Field-Based Assessment of Soil Salinity and Alkalinity Stress on Growth and Biochemical Responses in Eggplant (Solanum melongena L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Experimental Conditions and Soil–Plant Material
2.2. Methods
2.2.1. Soil Analyses
2.2.2. Plant Quality Parameters
2.2.3. Macro and Micro Element Analyses in Plants
2.2.4. Physiological Analyses
Hydrogen Peroxide (H2O2) Activity
Lipid Peroxidation (MDA) content
Antioxidant Enzyme Activities
Catalase (CAT; EC 1.11.1.6) Activity
Superoxide Dismutase (SOD; EC 1.15.1.1) Activity
Ascorbate Peroxidase (APX; EC 1.11.1.11) Activity
Proline
2.2.5. Statistical Analysis
3. Results
3.1. Plant Growth
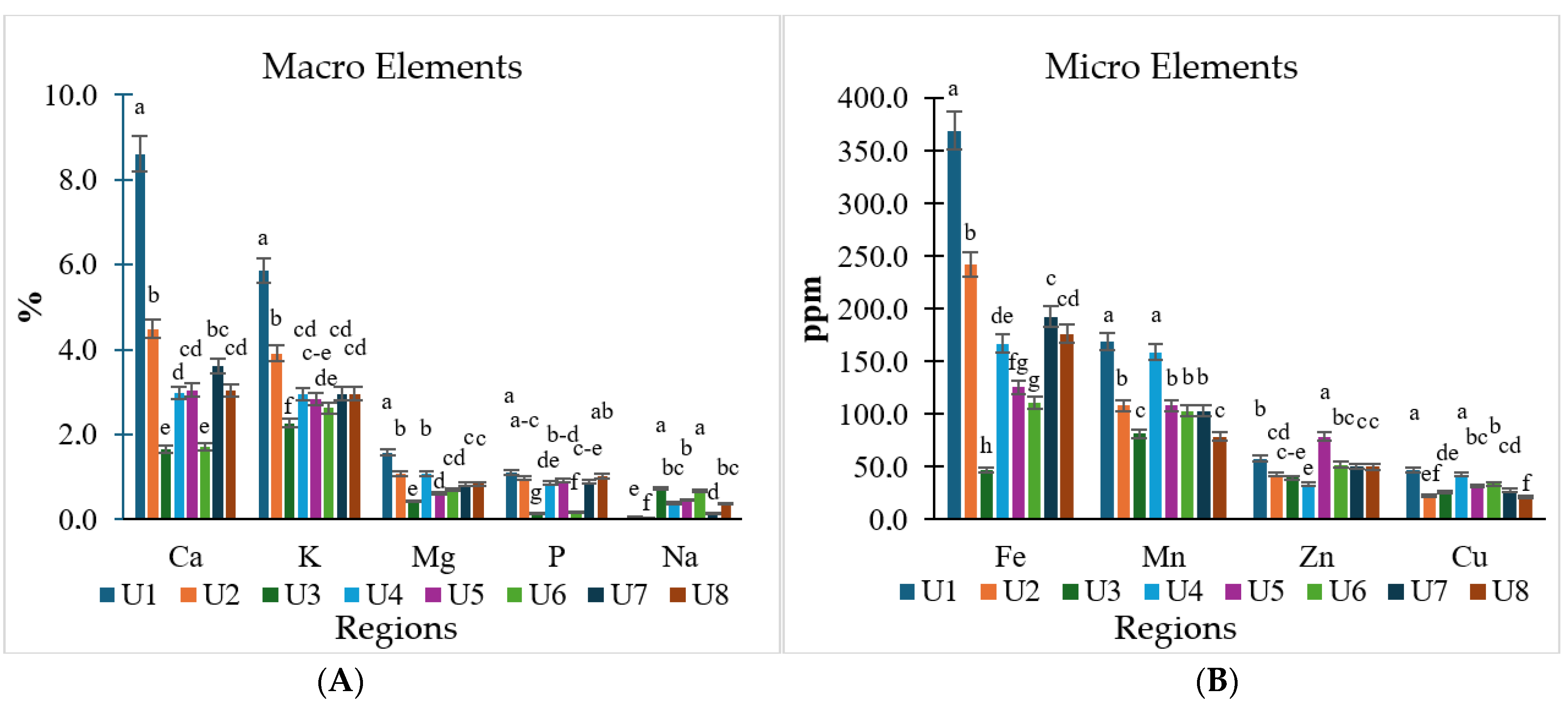
3.2. Nutrient Uptake
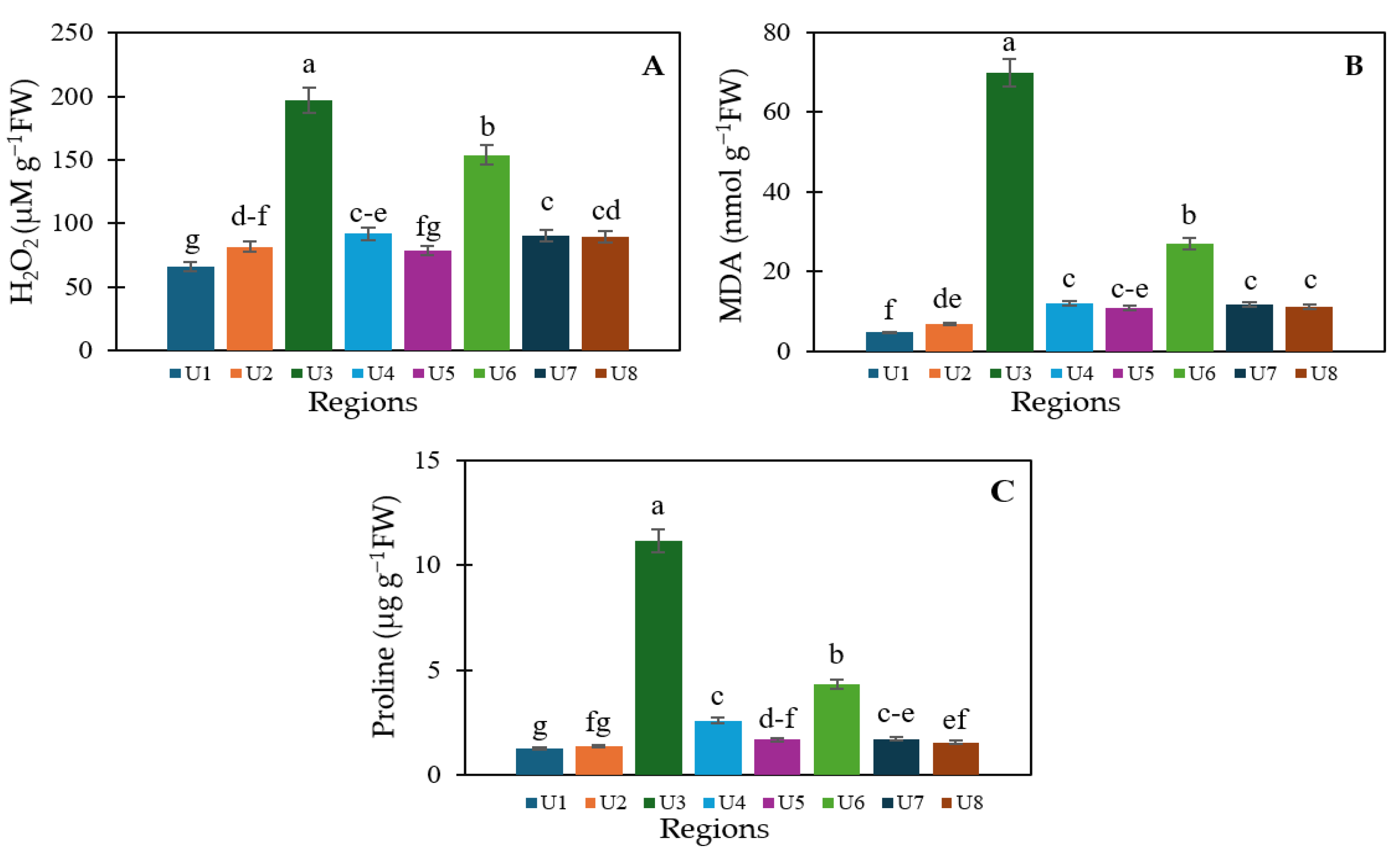
3.3. Cellular Damage Level
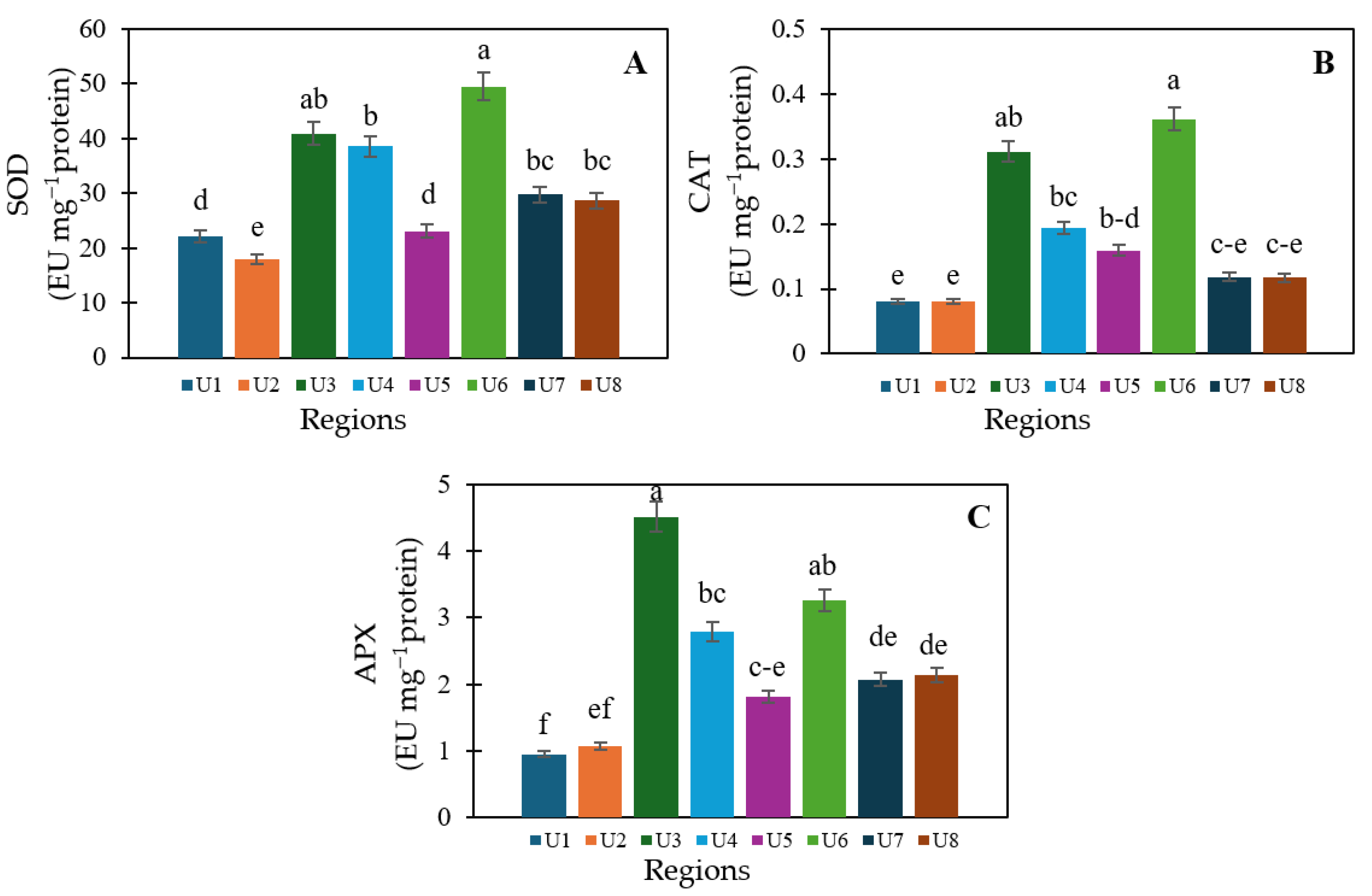
3.4. Antioxidant Capacity
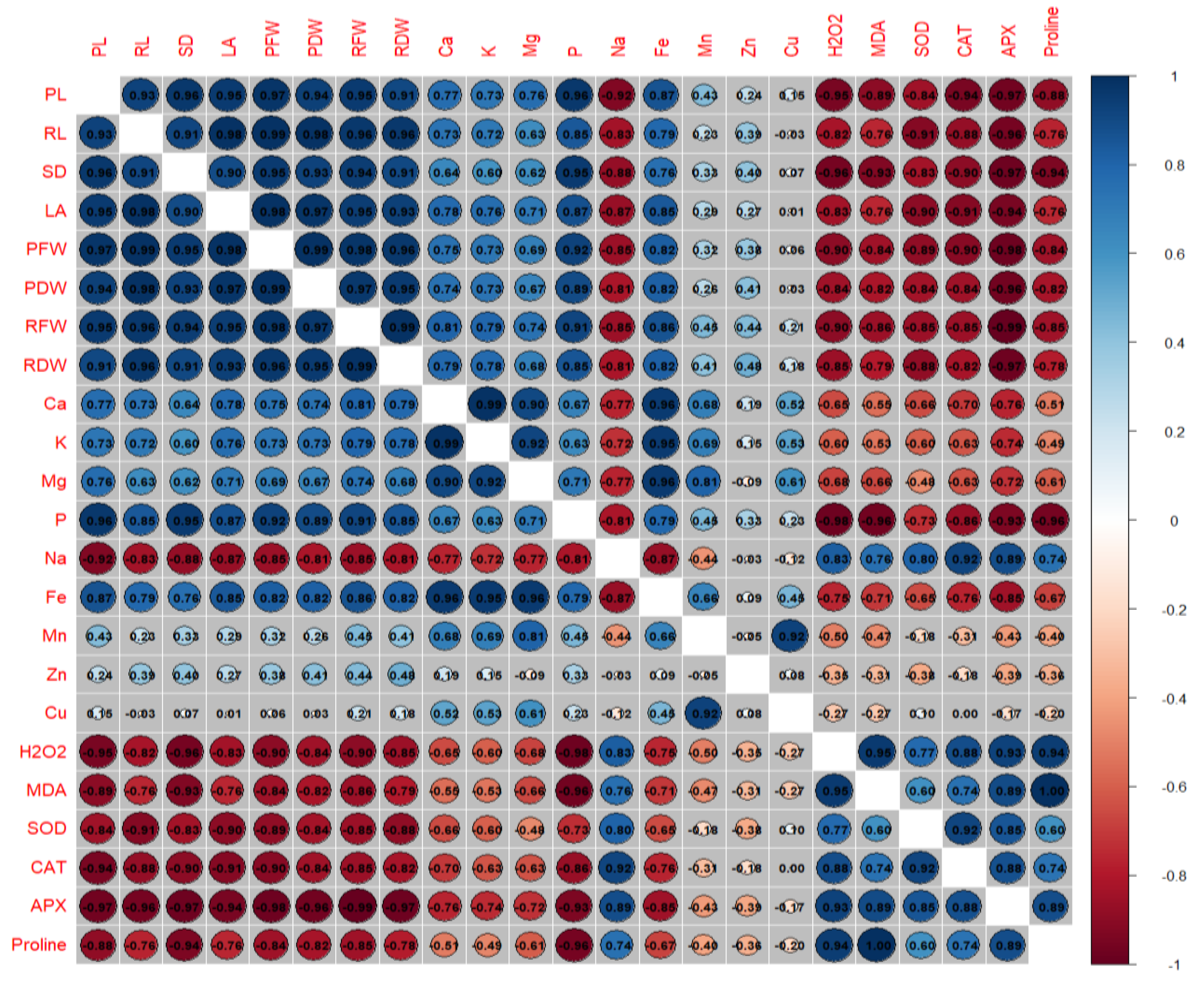
