Pecan Shell Extract Effectively Inhibits Listeria monocytogenes, E. coli O157:H7, and Pseudomonas spp. on Contaminated Lettuce Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Pecan Extract Preparation
2.1.1. Sample Preparation
2.1.2. Defatting of Shell Powder
2.1.3. Aqueous Extraction of PSE
2.2. Inoculum Preparation
2.2.1. Listeria monocytogenes
2.2.2. Escherichia coli (O157:H7)
2.2.3. Pseudomonas spp.
2.3. Seed Preparation and Inoculation
2.4. Seed Coating with PSE
2.5. Seed Priming with PSE
2.6. Seed Coating and Seed Priming for E. coli O157:H7 and Pseudomonas spp.
2.7. Germination Evaluation
- (a)
- Percentage of Seed Viability (PSV)
- (b)
- Germination Percentage (GP)
- (c)
- Germination rate (GR)where
- ni = number of seeds germinated on day i;
- ti = day number.
- (d)
- Mean Germination Time (MGT)
2.8. Experimental Design
3. Results
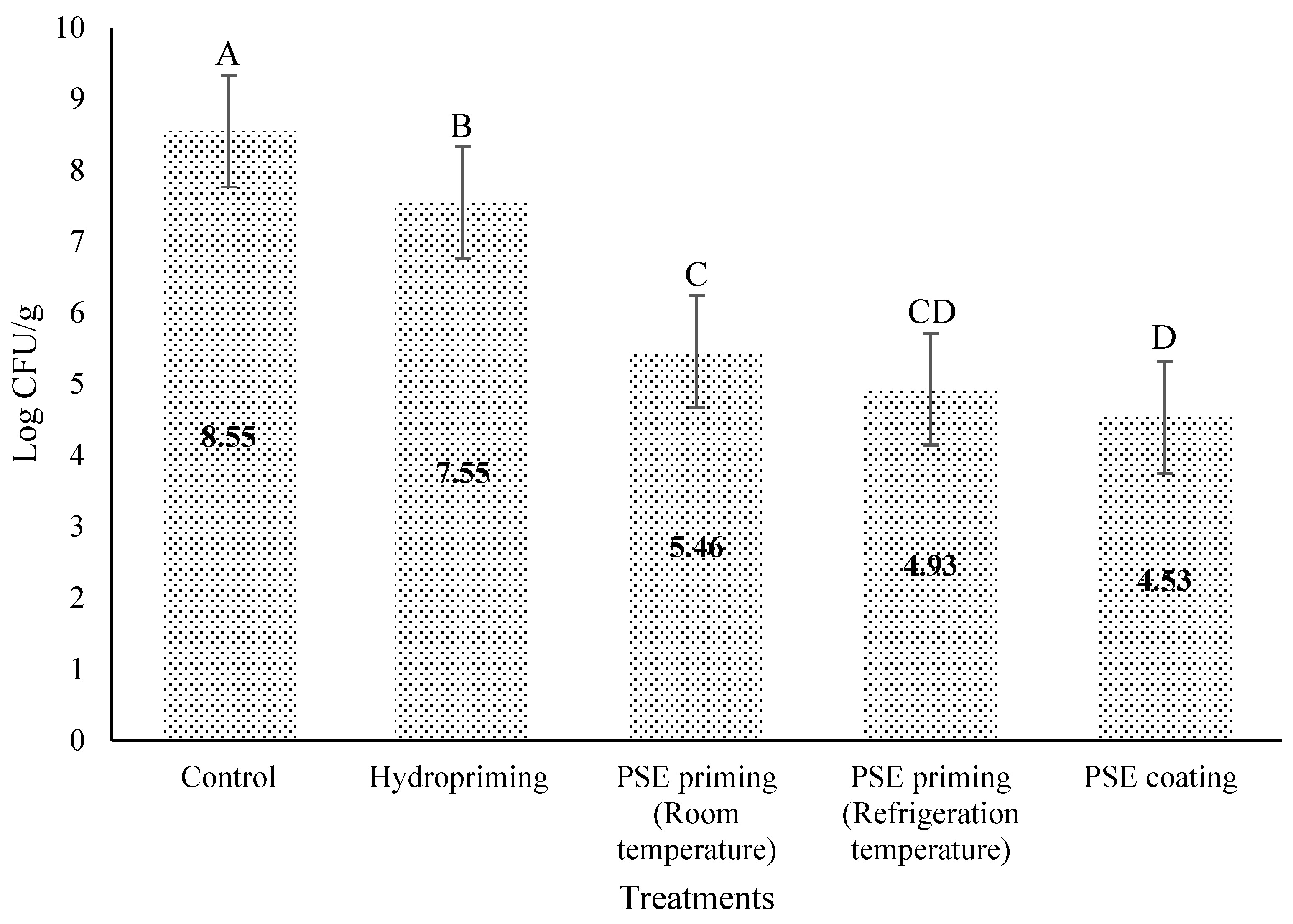
3.1. PSE Coating and Priming Effect on Listeria monocytogenes in Lettuce Seeds
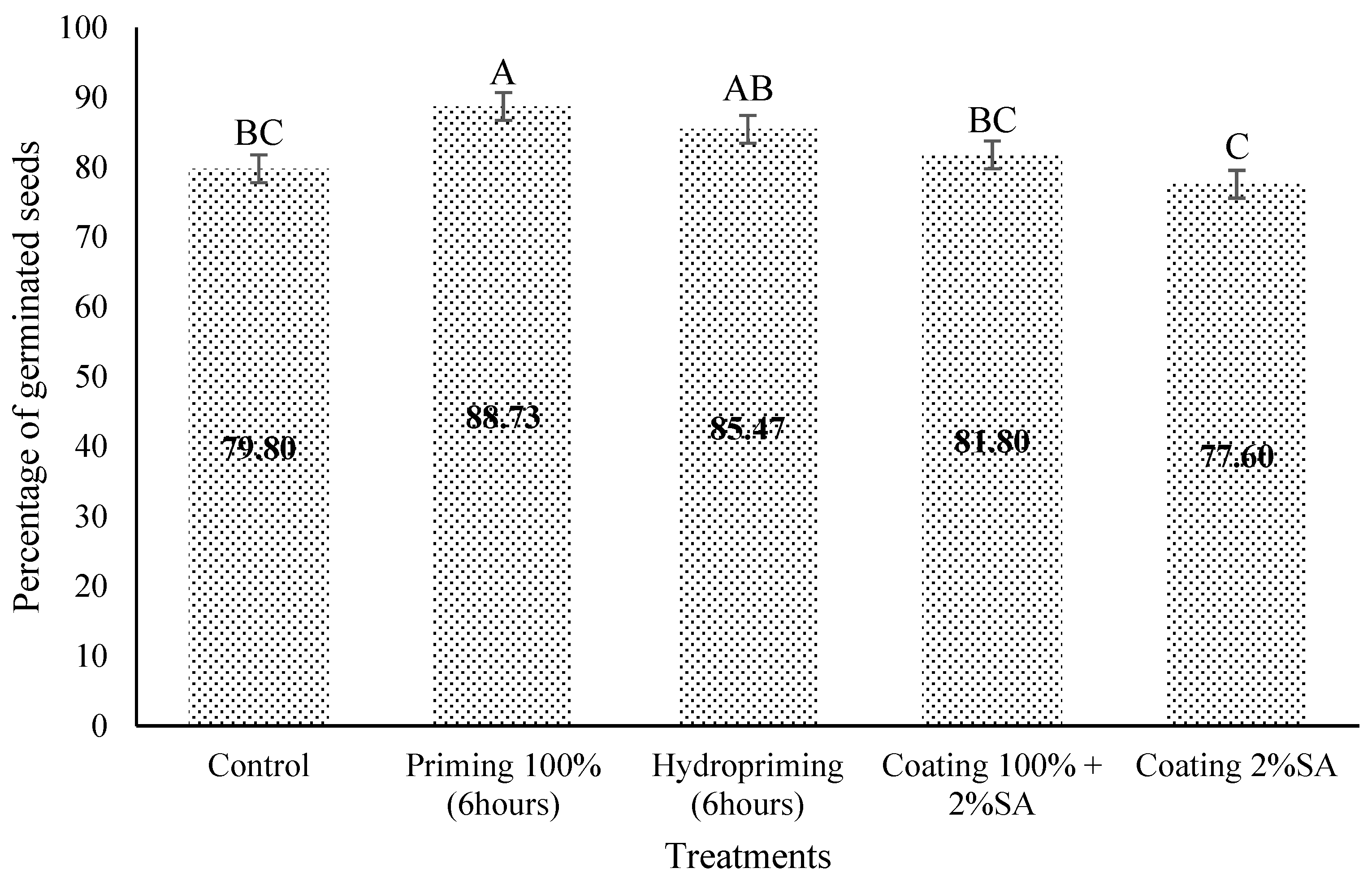
3.2. Germination Evaluation of PSE
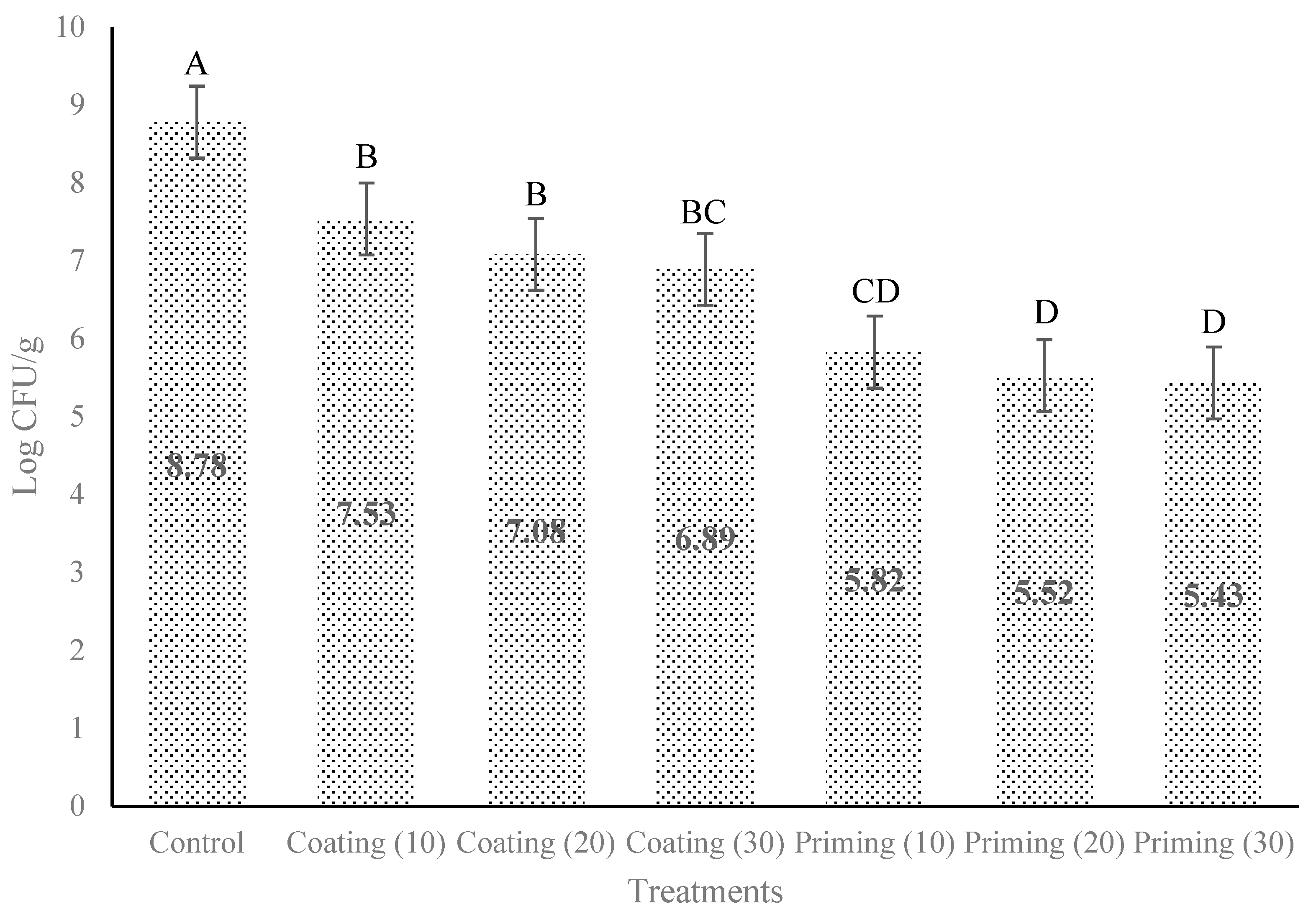
3.3. Effect of Different Concentrations of PSE for Coating and Priming on E. coli O157:H7 in Lettuce Seeds
