Abstract
The conversion of waste biomass into biochar through inert pyrolysis represents a promising strategy for carbon sequestration. However, biochar production is often accompanied by the release of small molecular chemical substances during pyrolysis, and the resulting biochar is susceptible to environmental degradation. To enhance the carbon retention rate of biochar during pyrolysis and its stability in the environment, this study explored the incorporation of various metal soluble salts (CaCl2, Ca(H2PO4)2, MgCl2, FeCl3) and clay minerals (quartz, goethite, bentonite, albite) with two types of waste biomass (phragmites and goldenrod) for pre-treatment to enhance both carbon retention and stability in the resulting biochar. Furthermore, to elucidate the regulatory mechanisms of minerals on biochar structural formation, the three primary components of raw biomass—hemicellulose, cellulose, and lignin—were individually mixed with the minerals at a ratio of 1:5 (mineral/biomass, w/w) to produce biochars for a comparative analysis. The experimental results demonstrated that metal soluble salts, particularly Ca(H2PO4)2, exhibited a superior performance in enhancing biochar’s carbon retention compared to clay minerals. Specifically, Ca(H2PO4)2 treatment resulted in a remarkable 15% increase in the carbon retention rate. Through K2Cr2O7 oxidation simulating soil aging conditions, Ca(H2PO4)2-treated biochar showed approximately 12% greater stability than the untreated samples. This enhanced stability was primarily attributed to the formation of stable chemical bonds (C–O–P and P–O), which facilitated the preservation of aromatic carbon structures and small molecular compounds including sugars, alcohols, and ethers. Mechanistic investigations revealed that Ca(H2PO4)2 significantly influenced the pyrolysis process by increasing the activation energy from 85.9 kJ mol−1 to 156.5 kJ mol−1 and introducing greater reaction complexity. During the initial pyrolysis stage (<300 °C), Ca(H2PO4)2 catalyzed depolymerization, ring-opening, and C–C bond cleavage in hemicellulose, enhanced cellulose depolymerization, and side-chain cleavage in lignin phenylpropanes. In the intermediate temperature range (300–400 °C), Ca(H2PO4)2 facilitated carboxylate nucleophilic addition reactions and promoted cyclization to form aromatic carbon structures. The innovative aspect of this work is that minerals can increase both the yield and carbon retention rate of biochar. Furthermore, it reveals the mechanisms underlying the improvements in pyrolysis, providing a scientific basis and theoretical foundation for better displaying the carbon sequestration potential of biochar in future applications.
1. Introduction
The 26th United Nations Climate Change Conference proposed a target of reducing the global CO2 emission by nearly half before 2030 [1]. Human-emitted CO2 should be offset through energy conservation, emission reduction, and carbon sequestration. Pyrolysis can convert waste biomass into valuable products and reduce environmental pollution [2]. The pyrolysis process generates biogas, bio-oil, and biochar [3]. Biochar not only contributes to carbon sequestration but also serves as a functional environmental material, such as an adsorbent for water and air purification, as well as an electrode material for electrochemical devices used in energy storage [2]. The production of biochar through biomass pyrolysis, has gradually become a powerful tool for carbon sequestration and CO2 reduction. Generally, only above half of the carbon would be retained in biochar after slow pyrolysis, with approximately 10% of the remaining carbon being further released through oxidation or mineralization in soil [4]. The carbon sequestration capacity of biochar depends on the factors including the rate of carbon retention after pyrolysis and its stability in environment. Previous studies attempted to enhance carbon retention and stability of biochar through artificial modification techniques [5,6,7]. Adding exogenous minerals, especially those containing P, Si, Ca, Mg, and Fe, to biomass before pyrolysis could increase carbon retention through chemical bonding, physical coverage, and adsorption of carbon-containing small molecules [1]. Pre-treating biomass with phosphorus compounds such as Ca(H2PO4)2 and superphosphate might improve carbon retention [6,8,9]. This improvement was likely due to the formation of C–O–PO3 or C–P bonds [9]. The addition of FeCl3 leads to the formation of new metal complexes or oxides, which provide physical isolation for sludge-based biochar, thereby enhancing the carbon sequestration rate [10]. Nan et al. [5] modified biochar with MgCl2 loading, which enhanced the carbon sequestration capacity by about 25%. This effect may be attributed to the physical barrier created by the newly formed MgO coating at higher temperature, which reduced the release of carbon-containing molecules and increased carbon retention. Phosphorus compounds were primarily formed through the chemical reaction between phosphates and the carbon skeleton of biomass, resulting in the establishment of stable C–O–PO3 or C–P covalent bonds, which effectively reduced carbon loss. In contrast, metal chlorides mainly generated metal oxide particles during the pyrolysis process, enhancing the carbon retention of biochar through physical encapsulation [1,5]. Despite previous studies demonstrated that mineral additives could enhance biochar stability, the underlying mechanisms governing their impact on biochar’s carbon structure remained unclear. This study aimed to address this knowledge gap by systematically evaluating.
The chemical structure evolution of biochar plays a critical role in determining its carbon sequestration capacity. Previous studies demonstrated that during pyrolysis, cellulose/hemicellulose initially experienced dehydration and polymerization, while lignin experienced side chain cleavage. Subsequent reactions involved ring-opening, cyclization, rearrangement, and polymerization of monomeric molecules, forming C=O, C=C, and C–OH structures that enhanced the degree of aromatization [11]. Partial depolymerization of hemicellulose generated molten phases that encapsulated cellulose and lignin, thereby suppressing the formation of small molecular products [12,13]. Mineral loading has been shown to regulate carbon evolution in biomass during pyrolysis, though related investigations remain limited. Dong et al. [14] revealed that metal oxide catalysts reduced activation energy for C–O bond cleavage in cellulose, starch, and hemicellulose by 20–50%, with Al2O3, Fe2O3, and sulfonic acid groups facilitating furan conversion to aromatic hydrocarbons. Xia et al. [15] observed that K2B4O7 addition in bamboo-processing-induced C–O–B bond formation between pyran rings and phenyl groups, enhancing carbon stability and impeding carbon release. Potassium was found to promote ring-opening and decarboxylation while inhibiting furan ring formation. Metal ions significantly influence pyrolysis product distribution and aromatic yield while also forming multiple interactions with hydroxyl and ether groups in cellulose structures [16]. Current research indicated that mineral additives catalyze diverse reaction pathways including dehydration, depolymerization, fragmentation, decarboxylation, and decarbonization [17]. However, the mechanistic understanding of how mineral-loaded pyrolysis regulates carbon retention and stabilization in biochar remained unclear, representing a critical knowledge gap in previous studies.
Previous studies demonstrated that metal salts and clay minerals could enhance the carbon sequestration capacity of biochar. However, the enhancement mechanism were not clarified. There is a lack of analyses from the perspective of pyrolysis kinetics and the evolution mechanisms of carbon structure. In this study, metal salts and clay minerals were applied to two types of biomass, phragmites and goldenrod, to prepare mineral-loaded biochar through pyrolysis. We assume that mineral loading could improve the carbon retention rate and aromatization structure of biochar. A series of experiments were conducted to (1) determine the effects of metal salts and clay minerals on carbon retention and stability in biochar using elemental analysis and K2Cr2O7 oxidation; (2) elucidate the pyrolysis kinetics and thermodynamics of mineral-loaded biochar through TGA analysis, conversion kinetics and thermodynamic analysis; and (3) investigate the microscopic structure of biochar using 13C-NMR and analyze the pyrolysis product components via PY-GC/MS to reveal the influencing mechanisms of minerals on the formation of carbon structure in biochar. Innovatively, this study proposed a technical pathway for carbon sequestration based on mineral-loaded reinforced biochar, offering a systematic solution to address the long-term stability challenges. The developed mineral-enhanced biochar technology established a theoretical foundation and practical framework for achieving durable carbon stabilization in biomass derived materials.
2. Materials and Methods
2.1. Biomass Collection and Pretreatment
This study selected common biomass species, phragmites and goldenrod, collected from Chong Ming Island in Shanghai, China, because both phragmites and goldenrod had high annual carbon sequestration rates. Clay minerals are widely available in nature, with a low cost. Since elements such as Fe, Ca, and Mg are naturally present, the stable minerals generated after pyrolysis are less likely to dissolve in natural water bodies, thereby minimizing the potential for secondary environmental pollution. In addition, in order to reveal the pyrolysis mechanism, hemicellulose, cellulose, and lignin were also used to produce biochar. The basic properties of all biochars are shown in Table 1. After air-drying, the phragmites and goldenrod were broken and passed through a 2 mm sieve. Then, the phragmites or goldenrod was impregnated in solutions containing metal salts (CaCl2, Ca(H2PO4)2, MgCl2, FeCl3) or minerals (quartz, goethite, bentonite, albite) with the ratio of 1:5 (mineral/biomass, w/w) [5]. The chemicals were bought from Shanghai Sinopharm company, China. The mixture was stirred and dried at 80 °C to remove moisture. A block diagram of the content of this study is shown in Figure 1.

