Optimizing Citrus aurantifolia (Christm. Swingle) Production Through Integrated Irrigation and Growth Regulation Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Study
2.2. Conditions of the Experiment
2.3. Site Characterization
Soil Characteristics (Soil Analysis)
2.4. Treatments, Experimental Design, and Experimental Unit
2.5. Application of Treatments
- Stage I: Fruit at “ping-pong ball” stage (approximately the size of a pea).
- Stage II: Fruit reaching approximately 90% of its final size.
2.6. Determination of Irrigation Levels
Irrigation Calculation Model for the Proposed Water Depths
2.7. Variables Evaluated
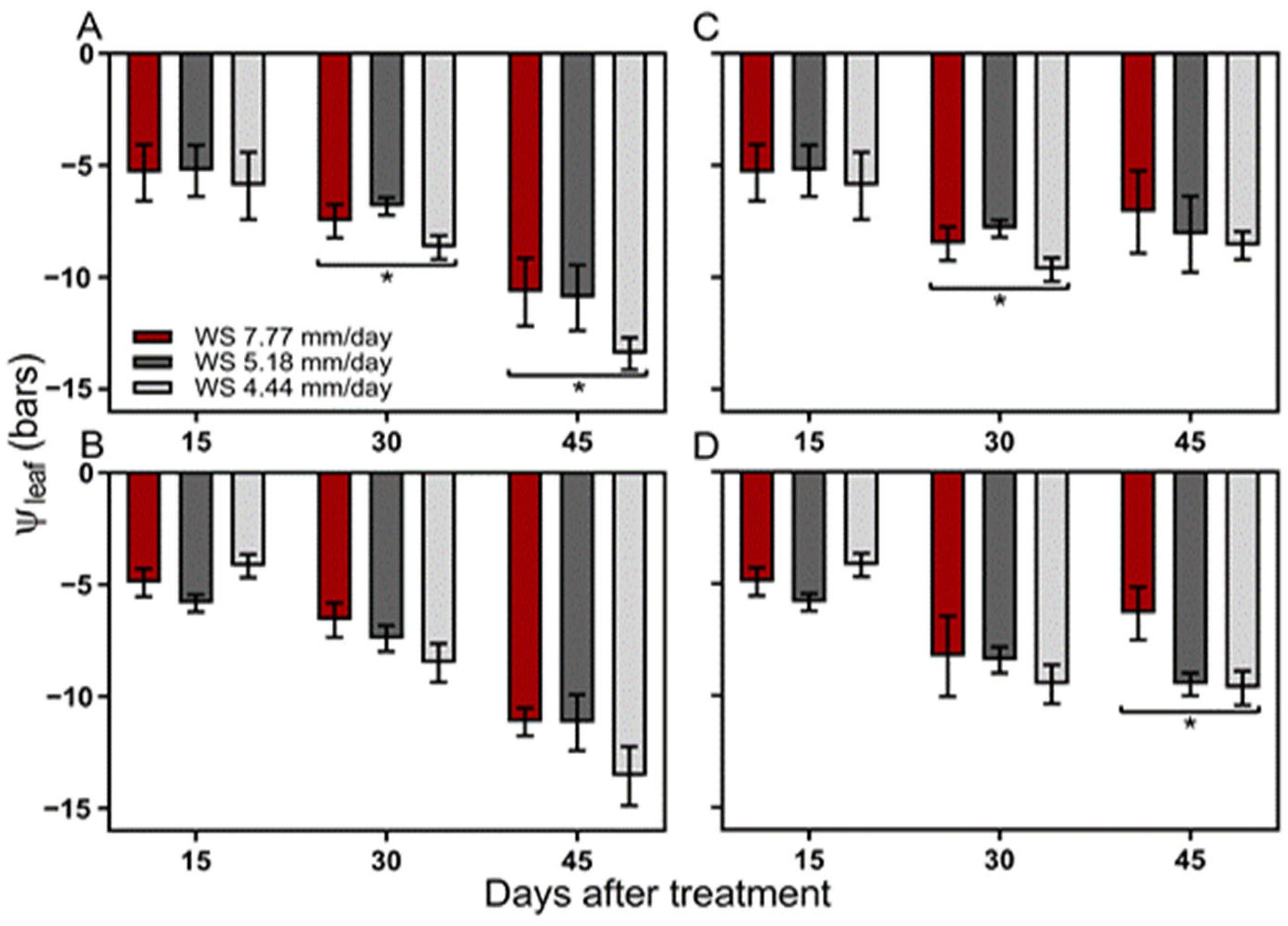
2.7.1. Leaf Water Potential (Ψh)
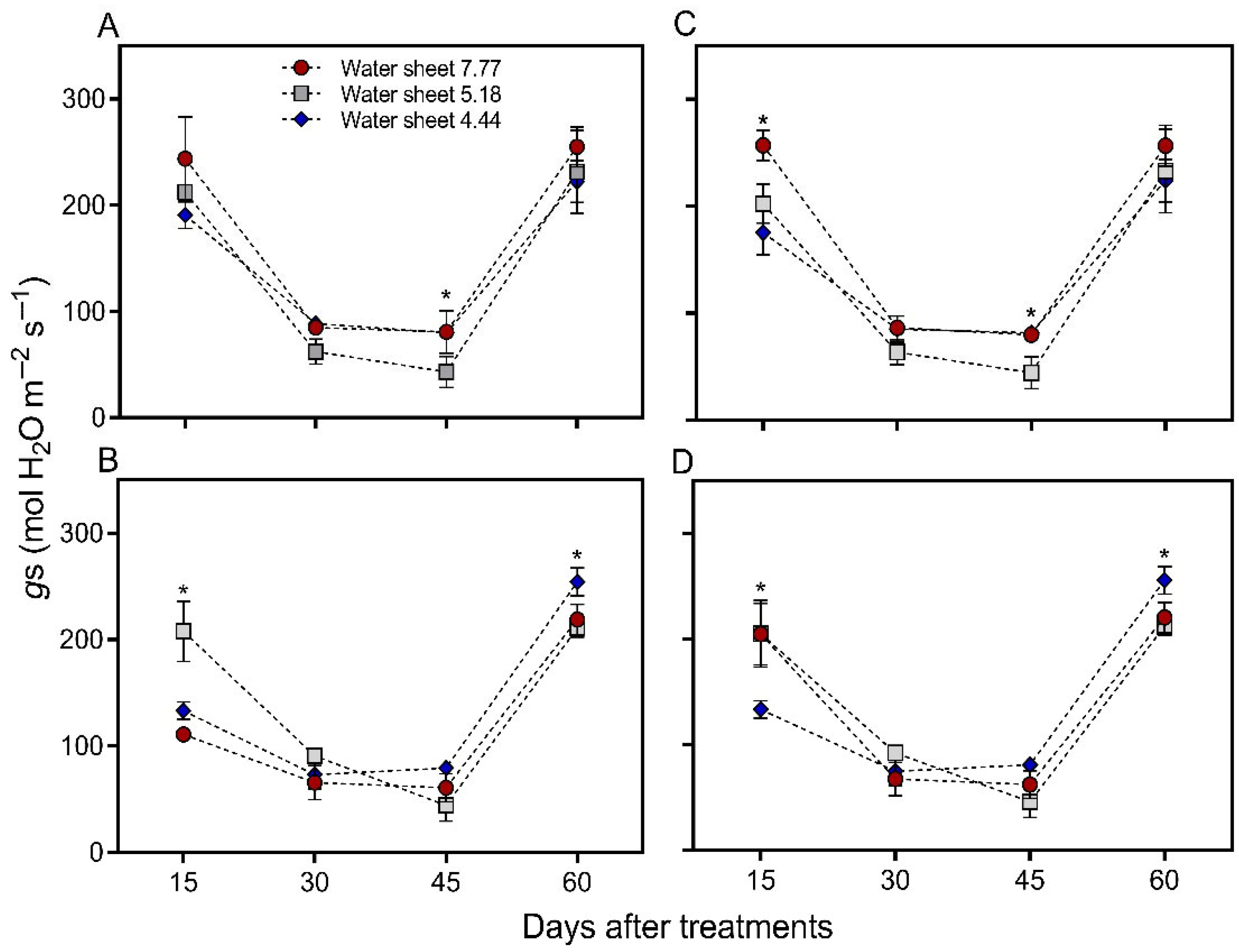
2.7.2. Stomatal Conductance
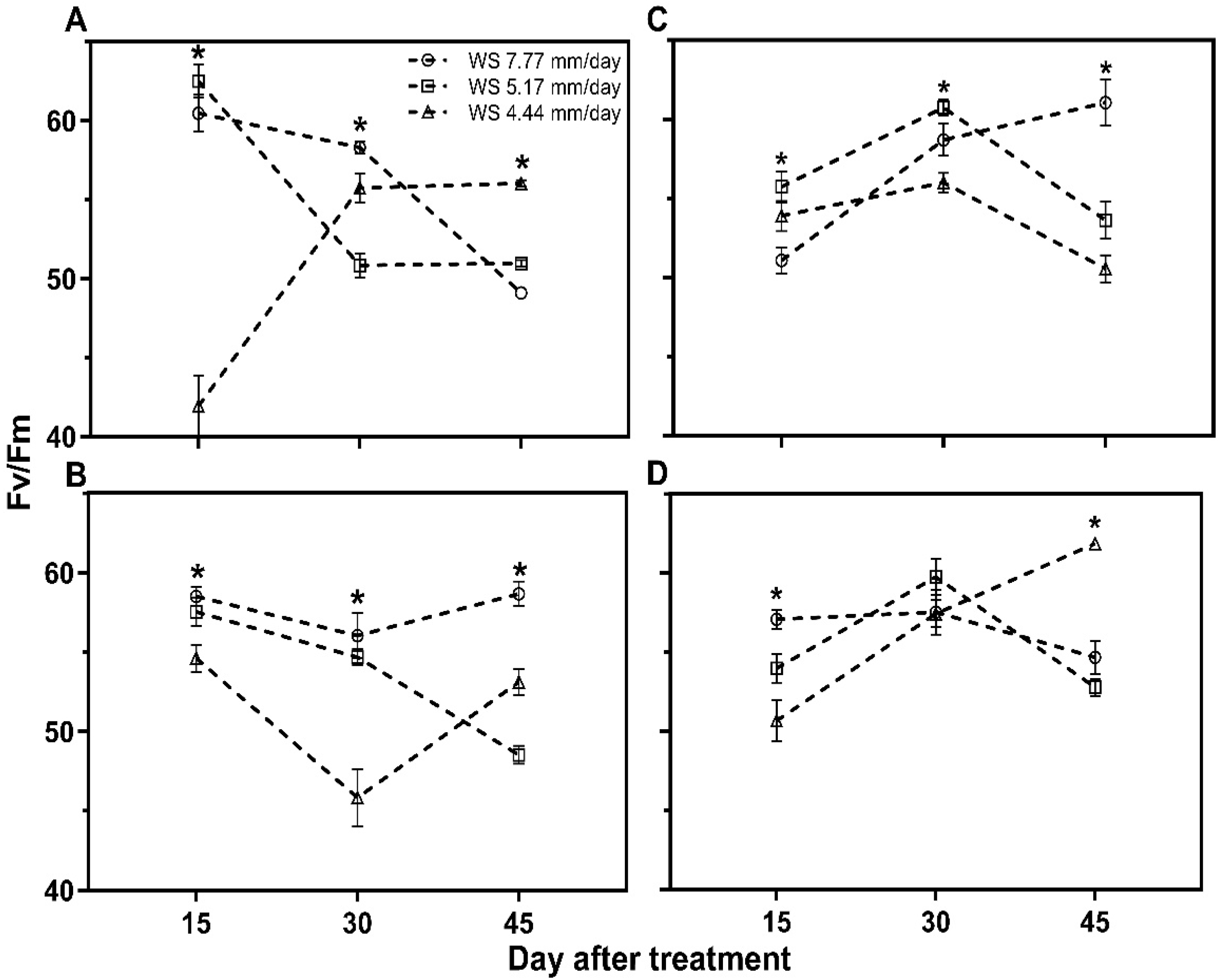
2.7.3. Maximum Quantum Efficiency of Photosystem II (Fv/Fm)
2.7.4. Chlorophyll Index
2.7.5. Fruit Yield (Kg Plant−1)
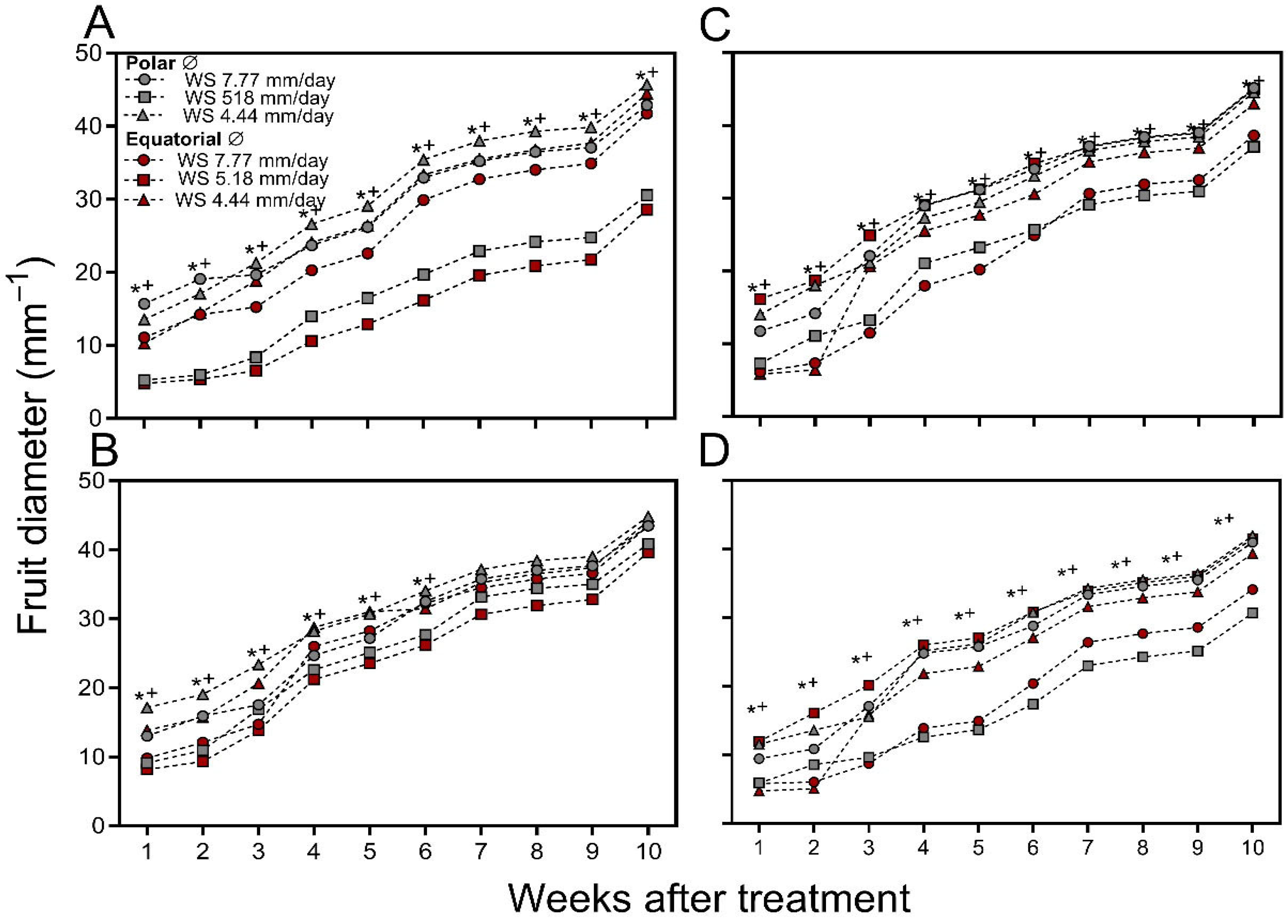
2.7.6. Polar and Equatorial Fruit Diameter (Mm)
2.7.7. Fruit Weight (G)
2.7.8. Pulp and Peel Weight
2.7.9. Juice Content (mL) and Number of Seeds
2.7.10. Total Soluble Solids (°Brix)
3. Results
Fruit Growth
4. Discussion
4.1. Stomatal Conductance (gₛ)
4.2. Leaf Water Potential (Ψh)
4.3. Chlorophyll Index