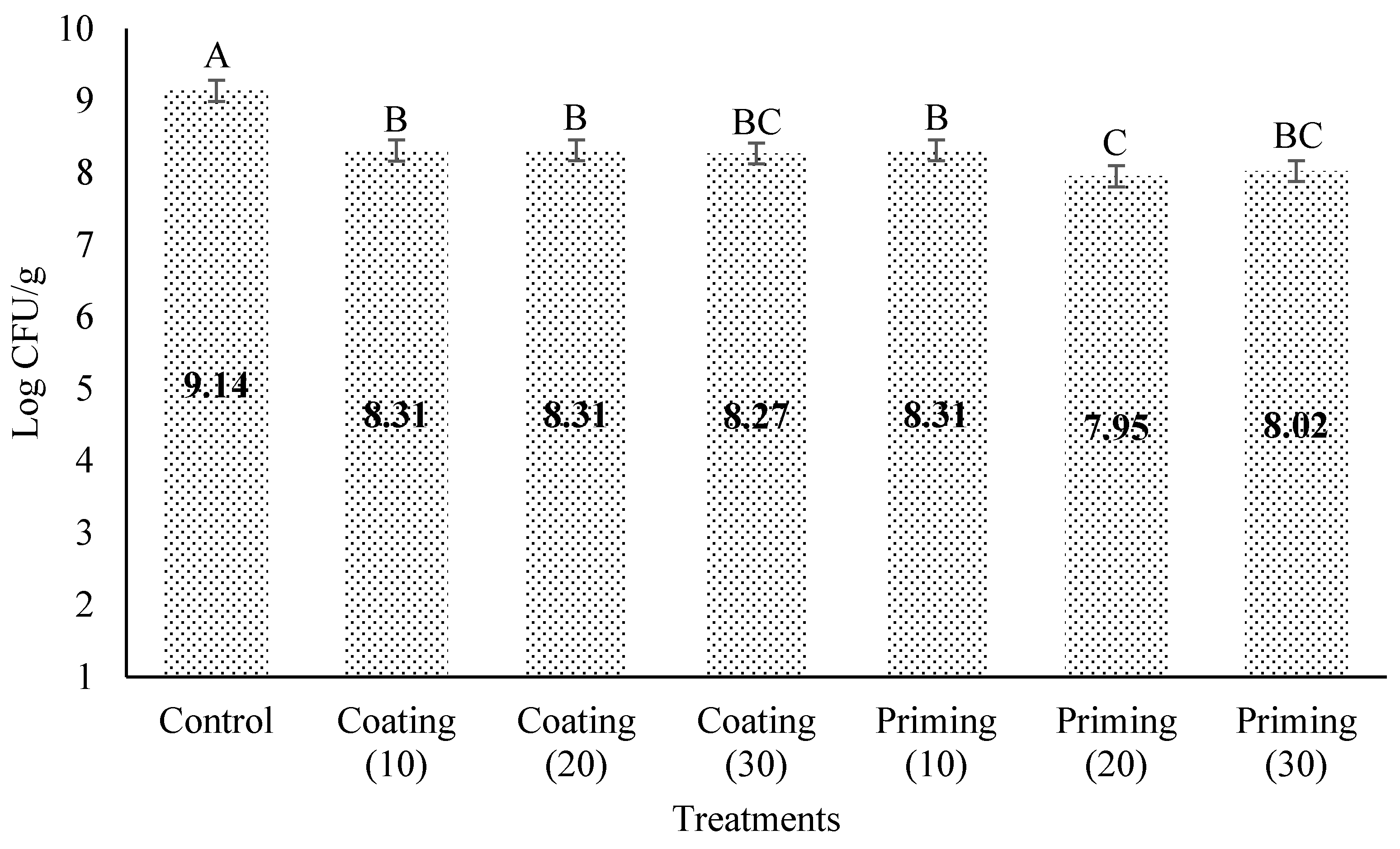
3.4. Effect of Different Concentrations of PSE for Coating and Priming on Pseudomonas spp. in Lettuce Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSE | Pecan Shell Extract |
| TSB | Tryptic Soy Broth |
| TSBYE | Tryptic Soy Broth supplemented with 6% extract |
| LB, Miller | Luria–Bertani, Miller broth/agar |
| SMAC | MacConkey Agar with Sorbitol |
| TTC | 2, 3, 5-triphenyltetrazolium chloride |
| SA | Sodium Alginate |
| DW | Distilled Water |
| PC | Pecan Shell Extract Coating |
| PP | Pecan Shell Extract Priming |
| PPR | Pecan Shell Extract Priming at Refrigeration |
References
- Rajaseger, G.; Chan, K.L.; Yee Tan, K.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Kadamb Haribhai, P. Hydroponics: Current trends in sustainable crop production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Khatri, L.; Kunwar, A.; Bist, D. Hydroponics: Advantages And Challenges in Soilless Farming. Big Data Agric. 2024, 6, 81–88. [Google Scholar] [CrossRef]
- Economic Research Service. U.S. Lettuce Production Shifts Regionally by Season. 2023. Available online: https://www.ers.usda.gov/data-products/charts-of-note/chart-detail?chartId=106516 (accessed on 15 July 2025).
- The Society of Tympanuchus Cupido Pinnatus, Ltd. Lettuce Market Insights, Size, Share, Growth Analysis, Trends, and Forecast 2024–2031. 2023. Available online: https://www.skyquestt.com/report/lettuce-market (accessed on 15 July 2025).
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Center for Crop Diversification, University of Kentucky. Hydroponic Lettuce (CCD-CP-063). 2024. Available online: https://publications.ca.uky.edu/ (accessed on 20 April 2025).
