Effects of Long-Term Cotton Straw Return on Soil Carbon and Bacterial Community in Topsoil and Deep Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experiment Design and Management
2.3. Soil Sampling and Analysis
2.4. Soil Incubation
2.5. Soil Extracellular Enzyme Activity
2.6. Total Soil Bacterial DNA Extraction and 16S rRNA Sequencing
2.7. Co-Occurrence Analysis
2.8. Data Analysis
3. Results
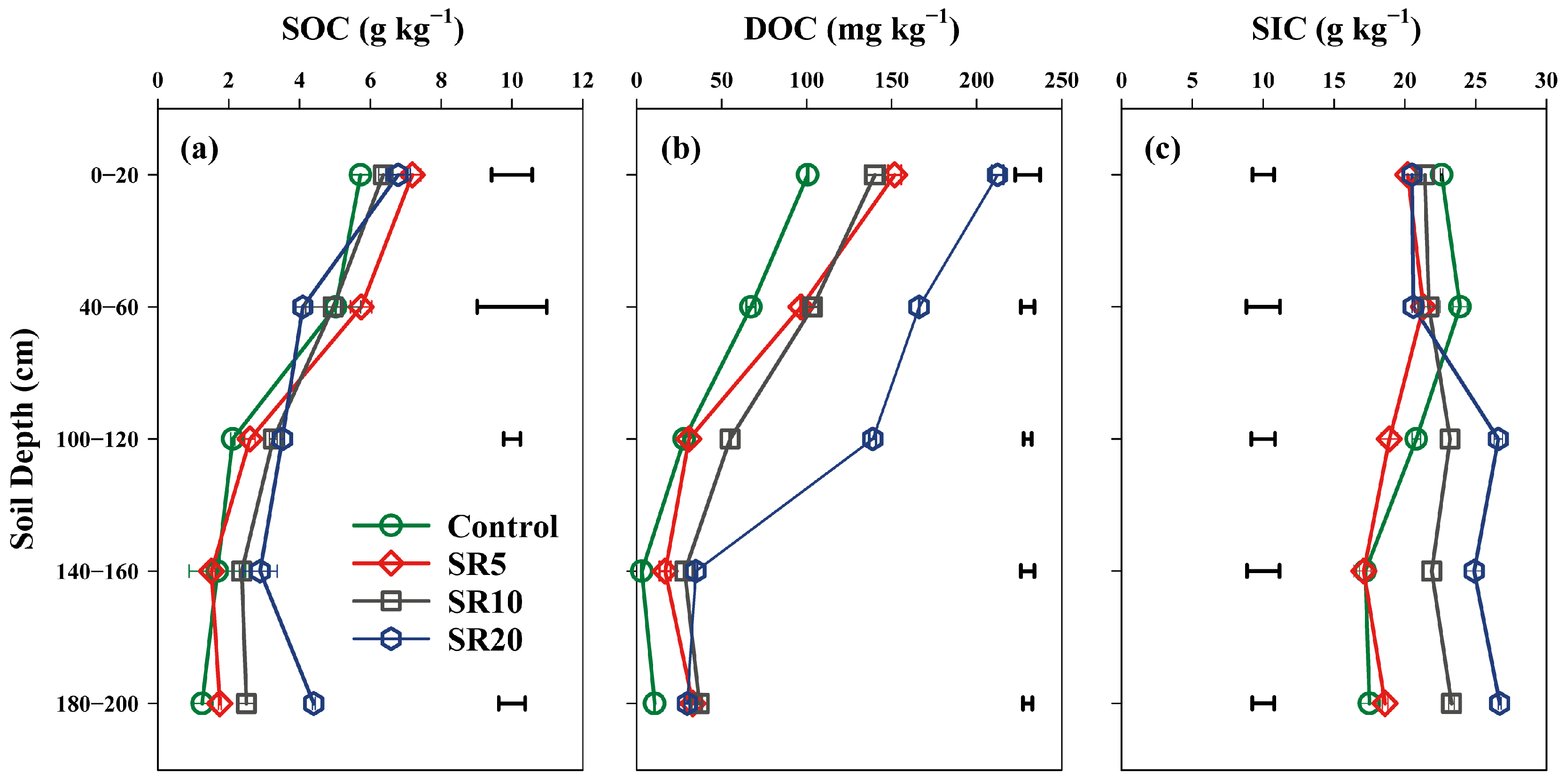
3.1. Impact of Straw Return Years on Soil Carbon
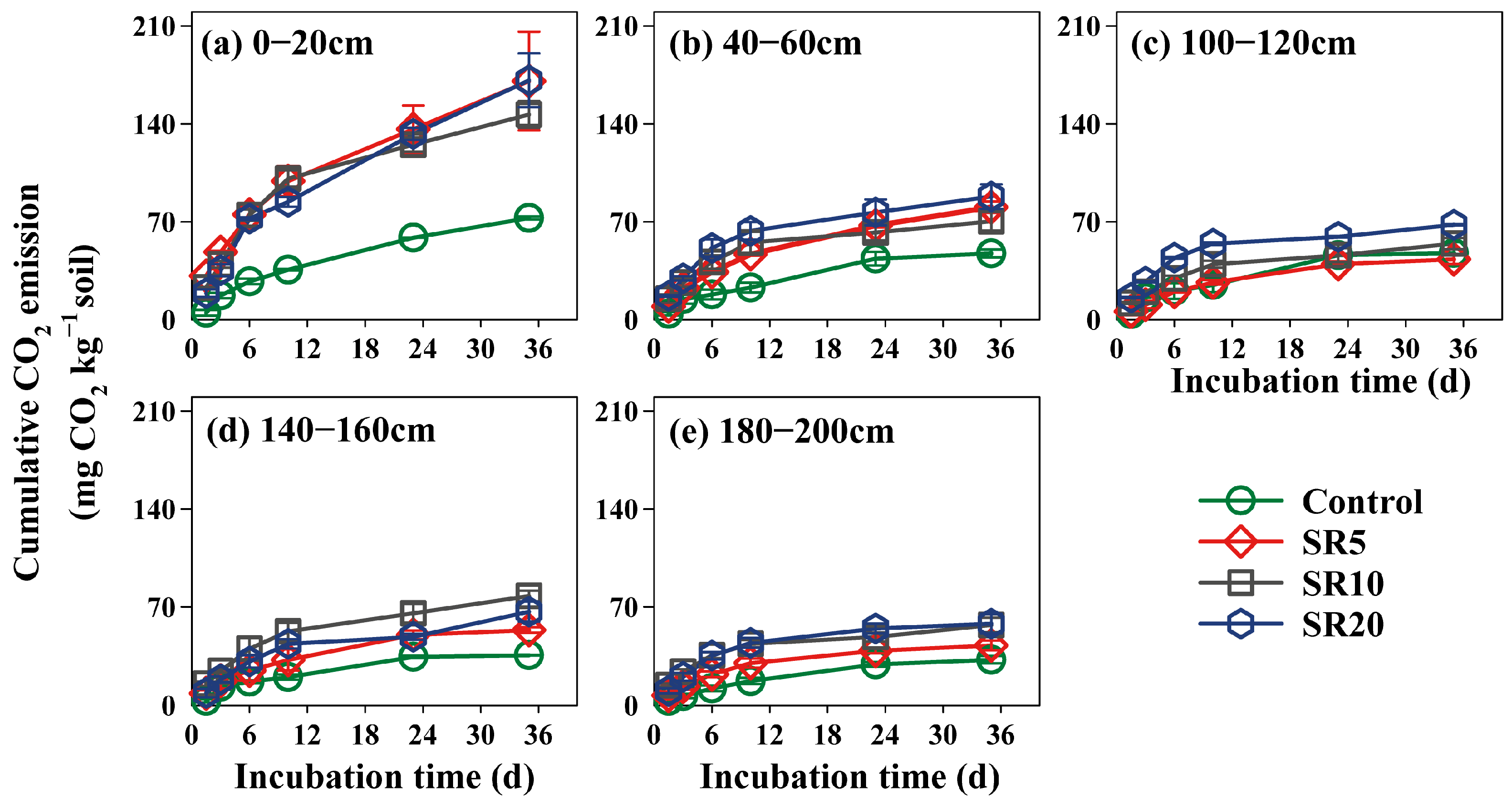
3.2. Cumulative CO2 Emission from Soil Incubation
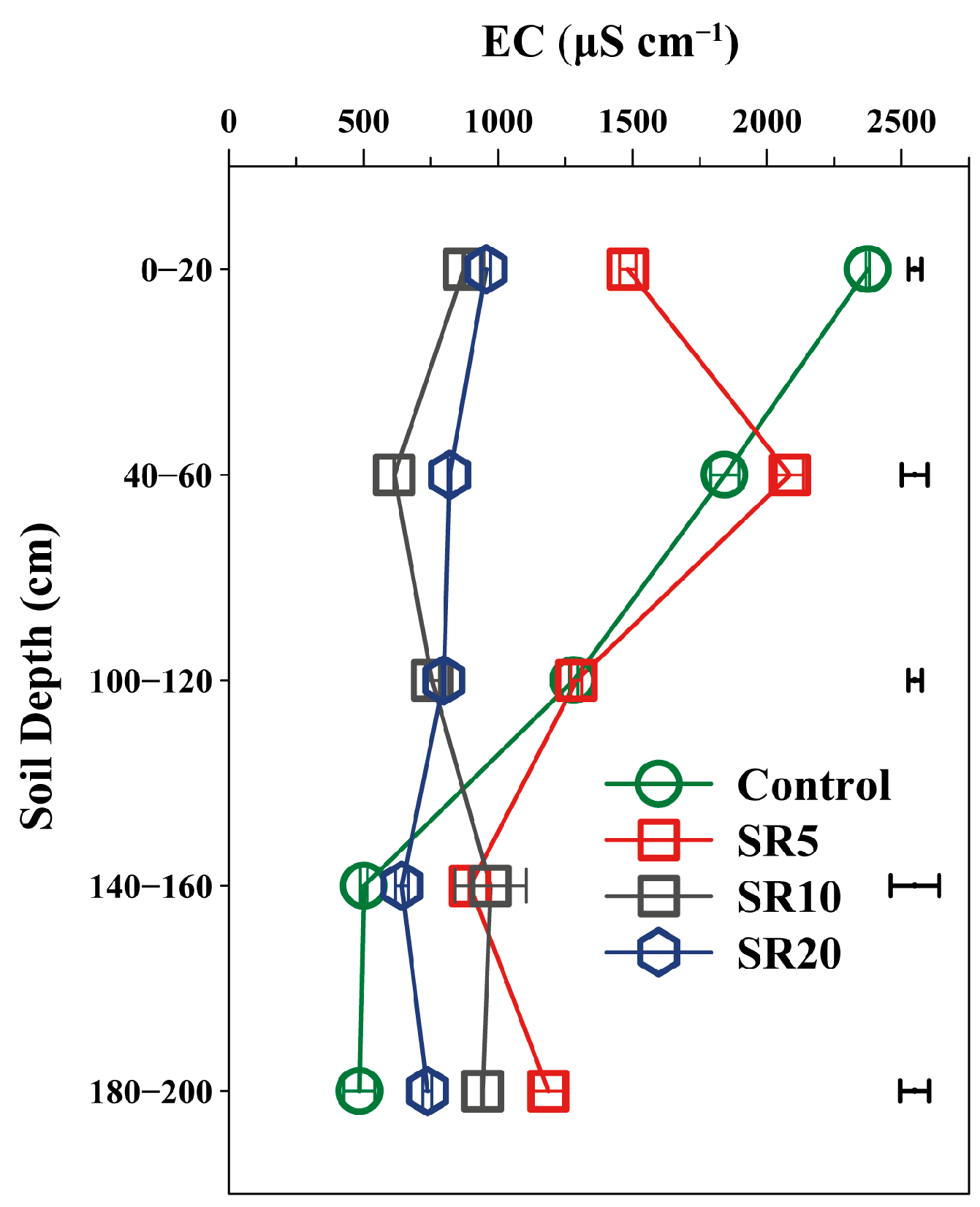
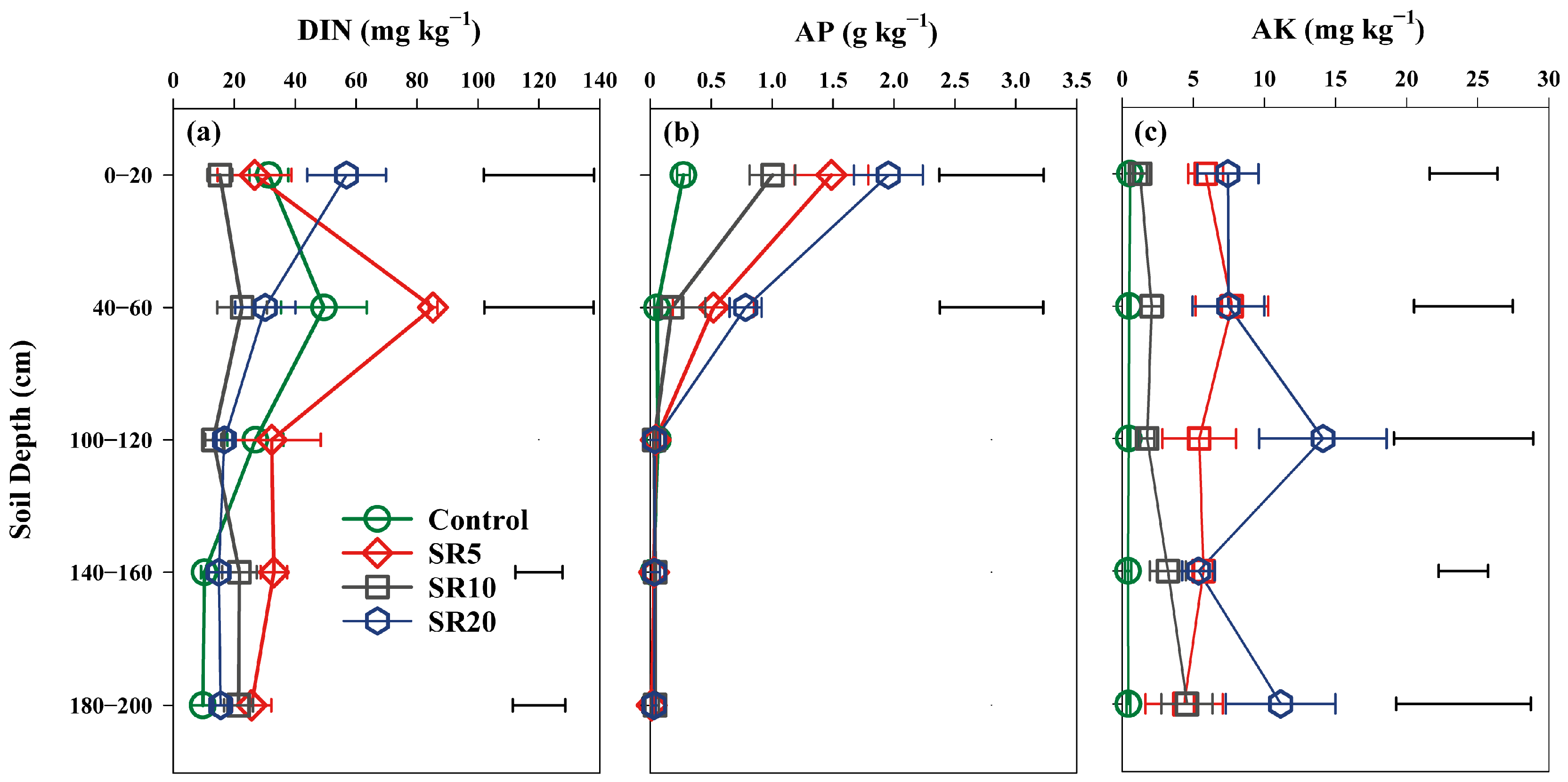
3.3. Effects of Straw Return on Soil Properties
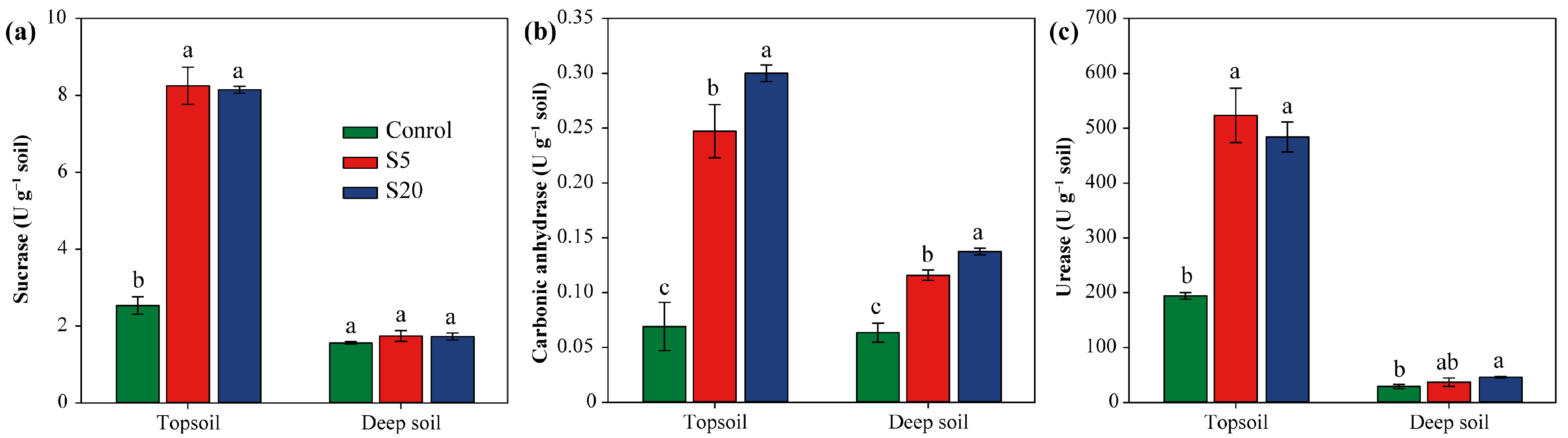
3.4. Soil Enzyme Activities of Topsoil and Deep Soil
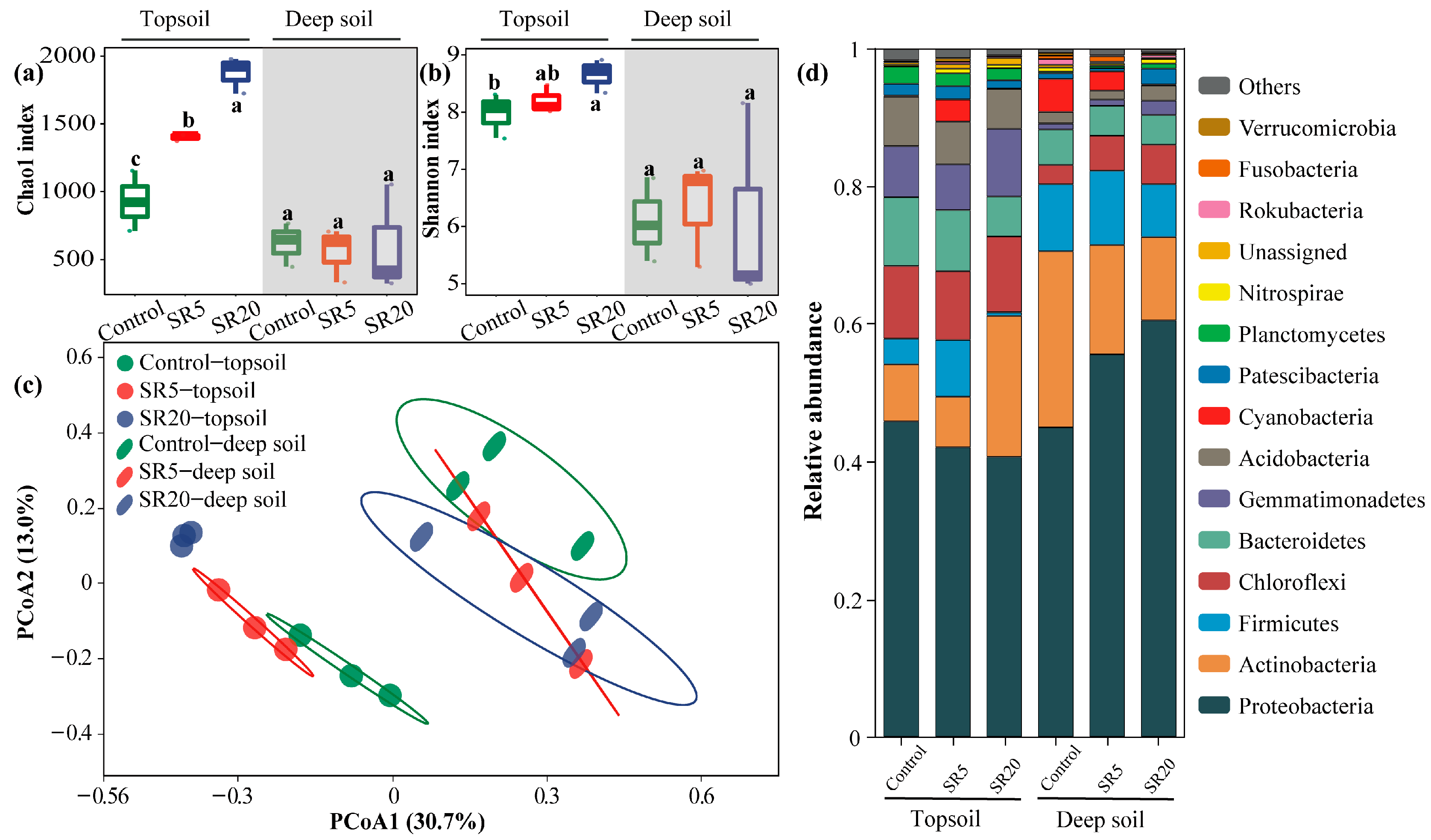
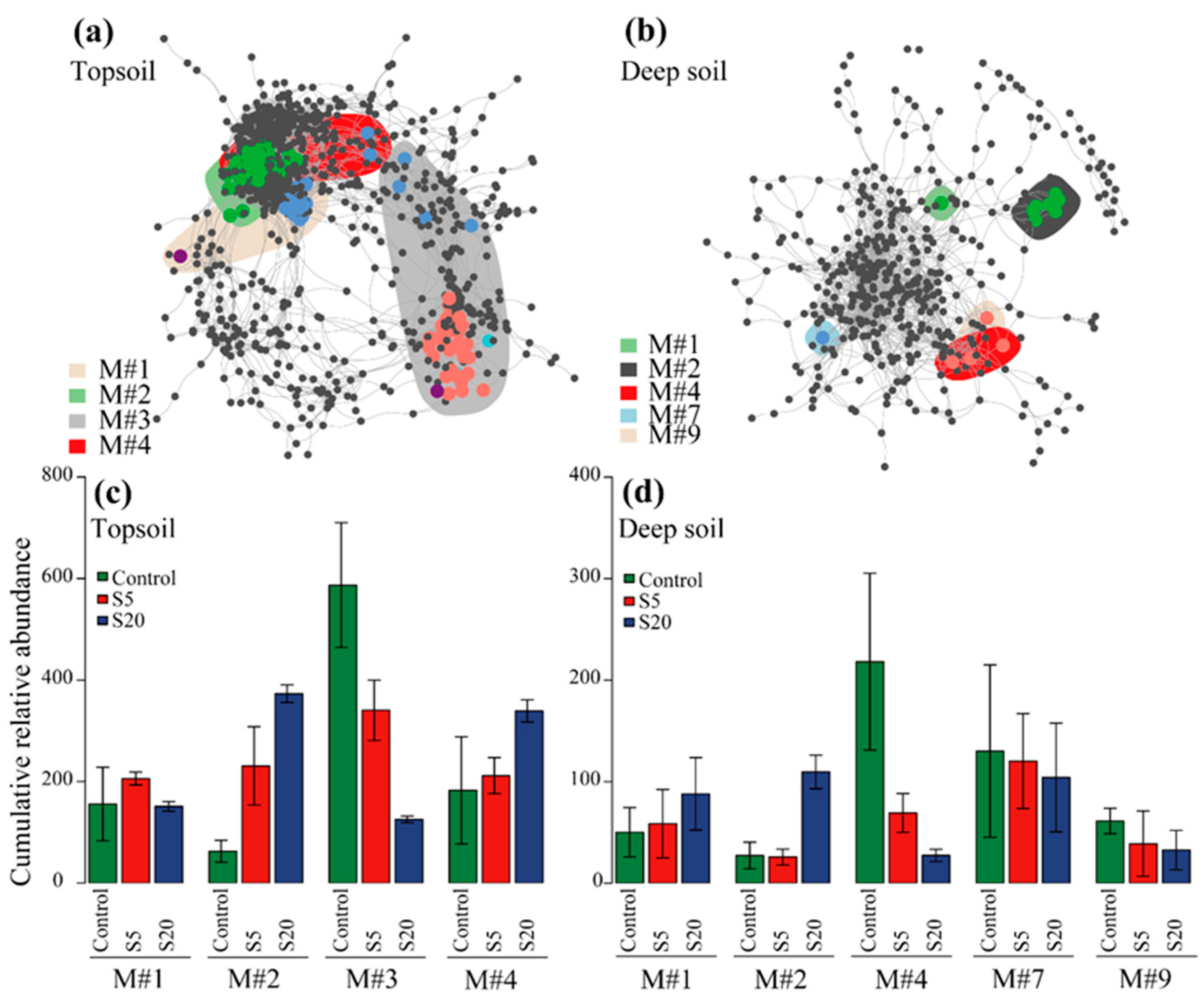
3.5. Soil Bacterial Communities of Topsoil and Deep Soil
3.6. Co-Occurrence Network Analysis
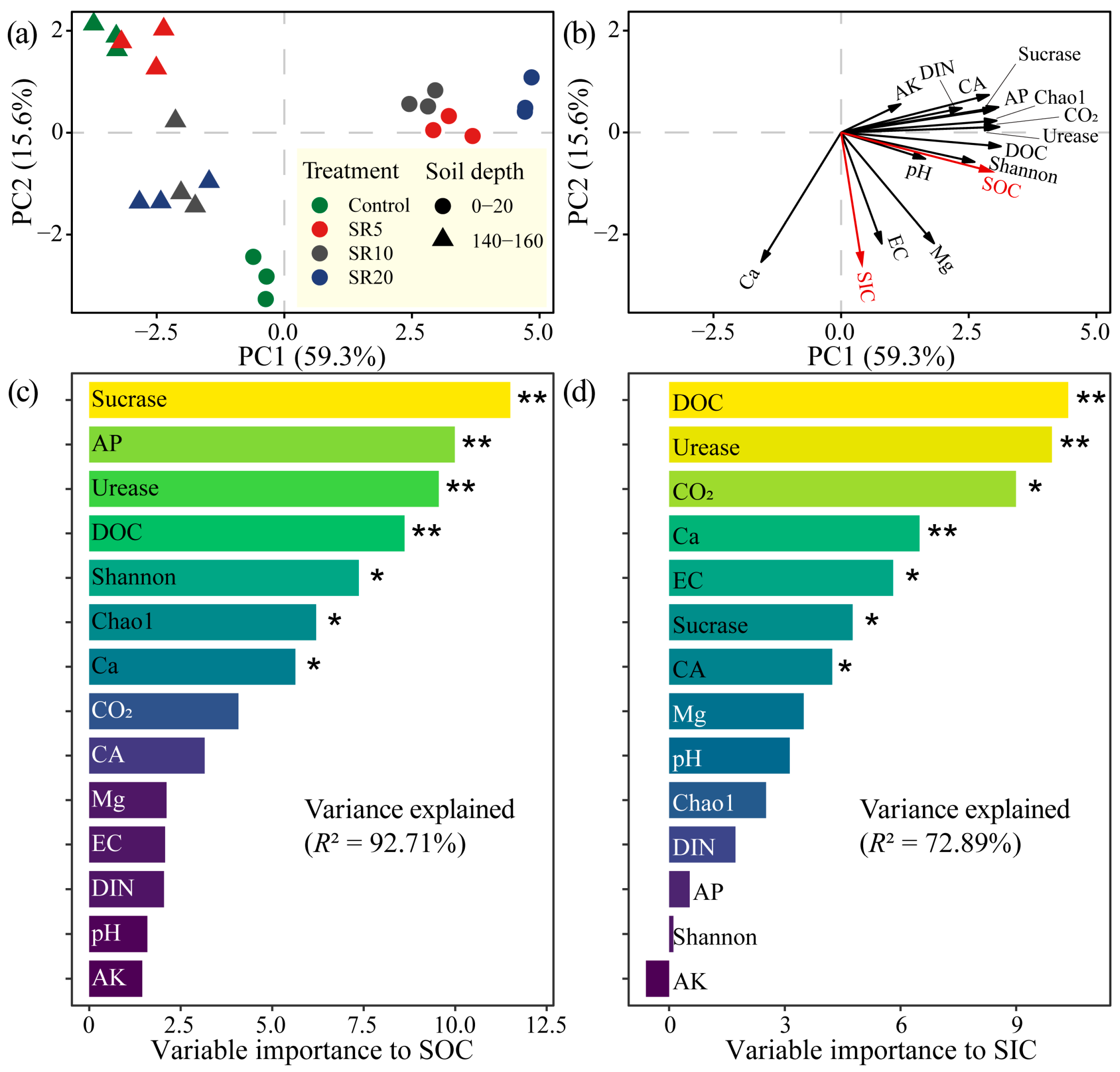
3.7. Factors Influencing SOC and SIC Sequestration
4. Discussion
4.1. Effects of Straw Return on SOC in Topsoil and Deep Soil Layers
4.2. Effects of Straw Return on SIC in Topsoil and Deep Soil Layers
4.3. Effects of Microbial Properties on Carbon Sequestration in Topsoil and Deep Soil Layers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Soil Texture
| Soil Depth cm | 2–0.05 mm | 0.05–0.002 mm | <0.002 mm | Soil Texture |
|---|---|---|---|---|
| g kg−1 | ||||
| 0–20 | 58.2 ± 0.3 | 431.0 ± 7.4 | 512.5 ± 42.1 | Silty clay |
| 40–60 | 16.3 ± 3.4 | 489.4 ± 16.7 | 496.4 ± 12.4 | Silty clay |
| 100–120 | 108.3 ± 4.0 | 429.4 ± 56.1 | 464.7 ± 16.0 | Silty clay |
| 140–160 | 491.5 ± 13.7 | 62.7 ± 0.8 | 451.8 ± 73.6 | Sandy clay |