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, H.B.; Chuc, L.Y.; Shao, M.A.; Jaleeld, C.A.; Mi, H.M. Higher plant antioxidants and redox signaling under environmental stresses. C. R. Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Pascual, L.S.; Segarra-Medina, C.; Gomez-Cadenas, A.; Lopez-Climent, M.F.; Vives-Peris, V.; Zandalinas, S.I. Climate change-associated multifactorial stress combination: A present challenge for our ecosystems. J. Plant Physiol. 2022, 276, 153764. [Google Scholar] [CrossRef]
- Greene, R.; Timms, W.; Rengasamy, P.; Arshad, M.; Cresswell, R. Soil and Aquifer Salinization: Toward an Integrated Approach for Salinity Management of Groundwater. In Integrated Groundwater Management; Jakeman, A.J., Barreteau, O., Hunt, R.J., Rinaudo, J.D., Ross, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 377–412. [Google Scholar] [CrossRef]
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Kol, C. Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Front. Plant Sci. 2022, 12, 787292. [Google Scholar] [CrossRef]
- Lopez-Delacall, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization of proline, ascorbate and oxidative stress pathways under the combination of salinity and heat in tomato plants. Environ. Exp. Bot. 2021, 183, 104351. [Google Scholar] [CrossRef]
- Raja, V.; Wani, U.M.; Wani, Z.A.; Jan, N.; Kottakota, C.; Reddy, M.K.; Kaul, T.; John, R. Pyramiding ascorbate–glutathione pathway in Lycopersicum Esculentum confers tolerance to drought and salinity stress. Plant Cell Rep. 2022, 41, 619–637. [Google Scholar] [CrossRef]
- Dustgeer, Z.; Seleiman, M.F.; Khan, I.; Chattha, M.U.; Alı, E.F.; Alhammad, B.A.; Jalal, R.S.; Hassan, M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12248. [Google Scholar] [CrossRef]
- Akbaba, M.; Özden, E. Salt tolerance of endophytic root bacteria and their effects on seed germination and viability on tomato plants. Braz. J. Microbiol. 2023, 54, 3147–3162. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.; Bor, M.; Ozdemir, F.; Türkan, I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 8, 2669–2681. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Mohammed, M.; Daba, A.W.; Hailu, B.; Belay, G.; Tesfaye, A.; Ertebo, T.M. Improving agricultural productivity on salt-affected soils in Ethiopia: Farmers’ perceptions and proposals. Afr. J. Agric. Res. 2019, 14, 897–906. [Google Scholar]
- Daliakopoulos, I.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.; Varouchakis, E.; Karatzas, G.; Ritsema, C. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Temel, S.; Şimşek, U. Desertification Process of Igdır Plain Soils and Solution Suggestions. Alinteri J. Agr. Sci. 2011, 21, 53–59. Available online: https://dergipark.org.tr/tr/download/article-file/26282 (accessed on 15 June 2025).
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stress: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Talhouni, M.; Sönmez, K.; Ellialtıoğlu, Ş.; Kuşvuran, Ş. Analysis of some plant and fruit characteristics of grafted eggplants grown under salinity stress. Acad. J. Agric. 2017, 6, 71–80. Available online: https://dergipark.org.tr/en/pub/azd/issue/32275/363327 (accessed on 15 June 2025).
- FAOSTAT. The Global Status of Vegetable Production, Trade and Utilization. Crop Production Manual. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 June 2025).