- Carvalho, R.; Luís, C.; Nunes, W.; Zanovello, C.; Gomes, M.; Luz, S.; Gadotti, G.I.; Luz, C.; Conill-Gomes, M. Hydroponic lettuce production and minimally processed lettuce. CIGR J. 2015, 2015, 290. [Google Scholar]
- Gillani, S.A.; Abbasi, R.; Martinez, P.; Ahmad, R. Comparison of Energy-use Efficiency for Lettuce Plantation under Nutrient Film Technique and Deep-Water Culture Hydroponic Systems. Procedia Comput. Sci. 2023, 217, 11–19. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L., var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Dankwa, A.S.; Machado, R.M.; Perry, J.J. Sources of food contamination in a closed hydroponic system. Lett. Appl. Microbiol. 2020, 70, 55–62. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Martín, I.; Gálvez, L.; Guasch, L.; Palmero, D. Fungal Pathogens and Seed Storage in the Dry State. Plants 2022, 11, 3167. [Google Scholar] [CrossRef]
- Cottyn, B.; Vanhouteghem, K.; Heyrman, J.; Bleyaert, P.; Vaerenbergh, J.; De Vos, P.; Höfte, M.; Maes, M. Pseudomonads associated with midrib rot and soft rot of butterhead lettuce and endive. Commun. Agric. Appl. Biol. Sci. 2005, 70, 101–109. [Google Scholar]
- Patel, N.; Patel, R.; Wyenandt, C.A.; Kobayashi, D.Y. First Report of Pseudomonas cichorii Causing Bacterial Leaf Spot on Romaine Lettuce (Lactuca sativa var. longifolia) and Escarole (Cichorium endivia) in New Jersey. Plant Dis. 2021, 105, 4150. [Google Scholar] [CrossRef] [PubMed]
- CDC. Listeria Outbreak Linked to Leafy Greens–February 2023. 2024. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.cdc.gov/listeria/outbreaks/monocytogenes-02-23/index.html&ved=2ahUKEwiNvPHZq96OAxU8s1YBHY7wDWQQFnoECBsQAQ&usg=AOvVaw3vnrVhZSNtn9ABLQaAjgLJ (accessed on 21 May 2025).
- CDC. Multistate Outbreak of E. coli O157:H7 Infections Linked to Romaine Lettuce (Final Update). 2019. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2019/o157h7-11-19/index.html (accessed on 21 May 2025).
- Aguilar-Rito, M.; Arzate-Fernández, A.; García-Núñez, H.; Héctor, T. Establishment of an efficient protocol for in vitro disinfection of seeds of seven Agave spp. species. Rev. Mex. De Fitopatol. Mex. J. Phytopathol. 2023, 42. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Diaz, A.; Bregoff, H.A. Seed Disinfestation Practices to Control Seed-Borne Fungi and Bacteria in Home Production of Sprouts. Foods 2023, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Bang, J.; Beuchat, L.R.; Ryu, J.-H. Synergistic Effect of Chlorine Dioxide and Drying Treatments for Inactivating Escherichia coli O157:H7 on Radish Seeds. J. Food Prot. 2010, 73, 1225–1230. [Google Scholar] [CrossRef]
- Sanna, M.; Gilardi, G.; Gullino, M.L.; Mezzalama, M. Evaluation of physical and chemical disinfection methods of Brassica oleracea seeds naturally contaminated with Xanthomonas campestris pv. campestris. J. Plant Dis. Prot. 2022, 129, 1145–1152. [Google Scholar] [CrossRef]
- Di Maro, M.; Gargiulo, L.; Gomez d’Ayala, G.; Duraccio, D. Exploring Antimicrobial Compounds from Agri-Food Wastes for Sustainable Applications. Int. J. Mol. Sci. 2024, 25, 13171. [Google Scholar] [CrossRef]
- CaxambÚ, S.; Biondo, E.; Kolchinski, E.; Padilha, R.; Brandelli, A.; Sant Anna, V. Evaluation of the antimicrobial activity of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell aqueous extract on minimally processed lettuce leaves. Ciência E Tecnol. De Aliment. 2016, 36, 42–45. [Google Scholar] [CrossRef]
