Genetic Parameters of Conilon Coffee Cultivated Under an Irrigation System in the Cerrado
Abstract
1. Introduction
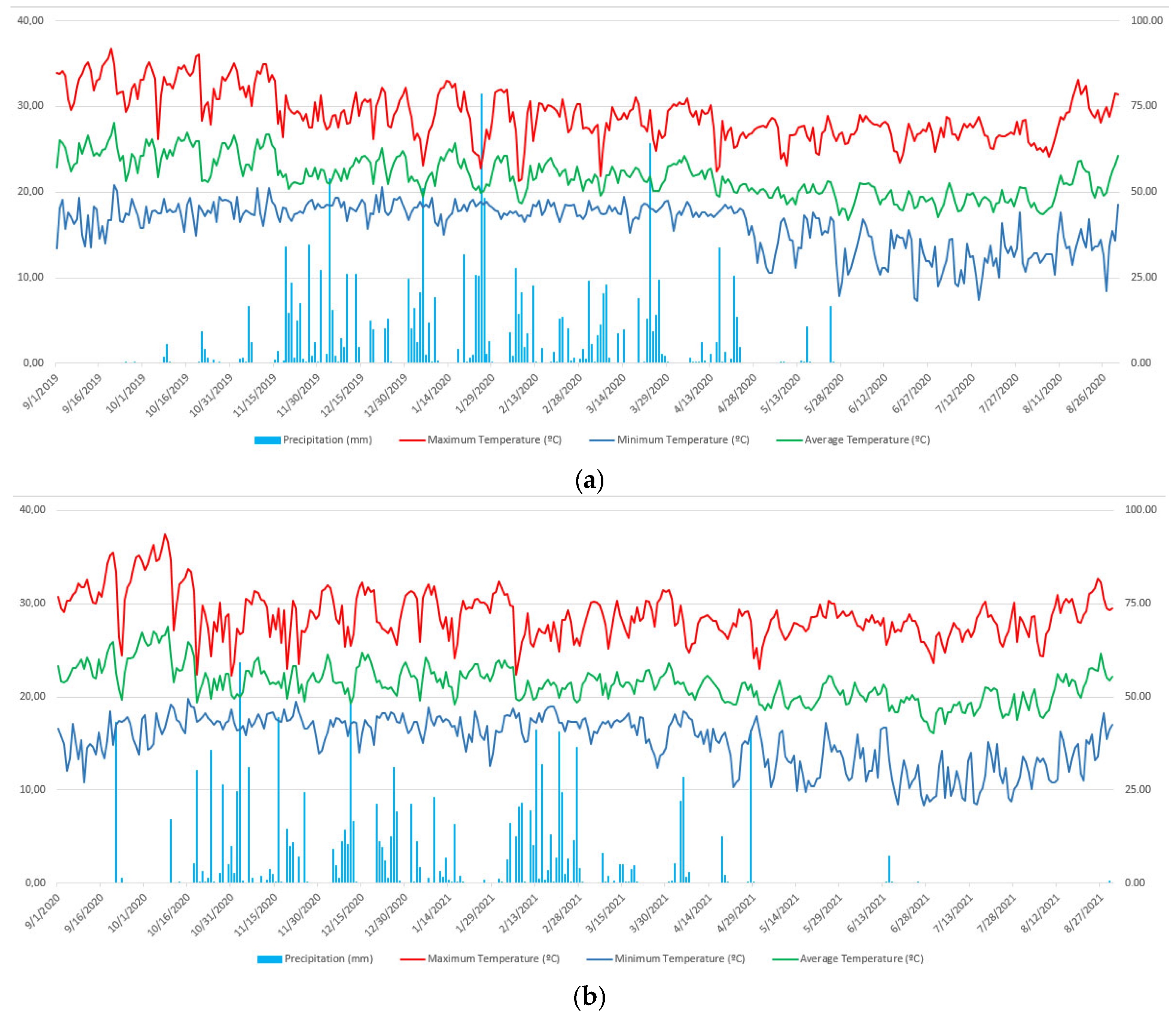
2. Materials and Methods
- Yijk = the relative observed value of the trait of the i-th genotype in the j-th repetition in the k-th year;
- µ = general average;
- Gi = effect of the i-th genotype (i = 1, 2, …, g);
- εij = random error a;
- Ak = effect of the k-th year (k = 1, 2, …, a);
- GAik = effect of the interaction between the i-th genotype and the k-th year;
- δijk = random error b.