Table 1.
Physicochemical properties of biochars.
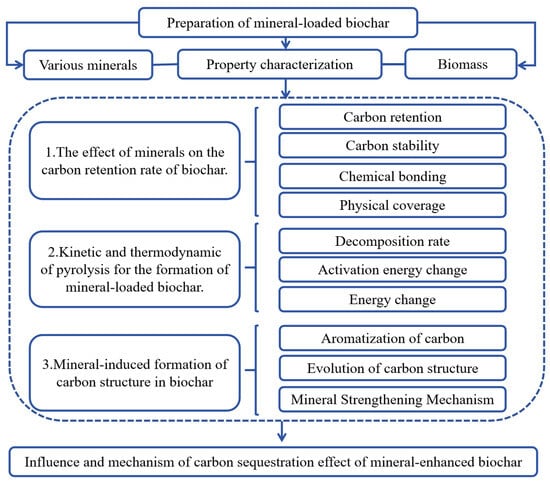
Figure 1.
A block diagram of the content of this study.
2.2. Biochar Production and Characterization
The pyrolysis process was carried out in a N2 atmosphere at a reaction pressure of 0.1 MPa, with a heating rate of 15 °C min−1, reaching a maximum temperature of 500 °C, maintained for 2 h [5]. The selection of temperature and heating rate takes into account the balance among carbonization degree, yield, and cost. The original biochar and biochars loaded with minerals derived from phragmites were labeled as PBC, Ca-PBC, Ca(P)-PBC, Mg-PBC, Fe-PBC, Quartz-PBC, Goethite-PBC, Bentonite-PBC and Albite-PBC. Similarly, as for goldenrod biochars, they were labeled as GBC, Ca-GBC, Ca(P)-GBC, Mg-GBC, Fe-GBC, Quartz-GBC, Goethite-GBC, Bentonite-GBC and Albite-GBC (Table S1). In addition, goldenrod, hemicellulose, cellulose, and lignin were also pyrolyzed at 300 °C and 400 °C, maintaining the temperature for 20 min with and without a Ca(H2PO4)2 addition. The pH of biochar was measured using a digital pH meter with a solid-to-liquid ratio of 1:20 (w/v) after vibration in deionized water for 10 min [5]. Elemental analysis (C/H/N/S) was performed using an elemental analyzer (Vario EL III, Elementar, Langenselbold, Germany). Ash content was determined by measuring weight loss after heating at 550 °C for 4 h at O2 atmosphere [5].
The mineral species in biochar were identified using X-ray diffraction (XRD, D/max-2200/PC, Rigaku, Tokyo, Japan) under 40 kV and 30 mA [9]. The chemical composition of the biochar powder was determined by XRF (Shimadzu XRF-1800, Kyoto, Japan). Functional groups on biochar surface were detected using FTIR (IR Prestige 21 FTIR, Shimadzu, Japan) [5]. Surface morphology of the biochar was observed using SEM (Nova Nano SEM 450, FEI Company, Hillsboro, OR, USA). Qualitative analyses of the fast pyrolysis volatiles biomass were conducted using a Curie point pyrolyzer (Frontier Laboratories Ltd., EGA/PY-3030D, Koriyama, Japan). Dry samples (50 μg) were wrapped in ferromagnetic foil with a Curie point temperature of 650 °C, then introduced into the furnace for heating at a rate of 3000 °C s−1, with a hold time of 10 s. Volatiles generated during pyrolysis were analyzed directly by GC/MS equipped with a chromatographic column (Agilent J&W DB-17 MS, Santa Clara, CA, USA) [18]. The spectra of solid-state 13C dipolar-decoupling magic-angle-spinning nuclear magnetic resonance (13C NMR) were recorded on a JEOL/ECA 400 spectrometer (Tokyo, Japan) operating at 100.53 MHz, with a total of 9032 scans accumulated per sample [6].
2.3. Carbon Retention and Stability During Biomass Pyrolysis
Carbon retention was calculated by comparing the carbon content of biochar with that in raw biomass. Calculation formula was as follows [5]:
where Ybiochar was the yield of biochar (%), Wbiochar and Wbiomass were the weights of biochar and biomass, respectively. Cbiochar and Cbiomass denoted the carbon contents (%) of biochar and biomass, respectively.
The stability of biochar was assessed using K2Cr2O7 oxidation, a method that simulated the decomposition of biochar under extreme oxidative conditions [19]. To correlate the organic carbon mass with the glucose standard solution. The chemicals were bought from Shanghai Sinopharm company, China. To investigate the kinetics and thermodynamics of the pyrolysis process, thermogravimetric analysis (TGA/DSC1, Netzsch, Selb, Germany) was used to simulate the biomass pyrolysis, and N2 at a flow rate of 50 mL min−1 was used as the carrier gas. At heating rates of 5, 15, and 30 °C min−1, the temperature was increased from room temperature to 600 °C under a pressure of 0.1 MPa [20]. The TGA tests were used for the pyrolysis kinetics and thermodynamic studies. The isoconversional method provides a means to determine kinetic parameters without assuming a specific kinetic model form, making it widely applicable for activation energy calculations [21]. This methodology is derived from two fundamental assumptions: (1) a single-step process occurs at each fixed conversion level, and (2) the reaction rate at a given conversion depends solely on temperature, allowing the Arrhenius kinetic law to be applied within the narrow temperature range corresponding to that conversion [22].
Under linear heating non-isothermal conditions, the reaction rate for solid-state processes can be expressed as:
where α represents the conversion degree, T is absolute temperature, A denotes the pre-exponential factor, E is activation energy, and f(α) represents the differential form of the kinetic model function.
From Equation (3), the following integrated form can be obtained [23]:
The integral in the right-hand side of Equation (4) is called the Arrhenius integral [24], which has no exact analytical solution. Many researchers proposed some approximations for the integral. The most common used approximation for the temperature integral is expressed as follows [25]:
Combining Equations (4) and (5) yields the following:
For a series of kinetic data at different heating rates and a given conversion rate, Equation (6) becomes
where α is the conversion rate, T is the absolute temperature, A is the pre-exponential factor, and E denotes the activation energy, the subscript α indicates values associated with the given conversion degree, and the subscript i denotes the heating rate in the i-th instance.
The activation energy obtained from the integral method could be used to calculate the pre-exponential factor:
where Tm represents the temperature at maximum conversion rate. Equation (4) formed the basis for estimating the pre-exponential factor using the Kissinger method.
Based on the obtained activation energy and the pre-exponential factor, the thermodynamic parameters, including changes in enthalpy (ΔH), Gibbs free energy (ΔG) and entropy (ΔS), were obtained. ΔH represents the total energy required for the decomposition of the sample into solid residues and volatiles. ΔG refers to the increase in the total energy of the system during the formation of the activated complex. ΔS indicates the change in the degree of disorder of the system throughout the pyrolysis process. They could be estimated using following equation [20]:
where ΔH, ΔG, and ΔS represent the changes in enthalpy, Gibbs free energy, and entropy, respectively. KB is the Boltzmann constant (1.38 × 10−23 J K−1), and h is the Planck constant (6.63 × 10−34 J s).
2.4. Statistical Analysis
Each experimental group was replicated three times, and the collected data were analyzed using Origin 2021(OriginPro 2021b SR1 v9.8.5.204 x64) software. All significant differences were reported at the p < 0.05 level, and the data from the K2Cr2O7 oxidation experiment is expressed as mean ± standard deviation (n = 3).