| 180–200 | 340.2 ± 0.5 | 109.3 ± 48.9 | 552.4 ± 69.6 | Clay |
Appendix A.2. Calculation of Cumulative CO2 Emission
Appendix A.3. Calculation of Chao1 and Shannon Indices
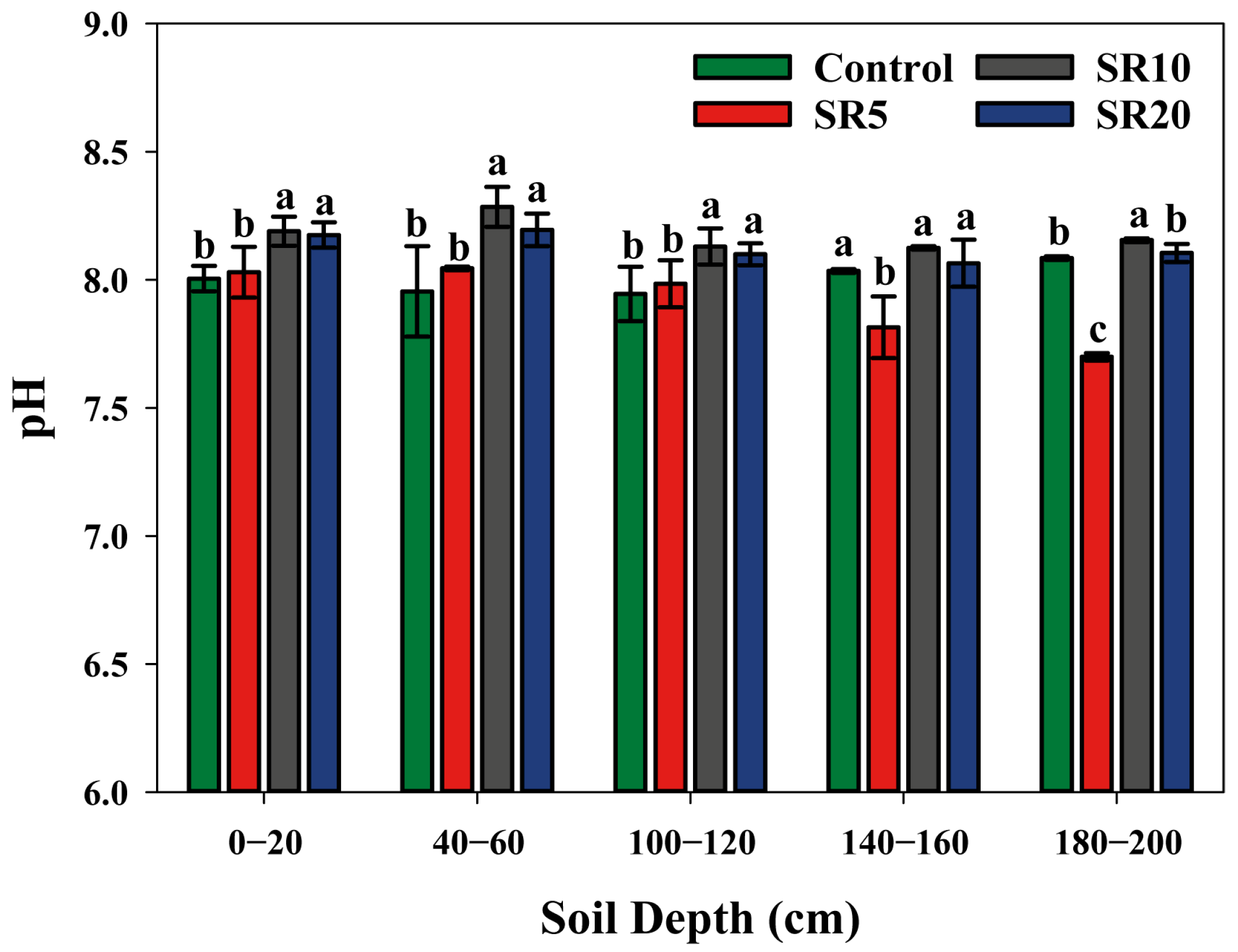
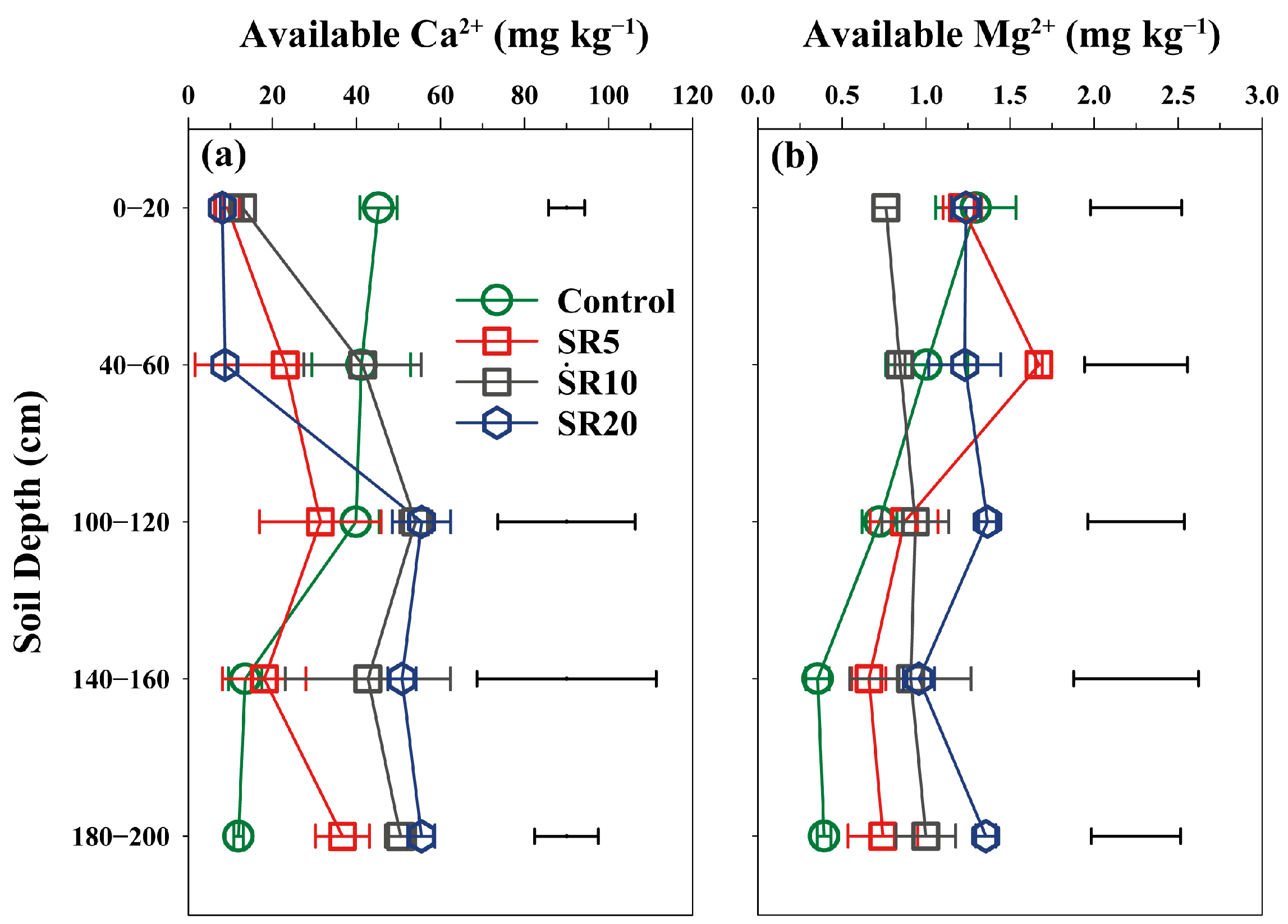
Appendix A.4. Soil pH Value and Available Ca and Mg
References
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Shang, Q.; Yang, X.; Gao, C.; Wu, P.; Liu, J.; Xu, Y.; Shen, Q.; Zou, J.; Guo, S. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: A 3-year field measurement in long-term fertilizer experiments. Glob. Change Biol. 2011, 17, 2196–2210. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Zhang, P.; Yu, Y.; Ding, F. A synthesis of change in deep soil organic carbon stores with afforestation of agricultural soils. For. Ecol. Manag. 2013, 296, 53–63. [Google Scholar] [CrossRef]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Press: London, UK, 2017. [Google Scholar]
- Yu, H.; Zha, T.; Zhang, X.; Ma, L. Vertical distribution and influencing factors of soil organic carbon in the Loess Plateau, China. Sci. Total Environ. 2019, 693, 133632. [Google Scholar] [CrossRef]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Emmerich, W.E. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric. For. Meteorol. 2003, 116, 91–102. [Google Scholar] [CrossRef]
- Saderne, V.; Cusack, M.; Almahasheer, H.; Serrano, O.; Masqué, P.; Arias-Ortiz, A.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Duarte, C.M. Accumulation of Carbonates Contributes to Coastal Vegetated Ecosystems Keeping Pace with Sea Level Rise in an Arid Region (Arabian Peninsula). J. Geophys. Res. Biogeosci. 2018, 123, 1498–1510. [Google Scholar] [CrossRef]