| SV | DF | MS | E (MS) | F |
|---|---|---|---|---|
| Genotype (G) | g − 1 | QMG | ||
| Error a | (r − 1)(g − 1) | QMEa | ||
| Year | a − 1 | QMA | ||
| G × A | (g − 1)(a − 1) | QMGA | ||
| Error b | (g − 1)(r − 1) | QMEb | ||
| Total | gar − 1 |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimenta, C.J.; Angélico, C.L.; Chalfoun, S.M. Challengs in coffee quality: Cultural, chemical and microbiological aspects. Ciência Agrotecnol. 2018, 42, 337–349. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Farah, A. Coffee as a speciality and functional beverage. In Functional and Speciality Beverage Technology; Woodhead Publishing: Cambridge, UK, 2009; pp. 370–395. [Google Scholar]
- Cheserek, J.J.; Ngugi, K.; Muthomi, J.W.; Omondi, C.O.; Kathurima, C.W. Genetic Variability and Correlation of Biochemical and Sensory Characteristics of Coffee. J. Agric. Sci. 2022, 14, 95. [Google Scholar] [CrossRef]
- Tran, H.T.; Lee, L.S.; Furtado, A.; Smyth, H.; Henry, R.J. Advances in genomics for the improvement of quality in coffee. J. Sci. Food Agric. 2016, 96, 3300–3312. [Google Scholar] [CrossRef] [PubMed]
- Gichimu, B.M.; Gichuru, E.K.; Mamati, G.E.; Nyende, A.B. Biochemical Composition within Coffea arabica cv. Ruiru 11 and its Relationship with Cup Quality. J. Food Res. 2014, 3, 31–44. [Google Scholar] [CrossRef]
- Sala, P.I.A.L. Biometria e Composição Química de Genótipos do café Conilon Irrigado no Cerrado do Planalto Central do Distrito Federal. Master’s Thesis, Faculdade de Agronomia e Medicina Veterinária, Universidade de Brasília, Brasília, Brazil, 2018. [Google Scholar]
- Brige, F.A.A. Variabilidade Genética e Caracterização Química de Acessos de Café Conilon em Sistema de Cultivo Irrigado no Cerrado. Master’s Thesis, Faculdade de Agronomia e Medicina Veterinária, Universidade de Brasília, Brasília, Brazil, 2016. [Google Scholar]
- Vencovsky, R.; Barriga, P. Genética Biométrica no Fitomelhoramento; Sociedade Brasileira de Genética: Ribeirão Preto, Brazil, 1992; 496p. [Google Scholar]
- Ferrão, R.G.; Volpi, P.; Senra, J.D.B.; Comério, M.; Ferrão, M.; Verdin Filho, A.C.; Da Fonseca, A.F.A. Comportamento e a variabilidade genética entre clones de café conilon em ambientes representativos e não irrigados do Espírito Santo. Multi-Sci. Res. 2023, 5, 6–21. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Weinheim, Germany, 2008; 361p. [Google Scholar]
- Ferrão, R.G.; Fonseca, A.F.A.d.; Ferrão, M.A.G.; Bragança, S.M. ‘Emcaper 8151’—Robusta Tropical: Primeira variedade melhorada de café conilon propagada por sementes para o Estado do Espírito Santo. In Resumos Expandidos; Simpósio de Pesquisa dos Cafés do Brasil; Embrapa Café/Minasplan: Brasília, Brazil, 2000; pp. 413–416. [Google Scholar]
- Köppen, W.; Geiger, R. Klimate der Erde; Verlag Justus Perthes: Gotha, Germany, 1928. [Google Scholar]