4.4. Quantum Efficiency of Photosystem II
4.5. Fruit Quality
4.6. Yield
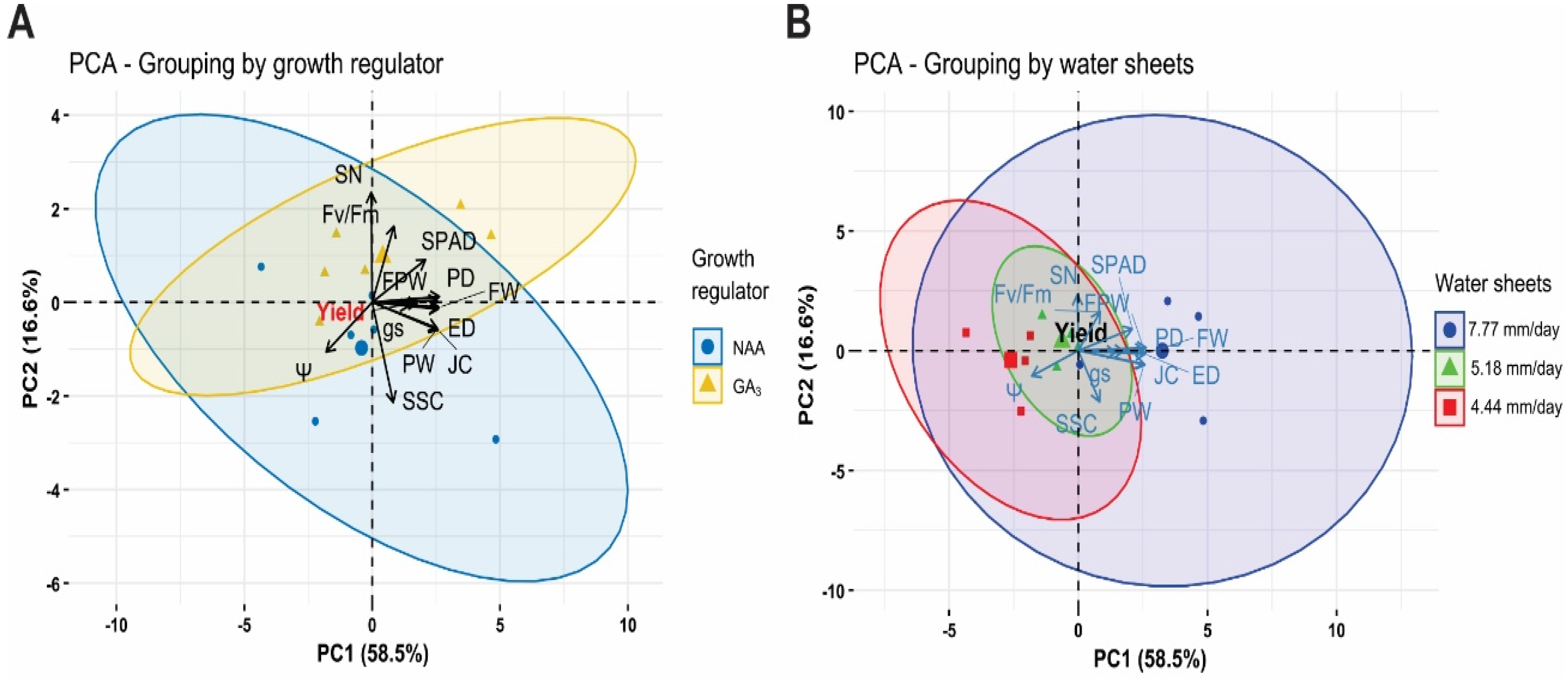
4.7. Principal Component Analysis (PCA)
5. Conclusions
6. Recommendation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asociación Citrícola del Noroeste Argentino (ACNOA). Europa Lidera la Producción Mundial de Limón. Available online: https://acnoa.com.ar/europa-lidera-la-produccion-mundial-de-limon/ (accessed on 19 February 2021).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56. Fao Rome 1998, 300, D05109. [Google Scholar]
- Instituto Nacional de Estadística y Censos (INEC), Ecuador. Encuesta de Superficie y Producción Agropecuaria Continua (ESPAC). Tabulados de ESPAC 2020. 2020. Available online: https://anda.inec.gob.ec/anda/index.php/catalog/912 (accessed on 18 June 2023).
- Valarezo, C.; Julca, A.; Rodríguez, A. Evaluación de la sustentabilidad de fincas productoras de limón en Portoviejo, Ecuador. Rev. Rivar 2020, 7, 108–120. [Google Scholar] [CrossRef]
- INEC (Instituto Nacional de Estadísticas y Censo), Quito, Ecuador. Información Ambiental Y Tecnificación Agropecuaria—Módulo Métodos de Producción y Ambiente. 2024. Available online: https://www.ecuadorencifras.gob.ec/informacion-agroambiental/ (accessed on 17 July 2025).
