Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands
Abstract
1. Introduction
2. Organic Matter and the Soil Microbial Engine
2.1. Composition and Fate of SOM in Croplands
2.2. Key Microbial Groups and Their Decomposition Roles
| Microbial Group | Primary Function | Habitat Preference | Response to Management | Influence of Climate and Soil Type | References |
|---|---|---|---|---|---|
| Proteobacteria | Rapid decomposition of labile organic compounds | Rhizosphere, nutrient-rich environments | Increases with fertilization and organic inputs | Favored by warmer temperatures and higher moisture in fertile, loamy soils. | [58,59,60,61] |
| Bacteroidetes | Decomposition of readily available substrates | Agricultural soils, organic-rich zones | Enhanced by agricultural practices | Prevalent in moist, neutral pH soils; sensitive to drought stress. | [62,63,64,65] |
| Ascomycetes | Decomposition of recalcitrant plant materials | Diverse soil environments | Sensitive to soil disturbance | Exhibit broad environmental tolerance; active across diverse soil types and moisture regimes, including dry conditions. | [66,67] |
| Basidiomycetes | Decomposition of complex organic compounds | Forest soils, organic matter-rich zones | Decreases with intensive cultivation | Prefer cooler, stable environments and higher moisture; dominant in forest soils with abundant lignin. | [68,69,70,71] |
| Actinobacteria | Decomposition of complex organic matter | Diverse soil conditions | Variable response to management | Resilient to desiccation and high pH; prominent in arid and alkaline soils. | [72,73,74] |
| Acidobacteria | Slow decomposition of stable organic compounds | Nutrient-poor, stable environments | Stable under low-input systems | Dominant in acidic, oligotrophic soils and stable, undisturbed environments; sensitive to rapid environmental shifts. | [60,75,76,77] |
2.3. Microbial Necromass and Its Contribution to Stable SOM
3. Climate Influences Decomposition Dynamics
3.1. Temperature Sensitivity of Microbial Processes and Enzyme Kinetics
3.2. Moisture Effects on Soil Aeration, Redox, and Microbial Activity
3.3. Climate Variability, Land Use Change, and Long-Term Shifts in OM Decomposition
4. Soil Properties as Contextual Modifiers
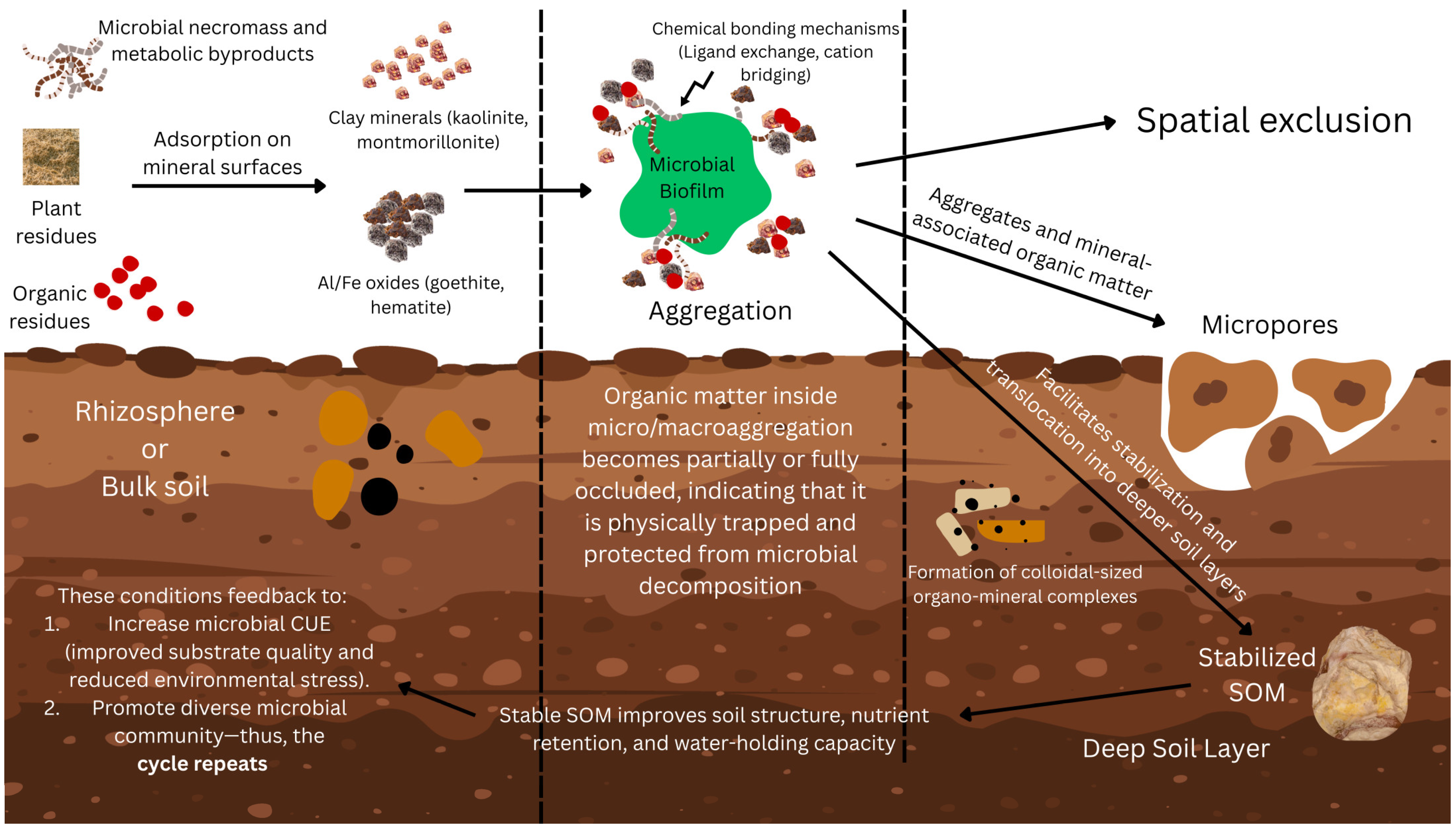
4.1. Soil Aggregation, Texture, and Porosity: Regulators of Microbial Habitat and OM Protection
4.2. Soil pH
4.3. Nutrient Stoichiometry and Microbial CUE
5. Cross-Scale Interactions and Emergent Feedback
5.1. Feedback Between Microbial Activity and Soil Microenvironment
5.2. Landscape-Level Implications of Local Decomposition Processes
6. Decomposition, Carbon Sequestration, and Cropland Sustainability
6.1. Balancing Decomposition and Carbon Stabilization
6.2. Role of Microbial Communities in SOC Formation and Loss
6.3. Effects of Agricultural Practices on Decomposition Dynamics
7. Future Research Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cotrufo, M.F.; Lavallee, J.M. Chapter One-Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 172, pp. 1–66. [Google Scholar]
- Zhao, X.; Liu, B.-Y.; Liu, S.-L.; Qi, J.-Y.; Wang, X.; Pu, C.; Li, S.-S.; Zhang, X.-Z.; Yang, X.-G.; Lal, R.; et al. Sustaining Crop Production in China’s Cropland by Crop Residue Retention: A Meta-Analysis. Land Degrad. Dev. 2020, 31, 694–709. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Liang, A.; Duan, Y.; Chen, H.; Yu, Z.; Fan, R.; Liu, H.; Pan, H. Chemical Composition of Plant Residues Regulates Soil Organic Carbon Turnover in Typical Soils with Contrasting Textures in Northeast China Plain. Agronomy 2022, 12, 747. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, H.; Zhang, Q.; Said Mgelwa, A.; Zhu, B.; Hu, Y. Negative Priming Effect from Tree Leaf and Root Residues with Contrasting Chemical Composition. Geoderma 2022, 427, 116118. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Pei, J.; Li, M.; Shan, T.; Zhang, W.; Wang, J. Below Ground Residues Were More Conducive to Soil Organic Carbon Accumulation than above Ground Ones. Appl. Soil Ecol. 2020, 148, 103509. [Google Scholar] [CrossRef]
- Luu, A.T.; Hoang, N.T.; Dinh, V.M.; Bui, M.H.; Grandy, S.; Hoang, D.T.T. Effects of Carbon Input Quality and Timing on Soil Microbe Mediated Processes. Geoderma 2022, 409, 115605. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial Decomposition of Soil Organic Matter Is Mediated by Quality and Quantity of Crop Residues: Mechanisms and Thresholds. Biol. Fertil. Soils. 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Miltner, A.; Zheng, T.; Liang, C.; Kästner, M. Microbial Necromass as a Source for Soil Organic Matter Formation-Implications for Soil Processes. In Proceedings of the Copernicus Meetings, Online, 4–8 May 2020. [Google Scholar]
- Yang, Y.; Gunina, A.; Cheng, H.; Liu, L.; Wang, B.; Dou, Y.; Wang, Y.; Liang, C.; An, S.; Chang, S.X. Unlocking Mechanisms for Soil Organic Matter Accumulation: Carbon Use Efficiency and Microbial Necromass as the Keys. Glob. Change Biol. 2025, 31, e70033. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef] [PubMed]
- Whalen, E.D.; Grandy, A.S.; Geyer, K.M.; Morrison, E.W.; Frey, S.D. Microbial Trait Multifunctionality Drives Soil Organic Matter Formation Potential. Nat. Commun. 2024, 15, 10209. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.; Błońska, E.; Piaszczyk, W. State of Soil Enzymatic Activity in Relationship to Some Chemical Properties of Brunic Arenosols. Soil Sci. Annu. 2021, 72, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in Soil Carbon Fractions and Enzyme Activities under Different Vegetation Types of the Northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Li, Z.; Zhang, Y.; Lu, L. Microbial Community and Soil Enzyme Activities Driving Microbial Metabolic Efficiency Patterns in Riparian Soils of the Three Gorges Reservoir. Front. Microbiol. 2023, 14, 1108025. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Jiang, W.; Zou, S.; Kang, D.; Yan, X. Microbial Carbohydrate-Active Enzymes Influence Soil Carbon by Regulating the of Plant- and Fungal-Derived Biomass Decomposition in Plateau Peat Wetlands under Differing Water Conditions. Front. Microbiol. 2023, 14, 1266016. [Google Scholar] [CrossRef] [PubMed]
- Weiler, D.A.; Bastos, L.M.; Schirmann, J.; Aita, C.; Giacomini, S.J. Changes in Chemical Composition of Cover Crops Residue during Decomposition. Cienc. Rural. 2021, 52, e20210357. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Q.; Wang, X.; Wang, Y.-P.; Ciais, P.; Zhang, H.; Goll, D.S.; Zhu, L.; Zhao, Z.; Guo, Z.; et al. Modeling Biochar Effects on Soil Organic Carbon on Croplands in a Microbial Decomposition Model (MIMICS-BC_v1.0). Geosci. Model Dev. 2024, 17, 4871–4890. [Google Scholar] [CrossRef]
- Hondroudakis, L.; Kopittke, P.M.; Dalal, R.C.; Barnard, M.; Weng, Z.H. The Influence of Land Use and Management on the Behaviour and Persistence of Soil Organic Carbon in a Subtropical Ferralsol. SOIL 2024, 10, 451–465. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, H.; Gao, X.; Huang, S.; Niu, S.; Lugato, E.; Xia, X. Short-Term Warming Supports Mineral-Associated Carbon Accrual in Abandoned Croplands. Nat. Commun. 2025, 16, 344. [Google Scholar] [CrossRef]
- Ling, J.; Dungait, J.A.J.; Delgado-Baquerizo, M.; Cui, Z.; Zhou, R.; Zhang, W.; Gao, Q.; Chen, Y.; Yue, S.; Kuzyakov, Y.; et al. Soil Organic Carbon Thresholds Control Fertilizer Effects on Carbon Accrual in Croplands Worldwide. Nat. Commun. 2025, 16, 3009. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Y.; Lou, M.; Cheng, Z.; Ma, R.; Guo, H.; Zhou, J.; Jia, H.; Fan, L.; Wang, T. Microbial Transformation Mechanisms of Particulate Organic Carbon to Mineral-Associated Organic Carbon at the Chemical Molecular Level: Highlighting the Effects of Ambient Temperature and Soil Moisture. Soil Biol. Biochem. 2024, 195, 109454. [Google Scholar] [CrossRef]