- Rocha, O.C.; Guerra, A.F.; Silva, F.A.M.; Machado Júnior, J.R.R.; Araújo, M.C.; Silva, H.C. Programa para monitoramento de irrigação do cafeeiro no Cerrado. In Proceedings of the VIII Simpósio Brasileiro de Pesquisa em Cafeicultura Irrigada, Araguari, Brazil, 23–26 October 2006; UFV: Viçosa, Brazil, 2006; pp. 61–64. [Google Scholar]
- Guerra, A.F.; Rocha, O.C.; Rodrigues, G.C.; Sanzonowicz, C.; Sampaio, J.B.R.; Silva, H.C.; Araújo, M.C. Irrigação do Cafeeiro no Cerrado: Estratégia de Manejo de água para Uniformização de Florada; Embrapa Cerrados. Comunicado técnico, 122; Embrapa Cerrados: Planaltina, Brazil, 2005; 4p. [Google Scholar]
- Cruz, C.D. GENES—A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. 2013, 35, 271–276. [Google Scholar]
- Rodrigues, M.; Machado, M.A.; Ferreira, L.F.; Silva, R.M. Use of NIR spectroscopy to discriminate coffee genotypes based on chemical composition. J. Food Sci. Technol. 2019, 56, 4204–4211. [Google Scholar]
- Mishra, S.; Bal, L.M.; Mohapatra, D. Advances in coffee breeding and quality assessment using omics and phenotyping tools. Plant Physiol. Biochem. 2022, 173, 12–25. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J.; Trugo, L.C. Effect of roasting on chlorogenic acids, caffeine, and other bioactive compounds in coffee. Food Chem. 2019, 302, 125281. [Google Scholar] [CrossRef]
- Ávila, F.A.; Santos, J.B.; Mendes, T.R. Influence of climatic factors on biochemical compounds of Coffea arabica L. during the development stages. Plant Physiol. Biochem. 2021, 158, 120–128. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Silva, J.A.G.; Ferreira, C.D. Genetic variability and stability of coffee genotypes for biochemical traits under varying environments. Crop Breed. Appl. Biotechnol. 2020, 20, e282120. [Google Scholar]
- Campa, C.R.; Machado, R.P.; Lima, L.S. Integrating environmental data and genotypic performance for improved coffee breeding strategies. Theor. Appl. Genet. 2022, 135, 2831–2845. [Google Scholar]
- Silva, R.S.; Barbosa, T.A.; Souza, L.A. Adaptability and resilience of coffee genotypes under climate change scenarios: Challenges and prospects. Front. Plant Sci. 2023, 14, 1156723. [Google Scholar]
- Cheserek, M.J.; Mwangi, M.; Kibet, L. Heritability and genetic variability of caffeine, sucrose and trigonelline contents in Coffea arabica and Coffea canephora hybrids. Plant Breed. 2022, 141, 453–463. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, R.; Singh, K. Genetic improvement of coffee quality traits: Recent advances and future prospects. Breed. Sci. 2022, 72, 1–15. [Google Scholar]
- Gichimu, B.M.; Wanyoko, J.K.; Kinyua, M.G. Influence of genetic and environmental factors on caffeine content in Arabica coffee grown in Kenya. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Silva, J.A.; Santos, F.C. Environmental modulation of caffeine biosynthesis and accumulation in Coffea arabica L. beans. Front Plant Sci. 2020, 11, 578682. [Google Scholar]
- Alves, R.M.; Pereira, G.E.; Oliveira, L.R. Variability in caffeine content among coffee genotypes and implications for breeding programs. Crop Sci. 2019, 59, 1551–1560. [Google Scholar]