- Baradas, M. Crop requirements of tropical crops. In Handbook of Agricultural Meteorology; Griffiths, J.F., Ed.; Oxford University Press: New York, NY, USA, 1994; pp. 189–202. [Google Scholar]
- Pérez, R.; Cabrera, E.; Hinostroza, M. The Irrigation Regime for Crops in Manabí, Ecuador: Climatological Study. Rev. Cienc. Técnicas Agropecu. 2018, 27, 5–12. [Google Scholar]
- Speer, M.; Hartigan, J.; Leslie, L. Machine Learning Identification of Attributes and Predictors for a Flash Drought in Eastern Australia. Climate 2024, 12, 49. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–774. [Google Scholar] [CrossRef]
- García-Tejero, I.; Durán-Zuazo, V.; Jiménez-Bocanegra, J.; Muriel-Fernández, J. Improved water-use efficiency by deficit-irrigation programmes: Implications for saving water in citrus orchards. Rev. Sci Hort. 2011, 128, 274–282. [Google Scholar] [CrossRef]
- Robles, J.; García-García, J.; Navarro, J.; Botía, P.; Pérez-Pérez, J. Changes in Drip Irrigation Water Distribution Patterns Improve Fruit Quality and Economic Water Productivity in Early-Season Lemon Trees. Rev. Agron. 2023, 13, 15–19. [Google Scholar] [CrossRef]
- Wagner, Y.; Pozner, E.; Bar-On, P.; Ramon, U.; Raveh, E.; Neuhaus, E.; Cohen, S.; Grünzweig, J.; Klein, T. Rapid stomatal response in lemon saves trees and their fruit yields under summer desiccation, but fails under recurring droughts. Rev. Agric. Water. Manag. 2021, 307, 108487. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.; Abbas, A.; Parveen, A.; Atiq, M.; Alshaya, H.; et al. Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future Perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef]
- Manzoor, M.; Xu, Y.; Lv, Z.; Xu, J.; Wang, Y.; Sun, W.; Liu, X.; Wang, L.; Wang, J.; Liu, R.; et al. Fruit crop abiotic stress management: A comprehensive review of plant hormones mediated responses. Fruit Res. 2023, 3, 30. [Google Scholar] [CrossRef]
- Bisht, T.S.; Rawat, L.; Chakraborty, B.; Yadav, V. A recent advance in use of plant growth regulators (PGRs) in fruit crops -A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1307–1336. [Google Scholar] [CrossRef]
- Brady, C.J. Fruit ripening. Ann. Rev. Plant. Physiol. 1987, 38, 155–178. [Google Scholar] [CrossRef]
- Choudhary, H.; Jain, M.; Sharma, M.; Singh, B. Yield and quality attributes of Nagpur mandarin as affected by use of different plant growth regulators. Environ. Ecol. 2014, 32, 41–45. [Google Scholar]
- Viteri-Díaz, P.; Vásquez-Castillo, W.; Sangotuña, M.; Villota, A.; Caiza, K.; Viera, W. El ácido giberélico mejora el peso del racimo y el número de bayas de uva (Vitis vinifera L.), cv. Marroo Seedless, cultivado en los Valles interandinos del Ecuador. Sci. Agrop. 2020, 11, 591–598. [Google Scholar] [CrossRef]
- Trigueros, C.; Cabañero, J.J.; Tortosa, P.A.; Gambín, J.M.; Maestre, J.F.; Nicolas, E. Midlong term efects of saline reclaimed water irrigation and regulated defcit irrigation on fruit quality of citrus. J. Sci. Food. Agric. 2020, 100, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and salinity in citri culture: Optimal practices to alleviate salinity and water stress. Rev. Agron. 2021, 11, 1283. [Google Scholar] [CrossRef]
- Huo, L.; Xie, Q.; Sun, L.; Song, L.; Tao, S.; Liu, S.; Wang, Z.; Li, Y. Estimation of agricultural flood irrigation water consumption in the Heihe River Basin, China, using satellite-based daily land surface evapotranspiration and soil moisture. J. Hydrol. Reg. Stud. 2025, 60, 102524. [Google Scholar] [CrossRef]
- Jamshidi, S.; Zand-Parsa, S.; Kamgar-Haghighi, A.A.; Shahsavar, A.R.; Niyogi, D. Evapotranspiration, crop coefficients, and physiological responses of citrus trees in semi-arid climatic conditions. Agric. Water Manag. 2020, 227, 105838. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Ganadería, Quito, Ecuador. Precios. (MAG). 2018. Available online: https:////www.agricultura.gob.ec (accessed on 17 July 2025).
- Taylor, N.; Mahohoma, W.; Vahrmeijer, J.; Gush, M.B.; Allen, R.G.; Annandale, J.G. Crop coefficient approaches based on fixed estimates of leaf resistance are not appropriate for estimating water use of citrus. Irrig. Sci. 2015, 33, 153–166. [Google Scholar] [CrossRef]
- Schroeder, J.; Kwak, J.; Allen, G. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Rev. Nat. 2001, 410, 327–330. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.; Primo, A.; Forner-Giner, M. Screening of ‘king’ mandarin hybrids as tolerant citrus rootstocks to flooding stress. Rev. Hortic. 2021, 7, 388. [Google Scholar] [CrossRef]
- Celi, A.; Mejía, M.S.; Ríos, L. Gas exchange and fluorescence in ‘sutil’ lime (Citrus aurantifolia Swingle) under different soil moisture levels. Rev. Bioagro 2022, 34, 195–206. [Google Scholar] [CrossRef]
- Vélez, J.; Álvarez-Herrera, J.; Alvarado-Sanabria, O. El estrés hídrico en cítricos (Citrus spp.): Una revisión. Rev. Orinoquia. 2012, 16, 32–39. [Google Scholar] [CrossRef]
- Oliveros, M.; Caicedo, J. La conductancia estomática (gs), importancia, función y factores de influencia. Medición de la conductancia estomática (gs) a través del porómetro de difusión estable en diferentes cultivos. ResearchGate 2023. Available online: https://n9.cl/86u9p (accessed on 17 July 2025).