- Hansen, P.M.; Even, R.; King, A.E.; Lavallee, J.; Schipanski, M.; Cotrufo, M.F. Distinct, Direct and Climate-Mediated Environmental Controls on Global Particulate and Mineral-Associated Organic Carbon Storage. Glob. Change Biol. 2024, 30, e17080. [Google Scholar] [CrossRef]
- Liang, Y.; Leifheit, E.F.; Lehmann, A.; Rillig, M.C. Soil Organic Carbon Stabilization Is Influenced by Microbial Diversity and Temperature. Sci. Rep. 2025, 15, 13990. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Influence of Water Availability and Temperature on Estimates of Microbial Extracellular Enzyme Activity. PeerJ 2021, 9, e10994. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Cotrufo, M.F. Mechanisms of Soil Organic Carbon and Nitrogen Stabilization in Mineral-Associated Organic Matter–Insights from Modeling in Phase Space. Biogeosciences 2024, 21, 4077–4098. [Google Scholar] [CrossRef]
- Dohnalkova, A.C.; Tfaily, M.M.; Chu, R.K.; Smith, A.P.; Brislawn, C.J.; Varga, T.; Crump, A.R.; Kovarik, L.; Thomashow, L.S.; Harsh, J.B.; et al. Effects of Microbial-Mineral Interactions on Organic Carbon Stabilization in a Ponderosa Pine Root Zone: A Micro-Scale Approach. Front. Earth Sci. 2022, 10, 799694. [Google Scholar] [CrossRef]
- Matus, F.J. Fine Silt and Clay Content Is the Main Factor Defining Maximal C and N Accumulations in Soils: A Meta-Analysis. Sci. Rep. 2021, 11, 6438. [Google Scholar] [CrossRef]
- Calero, J.; García-Ruiz, R.; Torrús-Castillo, M.; Vicente-Vicente, J.L.; Martín-García, J.M. Role of Clay Mineralogy in the Stabilization of Soil Organic Carbon in Olive Groves under Contrasted Soil Management. Minerals 2023, 13, 60. [Google Scholar] [CrossRef]
- Poeplau, C.; Dechow, R.; Begill, N.; Don, A. Towards an Ecosystem Capacity to Stabilise Organic Carbon in Soils. Glob. Change Biol. 2024, 30, e17453. [Google Scholar] [CrossRef] [PubMed]
- Kästner, M.; Miltner, A.; Thiele-Bruhn, S.; Liang, C. Microbial Necromass in Soils—Linking Microbes to Soil Processes and Carbon Turnover. Front. Environ. Sci. 2021, 9, 756378. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, D.; Wei, B.; Yang, Y. Dual Roles of Microbes in Mediating Soil Carbon Dynamics in Response to Warming. Nat. Commun. 2024, 15, 6439. [Google Scholar] [CrossRef]
- Kou, X.; Morriën, E.; Tian, Y.; Zhang, X.; Lu, C.; Xie, H.; Liang, W.; Li, Q.; Liang, C. Exogenous Carbon Turnover within the Soil Food Web Strengthens Soil Carbon Sequestration through Microbial Necromass Accumulation. Glob. Change Biol. 2023, 29, 4069–4080. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, J.; Wang, P.; Zhu, B. The Direct and Legacy Effects of Drying-Rewetting Cycles on Active and Relatively Resistant Soil Carbon Decomposition. Land Degrad. Dev. 2023, 34, 2124–2135. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A Global Meta-Analysis of Soil Organic Carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Zhu, G.; Lu, S.; Qiu, D.; Jiao, Y.; Meng, G.; Chen, L.; Li, R.; Zhang, W.; et al. Agricultural Activities Increased Soil Organic Carbon in Shiyang River Basin, a Typical Inland River Basin in China. Sci. Rep. 2025, 15, 11727. [Google Scholar] [CrossRef] [PubMed]
- Villat, J.; Nicholas, K.A. Quantifying Soil Carbon Sequestration from Regenerative Agricultural Practices in Crops and Vineyards. Front. Sustain. Food Syst. 2024, 7, 1234108. [Google Scholar] [CrossRef]
- Abrar, M.M.; Waqas, M.A.; Mehmood, K.; Fan, R.; Memon, M.S.; Khan, M.A.; Siddique, N.; Xu, M.; Du, J. Organic Carbon Sequestration in Global Croplands: Evidenced through a Bibliometric Approach. Front. Environ. Sci. 2025, 13, 1495991. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Narsing Rao, M.P.; Shi, Y.; Lian, Z.-H.; Jin, P.-J.; Wang, W.; Li, Y.-M.; Wang, K.-K.; Banerjee, A.; et al. The Shift of Soil Microbial Community Induced by Cropping Sequence Affect Soil Properties and Crop Yield. Front. Microbiol. 2023, 14, 1095688. [Google Scholar] [CrossRef]
- Wei, X.; Fu, T.; He, G.; Zhong, Z.; Yang, M.; Lou, F.; He, T. Characteristics of Rhizosphere and Bulk Soil Microbial Community of Chinese Cabbage (Brassica Campestris) Grown in Karst Area. Front. Microbiol. 2023, 14, 1241436. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Fu, Q.; Qiu, Y.; Zhao, J.; Li, J.; Wu, X.; Yang, Y.; Liu, H.; Yang, X.; et al. Critical Transition of Soil Microbial Diversity and Composition Triggered by Plant Rhizosphere Effects. Front. Plant Sci. 2023, 14, 1252821. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Elsgaard, L.; Du, T.; Siddique, K.H.M.; Kang, S.; Butterbach-Bahl, K. Diversified Crop Rotations Improve Soil Microbial Communities and Functions in a Six-Year Field Experiment. J. Environ. Manag. 2024, 370, 122604. [Google Scholar] [CrossRef] [PubMed]
- Speckert, T.C.; Wiesenberg, G.L.B. Source or Decomposition of Soil Organic Matter: What Is More Important with Increasing Forest Age in a Subalpine Setting? Front. For. Glob. Change 2023, 6, 1290922. [Google Scholar] [CrossRef]
- Chaudhari, Y.B.; Várnai, A.; Sørlie, M.; Horn, S.J.; Eijsink, V.G.H. Engineering Cellulases for Conversion of Lignocellulosic Biomass. Protein Eng. Des. Sel. 2023, 36, gzad002. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Paice, M.; Zhang, X. Enzymatic Oxidation of Lignin: Challenges and Barriers Toward Practical Applications. ChemCatChem 2020, 12, 401–425. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial Extracellular Enzymes in Biogeochemical Cycling of Ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Cheng, J.; Jin, H.; Zhang, J.; Xu, Z.; Yang, X.; Liu, H.; Xu, X.; Min, D.; Lu, D.; Qin, B. Effects of Allelochemicals, Soil Enzyme Activities, and Environmental Factors on Rhizosphere Soil Microbial Community of Stellera Chamaejasme L. along a Growth-Coverage Gradient. Microorganisms 2022, 10, 158. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, T.; Wang, L.; Liu, E. Drip Irrigation Affects Soil Bacteria Primarily through Available Nitrogen and Soil Fungi Mainly via Available Nutrients. Front. Microbiol. 2024, 15, 1453054. [Google Scholar] [CrossRef]
- Ahmed, N.; Li, J.; Li, Y.; Deng, L.; Deng, L.; Chachar, M.; Chachar, Z.; Chachar, S.; Hayat, F.; Raza, A.; et al. Symbiotic Synergy: How Arbuscular Mycorrhizal Fungi Enhance Nutrient Uptake, Stress Tolerance, and Soil Health through Molecular Mechanisms and Hormonal Regulation. IMA Fungus 2025, 16, e144989. [Google Scholar] [CrossRef]
- Bernier, L.S.; Estoppey, A.; Bindschedler, S.; Stan, G.-B.; Junier, P.; Stanley, C.E. Microfluidic Platform for Microbial Spore Germination Studies in Multiple Growth Conditions. BMC Methods 2024, 1, 12. [Google Scholar] [CrossRef]
- Hwang, Y.; Rahlff, J.; Schulze-Makuch, D.; Schloter, M.; Probst, A.J. Diverse Viruses Carrying Genes for Microbial Extremotolerance in the Atacama Desert Hyperarid Soil. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, J.A.W.W.; Seneviratne, G.; Wijepala, P.C. Application of Microbial Biofilms to Reinstate Lost Soil Microbial Diversity under Global Warming: A Case Study. In Proceedings of International Forestry and Environment Symposium; University of Sri Jayewardenepura: Nugegoda, Sri Lanka, 2022; Volume 26. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q.; et al. Environmental Behaviors of Bacillus Thuringiensis (Bt) Insecticidal Proteins and Their Effects on Microbial Ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Stefanova, V.V.; Petrov, P.G. Soil Development and Properties of Microbial Biomass Succession in Reclaimed Sites in Bulgaria. In CBU International Conference Proceedings; CBU Research Institute: Prague, Czech Republic, 2019; Volume 7, pp. 1008–1014. [Google Scholar] [CrossRef]
