Exogenous Application of Nano-Silicon and Melatonin Ameliorates Salinity Injury in Coix Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Material, Germination, and Seedling Establishment
2.2. Salinity Stress Treatment and Exogenous Chemical Application
2.3. Plant Measurements
2.4. Statistical Analysis
3. Results
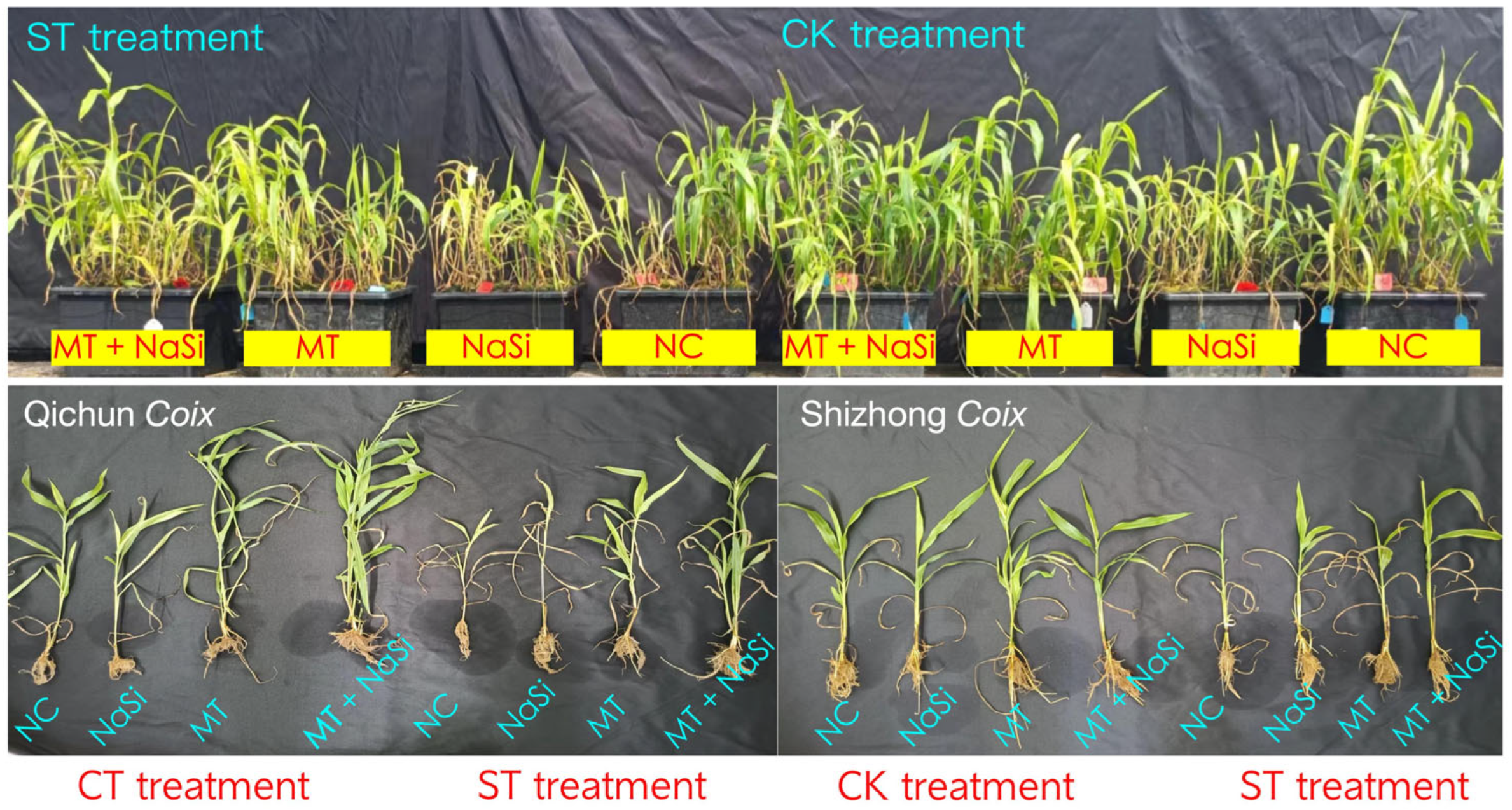
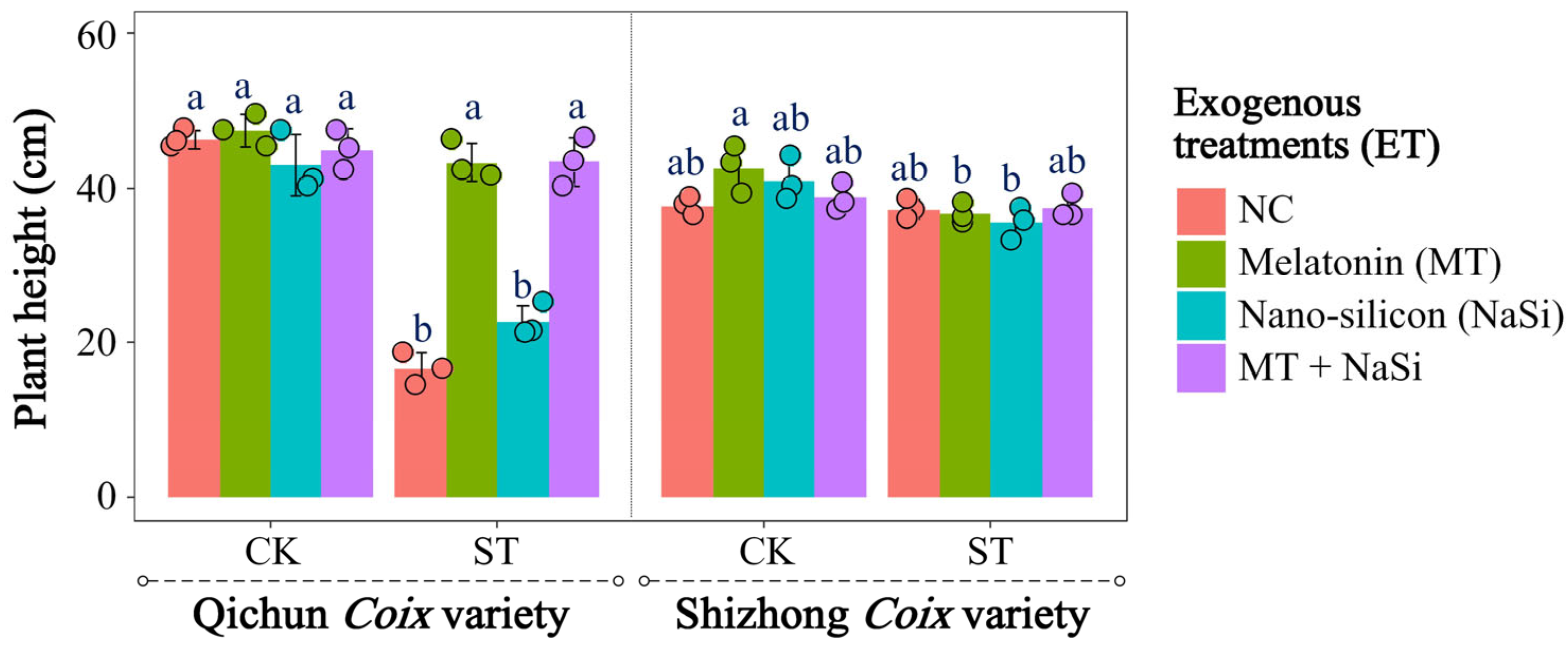
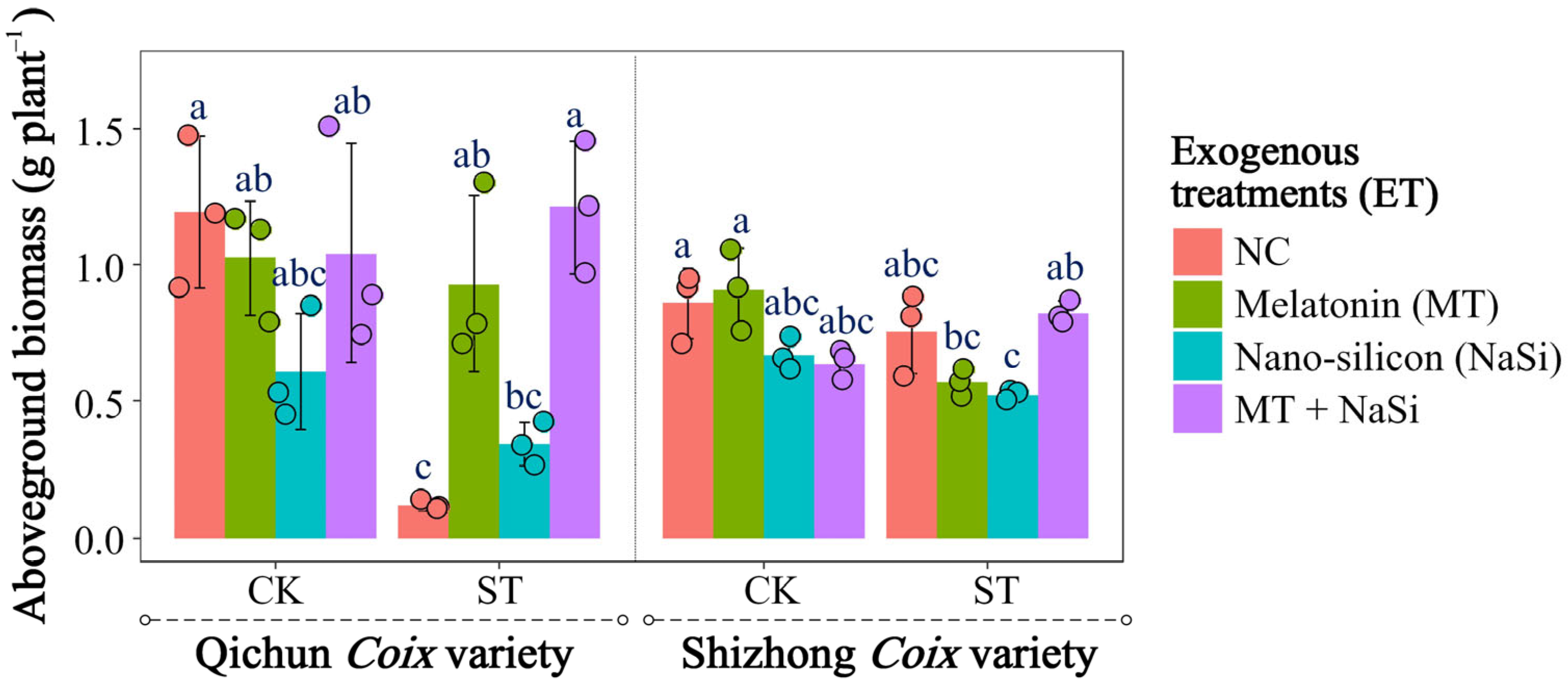
3.1. Effects of Exogenous Substances on Plant Height and Biomass Under Salinity Stress
3.2. Effects of Exogenous Substances on Photosynthesis Under Salinity Stress
3.3. Effects of Exogenous Substances on Physiological Traits Under Salinity Stress
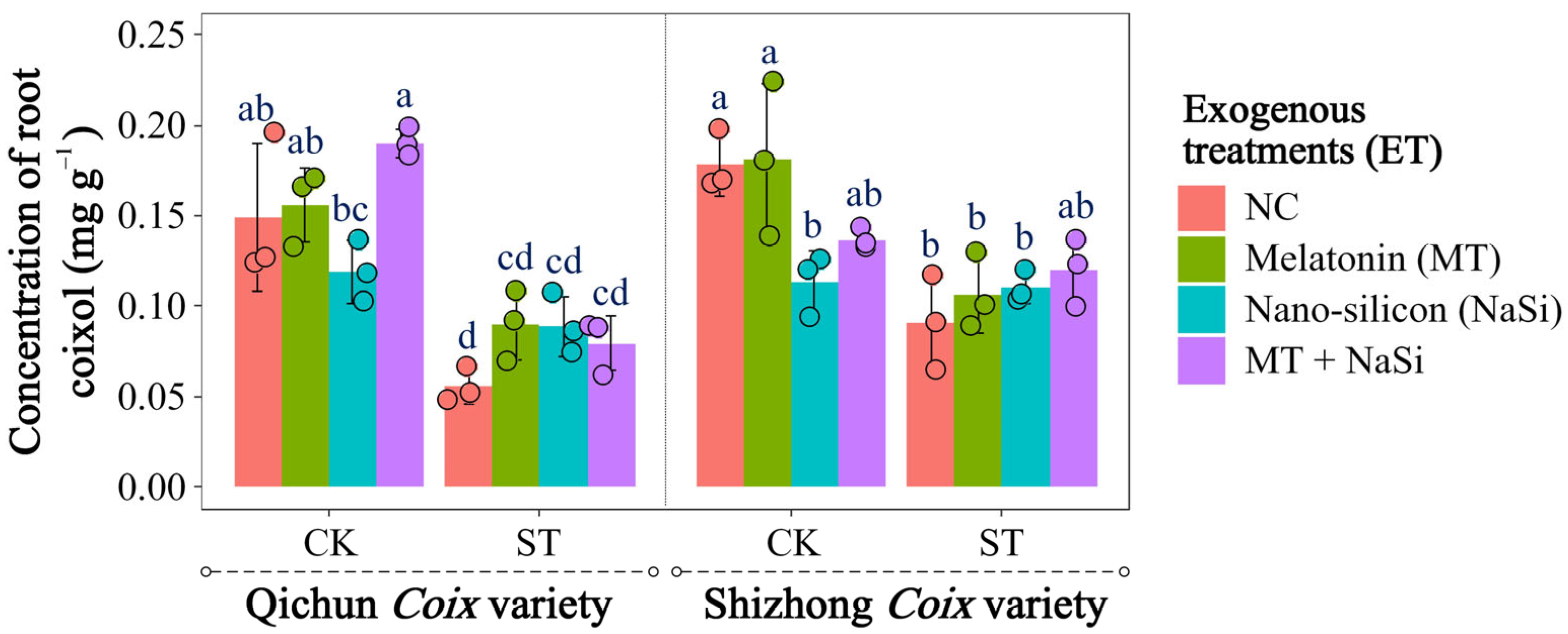
3.4. Effects of Exogenous Substances on Root Coixol Content Under Salinity Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.H.; Kim, B.; Choi, B.S.; Lee, H.O.; Kim, N.H.; Lee, S.J.; Kim, H.S.; Shin, M.J.; Kim, H.W.; Nam, K.; et al. Genome assembly and annotation of soft-shelled adlay (Coix lacryma-jobi variety ma-yuen), a cereal and medicinal crop in the Poaceae family. Front. Plant Sci. 2020, 11, 630. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Cheng, S.; Chen, Y.; Song, J.; Zhang, X.; Zhao, S.; Tang, H. Medicine and food homologous plant resources and their utilization in Guangxi: A review. Guihaia 2025, 45, 450–465. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Yin, F.; Fang, J.; Cai, L.; Zhang, C.; Xiang, Z.; Zhao, Y.; Zhang, S.; Sheng, H.; et al. Research on coix seed as a food and medicinal resource, it’s chemical components and their pharmacological activities: A review. J. Ethnopharmacol. 