3. Results
3.1. Effect of Metal Ions and Clay Minerals on Carbon Retention Rate of Biochar
Co-pyrolysis of minerals with raw biomass increased both ash contents and biochar yields (Table 1). It was primarily due to the fact that mineral components, when loaded with biomass, were retained in the form of pyrolysis residues within the biochar during the pyrolysis process, thereby enhancing both the yield and ash content of the biochar [1]. The mineral impregnation treatments exerted distinct effects on biochar production yields and pH characteristics. Notably, CaCl2-modified biochars demonstrated the most pronounced yield enhancement among all treatments, with the phragmites-derived biochar (PBC) showing a 82.9% increase from 28.6 wt% to 52.3 wt% (designated as Ca-PBC), while the goldenrod-based biochar (GBC) exhibited a 98.2% yield improvement from 28.3 wt% to 56.0 wt% (denoted as Ca-GBC). In striking contrast, bentonite-impregnated biochars displayed the least significant yield augmentation, registering 40.1 wt% for Bentonite-PBC and 36.8 wt% for Bentonite-GBC. Ca(H2PO4)2 and FeCl3 produced substantial pH reduction in both biochar types. Phragmites biochar underwent a pH decline from neutral 7.09 to strongly acidic 3.11 and 2.32 following Ca(H2PO4)2 and FeCl3 treatments, respectively. Similarly, goldenrod biochar’s alkaline nature (initial pH 11.0) was significantly neutralized to pH 3.60 and 2.76 by the respective treatments. Conversely, soil mineral amendments induced pH elevation, particularly in albite-treated biochars which maintained strongly alkaline conditions (Albite-PBC: pH 10.9; Albite-GBC: pH 11.0). As for carbon retention rate, approximately 50% of the carbon was retained in both PBC and GBC after pyrolysis (Figure 2). The incorporation of Ca(H2PO4)2 and FeCl3 significantly enhanced the carbon retention properties of both biochar materials. For PBC, the carbon retention rate increased from 47.5% to 61.5% and 62.8% with Ca(H2PO4)2 and FeCl3 impregnation, respectively. A similar enhancement was observed in GBC, where the carbon retention improved from 45.0% to 60.8% and 59.7% following Ca(H2PO4)2 and FeCl3 treatment, respectively. Notably, alkaline earth metal chlorides also demonstrated carbon stabilization effects. CaCl2 and MgCl2 treatments elevated the carbon retention rate of PBC to 55.7% and 61.7%, respectively, while for GBC, these modifiers increased the retention rates to 55.7% and 56.7%, respectively. Previous studies also reported that the addition of minerals could enhance the carbon retention rate of biochar, mainly because exogenous minerals covered biomass surface, providing physical and chemical intervention to carbon. Nan et al. [5] reported that the carbon retention rate in biochar increased by 14.3% after cow manure was pretreated with MgCl2. Other researchers found that zeolites and Ca(OH)2 could improve the carbon retention rate of biochar [26,27,28]. In contrast, among four clay mineral-modified biochars, only quartz and albite increased the carbon retention rate by 1.3–5.0%, while bentonite and goethite decreased the carbon retention rate by 0.6–9.5%. It seemed that Mg2+ and Fe3+ were more effective than Ca2+ in enhancing the carbon retention rate of biochar. Previous studies reported that during pyrolysis, alkaline earth metals (Ca, Mg) and transition metals (Fe, Cu) predominantly remained on biochar [29]. Mineral calcium could catalyze the decomposition of carbon at lower temperatures [1]. The formation of CaO, MgO, or CaCO3 inhibited the release of small organic molecules [5], while iron (Fe) could form metal complexes or oxides during pyrolysis, providing physical isolation [10]. Additionally, when comparing CaCl2 and Ca(H2PO4)2 treatments, it was observed that H2PO4− was more effective than Cl− in improving carbon retention rate of biochar. It was likely because H2PO4− reacted with biomass carbon to form C–O–PO3 and C–P bonds, thereby enhancing the carbon retention rate of biochar [9].
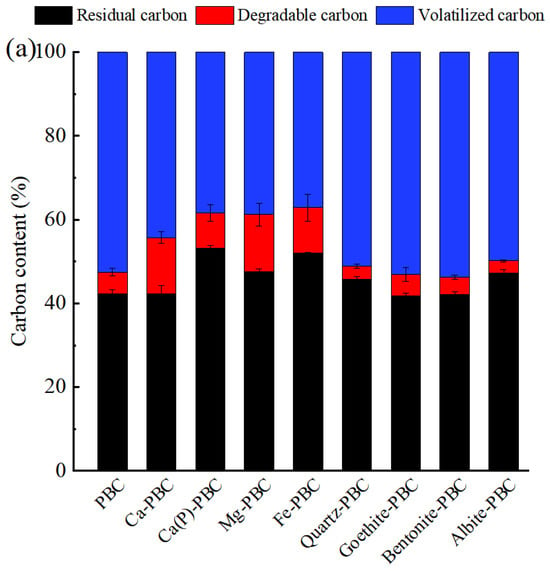
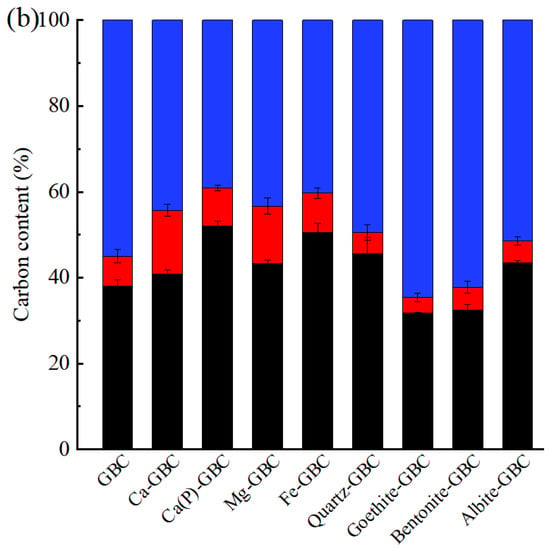
Figure 2.
Distribution of carbon in phragmite biochar (a) and goldenrod biochar (b). PBC and GBC represented the original biochars derived from phragmite and goldenrod, respectively. The prefix of Ca, Ca(P), Mg, Fe, Quartz, Goethite, Bentonite and Albite meant the CaCl2, Ca(H2PO4)2, MgCl2, FeCl3, quartz, goethite, bentonite and albite was added to raw biomass in the process of biochar production.
After K2Cr2O7 oxidation, the content of degradable carbon in modified biochars loaded with Ca(H2PO4)2 and FeCl3 (8.4–10.8%) were higher than that in original biochar (5.2% for PBC, and 6.9% for GBC) (Figure 2). In contrast, the degradable carbon content in biochars loaded with CaCl2 and MgCl2 reached up to 13.4–14.9%, suggesting that degradable carbon retained by soluble salts during pyrolysis was lost after K2Cr2O7 oxidation. Nan et al. [5] reported that doping with CaCl2 induced the formation of degradable carbon structure. Biochars loaded with Ca(H2PO4)2 exhibited the highest proportion of residual carbon. Compared to the original biochar, the residual carbon in Ca(P)-PBC increased from 42.3% to 53.1%, and in Ca(P)-GBC, it increased from 38.1% to 52.1%. Li et al. [8] reported that during pyrolysis, Ca(H2PO4)2 interacted with cellulose, hemicellulose, and lignin to form thermally stable phosphorus complexes, which hindered carbon decomposition and prevented active carbon sites from undergoing K2Cr2O7 oxidation, thereby enhancing carbon stability. As for quartz, goethite, bentonite, and albite, they were not effective in increasing the residual carbon rate of biochar. Previous studies reported that alkali metals (K, Na) exhibited stronger catalytic cracking effects, compared to alkaline earth metals (Mg, Ca), resulting in the production of a greater quantity of low molecular weight species. Furthermore, during the pyrolysis process, a significant portion of Mg and Ca remained in the solid products, while K and Na were markedly transferred into volatile fractions [29]. These minerals differed in their ability to enhance the stability of carbon in biochar, indicating that the interaction mechanisms between these minerals and carbon in biochar. Nan et al. [5] investigated the pyrolysis of cellulose loaded with CaCl2 and found that Ca2+ promoted the formation of unsaturated structures while inhibiting the cleavage of glycosidic bonds, thereby facilitating the condensation of unstable solid components. In contrast, relatively reactive elements such as K and Na in soil minerals directly formed organometallic complexes with carbon during pyrolysis, further hindering aromatization [30]. Additionally, some minerals that diffused into the interior of biochar altered the functional groups of the side chains, leading to differences in the reactivity of biochar [31].