- Furiosi, M.; Hasanaliyeva, G.; Caffi, T.; Rossi, V. Soil Covering and Biofumigant Effect of Armoracia Rusticana against Spore Dispersal and Viability of Downy Mildew Inoculum in Viticultural Systems-BIOVINE. BIO Web Conf. 2022, 50, 03004. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, J.; Ongena, M.; Liu, W.; Wei, D.; Zhao, B.; Guan, D.; Jiang, X.; Li, J. Effect of Long-Term Fertilization Strategies on Bacterial Community Composition in a 35-Year Field Experiment of Chinese Mollisols. AMB Express 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, X.; Xiang, Q.; Penttinen, P.; Gu, Y. Response of Soil Microbial Community Structure and Function to Different Altitudes in Arid Valley in Panzhihua, China. BMC Microbiol. 2022, 22, 86. [Google Scholar] [CrossRef]
- Wang, X.; Huang, T.; Li, Y.; Zhao, G.; Zhao, J. Elevational Characteristics of Soil Bacterial Community and Their Responses to Soil Translocation at a Mountainside in Northwest Sichuan, China. Sci. Rep. 2023, 13, 17906. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Yang, Y.; Wang, S.; Chen, K.; Zhang, N. The Response Mechanism of the cbbM Carbon Sequestration Microbial Community in the Alpine Wetlands of Qinghai Lake to Changes in Precipitation. Biology 2024, 13, 1090. [Google Scholar] [CrossRef]
- Cui, J.; Yang, B.; Zhang, M.; Song, D.; Xu, X.; Ai, C.; Liang, G.; Zhou, W. Investigating the Effects of Organic Amendments on Soil Microbial Composition and Its Linkage to Soil Organic Carbon: A Global Meta-Analysis. Sci. Total Environ. 2023, 894, 164899. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef]
- Roley, S.S. Diazotrophic Nitrogen Fixation in the Rhizosphere and Endosphere. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Singapore, 2021; pp. 93–108. ISBN 978-981-15-6125-2. [Google Scholar]
- Zhang, N.; Chen, K.; Wang, S.; Qi, D.; Zhou, Z.; Xie, C.; Liu, X. Dynamic Response of the cbbL Carbon Sequestration Microbial Community to Wetland Type in Qinghai Lake. Biology 2023, 12, 1503. [Google Scholar] [CrossRef]
- Shi, L.; Dossa, G.G.O.; Paudel, E.; Zang, H.; Xu, J.; Harrison, R.D. Changes in Fungal Communities across a Forest Disturbance Gradient. Appl. Environ. Microbiol. 2019, 85, e00080-19. [Google Scholar] [CrossRef]
- Das, S.K. Biochar Application Method and Amount Both Changed the Dynamics of Soil Temperature-Moisture-Metals in an Acidic Inceptisols. Water Air Soil Pollut. 2024, 235, 303. [Google Scholar] [CrossRef]
- Muneer, M.A.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.Z.; Wu, L.; Zheng, C. Response of Fungal Diversity, Community Composition, and Functions to Nutrients Management in Red Soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Pinotti, C.R.; Hirai, W.Y.; de Moraes, M.L.T.; Montanari, R.; Filho, M.C.M.T.; Milori, D.M.B.P.; Júnior, N.L.S.; Panosso, A.R. CO2 Emission and Its Relation to Soil Temperature, Moisture, and O2 Absorption in the Reforested Areas of Cerrado Biome, Central Brazil. Plant Soil 2019, 444, 193–211. [Google Scholar] [CrossRef]
- Gallo, A.C.; Holub, S.M.; Littke, K.; Lajtha, K.; Maguire, D.; Hatten, J.A. Short-Term Effects of Organic Matter and Compaction Manipulations on Soil Temperature, Moisture, and Soil Respiration for 2 Years in the Oregon Cascades. Soil Sci. Soc. Am. J. 2023, 87, 156–171. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The Sensitivity of Soil Respiration to Soil Temperature, Moisture, and Carbon Supply at the Global Scale. Glob. Change Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Cui, J. Microbial Community Structure and Carbon Transformation Characteristics of Different Aggregates in Black Soil. PeerJ 2024, 12, e17269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Tai, X.; Sun, L.; Wu, M.; Zhang, W.; Chen, X.; Zhang, G.; Chen, T.; Liu, G.; et al. Variation in Actinobacterial Community Composition and Potential Function in Different Soil Ecosystems Belonging to the Arid Heihe River Basin of Northwest China. Front. Microbiol. 2019, 10, 2209. [Google Scholar] [CrossRef]
- Fang, J.; Wei, S.; Shi, G.; Cheng, Y.; Zhang, X.; Zhang, F.; Lu, Z.; Zhao, X. Potential Effects of Temperature Levels on Soil Bacterial Community Structure. E3S Web Conf. 2021, 292, 01008. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, K.; Schneider, R.L.; Morreale, S.J.; Xie, Y. Comparison of Microbial Community Structures in Soils with Woody Organic Amendments and Soils with Traditional Local Organic Amendments in Ningxia of Northern China. PeerJ 2019, 7, e6854. [Google Scholar] [CrossRef]
- Li, M.; Dai, G.; Mu, L. Composition and Diversity of Soil Bacterial Communities under Identical Vegetation along an Elevational Gradient in Changbai Mountains, China. Front. Microbiol. 2022, 13, 1065412. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Rowińska, P.; Gutarowska, B.; Janas, R.; Szulc, J. Biopreparations for the Decomposition of Crop Residues. Microb. Biotechnol. 2024, 17, e14534. [Google Scholar] [CrossRef]
- Glassman, S.I.; Weihe, C.; Li, J.; Albright, M.B.N.; Looby, C.I.; Martiny, A.C.; Treseder, K.K.; Allison, S.D.; Martiny, J.B.H. Decomposition Responses to Climate Depend on Microbial Community Composition. Proc. Natl. Acad. Sci. USA 2018, 115, 11994–11999. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Du, M.; Chen, J.; Tie, L.; Zhou, S.; Buckeridge, K.M.; Cornelissen, J.H.C.; Huang, C.; Kuzyakov, Y. Microbial Necromass under Global Change and Implications for Soil Organic Matter. Glob. Change Biol. 2023, 29, 3503–3515. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; He, N.; Tiemann, L. Global Microbial Necromass Contribution to Soil Organic Matter. In Proceedings of the AGU Fall Meeting 2021, New Orleans, LA, USA, 13–17 December 2021. [Google Scholar]
- Jikai, S.; Yimei, H.; Qian, H.; Fengjing, X.U. Accumulation of Microbial Necromass Carbon and Their Contribution to Soil Organic Carbon in Different Vegetation Types on the Loess Plateau, Northwest China. Chin. J. Appl. Ecol./Yingyong Shengtai Xuebao 2024, 35, 124–132. [Google Scholar]
- Li, S.-J.; Sheng, M.-J.; Li, G.; Wang, R.; Li, J.; Zhang, G.-L.; Xiu, W.-M. Impacts of Land Use Intensification Level on Fluvo-aquic Cropland Soil Microbial Community Abundance and Necromass Accumulation in North China. Huan Jing Ke Xue 2023, 44, 4611–4622. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.; Tiemann, L.K.; Zhang, F.; Kuzyakov, Y.; Tian, J. Microbial Necromass in Cropland Soils: A Global Meta-Analysis of Management Effects. Glob. Change Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef] [PubMed]
- Beidler, K.V.; Huenupi, E.; DeLancey, L.C.; Maillard, F.; Zhang, B.; Persson, P.; Kennedy, P.G.; Phillips, R. Melanization Interacts with Soil Mineral and Microbial Properties to Determine Fungal Carbon and Nitrogen Persistence in Soils. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cotrufo, M.F.; Sun, L.; Jiang, P.; Liu, Z.; Bai, E. Elevated Temperature Increases the Accumulation of Microbial Necromass Nitrogen in Soil via Increasing Microbial Turnover. Glob. Change Biol. 2020, 26, 5277–5289. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.; Xiao, J.; Duan, P.; Tang, T.; et al. Lithological Controls on Soil Aggregates and Minerals Regulate Microbial Carbon Use Efficiency and Necromass Stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef] [PubMed]