- Buckley, T. Modeling Stomatal Conductance. Plant. Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Rai, A.K.; Kumar, R.; Kumar, M.; Singh, M.; Raj, A.; Yadav, A.K. Abscisic acid-mediated physiological and molecular mechanisms for drought tolerance in plants. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef]
- Sezena, S.; Yazar, A.; Tekin, S. Physiological response of red pepper to different irrigation regimes under drip irrigation in the Mediterranean region of Turkey. Rev. Sci. Hortic. 2019, 245, 280–288. [Google Scholar] [CrossRef]
- Panigrahi, P.; Srivastava, A. Effective management of irrigation water in citrus orchards under a water scarce hot sub-humid region. Rev. Sci. Hortic. 2016, 210, 6–13. [Google Scholar] [CrossRef]
- Puente-Ramírez, J.; Rivera-Ortiz, P.; Silva-Espinosa, J.; Andrade-Limas, E. Quelato EDDHA para corregir la deficiencia de hierro en árboles de limón italiano (Citrus limon (L.) Osbeck). Terra Latinoam. 40, 2–6. [CrossRef]
- Gil-Marín, J.; Cordova-Rodriguez, M.; Montaño-Mata, N. Efectos de los regímenes de riego sobre el rendimiento y el uso del agua del calabacín (Cucurbita pepo L.) en condiciones de campo. Anal. Cient. 2021, 82, 237–250. [Google Scholar] [CrossRef]
- Bermejo, A.; Granero, B.; Mesejo, C.; Reig, C.; Tejedo, V.; Agustí, M.; Primo-Millo, E.; Iglesias, D. Auxin and gibberellin interact in citrus fruit set. J. Plant. Growth. Regul. 2018, 37, 491–501. [Google Scholar] [CrossRef]
- Talón, M.; Zacarías, L.; Primo-Millo, E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenoarpic ability. Physiol. Plant. 1990, 79, 400–406. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Reig, C.; Martinez-Fuentes, A.; Iglesias, D.; Muñoz-Frambuena, N.; Bermejo, A.; Germanà, M.; Primo-Millo, E.; Agustí, M. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant. Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef]
- Suman, M.; Sangama, P.; Maghawal, D.; Sahu, O. Effect of plant growth regulators on fruit crops. J. Pharma. Phytochem. 2017, 6, 331–337. [Google Scholar]
- Muhammad, N.; Ghulam, N. Role of Auxin in vegetative growth, flowering, yield and fruit quality of Horticultural crops-A review. Pure Appl. Biol. 2023, 12, 1234–1241. [Google Scholar] [CrossRef]
- Conesa, M.; de la Rosa, J.; Fernández-Trujillo, J.; Domingo, R.; Pérez-Pastor, A. Deficit irrigation in commercial mandarin trees: Water relations, yield and quality responses at harvest and after cold storage. Span. J. Agric. Res. 2018, 16, 15. [Google Scholar] [CrossRef]
- Bhati, A.; Kanwar, J.; Naruka, I.; Tiwari, R.; Gallani, R.; Singh, O. Effect of plant growth regulators and zinc on fruiting and yield parameters of acid lime (Citrus aurantifolia swingle) under Malwa plateau conditions. Bioscan 2016, 11, 2665–2668. [Google Scholar]
- Bazurto, F.; Celi, A.; Corozo, L.; Solís, L. Importancia del paclobutrazol en la producción de cítricos fuera de temporada. Rev. Manglar. 2022, 19, 117–127. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.; Murphy, A. Plant Physiology and Development, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2015; 731p. [Google Scholar]
- Fawole, O.A.; Opara, U.L. Seasonal variation in chemical composition, aroma volatiles and antioxidant capacity of pomegranate juice (Punica granatum) from South Africa. Bioresour. Technol. 2013, 132, 109–117. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-López, A. Chapter 14: Texture. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Duxford, UK, 2019; pp. 293–314. [Google Scholar] [CrossRef]
- Talat, H.; Shafqat, W.; Qureshi, M.A.; Sharif, N.; Raza, M.; ud Din, S.; Ikram, S.; Jaskani, M.J. Effect of gibberellic acid on fruit quality of Kinnow mandarin. J. Glob. Innov. Agri. Soc. Sci. 2020, 8, 59–63. [Google Scholar] [CrossRef]
- Elmenofy, H.; Kheder, A.; Mansour, A. Improvement of fruit quality and marketability of “Washington Navel” orange fruit by cytokinin and gibberellin. Egypt. J. Hortic. 2021, 48, 141–156. [Google Scholar] [CrossRef]
- El-Khayat, H. Yield and fruit quality of Washington Navel orange as influenced by preharvest application of giberellic, citric, ascorbic and salicylic acids. J. Agric. Res. Adv. 2020, 2, 9–23. [Google Scholar]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant. Growth. Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant. Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Palma, J.; Parra, H.; Orduño, N. Análisis del ácido giberélico desde la cartografía conceptual con enfoque bioético y sustentable. Acta Univ. 2022, 32, 1–18. [Google Scholar] [CrossRef]
- Yang, X.; Jansen, M.; Zhang, Q.; Sergeeva, L.; Ligterink, W.; Mariani, C.; Rieu, I.; Visser, E. A disturbed auxin signaling affects adventitious root outgrowth in Solanum dulcamara under complete submergence. J. Plant. Physiol. 2018, 224, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T. Role of plant growth regulators on vegetative growth, yield and quality of sweet orange (Citrus sinensis L.) cv. Sathgudi. Pharma Innov. J. 2021, 10, 1007. [Google Scholar]
- Hiteshbhai, R.; Johar, V.; Singh, V. Effect of plant growth regulators on fruit set and quality of kinnow mandarin (Citrus reticulata Blanco). Rev. Environ. Ecol. 2023, 41, 7–12. [Google Scholar]
- Pinto, M.; Rivera, A.; Zulueta, C.; Mena, F.; Gardiazabal, F.; Torres, J.; Tarride, P. Efecto de la aplicación de auxinas y citoquininas de síntesis al final de la caída fisiológica de frutos sobre el calibre y productividad de mandarinos cv. W. Murcott. Rev. Citric. Eurek. 2021, 2, 42–50. [Google Scholar]
- Tapia, C. Ácido Giberélico en la Producción de Cerezas: Bases Teóricas y Experiencias en Chile. Cereza Inteligente. Available online: https://www.smartcherry.cl/opinion-expertos/acido-giberelico-en-la-produccion-de-cerezas-bases-teoricas-y-experiencias-en-chile/ (accessed on 22 October 2021).