2024, 319, 117309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, X.; Lu, J.; He, G.; Xia, J.; Li, Y.; Wu, Z. Effect of adlay (Coix lachryma-jobi L.) on growth, antioxidation, immune modulation, and intestinal health of crucian carp (Carassius auratus). Aquacult. Int. 2025, 33, 166. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Landi, S.; Hausman, J.-F.; Guerriero, G.; Esposito, S. Poaceae vs. abiotic stress: Focus on drought and salt stress, recent insights and perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Xin, T.; Cheng, Z.; Xingyuan, L.; Qin, Y. Effects of salt stress on seed germination and seedling growth of Coix. Crops 2015, 31, 140–143. [Google Scholar] [CrossRef]
- Cabusora, C.C. Developing climate-resilient crops: Adaptation to abiotic stress-affected areas. Technol. Agron. 2024, 4, e005. [Google Scholar] [CrossRef]
- Gharbi, P.; Amiri, J.; Mahna, N.; Naseri, L.; Sadaghiani, M.R. Silicon-induced mitigation of salt stress in GF677 and GN15 rootstocks: Insights into physiological, biochemical, and molecular mechanisms. BMC Plant Biol. 2025, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, L.; Wang, H.; Jiang, H.; Ding, Q.; Yang, D.; Yan, C.; Lu, X. The physiological mechanism of exogenous melatonin regulating salt tolerance in eggplant seedlings. Agronomy 2025, 15, 270. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef]