- Gies, H.; Hagedorn, F.; Lupker, M.; Montluçon, D.; Haghipour, N.; van der Voort, T.S.; Eglinton, T.I. Millennial-Age Glycerol Dialkyl Glycerol Tetraethers (GDGTs) in Forested Mineral Soils: 14C-Based Evidence for Stabilization of Microbial Necromass. Biogeosciences 2021, 18, 189–205. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Fan, X.; Sun, L.; Sang, C.; Wang, X.; Jiang, P.; Fang, Y.; Bai, E. Mineral Composition Controls the Stabilization of Microbially Derived Carbon and Nitrogen in Soils: Insights from an Isotope Tracing Model. Glob. Change Biol. 2024, 30, e17156. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Lynch, L.; Zheng, T.; Chen, F.; Liang, C. Factors Driving Microbial Biomass and Necromass Relationships Display Ecosystem-Dependent Responses. Eur. J. Soil Sci. 2024, 75, e13555. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, G.; Zhai, G.; Yi, W.; Ma, L.; Huang, Z.; Ye, X.; Ma, W.; Wang, Y.; Zhang, P.; et al. Root-Borne Microbial Necromass—An Overlooked Source of Grassland Soil Organic Carbon. Geophys. Res. Lett. 2024, 51, e2024GL110908. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Yang, Y.; Ren, L.; Wang, Z.; Liao, Y.; Yong, T. Global Synthesis on the Response of Soil Microbial Necromass Carbon to Climate-Smart Agriculture. Glob. Change Biol. 2024, 30, e17302. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Zhang, X.; Li, Y.; Virk, A.L.; Li, F.-M.; Yang, H.; Liu, S.; Kan, Z.-R. No-till Reduced Subsoil Organic Carbon Due to Decreased Microbial Necromass in Micro-Aggregates. Land Degrad. Dev. 2024, 35, 1792–1803. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, T.; Thomas, B.W.; Chen, J.; Xie, J.; Hu, Y.; Kong, F.; Yang, Y.; Chen, X.; Zhang, Y.; et al. Legume Cover Crops Enhance Soil Organic Carbon via Microbial Necromass in Orchard Alleyways. Soil Tillage Res. 2023, 234, 105858. [Google Scholar] [CrossRef]
- Si, Q.; Chen, K.; Wei, B.; Zhang, Y.; Sun, X.; Liang, J. Dissolved Carbon Flow to Particulate Organic Carbon Enhances Soil Carbon Sequestration. SOIL 2024, 10, 441–450. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart Grandy, A.; Liang, C.; Phillips, R.P. Fast-Decaying Plant Litter Enhances Soil Carbon in Temperate Forests but Not through Microbial Physiological Traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Abs, E.; Allison, S.D.; Tao, F.; Huang, Y.; Manzoni, S.; Abramoff, R.; Bruni, E.; Bowring, S.P.K.; Chakrawal, A.; et al. Emerging Multiscale Insights on Microbial Carbon Use Efficiency in the Land Carbon Cycle. Nat. Commun. 2024, 15, 8010. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, H.; Gu, X.; Li, N.; Zhao, X.; Lei, M.; Alharbi, H.; Megharaj, M.; He, W.; Kuzyakov, Y. Catalytic Efficiency of Soil Enzymes Explains Temperature Sensitivity: Insights from Physiological Theory. Sci. Total Environ. 2022, 822, 153365. [Google Scholar] [CrossRef]
- Woo, D.K.; Seo, Y. Effects of Elevated Temperature and Abnormal Precipitation on Soil Carbon and Nitrogen Dynamics in a Pinus Densiflora Forest. Front. For. Glob. Change 2022, 5, 1051210. [Google Scholar] [CrossRef]
- Adekanmbi, A.A.; Dale, L.; Shaw, L.; Sizmur, T. Differential Temperature Sensitivity of Intracellular Metabolic Processes and Extracellular Soil Enzyme Activities. Biogeosciences 2023, 20, 2207–2219. [Google Scholar] [CrossRef]
- Min, K.; Buckeridge, K.; Ziegler, S.E.; Edwards, K.A.; Bagchi, S.; Billings, S.A. Temperature Sensitivity of Biomass-Specific Microbial Exo-Enzyme Activities and CO2 Efflux Is Resistant to Change across Short- and Long-Term Timescales. Glob. Change Biol. 2019, 25, 1793–1807. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Heinzle, J.; Salas, E.; Kwatcho-Kengdo, S.; Borken, W.; Schindlbacher, A.; Wanek, W. Long-Term Soil Warming Decreases Soil Microbial Necromass Carbon by Adversely Affecting Its Production and Decomposition. Glob. Change Biol. 2024, 30, e17379. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, V.; Sharma, A.D.; Srinivasan, K. Evaluation of Seed Quality of Barley Varieties through Controlled Deterioration Test. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3123–3131. [Google Scholar] [CrossRef]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S.; et al. Soil Enzymes in Response to Climate Warming: Mechanisms and Feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Mayes, M.A.; Allison, S.D.; Frey, S.D.; Shi, Z.; Hu, X.-M.; Luo, Y.; Melillo, J.M. Reduced Carbon Use Efficiency and Increased Microbial Turnover with Soil Warming. Glob. Change Biol. 2019, 25, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial Temperature Sensitivity and Biomass Change Explain Soil Carbon Loss with Warming. Nat. Clim. Change. 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, B.; Liang, M.; Wu, Y.; Li, Y.; Zhao, Z.; Liu, G.; Xue, S. Temperature Sensitivity Response of Soil Enzyme Activity to Simulated Climate Change at Growth Stages of Winter Wheat. Agronomy 2025, 15, 106. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Yang, C.; Li, H.; Wu, J. Effects of Water Stress on Nutrients and Enzyme Activity in Rhizosphere Soils of Greenhouse Grape. Front. Microbiol. 2024, 15, 1376849. [Google Scholar] [CrossRef]
- Kumari, J.A.; Rao, P.C.; Padmaja, G.; Madhavi, M. Effect of Temperature on Soil Enzyme Acid Phosphatase. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2830–2845. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Wang, H.; Gao, D.; Hu, G.; Xu, W.; Zhuge, Y.; Bai, E. Drying–Rewetting Events Enhance the Priming Effect on Soil Organic Matter Mineralization by Maize Straw Addition. Catena 2024, 238, 107872. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A.; Niedźwiecki, J. Changes in Soil Enzymatic Activity Causedby Hydric Stress. Pol. J. Environ. Stud. 2020, 29, 2653–2660. [Google Scholar] [CrossRef]
- Dowdeswell-Downey, E.; Grabowski, R.C.; Rickson, R.J. Do Temperature and Moisture Conditions Impact Soil Microbiology and Aggregate Stability? J. Soils Sediments 2023, 23, 3706–3719. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; Van Nostrand, J.D.; Wu, L.; et al. Warming Enhances Old Organic Carbon Decomposition through Altering Functional Microbial Communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.M.; Hayer, M.; Koch, B.J.; Mau, R.L.; Blazewicz, S.J.; Dijkstra, P.; Mack, M.C.; Marks, J.C.; Morrissey, E.M.; Pett-Ridge, J.; et al. Decreased Growth of Wild Soil Microbes after 15 Years of Transplant-Induced Warming in a Montane Meadow. Glob. Change Biol. 2022, 28, 128–139. [Google Scholar] [CrossRef]
- Ruan, Y.; Kuzyakov, Y.; Liu, X.; Zhang, X.; Xu, Q.; Guo, J.; Guo, S.; Shen, Q.; Yang, Y.; Ling, N. Elevated Temperature and CO2 Strongly Affect the Growth Strategies of Soil Bacteria. Nat. Commun. 2023, 14, 391. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.; Sun, W.; Zhao, Q.; Liang, M.; Chen, W.; Guo, Z.; Jiang, Y.; Jiang, Y.; Liu, G.; et al. Temperature Sensitivity of Soil Enzyme Kinetics under N and P Fertilization in an Alpine Grassland, China. Sci. Total Environ. 2022, 838, 156042. [Google Scholar] [CrossRef]
- Gałęzewski, L.; Jaskulska, I.; Kotwica, K.; Lewandowski, Ł. The Dynamics of Soil Moisture and Temperature—Strip-Till vs. Plowing—A Case Study. Agronomy 2023, 13, 83. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Chen, J.; Castellano, M.J.; Ye, C.; Zhang, N.; Miao, Y.; Zheng, H.; Li, J.; Ding, W. Oxygen Availability Regulates the Quality of Soil Dissolved Organic Matter by Mediating Microbial Metabolism and Iron Oxidation. Glob. Change Biol. 2022, 28, 7410–7427. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial Carbon Use Efficiency Promotes Global Soil Carbon Storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.E.; Breecker, D.O.; Blackwood, C.B.; Gallagher, T.M. A New Approach to Continuous Monitoring of Carbon Use Efficiency and Biosynthesis in Soil Microbes from Measurement of CO2 and O2. Biogeosciences 2025, 22, 87–101. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture Is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Warren, C.R.; Swarbrick, B.; Minasny, B.; McBratney, A.B.; Young, I.M. Microbial Decomposition of Organic Matter and Wetting–Drying Promotes Aggregation in Artificial Soil but Porosity Increases Only in Wet-Dry Condition. Geoderma 2024, 447, 116924. [Google Scholar] [CrossRef]
- Murthy, R.K.; Raut, M. Impact of Climate Change on Soil Properties and Functions. Mysore J. Agric. Sci. 2024, 59, 34–49. [Google Scholar]