The crystals on the biochar surface were detected by XRD (Figure 3a,b), and the metal oxides in biochar were detected by XRF (Table S2), which revealed that compared to original biochars, the mineral-loaded biochars contained abundant minerals. CaCO3 was detected in biochar loaded with CaCl2, and Ca2P2O7 was detected in biochar loaded with Ca(H2PO4)2. MgO and MgO3(CO3)2 were detected in biochar loaded with MgCl2. Fe2O3 and Fe3O4 were detected in biochar loaded with FeCl3. Compared to the original biochar, the CaCO3 content in Ca-PBC and Ca-GBC increased from 1.2% wt and 6.2% wt to 16.4% wt and 20.6% wt, respectively. Similarly, the CaCO3 content in Ca(P)-PBC and Ca(P)-GBC rose to 9.2% wt and 11.3% wt, respectively. The loading of MgCl2 also resulted in an increase in MgO content in Mg-PBC and Mg-GBC from 0% wt to 6.3% wt and 7.8% wt, respectively. Furthermore, the addition of FeCl3 lead to an increase in Fe2O3 content in Fe-PBC and Fe-GBC from 0.01% wt and 0.02% wt to 10.3% wt and 9.4% wt, respectively, while the Fe3O4 content increased from 0 to 5.1% wt and 7.2% wt, respectively. Some studies also reported that MgO3(CO3)2 and CaCO3 generated from the conversion of MgCl2 and CaCl2 during pyrolysis [32,33]. Those newly formed minerals changed the evolution process of carbon structure. On the one hand, Ca2+ could catalyze biomass decomposition, and on the other, CaCO3 could act as a coating layer, suppressing the release of organic small molecules [1]. When the temperature reached 500 °C, a greater number of iron ions could capture oxygen atoms from the biochar, leading to the formation of various forms of iron oxides (such as Fe2O3 and Fe3O4) [34], Ca(H2PO4)2 decomposed into insoluble calcium pyrophosphate (Ca2P2O7) [9], and these newly formed metal complexes provided physical isolation to reduce carbon loss caused by high temperatures. Ren et al. [28] reported that Mg and Ca could form surface compounds that acted as a physical barriers. Phosphorus-containing substances with aromatic rings formed in pyrolysis were a crucial factor influencing the stability of biochar [35]. FTIR spectra of biochar loaded with CaCl2 exhibited an obvious peak at 3440 cm−1, corresponding to the stretching vibration of –OH groups, whereas no such peak was present in the spectra of biochars loaded with other soluble salts (Figure 3c,d). It was likely due to the formation of CaCl2·2H2O or Ca(OH)2 during the cooling stage [5], which did not occur in biochars loaded with Ca(H2PO4)2, MgCl2, or FeCl3. FTIR analysis revealed distinct chemical interactions in mineral-impregnated biochars. The Ca(H2PO4)2-modified biochar exhibited characteristic absorption bands at 974 cm−1 and 1065 cm−1, corresponding to P–O–C stretching vibrations, along with a prominent P–O vibration peak at 935 cm−1 [5]. Similarly, FeCl3-impregnated biochar demonstrated a distinct Fe–O–C stretching vibration at 1095 cm−1 [36]. These spectral features collectively demonstrate the formation of metal–carbon coordination bonds during pyrolysis, consistent with the carbon-mineral interaction mechanism proposed by Nan et al. [1], where phosphorus/silicon-containing compounds induce crosslinked networks through P–O–C, C–P, or C–Si–C bonds. Notably, the CaCl2-modified biochar showed significantly enhanced peak intensities compared to other modified counterparts (Figure 3c,d). Additional spectral signatures included a carboxylate (–COO−) stretching vibration at 1630 cm−1, Aliphatic C–H vibrations at 1100 cm−1 (CH deformation), and 1420 cm−1 (CH3 symmetric bending). The observed crosslinked structures, particularly the P–O–C and Fe–O–C configurations, are proposed to function as dense protective shells [5]. These architectures likely impede the outward diffusion of low-molecular-weight carbon species from pyrolyzing particles, thereby enhancing carbon sequestration efficiency through physical encapsulation and chemical stabilization mechanisms [1].
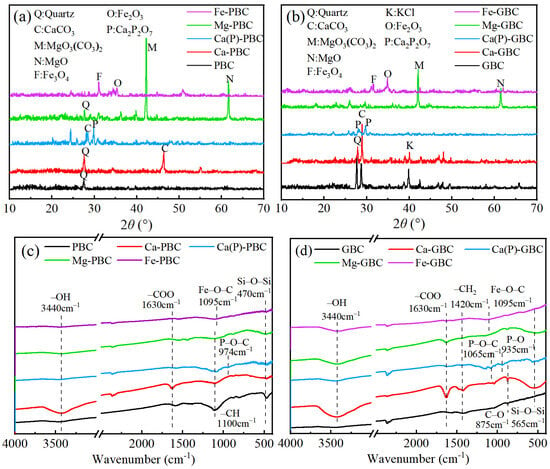
Figure 3.
XRD and FTIR spectra of phragmite biochar (a,c) and goldenrod biochar (b,d), respectively.
The above results demonstrated that the biochars loaded with Ca(H2PO4)2 exhibited the highest carbon retention capacity; thus, the subsequent research focused on the Ca(P)-PBC and Ca(P)-GBC. The SEM-EDS elemental mapping revealed compositional differences between the original biochar (PBC/SBC) and the biochar loaded with Ca(H2PO4)2 (Ca(P)-PBC/Ca(P)-SBC) (Figure 4a–d). The original biochar predominantly exhibited distributions of C, Si, and O, with a notable spatial overlap between the Si and O signals, suggesting the potential formation of SiO2 during the pyrolysis process. In contrast, the Ca(H2PO4)2 loading induced significant enrichment of Ca and P, with their surface concentrations markedly increasing compared to the original biochar. Notably, the distribution of Ca and P, along with their spatial correlation with O and Si, implies three possible mineralization pathways: (1) crystallization of calcium carbonate through chemical adsorption of carbon dioxide [5], (2) formation of calcium phosphate complexes (e.g., Ca2P2O7) via phosphoric bonding [9], and (3) interfacial reactions between Si-O and the biomass components and the modifying agent [1]. From the SEM images (Figure S1), it could be seen that PBC and GBC had porous structures, while after loading them with Ca(H2PO4)2, a significant amount of mineral particles adhering to the surface was present.
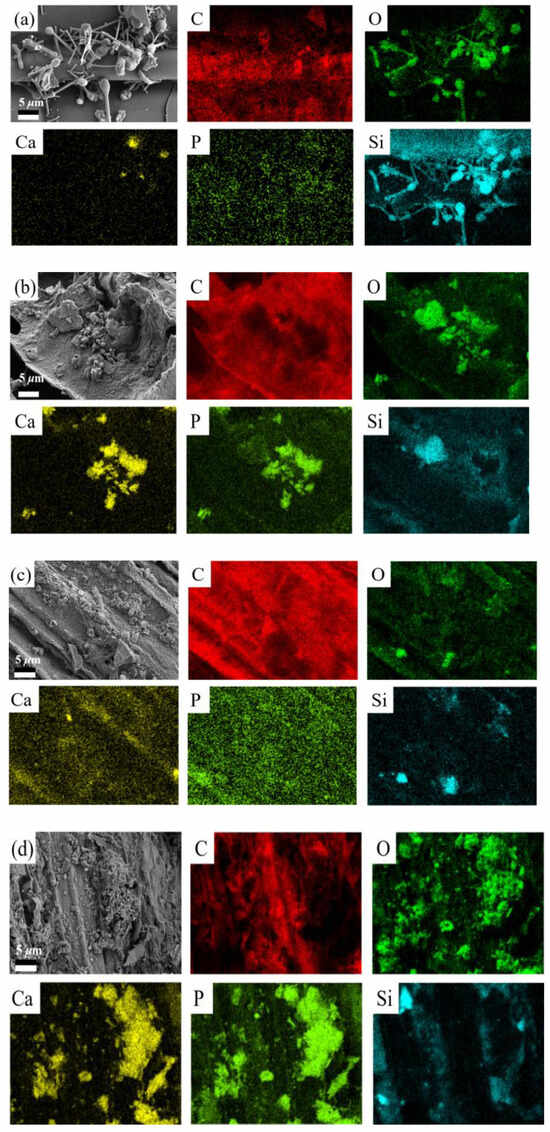
Figure 4.
The SEM mapping of PBC (a), Ca(P)-PBC (b), GBC (c), and C(P)-PBC (d).
3.2. Kinetic and Thermodynamic of Pyrolysis for the Formation of Mineral-Loaded Biochar
To elucidate the impact of Ca(H2PO4)2 on biomass during pyrolysis, TGA was conducted under a N2 atmosphere to simulate the thermal decomposition process of biomass (Figure 5). Both raw and Ca(H2PO4)2-loaded biomass exhibited three distinct peaks, corresponding to moisture evaporation, cellulose decomposition, and lignin decomposition [32]. For phragmites, the addition of Ca(H2PO4)2 promoted the decomposition of cellulose and lignin. The decomposition of cellulose occurred at 196 °C compared to 285 °C in raw biomass, and lignin decomposition occurred at 301 °C compared to 349 °C in raw biomass. Conversely, for goldenrod, the addition of Ca(H2PO4)2 suppressed the decomposition of cellulose and lignin, with the cellulose decomposition peak shifting from 281 °C to 287 °C and the lignin decomposition peak shifting from 327 °C to 351 °C. It was likely because the content of organic materials such as starch and proteins in goldenrod was higher than phragmites, and under the influence of Ca(H2PO4)2 the decomposition of organic materials was delayed. Yang et al. [36] reported that the C–C and C–H bonds in starch and lipids were stable and difficult to be broken. Additionally, CaO absorbed CO2 releasing during starch pyrolysis and formed CaCO3, reducing the weight loss. It was noteworthy that the maximum thermal decomposition rate of Ca(P)-PBC was only 0.42% s−1, compared to 0.75% s−1 for PBC. Similarly, the maximum thermal decomposition rate of Ca(P)-GBC was 0.36% s−1, whereas GBC had a maximum thermal decomposition rate of 0.73% s−1. It was likely due to the reaction between Ca(H2PO4)2 and biomass carbon, leading to the formation of C–O–PO3 and C–P bonds, which retained more carbon [9,30]. Additionally, the formation of Ca2P2O7 on the biochar surface created a physical coating that further reduced the decomposition of organic matter.