- Flores-Estrada, R.A.; Gámez-Meza, N.; Medina-Juárez, L.A.; Castillón-Campaña, L.G.; Molina-Domínguez, C.C.; Rascón-Valenzuela, L.A.; García-Galaz, A. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Wastes from Pecan Nut [Carya illinoinensis (Wagenh) K. Koch]. Waste Biomass Valorization 2020, 11, 3419–3432. [Google Scholar] [CrossRef]
- Chiao Ying, H.; Gerald, L.R.; Jennifer, C.; Ching Hsuan, L.; Jinn Tsyy, L.; Audrey Chingzu, C. Pecan shell by-products—Phenolic compound contents and antimicrobial properties. AIMS Agric. Food 2020, 5, 218–232. [Google Scholar]
- Cason, C.; Yemmireddy, V.K.; Moreira, J.; Adhikari, A. Antioxidant Properties of Pecan Shell Bioactive Components of Different Cultivars and Extraction Methods. Foods 2021, 10, 713. [Google Scholar] [CrossRef]
- do Prado, A.C.P.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.L.M.; Vieira, C.R.W.; Maraschin, M.; Ferreira, S.R.S.; Block, J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crops Prod. 2014, 52, 552–561. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Cason, C.; Moreira, J.; Adhikari, A. Effect of pecan variety and the method of extraction on the antimicrobial activity of pecan shell extracts against different foodborne pathogens and their efficacy on food matrices. Food Control 2020, 112, 107098. [Google Scholar] [CrossRef]
- Kharel, K.; Kraśniewska, K.; Gniewosz, M.; Prinyawiwatkul, W.; Fontenot, K.; Adhikari, A. Antimicrobial screening of pecan shell extract and efficacy of pecan shell extract-pullulan coating against Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus on blueberries. Heliyon 2024, 10, e29610. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Saqlain Zaheer, M.; Faheem, M.; Haider Ali, H.; Iqbal, J. Seed coating technology: An innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Soc. Agric. Sci. 2022, 21, 536–545. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Jarzembowski, Ł.; Izydorczyk, G.; Mikula, K.; Hoppe, V.; Mielko, K.A.; Pudełko-Malik, N.; Młynarz, P.; Chojnacka, K.; Witek-Krowiak, A. Hydrogel Alginate Seed Coating as an Innovative Method for Delivering Nutrients at the Early Stages of Plant Growth. Polymers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Szabó, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Durgadevi, P.; Girigoswami, K.; Girigoswami, A. Biodegradable nanomaterials in boosting seed vigor and germination: Seed coating towards sustainability. Discov. Appl. Sci. 2025, 7, 695. [Google Scholar] [CrossRef]
- Dutra, F.; Bianca, D.; Silva, J.M.; Shiguehara, A.P. Seeds technologies: Performance of sodium alginate and calcium chloride as seed coating. Acta Biológica Catarin. 2024, 11, 34–43. [Google Scholar] [CrossRef]
- Abdukerim, R.; Li, L.; Li, J.-H.; Xiang, S.; Shi, Y.-X.; Xie, X.-W.; Chai, A.L.; Fan, T.-F.; Li, B.-J. Coating seeds with biocontrol bacteria-loaded sodium alginate/pectin hydrogel enhances the survival of bacteria and control efficacy against soil-borne vegetable diseases. Int. J. Biol. Macromol. 2024, 279, 135317. [Google Scholar] [CrossRef] [PubMed]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Mohan, V.R. Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Curr. Issues Mol. Biol. 2025, 47, 177. [Google Scholar] [CrossRef]
- Ortiz-Quezada, A.G.; Lombardini, L.; Cisneros-Zevallos, L. Chapter 104—Antioxidants in Pecan Nut Cultivars [Carya illinoinensis (Wangenh.) K. Koch]. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 881–889. [Google Scholar]