- Fiza, A.; Chugh, V.; Mishra, A.C.; Purwaet, S.; Dwived, S.V.; Mishra, A.; Nagar, S.; Kharor, N.; Kumar, V. Physiological and biochemical adaptations for salt stress tolerance in eggplant (Solanum melongena). Plant Physiol. Rep. 2025, 30, 352–368. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; van Breusegem, F. Reactive oxygen species signaling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- USDA. Soil Survey Manual No: 18; Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 2017; pp. 120–130. [Google Scholar]
- USDA. Diagnosis and Improvement of Saline and Alkali Soils No: 60; Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1954; pp. 4–6. [Google Scholar]
- Gee, G.W.; Or, D. Particle-size analysis. In Methods of Soil Analysis, Part 4. Physical Methods, SSSA Book Series 5; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Chemical Methods “Carbonate and gypsum”. In Methods of Soil Analysis, Part 3. SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3. Chemical Methods, SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3. Chemical Methods, SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3. Chemical Methods, SSSA Book Series 5; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; Agronomy Monographs; American Society of Agronomy: Fitchburg, WI, USA, 1982; Volume 9, pp. 149–157. [Google Scholar]
- Rhoades, J.D. Exchangeable cations. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Fitchburg, WI, USA, 1982; Volume 9, pp. 159–164. [Google Scholar]
- Mertens, D. Plants Preparation of Laboratory Sample. In Official Methods of Analysis, AOAC Official Method 922.02, Chapter 3, 22nd ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC-International: Gaitherburg, MD, USA, 2005; pp. 1–2. [Google Scholar]
- Mertens, D. Metal in Plants and Pet Foods. In Official Methods of Analysis, AOAC Official Method 922.02, Chapter 3, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC-International: Gaitherburg, MD, USA, 2005; pp. 3–4. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes, Possible Mechanism of Water Stress Tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Liman, F.; Aouani, M.E. Changes in ascorbate peroxidase, catalase, guaicol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Abdalla, M.M.; El-Khoshiban, N.H. The influence of water stress on growth. relative water content, photosynthetic pigments, some metabolic and hormonal contents of two Triticium aestivum cultivars. J. Appl. Sci. Res. 2007, 3, 2062–2074. [Google Scholar]
- Kurum, R.; Ulukapı, K.; Aydınşakir, K.; Onus, N.A. The influence of salinity on seedling growth of some pumpkin varieties used as rootstock. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 219–225. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Jameel, J.; Anwar, T.; Majeed, S.; Qureshi, H.; Siddiqi, E.H.; Sana, S.; Zaman, W.; Ali, H.M. Effect of salinity on growth and biochemical responses of brinjal varieties: Implications for salt tolerance and antioxidant mechanisms. BMC Plant Biol. 2024, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.; Marco, A.; Ulery, A.P.; Zohrab, S.; Picchioni, G.; Flynn, R.P. Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: I growth and yield. Trop. Subtrop. Agroecosystems 2011, 14, 137–147. Available online: https://www.redalyc.org/articulo.oa?id=93915703012 (accessed on 18 June 2025).
- Kıran, S.; Ateş, Ç.; Kuşvuran, Ş.; Sönmez, K.; Ellialtıoğlu, Ş. Investigation of changes on some morphological characteristics in different eggplant scion/rootstock combinations under salt and drought stresses. J. Inst. Sci. Technol. 2017, 7, 43–54. Available online: https://dergipark.org.tr/en/pub/jist/issue/34625/389680 (accessed on 18 June 2025). [CrossRef]
- Giuffrida, F.; Martorana, M.; Leonardi, C. How sodium chloride concentration in the nutrient solution influences the mineral composition of tomato leaves and fruit. HortScience 2009, 44, 707–711. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Aust. J. Plant Physiol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Á.; Prohens, J.; Plazas, M. Physiological and Biochemical Responses to Salt Stress in Cultivated Eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy 2020, 10, 651. [Google Scholar] [CrossRef]
- Talubaghi, M.J.; Daliri, M.S.; Mazloum, P.; Rameeh, V.; Mousavi, A. Effect of salt stress on growth, physiological and biochemical parameters and activities of antioxidative enzymes of rice cultivars. Cereal Res. Commun. 2023, 51, 403–411. [Google Scholar] [CrossRef]
- Dikobe, T.B.; Mashile, B.; Sinthumule, R.R.; Ruzvidzo, O. Distinct morpho-physiological responses of maize to salinity stress. Am. J. Plant Sci. 2021, 12, 946–959. [Google Scholar] [CrossRef]
- Asraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora-Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Türkan, I.; Bor, M.; Ozdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought sensitive P. vulgaris L. subjected to polyethylene glycol mediates water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Hu, H.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Sharkey, T.D.; Lu, J. The reduction in leaf area precedes that in photosynthesis under potassium deficiency: The importance of leaf anatomy. New Phytol. 2020, 227, 1749–1763. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.; Gruda, N. Influence of Grafting on Growth, Development and some Physio-logical Parameters of Tomatoes under Controlled Heat Stress Conditions. Eur. J. Hortic. Sci. 2009, 74, 16–20. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20093109326 (accessed on 18 June 2025). [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodríguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific Hybridization between Eggplant and Wild Relatives from Different Genepools. J. Am. Soc. Hortic. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Mozafarian, M.; Hawrylak-Nowak, B.; Kappel, N. Effect of Different Rootstocks on the Salt Stress Tolerance and Fruit Quality of Grafted Eggplants (Solanum melongena L.). Plants 2023, 12, 3631. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Cebeci, E.; Boyaci, H.F.; Kiran, S.; Ellialtioglu, S.S. Comprehensive assessment to reveal the salt tolerance potential of cultivated eggplants and their wild relatives. Front. Plant Sci. 2025, 16, 1483409. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Albero, N.; Adalid-Martínez, A.M.; Castell-Zeising, V.; Raigón, M.D.; Rodríguez-Burruezo, A.; Fita, A. Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment. Agriculture 2024, 14, 871. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. A calcium sensor homolog required for plant salt tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Suarez, D.L.; Celis, N.; Ferreira, J.F.; Reynolds, T.; Sandhu, D. Linking genetic determinants with salinity tolerance and ion relationships in eggplant, tomato and pepper. Sci. Rep. 2021, 11, 16298. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H. Comparison of growth and mineral accumulation of two solanaceous species Solanum scabrum Mill. (huckleberry) and S. melongena L. (eggplant), under salinity stress. Soil Sci. Plant Nutr. 2013, 59, 912–920. [Google Scholar] [CrossRef]
- Hannachi, S.; van Labeke, M.C. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 181. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19950708651 (accessed on 18 June 2025).