- Guerra, D.; Grajales, L.; Ríos, L. Efecto del riego y la fertilización sobre el rendimiento y la calidad de la fruta de lima ácida Tahití Citrus latifolia Tanaka (Rutaceae). Cienc. Tecnol. Agropecu. 2015, 16, 87–93. [Google Scholar] [CrossRef]
- Díaz, L.; Barrera, J.; Pinilla, C. Efecto del Ácido Giberélico Sobre el Crecimiento y Desarrollo del Fruto de Banano (Musa AAA), en Uraba. Temas Agrar. 2003, 8, 30–36. [Google Scholar] [CrossRef][Green Version]
- Ali, A.; Khan, A.S.; Malik, A.U.; Anwar, R. Role of exogenous auxin in improving fruit quality and yield in citrus. Sci. Hortic. 2020, 267, 109331. [Google Scholar] [CrossRef]
- Vamshi, T.; Rajan, R.; Reddy, G.B.; Singh, T.; Chundurwar, K.; Kumar, A.; Ramprasad, R.R. Effect of Plant Growth Regulators for Improvement of the Quality and Shelf Life of Kinnow (Citrus nobilis x Citrus deliciosa): A Review. Int. J. Environ. Clim. Change. 2023, 13, 1111–1126. [Google Scholar] [CrossRef]
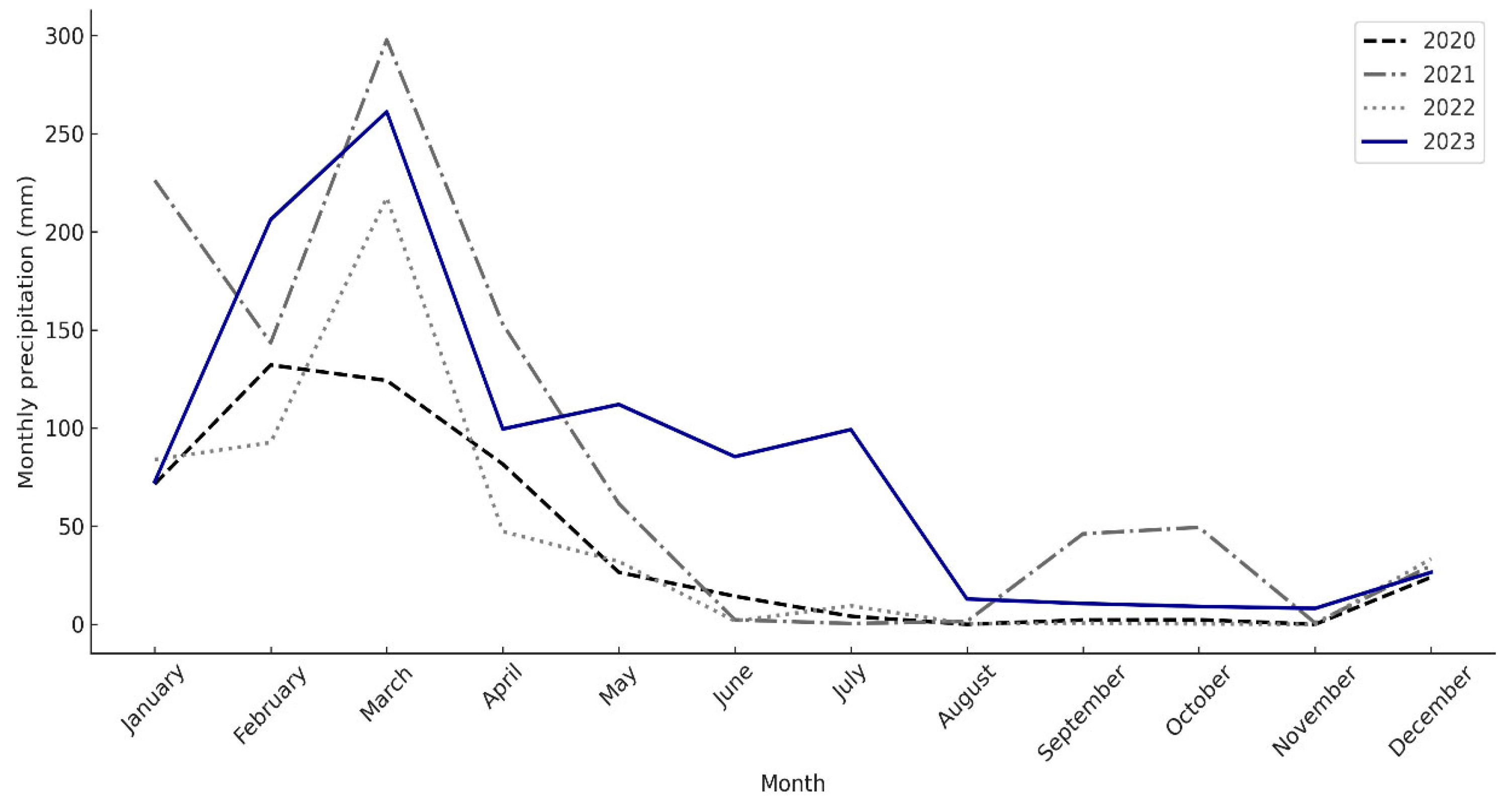

| E | F | M | Ab | M | Jn | Jl | Ag | S | O | N | D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.34 | 3.12 | 3.82 | 4.11 | 3.82 | 3.24 | 3.60 | 4.03 | 4.42 | 4.33 | 4.36 | 4.44 |
| Daily GID (mm/Days) | Gross Irrigation Depth (GID) Accumulated Every 15 Days of Application |
|---|---|
| 7.77 | 116.55 |
| 5.18 | 77.7 |
| 4.44 | 66.6 |
| Sheet Water | Yield Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit Weight (g−1) | Peel Weight (g−1) | Pulp Weight (g−1) | Juice Content (mL) | Soluble Solids % | Number of Seeds | |||||||
| NAA 100 mg L−1 | NAA 300 mg L−1 ns | NAA 100 mg L−1 | NAA 300 mg L−1 ns | NAA 100 mg L−1 | NAA 300 mg L−1 ns | NAA 100 mg L−1 | NAA 300 mg L−1 ns | NAA 100 mg L−1 ns | NAA 300 mg L−1 ns | NAA 100 mg L−1 ns | NAA 300 mg L−1 | |
| 7.77 mm | 50.53 ± 0.87 a | 42.27 ± 2.37 | 30.20 ± 0.31 a | 26.47 ± 27.60 | 21.80 ± 0.20 a | 16.60 ± 1.42 | 21.21 ± 0.94 a | 17.45 ± 0.83 | 7.99 ± 0.07 | 7.79 ± 0.10 | 5.37 + 0.07 | 5.30 ± 0.32 a |
| 5.17 mm | 41.53 ± 1.33 b | 43.93 ± 0.97 | 26.07 ± 0.87 b | 27.60 ± 0.53 | 15.53 ± 0.57 b | 16.60 ± 0.83 | 16.47 ± 0.58 b | 17.13 ± 0.18 | 7.84 ± 0.05 | 7.75 ± 0.02 | 5.77 ± 0.34 | 6.13 ± 0.15 ab |
| 4.44 mm | 37.60 ± 0.83 c | 41.00 ± 1.68 | 23.60 ± 0.69 c | 25.20 ± 0.92 | 14.30 ± 0.06 b | 15.80 ± 0.76 | 14.42 ± 0.12 c | 16.42 ± 0.88 | 7.64 ± 0.10 | 7.76 ± 0.00 | 6.33 ± 0.23 | 5. 00 ± 0.35 b |
| Sheet Water | Yield Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit Weight (g−1) | Peel Weight (g−1) | Pulp Weight (g−1) | Juice Content (mL) | Soluble Solids % | Number of Seeds | |||||||