- Shoukat, A.; Pitann, B.; Hossain, M.S.; Saqib, Z.A.; Nawaz, A.; Mühling, K.H. Zinc and silicon fertilizers in conventional and nano-forms: Mitigating salinity effects in maize (Zea mays L.). J. Plant Nutr. Soil Sci. 2024, 187, 678–689. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 2018, 255, 953–962. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.-G.; Tan, D.-X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Liu, J.; Lyu, P.; Wu, C.; Liu, F.; Zhao, X.; Tang, H. The effects of different soil substrates on the growth and root coixol content of local coix varieties in China. Agronomy 2024, 14, 1792. [Google Scholar] [CrossRef]
- Cui, X.; Xu, Y.; Chen, L.; Zhao, M.; Yang, S.; Wang, Y. Ultrafine Pd nanoparticles supported on zeolite-templated mesocellular graphene network via framework aluminum mediation: An advanced oxygen reduction electrocatalyst. App. Catal. B Environ. 2019, 244, 957–964. [Google Scholar] [CrossRef]
- Liang, H.; Liu, B.; Wu, C.; Zhang, X.; Wang, M.; Huang, X.; Wan, L.; Tang, H. Effects of light intensity on the growth of Polygala fallax Hemsl. (Polygalaceae). Front. Plant Sci. 2022, 13, 985628. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, F.; Wu, C.; Tang, H.; Xiong, D.; Cui, K.; Peng, S.; Huang, J. Nitrogen supply mitigates temperature stress effects on rice photosynthetic nitrogen use efficiency and water relations. Plants 2025, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Gris, T.; Pinheiro, M.V.M.; Thiesen, L.A.; Webler, A.R.; Junges, D.L.; Holz, E.; Naibo, I.; Batista, D.S.; Schmidt, D. Light quality and sealing type affect in vitro growth and development of Capsicum frutescens cultivars. An. Acad. Bras. Ciênc. 2021, 93, e20190061. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Van Den Brand, T. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; The R Foundation: Ames, IA, USA, 2007. [Google Scholar]