- Wang, Y.; Joseph, S.; Wang, X.; Weng, Z.H.; Mitchell, D.R.G.; Nancarrow, M.; Taherymoosavi, S.; Munroe, P.; Li, G.; Lin, Q.; et al. Inorganic Carbon Accrual in Subsoil through Biochar Application on Calcareous Topsoil. Environ. Sci. Technol. 2023, 57, 1837–1847. [Google Scholar] [CrossRef]
- Liebmann, P.; Mikutta, R.; Kalbitz, K.; Wordell-Dietrich, P.; Leinemann, T.; Preusser, S.; Mewes, O.; Perrin, E.; Bachmann, J.; Don, A.; et al. Biogeochemical limitations of carbon stabilization in forest subsoils. J. Plant Nutr. Soil Sci. 2022, 185, 35–43. [Google Scholar] [CrossRef]
- Button, E.S.; Pett-Ridge, J.; Murphy, D.V.; Kuzyakov, Y.; Chadwick, D.R.; Jones, D.L. Deep-C storage: Biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils. Soil Biol. Biochem. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Henneron, L.; Balesdent, J.; Alvarez, G.; Barré, P.; Baudin, F.; Basile-Doelsch, I.; Cécillon, L.; Fernandez-Martinez, A.; Hatté, C.; Fontaine, S. Bioenergetic control of soil carbon dynamics across depth. Nat. Commun. 2022, 13, 7676. [Google Scholar] [CrossRef]
- Qin, S.; Yuan, H.; Hu, C.; Li, X.; Wang, Y.; Zhang, Y.; Dong, W.; Clough, T.; Luo, J.; Zhou, S.; et al. Anthropogenic N input increases global warming potential by awakening the “sleeping” ancient C in deep critical zones. Sci. Adv. 2023, 9, eadd0041. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.; Han, B.; Ouyang, Z.; Duan, X.; Zheng, H.; Miao, H. Soil carbon sequestrations by nitrogen fertilizer application, straw return and no-tillage in China's cropland. Glob. Change Biol. 2009, 15, 281–305. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Gupta Choudhury, S.; Srivastava, S.; Singh, R.; Chaudhari, S.K.; Sharma, D.K.; Singh, S.K.; Sarkar, D. Tillage and residue management effects on soil aggregation, organic carbon dynamics and yield attribute in rice–wheat cropping system under reclaimed sodic soil. Soil Tillage Res. 2014, 136, 76–83. [Google Scholar] [CrossRef]