| GA3 80 mg L−1 | GA3 200 mg L−1 | GA3 80 mg L−1 | GA3 200 mg L−1 | GA3 80 mg L−1 | GA3 200 mg L−1 | GA3 80 mg L−1 | GA3 200 mg L−1 | GA3 80 mg L−1 ns | GA3 200 mg L−1 ns | GA3 80 mg L−1 ns | GA3 200 mg L−1 ns | |
| 7.77 mm | 48.33 ± 2.02 a | 49.20 ± 1.01 a | 28.67 ± 0.37 a | 30.27 ± 0.64 a | 19.67 ± 1.65 a | 18.93 ± 0.37 a | 19.45 ± 1.05 a | 19.18 ± 0.30 a | 7.66 ± 0.11 | 7.60 ± 0.06 | 6.17 ± 0.24 | 6.40 ± 0.21 |
| 5.17 mm | 40.67 ± 0.07 b | 42.47 ± 0.82 b | 25.87 ± 0.24 b | 26.00 ± 0.23 b | 14.80 ± 0.20 b | 16.47 ± 0.59 b | 15.70 ± 0.15 b | 16.32 ± 0.66 b | 7.65 ± 0.05 | 7.79 ± 0.02 | 6.13 ± 0.12 | 6.37 ± 0.26 |
| 4.44 mm | 41.07 ± 0.18 b | 40.07 ± 1.05 b | 25.47 ± 0.33 b | 24.73 ± 0.71 c | 15.60 ± 0.50 b | 15.33 ± 0.37 b | 15.45 ± 0.46 b | 15.52 ± 0.38 b | 7.59 ± 0.02 | 7.74 ± 0.03 | 6.23 ± 0.24 | 5.77 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, A.C.; Sánchez, D.P.; Sánchez, L.P.; Zambrano, L.A.; Zambrano, Á.S.; Ponce, C.V.; García, G.C.; Rezabala, L.S.; Quiñónez, L.C.; Alcívar, F.A.; et al. Optimizing Citrus aurantifolia (Christm. Swingle) Production Through Integrated Irrigation and Growth Regulation Strategies. Agronomy 2025, 15, 1853. https://doi.org/10.3390/agronomy15081853
Soto AC, Sánchez DP, Sánchez LP, Zambrano LA, Zambrano ÁS, Ponce CV, García GC, Rezabala LS, Quiñónez LC, Alcívar FA, et al. Optimizing Citrus aurantifolia (Christm. Swingle) Production Through Integrated Irrigation and Growth Regulation Strategies. Agronomy. 2025; 15(8):1853. https://doi.org/10.3390/agronomy15081853
Chicago/Turabian StyleSoto, Adriana Celi, Diana Pincay Sánchez, Laura Pincay Sánchez, Luis Alcívar Zambrano, Ángel Sabando Zambrano, Cristhian Vega Ponce, George Cedeño García, Luis Saltos Rezabala, Liliana Corozo Quiñónez, Francisco Arteaga Alcívar, and et al. 2025. "Optimizing Citrus aurantifolia (Christm. Swingle) Production Through Integrated Irrigation and Growth Regulation Strategies" Agronomy 15, no. 8: 1853. https://doi.org/10.3390/agronomy15081853
APA StyleSoto, A. C., Sánchez, D. P., Sánchez, L. P., Zambrano, L. A., Zambrano, Á. S., Ponce, C. V., García, G. C., Rezabala, L. S., Quiñónez, L. C., Alcívar, F. A., Cuenca, E. C., Arellano, R. J., García, G. C., & Demera, M. D. (2025). Optimizing Citrus aurantifolia (Christm. Swingle) Production Through Integrated Irrigation and Growth Regulation Strategies. Agronomy, 15(8), 1853. https://doi.org/10.3390/agronomy15081853