- Du, L.; Tan, K.; Zhou, S. Research analysis and development of soil saline-alkali land improvement. Hans J. Soil Sci. 2021, 9, 14–17. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.; Wu, J.; Chen, J.; Liu, D.; Wang, C.; Ma, S.; Guo, W.; Li, G.; Di, D.; et al. Variation in TaSPL6-D confers salinity tolerance in bread wheat by activating TaHKT1;5-D while preserving yield-related traits. Nat. Genet. 2024, 56, 1257–1269. [Google Scholar] [CrossRef]
- Yuan, J.; Cao, H.; Qin, W.; Yang, S.; Zhang, D.; Zhu, L.; Song, H.; Zhang, Q. Genomic and modern biotechnological strategies for enhancing salt tolerance in crops. New Crops 2025, 2, 100057. [Google Scholar] [CrossRef]
- Li, A.; Yang, Y.; Guo, Y.; Li, Q.; Zhou, A.; Wang, J.; Lu, R.; Shelden, M.C.; Wu, C.; Wu, J. ZmASR6 positively regulates salt stress tolerance in maize. New Crops 2025, 2, 100067. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Qi, B.; Cheng, S.; Song, Y.; Wu, C.; Yang, M. Hidden stigmas enhance heat resilience: A novel breeding trait for sustaining rice spikelet fertility under nocturnal heat stress. Agronomy 2025, 15, 982. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestic, M.; Xie, W. Silicon: An essential element for plant nutrition and phytohormones signaling mechanism under stressful conditions. Plant Growth Regul. 2023, 100, 301–319. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Ahmad, J.; Munir, M.; Alqahtani, N.; Alyas, T.; Ahmad, M.; Bashir, S.; Qurashi, F.; Ghafoor, A.; Ali–Dinar, H. Enhancing plant resilience to biotic and abiotic stresses through exogenously applied nanoparticles: A comprehensive review of effects and mechanism. Phyton-Int. J. Exp. Bot. 2025, 94, 281–302. [Google Scholar] [CrossRef]