- Kou, X.; Ma, N.; Zhang, X.; Xie, H.; Zhang, X.; Wu, Z.; Liang, W.; Li, Q.; Ferris, H. Frequency of stover mulching but not amount regulates the decomposition pathways of soil micro-foodwebs in a no-tillage system. Soil Biol. Biochem. 2020, 144, 107789. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Gupta, R.K.; Panwar, A.S.; Mahajan, N.C.; Singh, R.; Mandal, A. Effect of tillage and straw return on carbon footprints, soil organic carbon fractions and soil microbial community in different textured soils under rice–wheat rotation: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 103–115. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhang, Q.; Shao, H.; Gao, C.; Zhang, X. Effects of fertilization and straw return methods on the soil carbon pool and CO2 emission in a reclaimed mine spoil in Shanxi Province, China. Soil Tillage Res. 2019, 195, 104361. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Shi, J.; Tian, X.; Li, X.; Li, Y.; Zhao, H. Impact of Straw Return on Soil Carbon Indices, Enzyme Activity, and Grain Production. Soil Sci. Soc. Am. J. 2017, 81, 1475–1485. [Google Scholar] [CrossRef]
- Huggins, D.R.; Buyanovsky, G.A.; Wagner, G.H.; Brown, J.R.; Darmody, R.G.; Peck, T.R.; Lesoing, G.W.; Vanotti, M.B.; Bundy, L.G. Soil organic C in the tallgrass prairie-derived region of the corn belt: Effects of long-term crop management. Soil Tillage Res. 1998, 47, 219–234. [Google Scholar] [CrossRef]