- Horodecki, P.; Jagodziński, A.M. Site Type Effect on Litter Decomposition Rates: A Three-Year Comparison of Decomposition Process between Spoil Heap and Forest Sites. Forests 2019, 10, 353. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Lasota, J.; Błońska, E.; Foremnik, K. How Habitat Moisture Condition Affects the Decomposition of Fine Woody Debris from Different Species. Catena 2022, 208, 105765. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Zheng, L.; Yu, Y.; Mushinski, R.M.; Zhou, Y.; Xiao, C. Greater Soil Water and Nitrogen Availability Increase C:N Ratios of Root Exudates in a Temperate Steppe. Soil Biol. Biochem. 2021, 161, 108384. [Google Scholar] [CrossRef]
- Bian, H.; Li, C.; Zhu, J.; Xu, L.; Li, M.; Zheng, S.; He, N. Soil Moisture Affects the Rapid Response of Microbes to Labile Organic C Addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Runkov, R.A.; Ilyasov, D.V. Spatial Variability of Methane Emissions from Soils of Wet Forests: A Brief Review. Environ. Dyn. Glob. Clim. Change 2023, 14, 167–180. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, I. Sustainable Development Goal 13: Recent Progress and Challenges to Climate Action. Environ. Sustain. 2023, 6, 297–301. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, R.; Li, T.; Zhou, R.; Hou, Z.; Zhang, X. Interactions among Microorganisms Functionally Active for Electron Transfer and Pollutant Degradation in Natural Environments. Eco-Environ. Health 2023, 2, 3–15. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, S.; Niu, B.; Pei, Y.; Song, S.; Lei, T.; Yun, H. Soil Texture Influences Soil Bacterial Biomass in the Permafrost-Affected Alpine Desert of the Tibetan Plateau. Front. Microbiol. 2022, 13, 1007194. [Google Scholar] [CrossRef]
- Naylor, D.; Sadler, N.; Bhattacharjee, A.; Graham, E.B.; Anderton, C.R.; McClure, R.; Lipton, M.; Hofmockel, K.S.; Jansson, J.K. Soil Microbiomes Under Climate Change and Implications for Carbon Cycling. Annu. Rev. Environ. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Salinas, J.; Guardia, G.; Monistrol, A.; Iglesias-Díez, S.M.; García-Gutiérrez, S.; Jiménez-Horcajada, R.; Espín, G.; Vallejo, A. Can Soluble Organic Fertilizers and/or Manure Reach the Agronomic and Environmental Performance of Synthetic Fertilizers in Drip-Fertigated Crops? Soil Use Manag. 2024, 40, e13136. [Google Scholar] [CrossRef]
- Rashid, M.A.; Bastami, M.S.; Bakar, N.A.A.; Jumat, F.; Suptian, M.F.M.; Rahman, M.H.A.; Azmin, A.A.; Talib, S.A.A. Development of National Emission Factor for Rice Production System in Malaysia: A Step Toward Achieving Sustainable Development Goals. J. Lifestyle SDGs Rev. 2025, 5, e02774. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Mapfumo, P.; Kirchmann, H. Decomposition of Organic Matter in Soil as Influenced by Texture and Pore Size Distribution. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Academy Science Publishers: Karen, Nairobi, Kenya, 2004; Volume 261. [Google Scholar]
- Wahdan, S.F.M.; Ji, L.; Schädler, M.; Wu, Y.-T.; Sansupa, C.; Tanunchai, B.; Buscot, F.; Purahong, W. Future Climate Conditions Accelerate Wheat Straw Decomposition alongside Altered Microbial Community Composition, Assembly Patterns, and Interaction Networks. ISME J. 2023, 17, 238–251. [Google Scholar] [CrossRef]
- Lu, D.; Mao, Z.; Tang, Y.; Feng, B.; Xu, L. Driving Factors Influencing Soil Microbial Community Succession of Coal Mining Subsidence Areas during Natural Recovery in Inner Mongolia Grasslands. Microorganisms 2023, 12, 87. [Google Scholar] [CrossRef]
- Xie, S.; Tran, H.-T.; Pu, M.; Zhang, T. Transformation Characteristics of Organic Matter and Phosphorus in Composting Processes of Agricultural Organic Waste: Research Trends. Mater. Sci. Energy Technol. 2023, 6, 331–342. [Google Scholar] [CrossRef]
- Chirol, C.; Séré, G.; Redon, P.-O.; Chenu, C.; Derrien, D. Depth Dependence of Soil Organic Carbon Additional Storage Capacity in Different Soil Types by the 2050 Target for Carbon Neutrality. SOIL 2025, 11, 149–174. [Google Scholar] [CrossRef]
- Miguel, M.A.; Kim, S.-H.; Lee, S.-S.; Cho, Y.-I. Impact of Soil Microbes and Oxygen Availability on Bacterial Community Structure of Decomposing Poultry Carcasses. Animals 2021, 11, 2937. [Google Scholar] [CrossRef]
- Leul, Y.; Assen, M.; Damene, S.; Legass, A. Effects of Land-Use Dynamics on Soil Organic Carbon and Total Nitrogen Stock, Western Ethiopia. Appl. Environ. Soil Sci. 2023, 2023, 5080313. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil Carbon Debt of 12,000 Years of Human Land Use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Welty, A.; Blazewicz, S.J.; Bru, D.; Rouard, N.; Breuil, M.-C.; Gessler, A.; Galiano, L.; Miranda, J.C.; Spor, A.; et al. Depth Matters: Effects of Precipitation Regime on Soil Microbial Activity upon Rewetting of a Plant-Soil System. ISME J. 2018, 12, 1061–1071. [Google Scholar] [CrossRef]
- Merloti, L.F.; Mendes, L.W.; Pedrinho, A.; de Souza, L.F.; Ferrari, B.M.; Tsai, S.M. Forest-to-Agriculture Conversion in Amazon Drives Soil Microbial Communities and N-Cycle. Soil Biol. Biochem. 2019, 137, 107567. [Google Scholar] [CrossRef]
- Mandal, D.; Chandrakala, M.; Alam, N.M.; Roy, T.; Mandal, U. Assessment of Soil Quality and Productivity in Different Phases of Soil Erosion with the Focus on Land Degradation Neutrality in Tropical Humid Region of India. Catena 2021, 204, 105440. [Google Scholar] [CrossRef]
- Gao, T.; Tian, H.; Wang, Z.; Shi, J.; Yang, R.; Wang, F.; Xiang, L.; Dai, Y.; Megharaj, M.; He, W. Effects of Atrazine on Microbial Metabolic Limitations in Black Soils: Evidence from Enzyme Stoichiometry. Chemosphere 2023, 334, 139045. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Li, F.; Sun, M.; Yang, X.; Wang, G.; Ma, J.; Sun, W. Exchangeable Ca2+ Content and Soil Aggregate Stability Control the Soil Organic Carbon Content in Degraded Horqin Grassland. Ecol. Indic. 2022, 134, 108507. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Li, X.; Su, D.; He, J. Biotic Interactions Contribute More than Environmental Factors and Geographic Distance to Biogeographic Patterns of Soil Prokaryotic and Fungal Communities. Front. Microbiol. 2023, 14, 1134440. [Google Scholar] [CrossRef]
- Doetterl, S.; Berhe, A.A.; Heckman, K.; Lawrence, C.; Schnecker, J.; Vargas, R.; Vogel, C.; Wagai, R. A Landscape-Scale View of Soil Organic Matter Dynamics. Nat. Rev. Earth Environ. 2025, 6, 67–81. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, P.; Zheng, J.; Xue, L.; Zhu, Q.; Cai, X.; Cheng, S.; Zhang, Z.; Kong, F.; Zhang, J. Effects of Soil Texture and Nitrogen Fertilisation on Soil Bacterial Community Structure and Nitrogen Uptake in Flue-Cured Tobacco. Sci. Rep. 2021, 11, 22643. [Google Scholar] [CrossRef]
- Turner, B.L. Variation in pH Optima of Hydrolytic Enzyme Activities in Tropical Rain Forest Soils. Appl. Environ. Microbiol. 2010, 76, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, N.; Heitman, J.L.; Shi, W. Soil Pore Size Distribution Shaped Not Only Compositions but Also Networks of the Soil Microbial Community. Appl. Soil Ecol. 2022, 170, 104273. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil Microbial Diversity and Composition: Links to Soil Texture and Associated Properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Dowd, S.E.; Bell, C.W.; Lascano, R.; Booker, J.D.; Zobeck, T.M.; Upchurch, D.R. Microbial Community Composition as Affected by Dryland Cropping Systems and Tillage in a Semiarid Sandy Soil. Diversity 2010, 2, 910–931. [Google Scholar] [CrossRef]