- Zaki, F.S.A.; Elsayed, A.E.; Ahmed, A.M.A.; Khalid, K.A. Salinity stress and different types of nano silicon’s effects on lupine morphology and biochemical accumulations. Biocatal. Agr. Biotechnol. 2024, 55, 102997. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Bioch. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, X.; Guan, X.; Sun, L.; Yang, Z.; Wang, D.; Chen, Y.; Li, P.; Xie, Z. Nano-silicon enhances tomato growth and antioxidant defense under salt stress. Environ. Sci. Nano 2025, 12, 315–324. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Liu, Y.; Li, T.; Xu, H.; Wei, Q.; Zeng, H.; Ni, H.; Li, S. Multiple functions of exogenous melatonin in cucumber seed germination, seedling establishment, and alkali stress resistance. BMC Plant Biol. 2025, 25, 359. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G.; et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K(+) transporters and K(+) homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Askari, M.; Hamid, N.; Abideen, Z.; Zulfiqar, F.; Moosa, A.; Nafees, M.; El-Keblawy, A. Exogenous melatonin application stimulates growth, photosynthetic pigments and antioxidant potential of white beans under salinity stress. S. Afr. J. Bot. 2023, 160, 219–228. [Google Scholar] [CrossRef]
- Faisal, M.; Faizan, M.; Soysal, S.; Alatar, A.A. Synergistic application of melatonin and silicon oxide nanoparticles modulates reactive oxygen species generation and the antioxidant defense system: A strategy for cadmium tolerance in rice. Front. Plant Sci. 2024, 15, 1484600. [Google Scholar] [CrossRef]