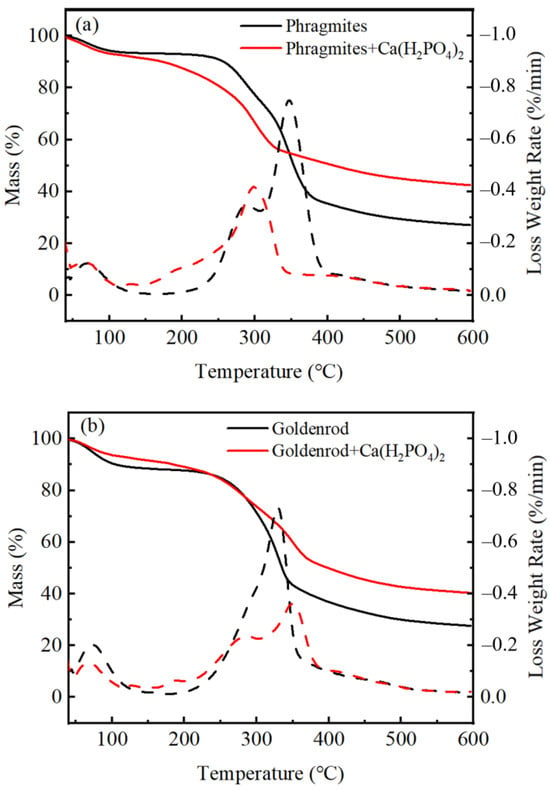
Figure 5.
The TGA and DTG curves of PBC and Ca(P)-PBC (a), and GBC and C(P)-PBC (b) under N2, at a heating rare of 15 °C min−1.
The conversion plots for the pyrolysis of phragmites and goldenrod were shown in Figure S2. It could be observed that for all heating rates, there was a high linear correlation between ln(βi/Tα,i2) and −1/R·Tα,i. This indicated that the obtained activation energy was independent of the heating rate. The activation energies (Eα) for the pyrolysis of phragmites and goldenrod were calculated using the KAS method and were shown in Figure 6a,b. The activation energy varied significantly with the conversion rate. For phragmites, Eα increased from 8.6 kJ mol−1 to 66.4 kJ mol−1 within the conversion range of 0.05 to 0.85, and then raised sharply to 161.3 kJ mol−1 within the conversion range of 0.85 to 0.95. For phragmites loaded with Ca(H2PO4)2, the Eα was lower than that of raw phragmites within the conversion range of 0.05 to 0.65, but subsequently increased to 199.8 kJ mol−1 within the conversion range of 0.65 to 0.95. For goldenrod, Eα increased from 2.7 kJ mol−1 to 62.8 kJ mol−1 within the conversion range of 0.05 to 0.90, and then rapidly increased to 85.9 kJ mol−1. For goldenrod loaded with Ca(H2PO4)2, the Eα was lower than that of raw goldenrod within the conversion range of 0.05 to 0.25, but subsequently increased to 156.5 kJ mol−1. The increase in activation energy indicates that the pyrolysis process of biomass evolves from a simple to a more complex mechanism, while also suggesting a reduction in the thermal degradation extent of the feedstock [20,30]. At a low temperature, easily decomposable components like polysaccharides in biomass decomposed first, while at high temperature, complex components such as lignin, lipids and aromatic substances began to decompose. Nie et al. [37] suggested that the increase in activation energy was related to the overlapping thermal decomposition reactions of cellulose, hemicellulose, and lignin in the biomass. After loading with Ca(H2PO4)2, the activation energy and corresponding pyrolysis temperature first decreased and then increased, indicated that the mineral initially catalyzed a portion of the reactions. Li et al. [38] reported that co-modification with Fe and Zn catalyzed the pyrolysis reaction of bamboo residue. Lu et al. [39] also reported that inherent minerals and Fe–Ca–Ni catalysts exhibited synergistic catalytic effects in the pyrolysis of wheat straw. The subsequent increase in activation energy indicated that the decomposition of biomass required more energy, which was essentially a comprehensive result of the structural aromatization, chemical bond reorganization, and changes in reaction pathways caused by the interaction between Ca(H2PO4)2 and biomass [8]. This phenomenon provided a theoretical basis for the enhanced yield of mineral-loaded biochar.
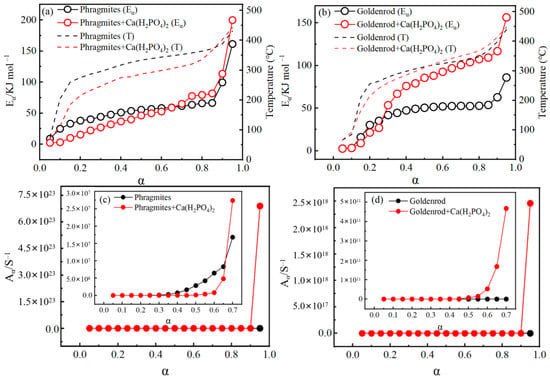
Figure 6.
The effect of the Ca(H2PO4)2 addition on the activation energy during pyrolysis of phragmite (a) and goldenrod (b) biochars, and the changes in the pre-exponential factor of phragmite (c) and goldenrod (d) biochars after the Ca(H2PO4)2 addition.
The pre-exponential factor values for the pyrolysis of phragmites and goldenrod were shown in Figure 6c,d. It could be observed that during the pyrolysis, Aα values increased with α. After loading with Ca(H2PO4)2, the pre-exponential factor for phragmites increased from 1.02 S−1–5.56 × 1019 S−1 to a range of 0.04 S−1–6.89 × 1023 S−1. For goldenrod, the pre-exponential factor increased from 2.1 S−1–1.31 × 1010 S−1 to a range of 0.05 S−1–2.46 × 1018 S−1. The increase in the pre-exponential factor might be attributed to the reaction between minerals and biomass. Saddawi et al. [40] reported that after the impregnation of metal into biomass, metal ions diffused from salt particles into chelate complexes, creating new active sites. These ions interacted with hydroxyl and ether bonds in cellulose, which significantly accelerated the pyrolysis reaction rate. Although the pre-exponential factor increased, the decomposition rate decreased substantially (Figure 5). The average activation energy calculated using the KAS method was used to determine the thermodynamic parameters such as ΔH, ΔG, ΔS at a heating rate of 15 °C min−1. The results were presented in Table 2 and Figure S4. ΔH represented the total energy required for the sample to decompose into solid residues and volatiles. ΔG indicated the increase in the system’s total energy when forming the activated complex. As the conversion rate increased, ΔH increased, while ΔG decreased gradually. The increase in ΔH suggested that the energy difference between the activated complex and the reactants became larger [41]. In the pyrolysis, positive entropy indicated that the disorder of reaction products was greater than that of the reactants [42]. The loading of Ca(H2PO4)2 increased ΔH, indicating that the mineral raised the energy required for biomass decomposition and hindered the conversion of carbon-containing components. The ΔS for biomass pyrolysis reactions increased from negative to positive. For phragmites, the ΔS changed from −249.3–121.7 J mol−1·K−1 to −276.9–199.9 J mol−1·K−1 with the addition of Ca(H2PO4)2. For goldenrod, ΔS shifted from −274.1–−62.8 J mol−1 K−1 to −274.7–95.5 J mol−1 K−1. During high-temperature pyrolysis, biomass decomposed into gaseous small molecules and solid biochar. The degrees of freedom of the gaseous products were significantly higher than those of the original solid biomass, resulting in a substantial increase in the system’s ΔS [43]. The primary reason for the decrease in ΔG was the dominant role of the entropy increase effect at elevated temperatures, complemented by the enhanced structural stability of the products. This process exemplified the principle of the second law of thermodynamics, which stated that a system tends toward a state of maximum entropy and minimum energy [44].

Table 2.
Changes in Gibbs free energy, enthalpy and entropy at various α for the pyrolysis of goldenrod with or without the addition of Ca(H2PO4)2.