- Oliveira, M.C.; Silva, J.A.G.; Ferreira, C.D. Genetic variability and selection potential for biochemical compounds in coffee under different environmental conditions. Euphytica 2021, 217, 125. [Google Scholar]

| SV | DF | MS | ||||
|---|---|---|---|---|---|---|
| 5-ACQ | CAFFEINE | SUCROSE | CITRIC ACID | TRIGONELLINE | ||
| Genotype | 17 | 0.3398 ns | 0.1450 ** | 1.2917 * | 0.1555 ns | 0.1384 ** |
| Error a | 36 | 0.0792 | 0.0087 | 0.1526 | 0.0309 | 0.0251 |
| Year | 1 | 2.9107 ** | 3.2691 ** | 32.2647 ** | 2.1028 ** | 2.0015 ** |
| G × A | 17 | 0.3376 ** | 0.0226 * | 0.4767 ** | 0.0807 ns | 0.0295 ns |
| Error b | 36 | 0.1223 | 0.0103 | 0.1389 | 0.0487 | 0.0272 |
| F GENOTYPE | 1.1085 | 4.4958 | 2.2735 | 1.8293 | 3.0302 | |
| F YEAR | 23.7949 | 318.449 | 232.2478 | 43.2063 | 73.6851 | |
| F G × A | 2.7601 | 2.2019 | 3.4313 | 1.6857 | 1.0861 | |
| Average | 4.3258 | 2.1383 | 4.7950 | 0.6626 | 1.2345 | |
| CVe GENOTYPE (%) | 6.5073 | 4.3650 | 8.1458 | 26.5231 | 12.8399 | |
| CVe YEAR (%) | 8.0851 | 4.7383 | 7.7731 | 33.2933 | 13.3505 | |
| 0.0075 | 0.0207 | 0.1336 | 0.0154 | 0.0185 | ||
| 0.0516 | 0.0603 | 0.5949 | 0.0380 | 0.0366 | ||
| 0.0678 | 0.0039 | 0.1063 | 0.0101 | 0.0007 | ||
| CVg (%) | 2.0068 | 6.7218 | 7.6217 | 18.7438 | 11.0128 | |
| CVr GENOTYPE | 0.3084 | 1.5399 | 0.9357 | 0.7067 | 0.8577 | |
| CVr—YEAR | 0.2482 | 1.4186 | 0.9805 | 0.5630 | 0.8249 | |
| H2 (average) (%) | 13.3088 | 85.4831 | 62.0400 | 59.5216 | 80.1514 | |
| 0.3128 | 0.8818 | 0.7484 | 0.6733 | 0.8185 | ||
| Genotype Names | 5-ACQ (%) | CAFFEINE (%) | SUCROSE (%) | CITRIC ACID (%) | TRIGONELLINE (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |||||||||||
| L1 L4 P139 | 4.140 | Bb | 5.280 | Aa | 1.787 | Bc | 2.015 | Ac | 4.642 | Ab | 3.947 | Bc | 0.730 | Ab | 0.725 | Aa | 1.159 | Ab | 1.122 | Ab |
| L1 L7 P80 | 4.363 | Ab | 4.163 | Ac | 2.053 | Bb | 2.540 | Aa | 5.514 | Aa | 4.464 | Bb | 0.508 | Ab | 0.415 | Aa | 1.330 | Ab | 1.195 | Aa |
| L2 L2 P42 | 4.477 | Ab | 4.157 | Ac | 1.927 | Bb | 2.260 | Ab | 4.754 | Ab | 4.833 | Ab | 0.754 | Ab | 0.436 | Aa | 1.214 | Ab | 1.025 | Ab |
| L2 L28 P100 | 4.210 | Ab | 4.050 | Ac | 1.777 | Bc | 2.180 | Ac | 4.273 | Ab | 3.704 | Ac | 0.697 | Ab | 0.431 | Aa | 1.315 | Ab | 1.084 | Ab |
| L2 L21 P20 | 4.553 | Ab | 3.655 | Bc | 1.717 | Bc | 2.150 | Ac | 4.967 | Ab | 3.629 | Bc | 0.660 | Ab | 0.127 | Ba | 1.144 | Ab | 0.856 | Bb |
| L2 L6 P35 | 4.510 | Ab | 3.933 | Bc | 1.980 | Bb | 2.413 | Aa | 4.961 | Ab | 3.560 | Bc | 0.941 | Aa | 0.585 | Ba | 1.433 | Ab | 1.082 | Bb |
| L2 L25 P123 | 5.167 | Aa | 4.595 | Bb | 1.920 | Bb | 2.385 | Aa | 4.921 | Ab | 3.995 | Bc | 1.029 | Aa | 0.452 | Ba | 1.522 | Aa | 1.210 | Ba |
| L2 L8 P42 | 4.140 | Ab | 4.250 | Ac | 2.050 | Bb | 2.253 | Ab | 5.838 | Aa | 4.383 | Bb | 0.961 | Aa | 0.412 | Ba | 1.368 | Ab | 1.025 | Bb |