- Zhan, X.Y.; Ren, Y.; Zhang, S.X.; Kang, R.F. Changes in Olsen phosphorus concentration and its response to phosphorus balance in the main types of soil in China. Sci. Agric. Sin. 2015, 48, 4728–4737. [Google Scholar]
- Kacar, B.; Katkat, A.V. Plant Nutrition, 5th ed.; Nobel Publications: Ankara, Türkiye, 2010; pp. 250–253. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Özdamar, F.Ö.; Furtana, G.B.; Tipirdamaz, R.; Duman, H. H2O2 and NO mitigate salt stress by regulating antioxidant enzymes in in two eggplant (Solanum melongena L.) genotypes. Soil Stud. 2022, 11, 1–6. [Google Scholar] [CrossRef]
- Perveen, T.; Nawaz, K. Modulation of morpho-physiological and biochemical attributes of Solanum melongena L.(brinjal) by exogenous application of thiourea under salinity stress. Pak. J. Bot. 2021, 53, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kul, R. Integrated application of plant growth promoting rhizobacteria and biochar improves salt tolerance in eggplant seedlings. Turk. J. Agric. For. 2022, 46, 677–702. [Google Scholar] [CrossRef]
- Yaşar, F.; Ellialtıoğlu, Ş.; Özpay, T.; Uzal, Ö. The Effect of Salt Stress on Antioksidative Enzyme (SOD, CAT, APX and GR) Activity in Watermelon (Citrullus lanatus (Thunb.) Mansf.). Yuz. Yıl Univ. J. Agric. Sci. 2008, 18, 61–65. Available online: https://dergipark.org.tr/en/pub/yyutbd/issue/21987/236079#article_cite (accessed on 15 June 2025).
- Ábrahám, E.; Rigó, G.; Székely, G.; Nagy, R.; Koncz, C. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef]
- Ortega-Albero, N.; González-Orenga, S.; Vicente, O.; Rodríguez-Burruezo, A.; Fita, A. Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants 2023, 12, 295. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt Stress and Phyto-biochemical Responses of Plant-a Review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N. Ameliorative Effects of Proline on Salt Stress-induced Lipid Peroxidation in Cell Lines of Groundnut (Arachis hypogaea L.). Plant Cell Rep. 2001, 20, 463–468. [Google Scholar] [CrossRef]
- Vijayan, K. Approaches for Enhancing Salt Tolerance in Mulberry (Morus L)-A review. Plant Omics J. 2009, 2, 41–59. Available online: https://www.pomics.com/Vijayan_January2009_2_1_41_59.pdf (accessed on 15 June 2025).