- Chiba, A.; Uchida, Y.; Kublik, S.; Vestergaard, G.; Buegger, F.; Schloter, M.; Schulz, S. Soil Bacterial Diversity Is Positively Correlated with Decomposition Rates during Early Phases of Maize Litter Decomposition. Microorganisms 2021, 9, 357. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.-C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the Dynamics of Soil Aggregation as Affected by Arbuscular Mycorrhizal Fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium Promotes Persistent Soil Organic Matter by Altering Microbial Transformation of Plant Litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N Ratio Determines Spatial Variations in Soil Microbial Communities and Enzymatic Activities of the Agricultural Ecosystems in Northeast China: Jilin Province Case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Ullah, S.; Raza, M.M.; Abbas, T.; Guan, X.; Zhou, W.; He, P. Responses of Soil Microbial Communities and Enzyme Activities under Nitrogen Addition in Fluvo-Aquic and Black Soil of North China. Front. Microbiol. 2023, 14, 1249471. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef] [PubMed]
- Carrino-Kyker, S.R.; Coyle, K.P.; Kluber, L.A.; Burke, D.J. Fungal and Bacterial Communities Exhibit Consistent Responses to Reversal of Soil Acidification and Phosphorus Limitation over Time. Microorganisms 2020, 8, 1. [Google Scholar] [CrossRef]
- Bai, T.; He, S.; Li, Y.; Deng, J.; Sang, F.; Zhao, Y.; Wang, H.; He, T.; Zhang, K.; Qiu, Y.; et al. Unexpected Suppressive Fungal Diversity and Stimulative Soil Carbon Loss under Soil Acidification in an Alkaline Grassland. Funct. Ecol. 2025, 39, 114–127. [Google Scholar] [CrossRef]
- Breugem, A.; Kros, H.; de Vries, W. Impacts of pH on Mechanisms and Rates of Carbon and Nitrogen Mineralisation: A Review; Wageningen Environmental Research: Wageningen, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, H.; Liu, B.; Zhang, M.; Zhang, C.; Chen, P.; Yang, B. Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil. Microorganisms 2022, 10, 977. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Wu, J.; Ma, D.; Zhang, C.; Zhang, B.; Liu, Y.; Zhang, Z.; Tian, F.; Zhao, H.; et al. Soil Fungal Community Is More Sensitive than Bacterial Community to Modified Materials Application in Saline–Alkali Land of Hetao Plain. Front. Microbiol. 2024, 15, 1255536. [Google Scholar] [CrossRef]
- Tang, S.; Pan, W.; Zhou, J.; Ma, Q.; Yang, X.; Wanek, W.; Marsden, K.A.; Kuzyakov, Y.; Chadwick, D.R.; Wu, L.; et al. Soil Nitrogen and Phosphorus Regulate Decomposition of Organic Nitrogen Compounds in the Rothamsted Experiment. Soil Biol. Biochem. 2024, 196, 109502. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, J.; Hao, J.; Li, C.; Quan, W. Decomposition Characteristics of Lignocellulosic Biomass in Subtropical Rhododendron Litters under Artificial Regulation. Metabolites 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz, T.S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington, S.P.; Leggieri, P.A.; Brown, J.L.; Swift, C.L.; Lipzen, A.; Na, H.; et al. Lignin Deconstruction by Anaerobic Fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef]
- Subedi, P.; Jokela, E.J.; Vogel, J.G.; Bracho, R.; Inglett, K.S. The Effects of Nutrient Limitations on Microbial Respiration and Organic Matter Decomposition in a Florida Spodosol as Influenced by Historical Forest Management Practices. For. Ecol. Manag. 2021, 479, 118592. [Google Scholar] [CrossRef]
- Pan, Y.; Fang, F.; Tang, H. Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis. Forests 2021, 12, 1544. [Google Scholar] [CrossRef]
- Soler-Bistué, A.; Couso, L.L.; Sánchez, I.E. The Evolving Copiotrophic/Oligotrophic Dichotomy: From Winogradsky to Physiology and Genomics. Environ. Microbiol. 2023, 25, 1232–1237. [Google Scholar] [CrossRef]
- Stone, B.W.G.; Dijkstra, P.; Finley, B.K.; Fitzpatrick, R.; Foley, M.M.; Hayer, M.; Hofmockel, K.S.; Koch, B.J.; Li, J.; Liu, X.J.A.; et al. Life History Strategies among Soil Bacteria—Dichotomy for Few, Continuum for Many. ISME J. 2023, 17, 611–619. [Google Scholar] [CrossRef]
- Lladó, S.; Baldrian, P. Community-Level Physiological Profiling Analyses Show Potential to Identify the Copiotrophic Bacteria Present in Soil Environments. PLoS ONE 2017, 12, e0171638. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.-B.; Zhang, X.-Y.; Dang, Y.-R.; Sun, L.-L.; Zhang, W.-P.; Fu, H.-H.; Yang, G.-P.; Wang, M.; McMinn, A.; et al. Experimental Evidence for Long-Term Coexistence of Copiotrophic and Oligotrophic Bacteria in Pelagic Surface Seawater. Environ. Microbiol. 2021, 23, 1162–1173. [Google Scholar] [CrossRef]
- Ntonta, S.; Zengeni, R.; Muchaonyerwa, P.; Chaplot, V. Variability in Decomposition Rate of Sorghum Cultivar Residues Linked to Lignin Content. Rhizosphere 2024, 29, 100850. [Google Scholar] [CrossRef]
- Wang, S.; Uhlgren, O.; Salonen, A.-R.; Heinonsalo, J. Soil Contents and Stoichiometry of Carbon, Nitrogen, and Phosphorus in Finnish Farmland and Feedbacks on Management Patterns. In Proceedings of the Copernicus Meetings, Online, 19–30 April 2021. [Google Scholar]
- Cheng, W.; Zhang, S.; Wang, Y.; Hong, L.; Qiu, M.; Wang, Y.; Luo, Y.; Zhang, Q.; Wang, T.; Jia, X.; et al. Dahongpao Mother Tree Affects Soil Microbial Community and Nutrient Cycling by Increasing Rhizosphere Soil Characteristic Metabolite Content. Front. Plant Sci. 2025, 16, 1508622. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B. Effects of Different Vegetation on Soil Microbial Biomass Carbon and Nitrogen in Newly Cultivated Land. Bangladesh J. Bot. 2021, 50, 879–886. [Google Scholar] [CrossRef]
- Manral, V.; Bargali, K.; Bargali, S.S.; Karki, H.; Chaturvedi, R.K. Seasonal Dynamics of Soil Microbial Biomass C, N and P along an Altitudinal Gradient in Central Himalaya, India. Sustainability 2023, 15, 1651. [Google Scholar] [CrossRef]
- Chakrawal, A.; Calabrese, S.; Herrmann, A.M.; Manzoni, S. Interacting Bioenergetic and Stoichiometric Controls on Microbial Growth. Front. Microbiol. 2022, 13, 859063. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Weintraub, M.N.; Moorhead, D. Estimating Microbial Carbon Use Efficiency in Soil: Isotope-Based and Enzyme-Based Methods Measure Fundamentally Different Aspects of Microbial Resource Use. Soil Biol. Biochem. 2022, 169, 108677. [Google Scholar] [CrossRef]
- Angst, G.; Angst, Š.; Frouz, J.; Jabinski, S.; Jílková, V.; Kukla, J.; Li, M.; Meador, T.B.; Angel, R. Stabilized Microbial Necromass in Soil Is More Strongly Coupled with Microbial Diversity than the Bioavailability of Plant Inputs. Soil Biol. Biochem. 2024, 190, 109323. [Google Scholar] [CrossRef]
- Wu, S.; Konhauser, K.O.; Chen, B.; Huang, L. “Reactive Mineral Sink” Drives Soil Organic Matter Dynamics and Stabilization. npj Mater. Sustain. 2023, 1, 3. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Yan, J.; Ma, Y.; Zhou, X.; Yu, W.; Kan, H.; Meng, Q.; Xie, R.; Dong, P. A Review on Adsorption Characteristics and Influencing Mechanism of Heavy Metals in Farmland Soil. RSC Adv. 2023, 13, 3505–3519. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qu, C.; Huang, Q.; Cai, P. Microbial Extracellular Polymeric Substances (EPS) in Soil: From Interfacial Behaviour to Ecological Multifunctionality. Geo-Bio Interfaces 2024, 1, e4. [Google Scholar] [CrossRef]
- Wang, X.-B.; Azarbad, H.; Leclerc, L.; Dozois, J.; Mukula, E.; Yergeau, É. A Drying-Rewetting Cycle Imposes More Important Shifts on Soil Microbial Communities than Does Reduced Precipitation. mSystems 2022, 7, e0024722. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y.; et al. Formation of Soil Organic Carbon Pool Is Regulated by the Structure of Dissolved Organic Matter and Microbial Carbon Pump Efficacy: A Decadal Study Comparing Different Carbon Management Strategies. Glob. Change Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, K.; Yang, Y.; Gao, B.; Zheng, H. Effects of Biochar on the Accumulation of Necromass-Derived Carbon, the Physical Protection and Microbial Mineralization of Soil Organic Carbon. Crit. Rev. Environ. Sci. Technol. 2024, 54, 39–67. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, Y.; Morrissey, E.; Liu, Y.; Sun, L.; Qu, L.; Sang, C.; Zhang, H.; Li, G.; et al. Integrating Microbial Community Properties, Biomass and Necromass to Predict Cropland Soil Organic Carbon. ISME Commun. 2023, 3, 86. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, G.; Zhao, B.; Cong, N.; Zheng, Z.; Zhu, J.; Duan, X.; Zhang, Y. Climate Warming Alters the Relative Importance of Plant Root and Microbial Community in Regulating the Accumulation of Soil Microbial Necromass Carbon in a Tibetan Alpine Meadow. Glob. Change Biol. 2023, 29, 3193–3204. [Google Scholar] [CrossRef]