- Lyalina, T.; Shagdarova, B.; Zhuikova, Y.; Il’ina, A.; Lunkov, A.; Varlamov, V. Effect of Seed Priming with Chitosan Hydrolysate on Lettuce (Lactuca sativa) Growth Parameters. Molecules 2023, 28, 1915. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Van Loo, E.J.; Babu, D.; Crandall, P.G.; Ricke, S.C. Screening of Commercial and Pecan Shell-Extracted Liquid Smoke Agents as Natural Antimicrobials against Foodborne Pathogens. J. Food Prot. 2012, 75, 1148–1152. [Google Scholar] [CrossRef]
- Worrall, D.; Holroyd, G.H.; Moore, J.P.; Glowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef]
- Carmello, C.R.; Cardoso, J.C. Effects of plant extracts and sodium hypochlorite on lettuce germination and inhibition of Cercospora longissima in vitro. Sci. Hortic. 2018, 234, 245–249. [Google Scholar] [CrossRef]
- Raza, A.; Tahir, M.; Noor Us, S.; Shah, S.H.; Sarwar, G.; Manzoor, M. Seed priming with zinc ion on growth performance and nutrient acquisition of maize in aridisols. Pak. J. Bot. 2023, 55, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.B.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed Priming with Sorghum Water Extract Improves the Performance of Camelina (Camelina sativa (L.) Crantz.) under Salt Stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Akhtar, S.; Imran, S.; Dar, A.H.; Raza, A.; Masroor, A. Seed Priming with Plant Extracts Gives Enhanced Resistance Against Alternaria Solani in Tomato. Pak. J. Phytopathol. 2024, 36, 2411–2422. [Google Scholar]
- Gassoumi, W.; Ellouzi, H.; Slimene, I.B.; Kalai, F.Z.; Ayed, R.B.; Zorrig, W.; Debez, A.; Abdelly, C.; Oueslati, S. Plant extracts and plant growth promoting rhizobacteria: Seed bio-priming approach to improve tolerance of Lactuca sativa L. under salt stress. Euro-Mediterr. J. Environ. Integr. 2024, 10, 1901–1918. [Google Scholar] [CrossRef]
| Label | Pecan Extract Concentration | SA Concentration |
|---|---|---|
| A-coating | 100% | 2% |
| B | Untreated Seeds | |
| Label | Pecan Extract Concentration | Soaking Time | Temperature °C |
|---|---|---|---|
| A | 100% | 6 h | 25 |
| B | 100% | 6 h | 4 |
| C | Sterilized distilled water | 6 h | 25 |
| E | Untreated seeds | ||
| Treatment | GP | EP | GE-DAY4 | GR | MGT |
|---|---|---|---|---|---|
| Control (No treatment) | 80 | 2 | 72 | 0.38 | 2.66 |
| PC (2%SA) | 81 | 2 | 71 | 0.35 | 2.85 |
| Coating (2%SA) | 77 | 2 | 67 | 0.35 | 2.84 |
| PP (6 h) | 90 | 2 | 84 | 0.45 | 2.20 |
| Hydropriming (6 h) | 88 | 2 | 82 | 0.44 | 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lituma, I.; Valle, F.; Ham, J.H.; Adhikari, A. Pecan Shell Extract Effectively Inhibits Listeria monocytogenes, E. coli O157:H7, and Pseudomonas spp. on Contaminated Lettuce Seeds. Agronomy 2025, 15, 1865. https://doi.org/10.3390/agronomy15081865
Lituma I, Valle F, Ham JH, Adhikari A. Pecan Shell Extract Effectively Inhibits Listeria monocytogenes, E. coli O157:H7, and Pseudomonas spp. on Contaminated Lettuce Seeds. Agronomy. 2025; 15(8):1865. https://doi.org/10.3390/agronomy15081865
Chicago/Turabian StyleLituma, Ivannova, Francisco Valle, Jong Hyun Ham, and Achyut Adhikari. 2025. "Pecan Shell Extract Effectively Inhibits Listeria monocytogenes, E. coli O157:H7, and Pseudomonas spp. on Contaminated Lettuce Seeds" Agronomy 15, no. 8: 1865. https://doi.org/10.3390/agronomy15081865
APA StyleLituma, I., Valle, F., Ham, J. H., & Adhikari, A. (2025). Pecan Shell Extract Effectively Inhibits Listeria monocytogenes, E. coli O157:H7, and Pseudomonas spp. on Contaminated Lettuce Seeds. Agronomy, 15(8), 1865. https://doi.org/10.3390/agronomy15081865