3.3. The Mechanism of Mineral-Induced Formation of Carbon Structure in Biochar
The carbon compositions of PBC, Ca(P)-PBC, GBC, and Ca(P)-GBC were analyzed using solid-state 13C NMR and peak deconvolution (Figure S3 and Table 3). Four biochars were predominantly aromatic compounds (peak (d) in Figure S3). After loading with Ca(H2PO4)2, the peaks associated with (a) ketones, quinones, aldehydes (C=O, HC=O) and (c) O-substituted aromatics (C–O, C–OH) decreased, while the peak for (b) carboxyl esters (COO, COOH) disappeared. This is because minerals promote the decarboxylation reaction by reducing the pyrolysis activation energy of biomass in the early pyrolysis phase [18,45]. Conversely, the (d) aromatic peaks (CH, C) were enhanced, confirming that Ca(H2PO4)2 strengthened the aromaticity of biochar through dehydrogenation and deoxygenation. Additionally, the addition of minerals significantly increased the (f) peaks for sugars, alcohols, and ethers (CHOH, CH2OH, CH2−O−), g. methoxy, methyl, amino groups (CH3O−, CH−, NH−, C), h. methylene groups (−CH2−) and i. methyl groups (CH3−). It indicated that while Ca(H2PO4)2 increased the aromatic carbon content in biochar, meanwhile fixed volatile carbon components such as sugars, alcohols and ethers within biochar.

Table 3.
Peak areas of the 13C NMR spectrum of biochar. The deconvoluted NMR signals are assigned to a. ketone, quinone, aldehyde (C=O, HC=O); b. carboxyl ester (COO, COOH); c. O-substituted aromatic (C–O, C–OH); d. aromatic (CH, C); e. aromatic (CH); f. saccharide, alcohol, ether (CHOH, CH2OH, CH2−O−); g. methoxy, methyne, quaternary (CH3O−, CH−, NH−, C); h. methylene (−CH2−); and i. methyl (−CH3).
Based on DTG curves, the pyrolysis temperature range was primarily between 250 °C and 400 °C (Figure 5). Therefore, PY-GC/MS analysis was performed at 300 °C and 400 °C. For hemicellulose derivatives (Figure 6a), thermal treatment facilitated the conversion of 1-vinyl-1H-pyrrole-2,5-dione and furfural to 2-ethoxy-2-cyclohexen-1-one. The mineral additive notably enhanced this process at 400 °C, elevating the corresponding peak area from 0.12 × 107 to 0.16 × 107. Simultaneously, Ca(H2PO4)2 promoted the ring-opening reaction of 1,4:3,6-dianhydro-α-D-glucopyranose (No. 3) and catalyzed the cyclization of stearic acid and hexadecanamide into 2-amino-1-phenylethanol (No. 8), increasing its peak intensity from 0.24 × 107 to 0.35 × 107 (Table S4). These transformations were accompanied by characteristic cleavage patterns of long-chain alkanes. The cellulose-derived biochar generated at 300 °C (Figure 6b) exhibited a predominant composition of nitrogen-containing compounds, aldehydes, phenolic derivatives, and saccharides. Co-pyrolysis with Ca(H2PO4)2 induced significant structural transformations: 2-methyl-1,3,4-oxadiazole (No. 12) and furfural (No. 13) underwent conversion to styrene (No. 14), accompanied by the disappearance of catechol (No. 19) and emergence of naphthalene (No. 18) characteristic peaks. Thermal elevation to 400 °C further enhanced styrene formation, with its peak area demonstrating a marked increase from 0.74 × 108 to 1.60 × 108 (Table S5), indicative of calcium phosphate’s catalytic effect on aromatic carbon formation during biomass pyrolysis. Lignin decomposition at 300 °C primarily yielded phenolic and acidic compounds (Figure 6c). The mineral additive significantly enhanced phenol (No. 23) and catechol (No. 19) production, with their peak areas increasing from 0.87 × 107 and 2.20 × 107 to 0.89 × 107 and 3.38 × 107, respectively (Table S6). Subsequent temperature elevation promoted phenolic ring-opening reactions coupled with dehydroxylation/decarboxylation processes. This synergistic effect between calcium phosphate catalysis and thermal treatment ultimately facilitated the structural reorganization of carbon skeletons toward aromatic configurations (Figure 7).
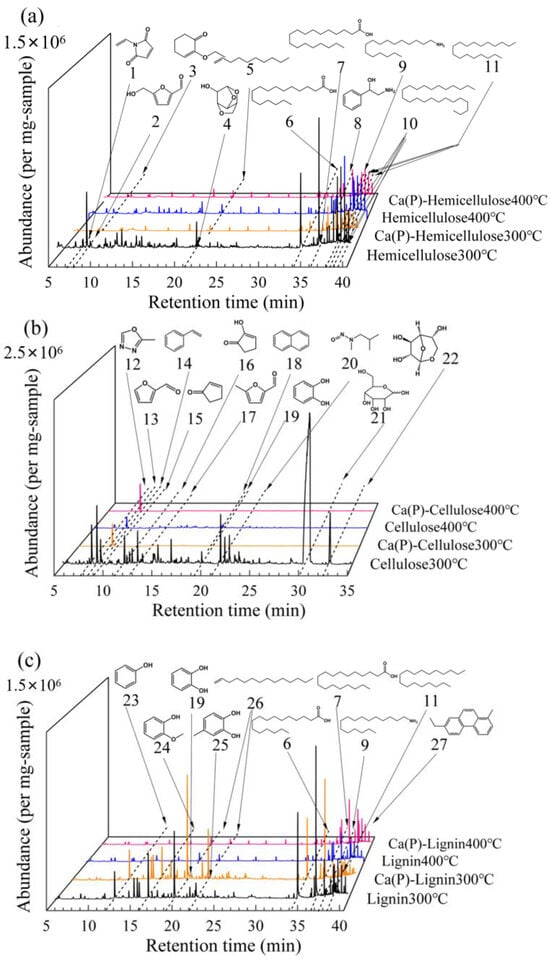
Figure 7.
PY-GC-MS spectra of hemicellulose (a), cellulose (b), and lignin (c) biochars produced at 300 °C and 400 °C, with and without a Ca(H2PO4)2 addition. Main peaks were assigned to 1. 1-vinyl-1H-pyrrole-2,5-dione; 2. 5-hydroxymethylfurfural; 3. 2-ethoxy-2-cyclohexen-1-one; 4. 1,4:3,6-Dianhydro-ALPHA-D-glucopyranose; 5. 1-Decene; 6. palmitic acid; 7. octadecanoic acid; 8. 2-amino-1-phenylethanol; 9. hexadecanamide; 10. n-24 alkane; 11. n-18 alkane; 12. 2-Methyl-1,3,4-oxadiazole; 13. furfural; 14. styrene; 15. 2-cyclopenten-1-one; 16. 2-hydroxy-2-cyclopenten-1-one; 17. 5-methyl-2-furaldehyde; 18. naphthalene; 19. catechol; 20. N,2-dimethyl-N-nitroso-1-propylamine; 21. D-allose; 22. 1,6-anhydro-Β-D-glucofuranose; 23. phenol; 24. O-methoxyphenol; 25. 4-methylcatechol; 26. 1-tetradecene; 27. Phenanthrene.
Biochar derived from goldenrod at 300 °C (Figure 8) primarily contained phenols, aromatic hydrocarbons, aldehydes, and sugars. After co-pyrolysis with Ca(H2PO4)2, the peak area of D-allose (No. 11) increased significantly from 0.10 × 107 to 3.26 × 107, and the peak area of styrene (No. 1) increased from 0.89 × 107 to 1.59 × 107 (Table S7), indicating that Ca(H2PO4)2 enhanced the formation of aromatic carbon and promoted the decomposition of substances such as cellulose into D-allose. As the temperature increased to 400 °C, the peak area of phenol (No. 3) decreased from 0.69 × 107 to 0.49 × 107, while a substantial amount of benzaldehyde (No. 2) was produced. The unstable phenol, catechol (No. 7), disappeared, and a more stable compound, naphthalene (No. 6), appeared. Similarly, 2,6-dimethylphenol (No. 10) was converted to naphthalene-1,7-dimethyl (No. 11). The biochar produced at 400 °C, loaded with Ca(H2PO4)2, showed a significant reduction in pyrolytic substances compared to original biochar, likely because the modified biochar was more stable and had fewer volatile substances. As shown in Figure 8, at the same temperature, the contents of phenolic and aldehyde substances in biochar loaded with Ca(H2PO4)2 were significantly lower, suggesting that Ca(H2PO4)2 promoted dehydrogenation and deoxygenation, leading the aromatization of biochar.