- Xu, L.; Xue, X.; Yan, Y.; Zhao, X.; Li, L.; Sheng, K.; Zhang, Z. Silicon combined with melatonin reduces Cd absorption and translocation in maize. Plants 2023, 12, 3537. [Google Scholar] [CrossRef]
- Gatasheh, M.K.; Shah, A.A.; Ali, S.; Ramzan, M.; Javad, S.; Waseem, L.; Noor, H.; Ahmed, S.; Wahid, A. Synergistic application of melatonin and silicon alleviates chromium stress in Brassica napus through regulation of antioxidative defense system and ethylene metabolism. Sci. Hortic. 2023, 321, 112280. [Google Scholar] [CrossRef]
- Li, H. Effects of exogenous melatonin and silicon on the growth and physiological characteristics of celery (Apium graveolens) seedlings under salt stress. J. Henan Agr. Sci. 2020, 49, 96–102. [Google Scholar] [CrossRef]
- Hua, Y.; Pan, X.; Tian, L.; Xu, Y.; Yang, M.; Deng, R. Application of salicylic acid improves the production of medicinal components in Mucuna macrocarpa wall by regulating endogenous hormone and nutrient balance. Plants 2025, 14, 1023. [Google Scholar] [CrossRef]
| Variety | Salinity Treatment | Exogenous Treatment | A (μmol m−2 s−1) | gs (mol m−2 s−1) | Ci (μmol mol−1) | E (mmol m−2 s−1) |
|---|---|---|---|---|---|---|
| Qichun Coix | CK | NC | 11.4 ± 0.69 c | 0.10 ± 0.00 a | 132 ± 9.50 ab | 1.22 ± 0.02 c |
| MT | 14.6 ± 0.97 b | 0.11 ± 0.00 a | 97.8 ± 22.2 ab | 2.36 ± 0.12 ab | ||
| NaSi | 11.2 ± 0.86 c | 0.10 ± 0.00 a | 131 ± 14.6 ab | 1.82 ± 0.11 bc | ||
| MT + NaSi | 17.1 ± 0.29 a | 0.13 ± 0.01 a | 78.0 ± 24.9 ab | 2.98 ± 0.24 a | ||
| ST | NC | 3.44 ± 0.53 e | 0.04 ± 0.02 b | 155 ± 41.0 a | 1.17 ± 0.46 c | |