- Reicosky, D.; Evans, S.D.; Cambardella, C.A.; Allmaras, R.R.; Wilts, A.; Huggins, D.R. Continuous corn with moldboard tillage: Residue and fertility effects on soil carbon. J. Soil Water Conserv. 2002, 57, 277–284. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Evaluation and corroboration by long-term incubations. Soil Biol. Biochem. 2008, 40, 1741–1750. [Google Scholar] [CrossRef]
- Mao, L.; Guo, W.; Yuan, Y.; Qin, D.; Wang, S.; Nie, J.; Zhao, N.; Song, X.; Sun, X. Cotton stubble effects on yield and nutrient assimilation in coastal saline soil. Field Crops Res. 2019, 239, 71–81. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, L.; Zhang, Y.; Sun, L.; Yu, X.; Yu, Y. Can climate change influence agricultural GTFP in arid and semi-arid regions of Northwest China? J. Arid. Land 2020, 12, 837–853. [Google Scholar] [CrossRef]
- Veroustraete, F.; Li, Q.; Verstraeten, W.; Chen, X.; Bao, A.; Dong, Q.; Liu, T.; Willems, P. Soil moisture content retrieval based on apparent thermal inertia for Xinjiang province in China. Int. J. Remote Sens. 2012, 33, 3870–3885. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook 2023; China Statistics Press: Beijing, China, 2023. [Google Scholar]
- Liu, J.; Jing, F.; Jiang, G.; Liu, J. Effects of Straw Incorporation on Soil Organic Carbon Density and the Carbon Pool Management Index under Long-Term Continuous Cotton. Commun. Soil Sci. Plant Anal. 2017, 48, 412–422. [Google Scholar] [CrossRef]
- Guo, H.; Shen, C.; Dai, Y.; Li, F.; Jin, X.; Shen, L.; Deng, Y. Current Situation and Development Countermeasure of Cotton Straw Utilization in Xinjiang. Agric. Eng. 2023, 13, 48–53. [Google Scholar] [CrossRef]