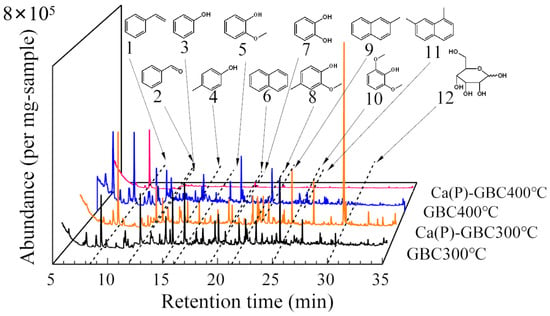
Figure 8.
PY-GC-MS spectra of goldenrod biochars produced at 300 °C and 400 °C with and without a Ca(H2PO4)2 addition. Main peaks were assigned to 1. styrene; 2. benzaldehyde; 3. phenol; 4. p-cresol; 5. 2-methoxyphenol; 6. naphthalene; 7. catechol; 8. 2-methoxy-4-methylphenol; 9. 2-methylnaphthalene; 10. 2,6-dimethylphenol; 11. naphthalene-1,7-dimethyl; 12. D-allose.
Based on analyses of the elemental composition, surface functional groups, carbon structural composition and volatiles of biochar, the interaction mechanisms governing the evolution of biochar’s carbon structure were summarized in Figure 9. Ca(H2PO4)2 enhanced dehydration and depolymerization at 300 °C. As the temperature increased, hemicellulose experienced depolymerization, ring-opening and C–C bond cleavage, resulting in the formation of small volatile acids and ketones. Simultaneously, hemicellulose inhibited these small molecules from undergoing secondary processes such as cyclization, recombination and polymerization. However, the addition of minerals catalyzed carboxyl group nucleophilic addition reactions, promoting cyclization and the formation of C=O and C=C structures, also protecting the small molecules. Some depolymerized hemicellulose products encapsulated around cellulose and lignin, thereby suppressing their degradation [11]. At 400 °C, Ca(H2PO4)2 primarily promoted the depolymerization of cellulose and the cleavage of lignin’s phenylpropane side chains [14]. Cellulose was decomposed to D-allose first and then continued to form saccharides and phenolics. Ca(H2PO4)2 significantly accelerated this process and promoted the cyclization of aldehydes and the formation of furans through dehydroxylation and decarboxylation [16]. As the temperature rose, reactions such as ring-opening and C–C bond cleavage also occurred. Minerals protected the generated small molecules such as aldehydes, ketones and alcohols, inducing the cyclization of chain alkanes. Under the combined influence of high temperature and minerals, aromatization reactions occurred and formed aromatic carbons such as naphthalene and anthracene. The depolymerization of lignin was accompanied by generation of phenolics and acids, which was primarily due to interactions with cellulose [12]. Ca(H2PO4)2 promoted the dehydration and cyclization of phenolics, as well as the cyclization of macromolecular acids, facilitating the formation of aromatic carbons. All aromatic carbon components polymerized and reorganized to form biochar with a graphite-like structure. The surface encapsulation of CaO, Ca2P2O7, and chemical bonding of C–O–PO3 and C–P, significantly enhanced the stability of biochar.
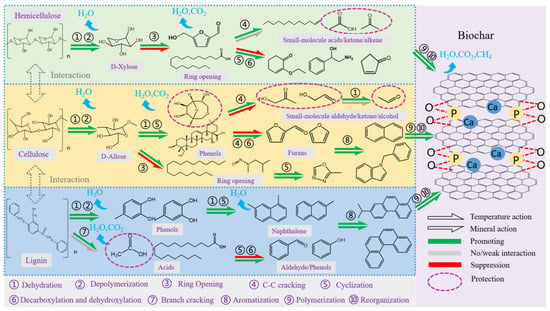
Figure 9.
Schematic figure of the effect of Ca(H2PO4)2 on the formation of carbon structures in biochar during pyrolysis.
4. Conclusions
This investigation significantly enhanced the carbon sequestration potential of biochar through mineral-mediated regulation, elucidating the underlying mechanisms involved. Among various additives, Ca(H2PO4)2 exhibited optimal performance, resulting in a 15% increase in biochar’s carbon retention and a 12% enhancement in stable carbon content. A comprehensive thermal analysis revealed a distinctive activation energy profile: the addition of minerals initially reduced, followed by an increase in activation energy throughout the pyrolysis process. Specifically, for goldenrod biomass, the activation energy rose from 85.9 kJ mol−1 to 156.5 kJ mol−1. Furthermore, Ca(H2PO4)2 significantly influenced pyrolysis dynamics by increasing reaction frequency, elevating energy barriers, and introducing greater reaction complexity. The mechanistic study identified a temperature-dependent catalytic behavior of Ca(H2PO4)2: during the initial stages of pyrolysis, it primarily facilitated biomass dehydration and depolymerization, while at elevated temperatures, it promoted ring-opening and C–C bond cleavage. The stabilization mechanism involved the following protection measures: (1) physical encapsulation through the deposition of CaO and Ca2P2O7 on the surface, and (2) chemical stabilization via the formation of C–O–PO3 and C–P bonds, effectively preserving volatile small molecules such as sugars, ketones, and ethers. Additionally, Ca(H2PO4)2 catalyzed critical structural transformations, including chain alkane cyclization and aromatization, leading to the formation of stable aromatic carbon networks through subsequent polymerization and reorganization processes. These findings established a scientifically grounded framework for developing high-performance biochar with enhanced carbon sequestration capacity, providing both practical methodologies and theoretical insights for sustainable carbon management strategies. Biochar enhanced soil hydration and fertility via porous structure-mediated nutrient retention, which qualifies for carbon credit certification due to its millennial-scale stability, and effectively immobilized aquatic heavy metals/organic pollutants through surface complexation and redox reactions. However, the costs of the collection of raw materials for biochar, along with the costs associated with pretreatment, pyrolysis energy consumption, and equipment maintenance, could be high. Additionally, pyrolysis released some greenhouse gases such as CH4 and CO. Future work will systematically reveal the carbon sequestration mechanisms of mineral-modified biochar. This research aimed to provide a theoretical basis for the development of an efficient and cost-effective carbon storage technologies, thereby contributing to sustainable agricultural development and the achievement of carbon neutrality goals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15040943/s1. Table S1. Sources of materials for different biochars; Table S2. Content of metal oxides in biochar (wt%); Table S3. Changes in Gibbs free energy, enthalpy and entropy at various α for the pyrolysis of phragmites with or without Ca(H2PO4)2; Table S4. Areas of major peaks detected by PY-GC-MS for hemicellulosic biochars; Table S5. Areas of major peaks detected by PY-GC-MS for cellulosic biochars; Table S6. Areas of major peaks detected by PY-GC-MS for lignin biochars; Table S7. Areas of major peaks detected by PY-GC-MS for goldenrod biochars; Figure S1. SEM images of biochars produced by phragmites (a), phragmites + Ca(H2PO4)2 (b), goldenrod (c) and goldenrod + Ca(H2PO4)2 (d); Figure S2. Isoconversional plots for the pyrolysis of phragmites (a), phragmites + Ca(H2PO4)2 (b), goldenrod (c) and goldenrod + Ca(H2PO4)2 (d); Figure S3. 13C NMR spectra of PBC (a) and Ca(P)-PBC (b), SBC (c) and Ca(P)-SBC (d). The deconvoluted NMR signals were assigned to a. ketone, quinone, aldehyde (C=O, HC=O); b. carboxyl ester (COO, COOH); c. O-substituted aromatic (C–O, C–OH); d. aromatic (CH, C); e. aromatic (CH); f. saccharide, alcohol, ether (CHOH, CH2OH, CH2−O−); g. methoxy, methyne, quaternary (CH3O−, CH−, NH−, C); h. methylene (−CH2−); and i. methyl (−CH3).