- Campeau, A.B.; Lafleur, P.M.; Humphreys, E.R. Landscape-Scale Variability in Soil Organic Carbon Storage in the Central Canadian Arctic. Can. J. Soil. Sci. 2014, 94, 477–488. [Google Scholar] [CrossRef]
- Qu, R.; Liu, G.; Yue, M.; Wang, G.; Peng, C.; Wang, K.; Gao, X. Soil Temperature, Microbial Biomass and Enzyme Activity Are the Critical Factors Affecting Soil Respiration in Different Soil Layers in Ziwuling Mountains, China. Front. Microbiol. 2023, 14, 1105723. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bhattacharyya, R.; Jiménez-Ballesta, R.; Dey, A.; Saha, N.D.; Kumar, S.; Nath, C.P.; Prakash, V.; Jatav, S.S.; Patra, A. Conventional and Zero Tillage with Residue Management in Rice–Wheat System in the Indo-Gangetic Plains: Impact on Thermal Sensitivity of Soil Organic Carbon Respiration and Enzyme Activity. Int. J. Environ. Res. Public Heal. 2023, 20, 810. [Google Scholar] [CrossRef]
- Valentine, K.; Herbert, E.R.; Walters, D.C.; Chen, Y.; Smith, A.J.; Kirwan, M.L. Climate-driven tradeoffs between landscape connectivity and the maintenance of the coastal carbon sink. Nat. Commun. 2023, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.C.; Ryu, D.; Sheridan, G.J.; Lane, P.N.J. Modeling Vegetation Water Stress over the Forest from Space: Temperature Vegetation Water Stress Index (TVWSI). Remote Sens. 2021, 13, 4635. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, L.; Bao, Y.; Zhang, J.; Yao, F.; Bai, L.; Zheng, P. Prediction of Soil Organic Carbon Content in Complex Vegetation Areas Based on CNN-LSTM Model. Land 2024, 13, 915. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chen, X. Land Use Transitions and the Associated Impacts on Carbon Storage in the Poyang Lake Basin, China. Remote Sens. 2023, 15, 2703. [Google Scholar] [CrossRef]
- Jiang, Q.; Jia, L.; Chen, Y.; Yang, Z.; Chen, S.; Xiong, D.; Liu, X.; Wang, X.; Yao, X.; Chen, T.; et al. Substrate and Adenylate Limit Subtropical Tree Fine-Root Respiration under Soil Warming. Plant Cell Environ. 2023, 46, 2827–2840. [Google Scholar] [CrossRef]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz-Horta, L.A.; Yang, X.; Du, H.; Wang, K.; Li, D. Tree Species Diversity Increases Soil Microbial Carbon Use Efficiency in a Subtropical Forest. Glob. Change Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Feng, J.; Li, X.; Zhang, Z.; He, J.-S.; Schimel, J.P.; Zhu, B. Whole-Soil-Profile Warming Does Not Change Microbial Carbon Use Efficiency in Surface and Deep Soils. Proc. Natl. Acad. Sci. USA 2023, 120, e2302190120. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Shen, Q.; Ling, N.; Nan, Z. Microbial Carbon Use Efficiency in Different Ecosystems: A Meta-Analysis Based on a Biogeochemical Equilibrium Model. Glob. Change Biol. 2023, 29, 4758–4774. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, G.; Zhang, T.; Fan, X.; Liu, Z.; Bai, E. Erosion Effects on Soil Microbial Carbon Use Efficiency in the Mollisol Cropland in Northeast China. Soil Ecol. Lett. 2023, 5, 230176. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, W.; Wang, W.; Li, X.; Sheng, Z. Microbial Assemblies with Distinct Trophic Strategies Drive Changes in Soil Microbial Carbon Use Efficiency along Vegetation Primary Succession in a Glacier Retreat Area of the Southeastern Tibetan Plateau. Sci. Total Environ. 2023, 867, 161587. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Su, Y.; Wang, J.; Lin, S.; Huang, Z.; Huang, G. Elevational Patterns of Microbial Carbon Use Efficiency in a Subtropical Mountain Forest. Biol. Fertil. Soils 2024, 60, 5–15. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; McNamara, N.P.; Ostle, N.; Puissant, J.; Goodall, T.; Griffiths, R.I.; Stott, A.W.; Whitaker, J. Environmental and Microbial Controls on Microbial Necromass Recycling, an Important Precursor for Soil Carbon Stabilization. Commun. Earth Environ. 2020, 1, 36. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.-J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial Diversity Drives Carbon Use Efficiency in a Model Soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining Trait-Based Microbial Strategies with Consequences for Soil Carbon Cycling under Climate Change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Ding, J.; Li, C.-Y.; Yan, Z.-Z.; He, J.-Z.; Hu, H.-W. Microbial Functional Attributes, Rather than Taxonomic Attributes, Drive Top Soil Respiration, Nitrification and Denitrification Processes. Sci. Total Environ. 2020, 734, 139479. [Google Scholar] [CrossRef] [PubMed]
- Raczka, N.C.; Piñeiro, J.; Tfaily, M.M.; Chu, R.K.; Lipton, M.S.; Pasa-Tolic, L.; Morrissey, E.; Brzostek, E. Interactions between Microbial Diversity and Substrate Chemistry Determine the Fate of Carbon in Soil. Sci. Rep. 2021, 11, 19320. [Google Scholar] [CrossRef]
- Qi, Q.; Haowei, Y.; Zhang, Z.; Van Nostrand, J.D.; Wu, L.; Guo, X.; Feng, J.; Wang, M.; Yang, S.; Zhao, J.; et al. Microbial Functional Responses Explain Alpine Soil Carbon Fluxes under Future Climate Scenarios. mBio 2021, 12, e00761-20. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Tarkka, M.; Knief, C.; Koller, R.; Peth, S.; Schmidt, V.; Spielvogel, S.; Uteau, D.; Weber, M.; Razavi, B.S. Bridging Microbial Functional Traits With Localized Process Rates at Soil Interfaces. Front. Microbiol. 2021, 12, 625697. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Shinfuku, M.; Junier, P.; Poirier, S.; Verrecchia, E.; Sebag, D.; DeAngelis, K.M. Direct Evidence for the Role of Microbial Community Composition in the Formation of Soil Organic Matter Composition and Persistence. ISME Commun. 2021, 1, 64. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wang, Y.; Chen, X.; Tan, H.; Jin, X.; Lu, Q.; He, S.; Long, M.-X. Cover Cropping Increases Soil Fungal-Bacterial Community Diversity and Network Complexity in Apple Orchards on the Loess Plateau, China. Front. Environ. Sci. 2022, 10, 916288. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, J.; Du, T.; Ju, X.; Gan, Y.; Li, S.; Xia, L.; Shen, Y.; Pacenka, S.; Steenhuis, T.S.; et al. Diversifying Crop Rotation Increases Food Production, Reduces Net Greenhouse Gas Emissions and Improves Soil Health. Nat. Commun. 2024, 15, 198. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Qiu, G.; Yu, H.; Liu, F.; Wang, G.; Duan, Y. Conservation Tillage Facilitates the Accumulation of Soil Organic Carbon Fractions by Affecting the Microbial Community in an Eolian Sandy Soil. Front. Microbiol. 2024, 15, 1394179. [Google Scholar] [CrossRef]
- Joshi, D.R.; Clay, D.E.; Alverson, R.; Clay, S.A.; Westhoff, S.; Johnson, J.M.F.; Wang, T.; Sieverding, H. Tillage Intensity Reductions When Combined with Yield Increases May Slow Soil Carbon Saturation in the Central United States. Sci. Rep. 2025, 15, 10697. [Google Scholar] [CrossRef]