- Gee, G.W.; Or, D. 2.4 Particle-Size Analysis. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- Dong, X.; Guan, T.; Li, G.; Lin, Q.; Zhao, X. Long-term effects of biochar amount on the content and composition of organic matter in soil aggregates under field conditions. J. Soils Sediments 2016, 16, 1481–1497. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and Bacterial Diversity: To What Extent Does the Concentration of Salt Affect the Bacterial Community in a Saline Soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi Binu, M.; Chu, H. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. mSystems 2019, 4, e0025-18. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Van Der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, M.; Assouline, S. Tillage Effects on Water and Salt Distribution in a Vertisol during Effluent Irrigation and Rainfall. Agron. J. 2002, 94, 1295–1304. [Google Scholar] [CrossRef]
- Huang, Z.B.; Assouline, S.; Zilberman, J.; Ben-Hur, M. Tillage and Saline Irrigation Effects on Water and Salt Distribution in a Sloping Field. Soil Sci. Soc. Am. J. 2000, 64, 2096–2102. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Wu, Y.; Du, T.; Ding, R.; Yuan, Y.; Li, S.; Tong, L. An isotope method to quantify soil evaporation and evaluate water vapor movement under plastic film mulch. Agric. Water Manag. 2017, 184, 59–66. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, T.; Liu, S.; Liu, Z.; Wang, Z. Profile soil organic and inorganic carbon sequestration in maize cropland after long-term straw return. Front. Environ. Sci. 2023, 11, 1095401. [Google Scholar] [CrossRef]
- Akala, V.A.; Lal, R. Potential of mine land reclamation for soil organic carbon sequestration in Ohio. Land Degrad. Dev. 2000, 11, 289–297. [Google Scholar] [CrossRef]