Author Contributions
All authors contributed to the study conception and design. F.Y. and L.C. provided the financial support for the project leading to this publication; F.Y. and P.G. performed the experiment; L.C. designed the methodology; X.L. and Z.G. provided the reagents and materials; Y.W. and L.L. wrote the initial draft; B.L. and J.S. performed review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (No. 22dz1209402), National Natural Science Foundation of China (No. 42477036), Open Funding of State Environmental Protection Engineering Center for Urban Soil Contamination Control and Remediation (No. USCR-202201), and National Natural Science Foundation of China (No. 52208269).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors extend their gratitude to Shuchun Wang from Shiyanjia Lab (www.shiyanjia.com) for providing invaluable assistance with the SEM analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nan, H.; Mašek, O.; Yang, F.; Xu, X.; Qiu, H.; Cao, X.; Zhao, L. Minerals: A missing role for enhanced biochar carbon sequestration from the thermal conversion of biomass to the application in soil. Earth-Sci. Rev. 2022, 234, 104215. [Google Scholar] [CrossRef]
- Pstrowska, K.; Łużny, R.; Fałtynowicz, H.; Jaroszewska, K.; Postawa, K.; Pyshyev, S.; Krowiak, A. Unlocking sustainability: A comprehensive review of up-recycling biomass waste into biochar for environmental solutions. Chem. Chem. Technol. 2024, 18, 211–231. [Google Scholar] [CrossRef]
- Pyshye, S.; Lypko, Y.; Demchuk, Y.; Kukhar, O.; Korchak, B.; Pochapska, I.; Zhytnetskyi, I. Characteristics and applications of waste tire pyrolysis products: A review. Chem. Chem. Technol 2024, 18, 244–257. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, J.-G.; Kim, T.; Alessi, D.S.; Baek, K. Interaction of biochar stability and abiotic aging: Influences of pyrolysis reaction medium and temperature. Chem. Eng. J. 2021, 411, 128441. [Google Scholar] [CrossRef]
- Nan, H.; Zhao, L.; Yang, F.; Liu, Y.; Xiao, Z.; Cao, X.; Qiu, H. Different alkaline minerals interacted with biomass carbon during pyrolysis: Which one improved biochar carbon sequestration? J. Clean. Prod. 2020, 255, 120162. [Google Scholar] [CrossRef]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of mineral additives on biochar formation: Carbon Retention, stability, and properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Park, J.-H.; Li, R.; Dodla, S.K.; Zhang, Z. Biochar produced from mineral salt-impregnated chicken manure: Fertility properties and potential for carbon sequestration. Waste Manag. 2018, 78, 802–810. [Google Scholar] [CrossRef]
- Li, F.; Gui, X.; Ji, W.; Zhou, C. Effect of calcium dihydrogen phosphate addition on carbon retention and stability of biochars derived from cellulose, hemicellulose, and lignin. Chemosphere 2020, 251, 126335. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Shen, M.; Zhu, X.; Zhang, S. Extraneous Fe increased the carbon retention of sludge-based biochar. Bull. Env. Contam. Toxicol. 2021, 106, 198–204. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y. Simulating the pyrolysis interactions among hemicellulose, cellulose and lignin in wood waste under real conditions to find the proper way to prepare bio-oil. Renew. Energy 2023, 205, 851–863. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Yang, H.; Jiang, H.; Chen, Y.; Zhang, S.; Chen, H. Impact of biomass constituent interactions on the evolution of char’s chemical structure: An organic functional group perspective. Fuel 2022, 319, 123772. [Google Scholar] [CrossRef]
- Zhang, S.; Mei, Y.; Lin, G. Pyrolysis interaction of cellulose, hemicellulose and lignin studied by TG-DSC-MS. J. Energy Inst. 2024, 112, 101479. [Google Scholar] [CrossRef]
- Dong, Z.; Rene, E.R.; Zhang, P.; Hu, Q.; Ma, W. Design and Preparation of carbon material catalyst modified with metal framework and sulfonate for biochar generation from low-temperature directional pyrolysis of kitchen waste: Mechanism and performance. Bioresour. Technol. 2023, 371, 128616. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Z.; Chen, Y.; Yang, H.; Chen, W.; Chen, H. Effect of Various potassium agents on product distributions and biochar carbon sequestration of biomass pyrolysis. Energy 2024, 289, 130012. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Yang, F.; Zuo, X.; Yang, H.; Ke, Q.; Huang, Y.; Cao, X.; Zhao, L. Ionic liquid-assisted production of high-porosity biochar with more surface functional groups: Taking cellulose as attacking target. Chem. Eng. J. 2022, 433, 133811. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Z.; Zheng, X.; Jin, X.; Cai, J. Understanding pyrolysis mechanisms of corn and cotton stalks via kinetics and thermodynamics. J. Anal. Appl. Pyrolysis 2022, 164, 105521. [Google Scholar] [CrossRef]
- Cai, J.; Alimujiang, S. Kinetic analysis of wheat straw oxidative pyrolysis using thermogravimetric analysis: Statistical description and isoconversional kinetic analysis. Ind. Eng. Chem. Res. 2009, 48, 619–624. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A Simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Z.; Li, C.; He, F.; Zhang, X.; Cai, J. Insight into master plots method for kinetic analysis of lignocellulosic biomass pyrolysis. Energy 2021, 233, 121194. [Google Scholar] [CrossRef]
- Cai, J.; Yao, F.; Yi, W.; He, F. New temperature integral approximation for nonisothermal kinetics. AIChE J. 2006, 52, 1554–1557. [Google Scholar] [CrossRef]
- Deng, C.; Cai, J.; Liu, R. Kinetic analysis of solid-state reactions: Evaluation of approximations to temperature integral and their applications. Solid. State Sci. 2009, 11, 1375–1379. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.; Ok, Y.S.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Env. Geochem. Health 2019, 41, 1687–1704. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, C.; Wang, Y.; He, L.; Lu, H.; Yang, S. Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J. Clean. Prod. 2020, 254, 120111. [Google Scholar] [CrossRef]
- Ren, N.; Tang, Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf. Environ. Prot. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Leijenhorst, E.J.; Wolters, W.; Van De Beld, L.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-assisted biomass thermal conversion: Reducing carbon loss and improving biochar stability. PLoS ONE 2014, 9, e115373. [Google Scholar] [CrossRef]
- Ren, J.; Wang, F.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Li, C.; Zeng, G. Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil. Chemosphere 2017, 189, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.; Carpenter, R.W.; Windman, T.; Kim, Y.; Nunez, R.; Alawneh, F. Reaction mechanisms for enhancing mineral sequestration of CO2. Environ. Sci. Technol. 2009, 43, 6314–6319. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Jiang, H.; Tian, K.; Ding, Y.-W.; Yu, H.-Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qian, F.; Liu, Y.; Matera, D.; Wu, G.; Zhang, S.; Chen, J. Controllable synthesis of magnetic carbon composites with high porosity and strong acid resistance from hydrochar for efficient removal of organic pollutants: An overlooked influence. Carbon. 2016, 99, 338–347. [Google Scholar] [CrossRef]
- Qian, T.-T.; Li, D.-C.; Jiang, H. Thermochemical behavior of tris(2-Butoxyethyl) phosphate (TBEP) during Co-pyrolysis with biomass. Environ. Sci. Technol. 2014, 48, 10734–10742. [Google Scholar] [CrossRef]
- Qiu, B. Synthesis of industrial solid wastes/biochar composites and their use for adsorption of phosphate_from surface properties to sorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 86–93. [Google Scholar] [CrossRef]
- Nie, N.; Wang, Y.; Yellezuome, D.; Liu, X.; Wang, P.; Wang, X.; Zhu, C.; Xiao, J.; Cai, J. Exploring kinetic and thermodynamic mechanisms of switchgrass pyrolysis using iterative linear integral isoconversional method and master plots approach. Fuel 2023, 338, 127266. [Google Scholar] [CrossRef]
- Li, C.; Yellezuome, D.; Li, Y.; Liu, R.; Cai, J. Enhancing bio-aromatics yield in bio-oil from catalytic fast pyrolysis of bamboo residues over bi-metallic catalyst and reaction mechanism based on quantum computing. Fuel 2023, 336, 127158. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, L.; Chen, X.; Li, K.; Meng, L.; Xie, X.; Yuan, S.; Gao, Y.; Zhou, X. Synergistic effect of volatile inherent minerals on catalytic pyrolysis of wheat straw over a Fe–Ca–Ni catalyst. Energy 2022, 253, 124216. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass. Fuel Process. Technol. 2012, 104, 189–197. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Lang, X.; Ren, X.; Fan, S. Evaluation of agricultural residues pyrolysis under non-isothermal conditions: Thermal behaviors, kinetics, and thermodynamics. Bioresour. Technol. 2017, 241, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Collazzo, G.C.; Broetto, C.C.; Perondi, D.; Junges, J.; Dettmer, A.; Dornelles Filho, A.A.; Foletto, E.L.; Godinho, M. A detailed non-isothermal kinetic study of elephant grass pyrolysis from different models. Appl. Therm. Eng. 2017, 110, 1200–1211. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and Inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Luo, L.; Yellezuome, D.; Rahman, M.M.; Zou, J.; Hu, H.; Cai, J. Insight into kinetic and thermodynamic analysis methods for lignocellulosic biomass pyrolysis. Renew. Energy 2023, 202, 154–171. [Google Scholar] [CrossRef]
- Huang, X.; Yamasaki, K.; Kudo, S.; Sperry, J.; Hayashi, J. Influence of ionic liquid type on porous carbon formation during the ionothermal pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2020, 145, 104728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).