| L2 L2 P24 | 4.437 | Ab | 4.475 | Ab | 1.697 | Bc | 2.220 | Ab | 6.451 | Aa | 4.462 | Bb | 0.716 | Ab | 0.935 | Aa | 1.415 | Ab | 1.260 | Aa |
| L2 L16 P51 | 4.283 | Ab | 4.050 | Ac | 2.140 | Ba | 2.447 | Aa | 5.552 | Aa | 3.684 | Bc | 0.532 | Ab | 0.513 | Aa | 1.327 | Ab | 1.062 | Ab |
| L3 L13 P39 | 4.887 | Aa | 4.217 | Bc | 1.940 | Bb | 2.160 | Ac | 5.396 | Aa | 4.461 | Bb | 0.811 | Ab | 0.648 | Aa | 1.331 | Ab | 1.048 | Bb |
| L3 L19 P28 | 4.657 | Aa | 4.137 | Ac | 1.980 | Bb | 2.307 | Ab | 5.413 | Aa | 3.775 | Bc | 0.535 | Ab | 0.318 | Aa | 1.332 | Ab | 0.921 | Bb |
| L3 L16 P6 | 4.843 | Aa | 3.945 | Bc | 2.260 | Ba | 2.575 | Aa | 5.835 | Aa | 4.636 | Bb | 1.226 | Aa | 0.700 | Ba | 1.789 | Aa | 1.421 | Ba |
| L3 L16 P112 | 4.495 | Ab | 4.043 | Ac | 2.040 | Bb | 2.280 | Ab | 5.957 | Aa | 4.688 | Bb | 1.060 | Aa | 0.459 | Ba | 1.609 | Aa | 1.044 | Bb |
| L3 L16 P51 | 4.437 | Ab | 4.107 | Ac | 1.957 | Bb | 2.463 | Aa | 5.573 | Aa | 3.964 | Bc | 0.957 | Aa | 0.692 | Aa | 1.388 | Ab | 1.112 | Bb |
| L4 L11 P55 | 3.985 | Ab | 3.973 | Ac | 2.315 | Aa | 2.423 | Aa | 5.418 | Aa | 4.818 | Ab | 0.538 | Ab | 0.361 | Aa | 1.252 | Ab | 1.032 | Ab |
| L4 L25 P123 | 4.870 | Aa | 4.243 | Bc | 2.013 | Bb | 2.490 | Aa | 5.698 | Aa | 5.606 | Aa | 0.793 | Ab | 0.683 | Aa | 1.495 | Aa | 1.488 | Aa |
| L4 L21 P20 | 4.367 | Ab | 3.637 | Bc | 1.807 | Bc | 2.060 | Ac | 4.987 | Ab | 3.864 | Bc | 0.992 | Aa | 0.526 | Ba | 1.250 | Ab | 0.784 | Bb |
| 5-ACQ | Caffeine | Sucrose | Citric Acid | Trigonelline | |
|---|---|---|---|---|---|
| Xo | 4.326 | 2.138 | 4.795 | 0.662 | 1.235 |
| Xs | 4.175 | 1.966 | 5.227 | 0.774 | 1.362 |
| H2 | 0.133 | 0.855 | 0.620 | 0.595 | 0.802 |
| DS | −0.151 | −0.172 | 0.432 | 0.112 | 0.128 |
| GS | −0.020 | −0.147 | 0.268 | 0.066 | 0.102 |
| GS% | −0.466 | −6.879 | 5.582 | 10.014 | 8.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brige, F.A.A.; Amabile, R.F.; Malaquias, J.V.; Veiga, A.D.; Santos, G.B.C.; Fialho, A.R.; Fagioli, M. Genetic Parameters of Conilon Coffee Cultivated Under an Irrigation System in the Cerrado. Agronomy 2025, 15, 1863. https://doi.org/10.3390/agronomy15081863
Brige FAA, Amabile RF, Malaquias JV, Veiga AD, Santos GBC, Fialho AR, Fagioli M. Genetic Parameters of Conilon Coffee Cultivated Under an Irrigation System in the Cerrado. Agronomy. 2025; 15(8):1863. https://doi.org/10.3390/agronomy15081863
Chicago/Turabian StyleBrige, Felipe Augusto Alves, Renato Fernando Amabile, Juaci Vitória Malaquias, Adriano Delly Veiga, Gustavo Barbosa Cobalchini Santos, Arlini Rodrigues Fialho, and Marcelo Fagioli. 2025. "Genetic Parameters of Conilon Coffee Cultivated Under an Irrigation System in the Cerrado" Agronomy 15, no. 8: 1863. https://doi.org/10.3390/agronomy15081863
APA StyleBrige, F. A. A., Amabile, R. F., Malaquias, J. V., Veiga, A. D., Santos, G. B. C., Fialho, A. R., & Fagioli, M. (2025). Genetic Parameters of Conilon Coffee Cultivated Under an Irrigation System in the Cerrado. Agronomy, 15(8), 1863. https://doi.org/10.3390/agronomy15081863