- Leinemann, T.; Mikutta, R.; Kalbitz, K.; Schaarschmidt, F.; Guggenberger, G. Small scale variability of vertical water and dissolved organic matter fluxes in sandy Cambisol subsoils as revealed by segmented suction plates. Biogeochemistry 2016, 131, 1–15. [Google Scholar] [CrossRef]
- Lajtha, K.; Townsend, K.L.; Kramer, M.G.; Swanston, C.; Bowden, R.D.; Nadelhoffer, K. Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 2014, 119, 341–360. [Google Scholar] [CrossRef]
- Mikutta, R.; Turner, S.; Schippers, A.; Gentsch, N.; Meyer-Stüve, S.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Eger, A.; Hempel, G.; et al. Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 2019, 9, 10294. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Guggenberger, G.; Kaiser, K. Dissolved organic matter in soil: Challenging the paradigm of sorptive preservation. Geoderma 2003, 113, 293–310. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of northwest China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Zamanian, K.; Zarebanadkouki, M.; Kuzyakov, Y. Nitrogen fertilization raises CO2 efflux from inorganic carbon: A global assessment. Glob. Change Biol. 2018, 24, 2810–2817. [Google Scholar] [CrossRef]
- Deng, F.; Wang, H.; Xie, H.; Bao, X.; He, H.; Zhang, X.; Liang, C. Low-disturbance farming regenerates healthy deep soil toward sustainable agriculture—Evidence from long-term no-tillage with stover mulching in Mollisols. Sci. Total Environ. 2022, 825, 153929. [Google Scholar] [CrossRef]
- Manning, D. Biological enhancement of soil carbonate precipitation: Passive removal of atmospheric CO2. Mineral. Mag.-Min. MAG 2008, 72, 639–649. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, M.G.; Wang, J.P.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Liu, H. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in northern China. Plant Soil 2014, 380, 89–100. [Google Scholar] [CrossRef]