| MT | 12.3 ± 0.57 bc | 0.10 ± 0.02 a | 82.5 ± 38.4 ab | 3.06 ± 0.61 a | ||
| NaSi | 5.95 ± 0.33 d | 0.04 ± 0.01 b | 84.3 ± 37.3 ab | 1.37 ± 0.19 c | ||
| MT + NaSi | 13.3 ± 0.76 bc | 0.10 ± 0.01 a | 62.9 ± 29.5 b | 3.16 ± 0.33 a | ||
| Shizhong Coix | CK | NC | 13.2 ± 0.73 cd | 0.10 ± 0.00 a | 104 ± 13.7 a | 1.54 ± 0.08 d |
| MT | 17.2 ± 0.90 ab | 0.12 ± 0.01 a | 71.0 ± 4.11 abc | 2.66 ± 0.10 abc | ||
| NaSi | 15.4 ± 0.98 bc | 0.12 ± 0.02 a | 83.8 ± 19.6 ab | 2.16 ± 0.00 cd | ||
| MT + NaSi | 19.6 ± 0.57 a | 0.14 ± 0.01 a | 51.1 ± 6.21 bc | 3.14 ± 0.15 ab | ||
| ST | NC | 9.11 ± 0.34 e | 0.06 ± 0.00 a | 39.7 ± 15.4 c | 1.70 ± 0.06 d | |
| MT | 14.2 ± 1.01 bcd | 0.36 ± 0.44 a | 54.2 ± 3.82 bc | 3.18 ± 0.32 a | ||
| NaSi | 11.3 ± 1.85 de | 0.08 ± 0.02 a | 49.6 ± 17.3 bc | 2.33 ± 0.56 bcd | ||
| MT + NaSi | 14.6 ± 0.80 bcd | 0.10 ± 0.01 a | 55.5 ± 16.6 bc | 3.43 ± 0.20 a |
| Variety | Salinity Treatment | Exogenous Treatment | SPAD | Leaf Nitrogen Concentration | Relative Conductivity | Leaf Water Content (%) |
|---|---|---|---|---|---|---|
| Qichun Coix | CK | NC | 39.6 ± 0.67 bc | 12.4 ± 0.20 bc | 0.14 ± 0.01 b | 85.8 ± 2.47 a |
| MT | 46.6 ± 2.57 a | 14.9 ± 0.81 a | 0.12 ± 0.03 b | 83.0 ± 2.11 a | ||
| NaSi | 36.6 ± 1.49 c | 11.5 ± 0.44 c | 0.12 ± 0.03 b | 84.9 ± 0.27 a | ||
| MT + NaSi | 50.1 ± 0.91 a | 15.6 ± 0.31 a | 0.10 ± 0.01 b | 85.0 ± 1.82 a | ||
| ST | NC | 15.9 ± 0.62 e | 5.3 ± 0.15 e | 0.45 ± 0.23 a | 60.8 ± 2.87 c | |
| MT | 41.4 ± 1.64 b | 13.0 ± 0.47 b | 0.18 ± 0.08 b | 83.4 ± 0.90 a | ||
| NaSi | 22.2 ± 2.21 d | 7.2 ± 0.66 d | 0.27 ± 0.07 ab | 69.0 ± 1.57 b | ||
| MT + NaSi | 46.7 ± 1.46 a | 14.6 ± 0.40 a | 0.22 ± 0.05 ab | 84.1 ± 1.34 a | ||