- Lima-Costa, M.E.; Ferreira, S.; Duarte, A.; Ferreira, A.L. Alleviation of Salt Stress Using Exogenous Proline on a Citrus Cell Line. Acta Hortic. 2010, 868, 109–112. [Google Scholar] [CrossRef]
- Al Hassan, M.; López-Gresa, M.D.P.; Boscaiu, M.; Vicente, O. Stress Tolerance Mechanisms in Juncus: Responses to Salinity and Drought in Three Juncus Species Adapted to Different Natural Environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef]
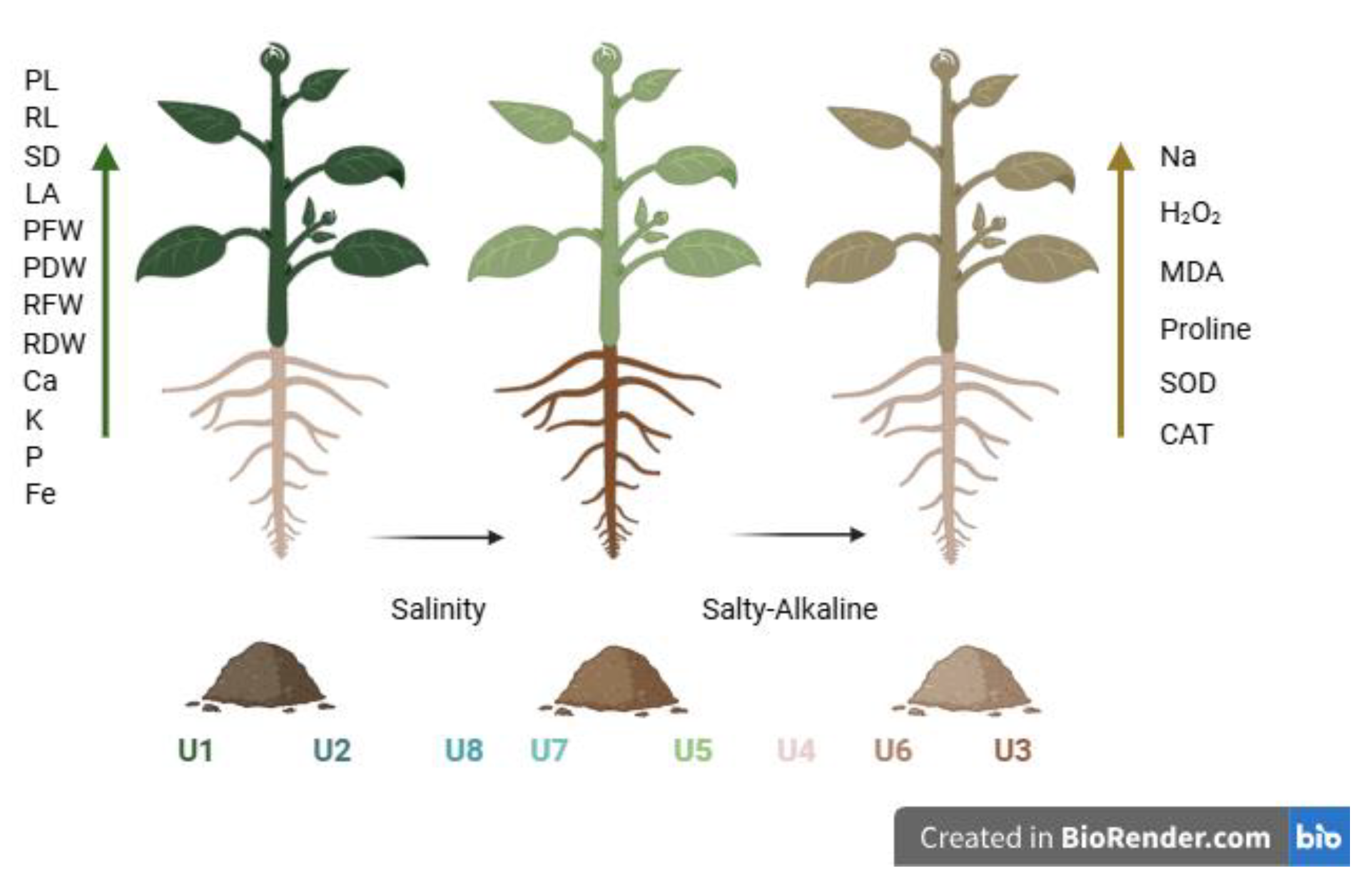
| Clay (%) | Silt (%) | Sand (%) | Texture Class ▲ | CaCO3 (%) | OM (%) | CEC, (me100 g−1) | Exchangeable Cations (me100 g−1) | Soluble Na (me100 g−1) | pHe Ψ | ECe Ψ (dS m−1) | ESP (%) | Salinity Class § | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | |||||||||||||
| U1 | 36.6 | 39.0 | 24.4 | Clay Loam | 11.12 | 1.32 | 28.2 | 1.3 | 1.7 | 20.8 | 9.1 | 0.18 | 8.27 | 0.88 | 3.8 | Normal |
| U2 | 34.5 | 41.1 | 24.4 | Clay Loam | 10.93 | 1.18 | 26.6 | 2.3 | 1.6 | 19.6 | 1.0 | 1.0 | 8.10 | 3.60 | 4.9 | Normal |
| U3 | 32.5 | 43.2 | 24.3 | Clay Loam | 13.81 | 0.62 | 21.4 | 45.6 | 1.9 | 14.1 | 3.0 | 30.2 | 10.41 | 67.80 | 71.9 | Saline-sodic |
| U4 | 24.3 | 47.4 | 28.3 | Loam | 11.22 | 0.15 | 28.2 | 8.9 | 1.4 | 16.7 | 6.8 | 3.8 | 8.68 | 7.88 | 18.0 | Saline-sodic |
| U5 | 30.6 | 39.1 | 30.3 | Clay Loam | 10.41 | 0.89 | 23.7 | 4.7 | 1.6 | 14.6 | 7.1 | 1.72 | 8.39 | 6.44 | 12.6 | Saline |
| U6 | 28.5 | 37.1 | 34.4 | Clay Loam | 11.96 | 0.78 | 21.8 | 14.9 | 1.9 | 16.4 | 6.1 | 3.9 | 11.72 | 11.87 | 50.5 | Saline-sodic |
| U7 | 18.1 | 37.2 | 44.7 | Loam | 11.38 | 0.49 | 19.5 | 2.1 | 0.7 | 16.9 | 6.4 | 0.1 | 8.35 | 7.5 | 10.3 | Saline |
| U8 | 26.4 | 39.2 | 34.3 | Loam | 12.09 | 0.92 | 24.2 | 1.6 | 1.0 | 14.4 | 6.2 | 0.1 | 8.42 | 4.9 | 6.3 | Saline |
| Region | PL (cm) | Change (%) | RL (cm) | Change (%) | SD (cm) | Change (%) | LA (mm2) | Change (%) |
|---|---|---|---|---|---|---|---|---|
| U1 | 153.1 a | 0.0 | 73.9 a | 0.00 | 1.49 a | 0.00 | 406.9 a | 0.00 |
| U2 | 143.5 b | −6.27 | 77.6 a | +5.01 | 1.52 a | +2.01 | 397.7 a | −2.26 |
| U3 | 34.6 h | −77.40 | 20.3 e | −72.53 | 0.81 e | −45.64 | 23.0 f | −94.35 |
| U4 | 101.9 ef | −33.44 | 33.1 d | −55.21 | 1.22 c | −18.12 | 135.5 d | −66.70 |