- Schlesinger, W.H. An evaluation of abiotic carbon sinks in deserts. Glob. Change Biol. 2017, 23, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jobbágy, E.G.; Richter, D.D.; Trumbore, S.E.; Jackson, R.B. Agricultural acceleration of soil carbonate weathering. Glob. Change Biol. 2020, 26, 5988–6002. [Google Scholar] [CrossRef] [PubMed]
- Whalen, E.D.; Grandy, A.S.; Sokol, N.W.; Keiluweit, M.; Ernakovich, J.; Smith, R.G.; Frey, S.D. Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding. Glob. Change Biol. 2022, 28, 7167–7185. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Ji, D.; Wang, Y.; Liu, W.; Wang, X.; Liu, K.; Shang, J. Effects of Long-Term Cotton Straw Return on Soil Carbon and Bacterial Community in Topsoil and Deep Soil. Agronomy 2025, 15, 1940. https://doi.org/10.3390/agronomy15081940
Yin Y, Ji D, Wang Y, Liu W, Wang X, Liu K, Shang J. Effects of Long-Term Cotton Straw Return on Soil Carbon and Bacterial Community in Topsoil and Deep Soil. Agronomy. 2025; 15(8):1940. https://doi.org/10.3390/agronomy15081940
Chicago/Turabian StyleYin, Yingjie, Dechang Ji, Yang Wang, Weiyang Liu, Xiang Wang, Kesi Liu, and Jianying Shang. 2025. "Effects of Long-Term Cotton Straw Return on Soil Carbon and Bacterial Community in Topsoil and Deep Soil" Agronomy 15, no. 8: 1940. https://doi.org/10.3390/agronomy15081940
APA StyleYin, Y., Ji, D., Wang, Y., Liu, W., Wang, X., Liu, K., & Shang, J. (2025). Effects of Long-Term Cotton Straw Return on Soil Carbon and Bacterial Community in Topsoil and Deep Soil. Agronomy, 15(8), 1940. https://doi.org/10.3390/agronomy15081940