- López i Losada, R.; Hedlund, K.; Haddaway, N.R.; Sahlin, U.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Jørgensen, H.B.; Isberg, P.-E. Synergistic Effects of Multiple “Good Agricultural Practices” for Promoting Organic Carbon in Soils: A Systematic Review of Long-Term Experiments. Ambio 2025. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Li, Y.; Jiao, Y.; Fan, L.; Jiang, Y.; Qu, C.; Filimonenko, E.; Jiang, Y.; Tian, X.; et al. Nitrogen Fertilizer Builds Soil Organic Carbon under Straw Return Mainly via Microbial Necromass Formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanski, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and A Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, C.; Zhang, R.; Jiao, N.; Tian, J.; Lambers, H.; Liang, C.; Cong, W.-F.; Zhang, F. Intercropping Increases Soil Macroaggregate Carbon through Root Traits Induced Microbial Necromass Accumulation. Soil Biol. Biochem. 2023, 185, 109146. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of Rhizobium-Soybean Symbiosis and Nitrogen Fixation under Drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef]
- Yang, X.; Hu, H.-W.; Yang, G.-W.; Cui, Z.-L.; Chen, Y.-L. Crop rotational diversity enhances soil microbiome network complexity and multifunctionality. Geoderma 2023, 436, 116562. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil Organic Carbon Sequestration Rates in Vineyard Agroecosystems under Different Soil Management Practices: A Meta-Analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-Analysis of the Impacts of Global Change Factors on Soil Microbial Diversity and Functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, W.; Zhang, Y.; Liu, X.; Zhang, Y.; Zheng, X.; Luo, J.; Zou, J. Effects of No-till on Upland Crop Yield and Soil Organic Carbon: A Global Meta-Analysis. Plant Soil 2024, 499, 363–377. [Google Scholar] [CrossRef]
- Prairie, A.M.; King, A.E.; Cotrufo, M.F. Restoring Particulate and Mineral-Associated Organic Carbon through Regenerative Agriculture. Proc. Natl. Acad. Sci. USA 2023, 120, e2217481120. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Gao, H.; Jia, A.; Gao, Q. Long-Term Conservation Tillage Increases Soil Organic Carbon Stability by Modulating Microbial Nutrient Limitations and Aggregate Protection. Agronomy 2025, 15, 1571. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-Term Use of Cover Crops and No-till Shift Soil Microbial Community Life Strategies in Agricultural Soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef] [PubMed]
- Seabloom, E.W.; Borer, E.T.; Hobbie, S.E.; MacDougall, A.S. Soil Nutrients Increase Long-Term Soil Carbon Gains Threefold on Retired Farmland. Glob. Change Biol. 2021, 27, 4909–4920. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, M.; Wang, S.; Lu, X. Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield. Agronomy 2024, 14, 1348. [Google Scholar] [CrossRef]
- Nghia, N.K.; Robatjazi, J.; Vy, V.D.T.; Tecimen, H.B.; Lasar, H.G.W.; Lesueur, D.; Bai, S.H.; Tran, H.-T.; Thien, N.H.; Luan, D.T. Effect of Organic Farming Practices on Soil Health Improvement of Coconut Farms. Environ. Technol. Innov. 2025, 38, 104067. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Chapter 2—Influence of Synthetic Fertilizers and Pesticides on Soil Health and Soil Microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. ISBN 978-0-08-103017-2. [Google Scholar]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The Effect of Application of Organic Manures and Mineral Fertilizers on the State of Soil Organic Matter and Nutrients in the Long-Term Field Experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Mcinga, S.; Muzangwa, L.; Janhi, K.; Mnkeni, P.N.S. Conservation Agriculture Practices Can Improve Earthworm Species Richness and Abundance in the Semi-Arid Climate of Eastern Cape, South Africa. Agriculture 2020, 10, 576. [Google Scholar] [CrossRef]
- Sazykina, M.; Minkina, T.; Konstantinova, E.; Khmelevtsova, L.; Azhogina, T.; Antonenko, E.; Karchava, S.; Klimova, M.; Sushkova, S.; Polienko, E.; et al. Pollution Impact on Microbial Communities Composition in Natural and Anthropogenically Modified Soils of Southern Russia. Microbiol. Res. 2022, 254, 126913. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Geng, S.; Cao, W.; Zuo, R.; Teng, Y.; Ding, A.; Fan, F.; Dou, J. Vertical Distribution Characteristics and Interactions of Polycyclic Aromatic Compounds and Bacterial Communities in Contaminated Soil in Oil Storage Tank Areas. Chemosphere 2022, 301, 134695. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Russo, F.; Ceci, A.; Pinzari, F.; Siciliano, A.; Guida, M.; Malusà, E.; Tartanus, M.; Miszczak, A.; Maggi, O.; Persiani, A.M. Bioremediation of Dichlorodiphenyltrichloroethane (DDT)-Contaminated Agricultural Soils: Potential of Two Autochthonous Saprotrophic Fungal Strains. Appl. Environ. Microbiol. 2019, 85, e01720-19. [Google Scholar] [CrossRef]
- Ebsa, G.; Gizaw, B.; Admassie, M.; Degu, T.; Alemu, T. The Role and Mechanisms of Microbes in Dichlorodiphenyltrichloroethane (DDT) and Its Residues Bioremediation. Biotechnol. Rep. 2024, 42, e00835. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Liu, P.; Wen, S.; Zhu, S.; Hu, X.; Wang, Y. Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes 2025, 13, 916. [Google Scholar] [CrossRef]
- Sekiya, N.; Mae, A.; Murai, A.; Peter, M.A.; Goto, M.; Kato, H.; Ichikawa, S.; Watanabe, K. Soil Microbes and Organic Fertilizer Efficiency Are Associated with Rice Field Topography. Sci. Rep. 2025, 15, 24939. [Google Scholar] [CrossRef]


| Soil Texture | Microbial Community Dominance | Impact on Organic Matter Decomposition | References |
|---|---|---|---|
| Clay-rich soils (fine-textured) | Actinobacteria, Mycorrhizal fungi | Slower decomposition due to microbial protection by clay particles | [134] |
| Sandy soils (coarse-textured) | Copiotrophs (for example, Proteobacteria) | Faster decomposition due to increased aeration and lower stabilization | [158] |
| Silt-loam soils | Balanced bacterial and fungal diversity | Moderate decomposition rate with good organic matter retention | [159] |
| High-clay aggregated soils | Fungal-dominated communities | Enhanced carbon stabilization through macroaggregate formation | [160] |
| Scale | Time Frame/Spatial Resolution | Key Processes | Dominant Drivers | Microbial–Mineral Interactions | References |
|---|---|---|---|---|---|
| Micro-scale | Seconds to days/µm–mm | Enzyme production, microbial respiration, and necromass formation | Temperature, pH, substrate quality | Enzyme-substrate binding, organo–mineral associations | [22,99] |
| Meso-scale | Weeks to seasons/cm–m | Root exudation, aggregate formation, CUE dynamics | Moisture cycles, root turnover | Rhizosphere activity, necromass input | [108,125] |
| Field-scale | Annual/plot–field (~10–1000 m2) | Litter decomposition, SOM pool turnover | Crop management, tillage, fertilization | Microbial diversity shifts, aggregate dynamics | [40,140] |
| Landscape-scale | Multi-year to decades/km2 | Land use transitions, erosion, SOC sequestration | Climate variability, land cover change | Lateral carbon fluxes, ecosystem connectivity | [135,145] |
| Regional/Global | Decades to centuries/>103 km2 | Carbon modeling, global sequestration trends | Climate change, policy, socioeconomic factors | Macroecological trends, soil carbon debt | [36,122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.T.; Supronienė, S.; Žvirdauskienė, R.; Aleinikovienė, J. Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands. Agronomy 2025, 15, 1928. https://doi.org/10.3390/agronomy15081928
Khan MT, Supronienė S, Žvirdauskienė R, Aleinikovienė J. Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands. Agronomy. 2025; 15(8):1928. https://doi.org/10.3390/agronomy15081928
Chicago/Turabian StyleKhan, Muhammad Tahir, Skaidrė Supronienė, Renata Žvirdauskienė, and Jūratė Aleinikovienė. 2025. "Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands" Agronomy 15, no. 8: 1928. https://doi.org/10.3390/agronomy15081928
APA StyleKhan, M. T., Supronienė, S., Žvirdauskienė, R., & Aleinikovienė, J. (2025). Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands. Agronomy, 15(8), 1928. https://doi.org/10.3390/agronomy15081928






