0 a | 2.8 ± 1.7 b | 44.5 ± 15.4 a | 17.1 ± 8.2 b |
| Magnesium | 2.5 ± 0.7 a | 2.3 ± 0.3 a | 14.3 ± 3.2 a | 14.6 ± 2.6 a |
| mg kg−1 | g ha−1 | |||
| Boron | 16.1 ± 0.7 a | 8.4 ± 1.1 b | 94.3 ± 2.5 a | 52.4 ± 6.7 b |
| Iron | 133.3 ± 14.3 a | 92.8 ± 68.5 a | 783.9 ± 100.5 a | 554.5 ± 345.2 a |
| Manganese | 288.6 ± 112.6 a | 43.7 ± 7.8 b | 1706.0 ± 710.6 a | 271.4 ± 23.8 b |
| Zinc | 27.1 ± 8.1 a | 31.6 ± 8.9 a | 157.0 ± 40.2 a | 200.8 ± 64.8 a |
| Copper | 6.8 ± 1.1 a | 8.9 ± 2.3 a | 39.6 ± 5.4 a | 56.3 ± 16.5 a |
| 13 June 2019 | 31 October 2019 | |||||
|---|---|---|---|---|---|---|
| N Kjeldahl | EOC | TOC | N Kjeldahl | EOC | TOC | |
| g kg−1 | g kg−1 | |||||
| Control | 1.0 ± 0.09 b | 17.6 ± 0.48 b | 20.1 ± 2.30 b | 1.1 ± 0.10 b | 23.2 ± 0.67 a | 24.5 ± 1.62 b |
| Lupine | 1.3 ± 0.10 a | 22.4 ± 1.25 a | 25.3 ± 0.73 a | 1.6 ± 0.07 a | 24.2 ± 0.79 a | 27.7 ± 1.31 a |
| Vetch | 1.3 ± 0.03 a | 22.0 ± 1.19 a | 26.0 ± 1.80 a | 1.5 ± 0.09 a | 24.0 ± 0.44 a | 27.6 ± 0.88 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimundo, S.; Arrobas, M.; Ribeiro, A.C.; Rodrigues, M.Â. White Lupin and Hairy Vetch as Green Manures: Impacts on Yield and Nutrient Cycling in an Organic Almond Orchard. Agronomy 2025, 15, 1974. https://doi.org/10.3390/agronomy15081974
Raimundo S, Arrobas M, Ribeiro AC, Rodrigues MÂ. White Lupin and Hairy Vetch as Green Manures: Impacts on Yield and Nutrient Cycling in an Organic Almond Orchard. Agronomy. 2025; 15(8):1974. https://doi.org/10.3390/agronomy15081974
Chicago/Turabian StyleRaimundo, Soraia, Margarida Arrobas, António Castro Ribeiro, and Manuel Ângelo Rodrigues. 2025. "White Lupin and Hairy Vetch as Green Manures: Impacts on Yield and Nutrient Cycling in an Organic Almond Orchard" Agronomy 15, no. 8: 1974. https://doi.org/10.3390/agronomy15081974
APA StyleRaimundo, S., Arrobas, M., Ribeiro, A. C., & Rodrigues, M. Â. (2025). White Lupin and Hairy Vetch as Green Manures: Impacts on Yield and Nutrient Cycling in an Organic Almond Orchard. Agronomy, 15(8), 1974. https://doi.org/10.3390/agronomy15081974