| Shizhong Coix | CK | NC | 48.9 ± 2.11 abc | 15.2 ± 0.67 abc | 0.11 ± 0.03 bc | 85.8 ± 1.53 a |
| MT | 52.8 ± 1.64 ab | 16.4 ± 0.46 ab | 0.08 ± 0.03 c | 83.8 ± 2.58 a | ||
| NaSi | 47.6 ± 2.48 bc | 14.8 ± 0.76 bc | 0.09 ± 0.02 c | 84.2 ± 1.62 a | ||
| MT + NaSi | 53.2 ± 2.86 a | 16.5 ± 0.85 a | 0.11 ± 0.01 bc | 85.0 ± 0.89 a | ||
| ST | NC | 34.2 ± 1.87 e | 10.8 ± 0.53 e | 0.35 ± 0.18 a | 77.8 ± 1.79 b | |
| MT | 44.1 ± 0.65 cd | 13.8 ± 0.20 cd | 0.32 ± 0.06 ab | 82.8 ± 3.22 ab | ||
| NaSi | 39.1 ± 1.88 de | 12.3 ± 0.56 de | 0.27 ± 0.08 abc | 81.9 ± 2.66 ab | ||
| MT + NaSi | 50.7 ± 0.51 ab | 15.7 ± 0.15 ab | 0.21 ± 0.03 abc | 85.0 ± 0.85 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, B.; Liu, J.; Zheng, R.; Huang, J.; Wu, C. Exogenous Application of Nano-Silicon and Melatonin Ameliorates Salinity Injury in Coix Seedlings. Agronomy 2025, 15, 1862. https://doi.org/10.3390/agronomy15081862
Qi B, Liu J, Zheng R, Huang J, Wu C. Exogenous Application of Nano-Silicon and Melatonin Ameliorates Salinity Injury in Coix Seedlings. Agronomy. 2025; 15(8):1862. https://doi.org/10.3390/agronomy15081862
Chicago/Turabian StyleQi, Beibei, Junkai Liu, Ruixue Zheng, Jiada Huang, and Chao Wu. 2025. "Exogenous Application of Nano-Silicon and Melatonin Ameliorates Salinity Injury in Coix Seedlings" Agronomy 15, no. 8: 1862. https://doi.org/10.3390/agronomy15081862
APA StyleQi, B., Liu, J., Zheng, R., Huang, J., & Wu, C. (2025). Exogenous Application of Nano-Silicon and Melatonin Ameliorates Salinity Injury in Coix Seedlings. Agronomy, 15(8), 1862. https://doi.org/10.3390/agronomy15081862