| U5 | 111.4 de | −27.24 | 59.1 b | −20.03 | 1.43 ab | −4.03 | 239.3 c | −41.19 |
| U6 | 62.2 g | −59.37 | 31.0 d | −58.05 | 1.08 d | −27.52 | 67.5 e | −83.41 |
| U7 | 120.0 c–e | −21.62 | 49.9 c | −32.48 | 1.44 ab | −3.36 | 221.1 c | −45.66 |
| U8 | 128.4 bc | −16.13 | 61.2 b | −17.19 | 1.39 ab | −6.71 | 325.6 b | −19.98 |
| Region | PFW (g) | Change (%) | PDW (g) | Change (%) | RFW (g) | Change (%) | RDW (g) | Change (%) |
|---|---|---|---|---|---|---|---|---|
| U1 | 403.7 a | 0.00 | 86.9 a | 0.00 | 113.8 a | 0.00 | 31.5 a | 0.00 |
| U2 | 392.3 ab | −2.82 | 82.6 ab | −4.95 | 100.7 b | −11.51 | 29.6 a | −6.03 |
| U3 | 16.9 h | −95.81 | 5.2 h | −94.02 | 6.0 g | −94.73 | 1.9 e | −93.97 |
| U4 | 170.1 f | −57.87 | 29.8 g | −65.71 | 49.4 ef | −56.59 | 11.5 d | −63.49 |
| U5 | 298.8 d | −25.99 | 61.4 cd | −29.34 | 83.4 bc | −26.71 | 25.0 b | −20.64 |
| U6 | 115.4 g | −71.41 | 34.2 fg | −60.64 | 40.8 f | −64.13 | 9.9 d | −68.57 |
| U7 | 250.7 e | −37.90 | 52.7 e | 39.36 | 68.0 d | −40.25 | 16.6 c | −47.30 |
| U8 | 328.5 cd | −22.89 | 71.2 bc | −18.07 | 72.0 cd | −36.73 | 18.0 bc | −42.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özden, E.; Tohumcu, F.; Sarı, S. Field-Based Assessment of Soil Salinity and Alkalinity Stress on Growth and Biochemical Responses in Eggplant (Solanum melongena L.). Agronomy 2025, 15, 1945. https://doi.org/10.3390/agronomy15081945
Özden E, Tohumcu F, Sarı S. Field-Based Assessment of Soil Salinity and Alkalinity Stress on Growth and Biochemical Responses in Eggplant (Solanum melongena L.). Agronomy. 2025; 15(8):1945. https://doi.org/10.3390/agronomy15081945
Chicago/Turabian StyleÖzden, Eren, Faruk Tohumcu, and Serdar Sarı. 2025. "Field-Based Assessment of Soil Salinity and Alkalinity Stress on Growth and Biochemical Responses in Eggplant (Solanum melongena L.)" Agronomy 15, no. 8: 1945. https://doi.org/10.3390/agronomy15081945
APA StyleÖzden, E., Tohumcu, F., & Sarı, S. (2025). Field-Based Assessment of Soil Salinity and Alkalinity Stress on Growth and Biochemical Responses in Eggplant (Solanum melongena L.). Agronomy, 15(8), 1945. https://doi.org/10.3390/agronomy15081945






