Postharvest 2,4-Epibrassinolide Treatment Delays Senescence and Increases Chilling Tolerance in Flat Peach
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Sampling
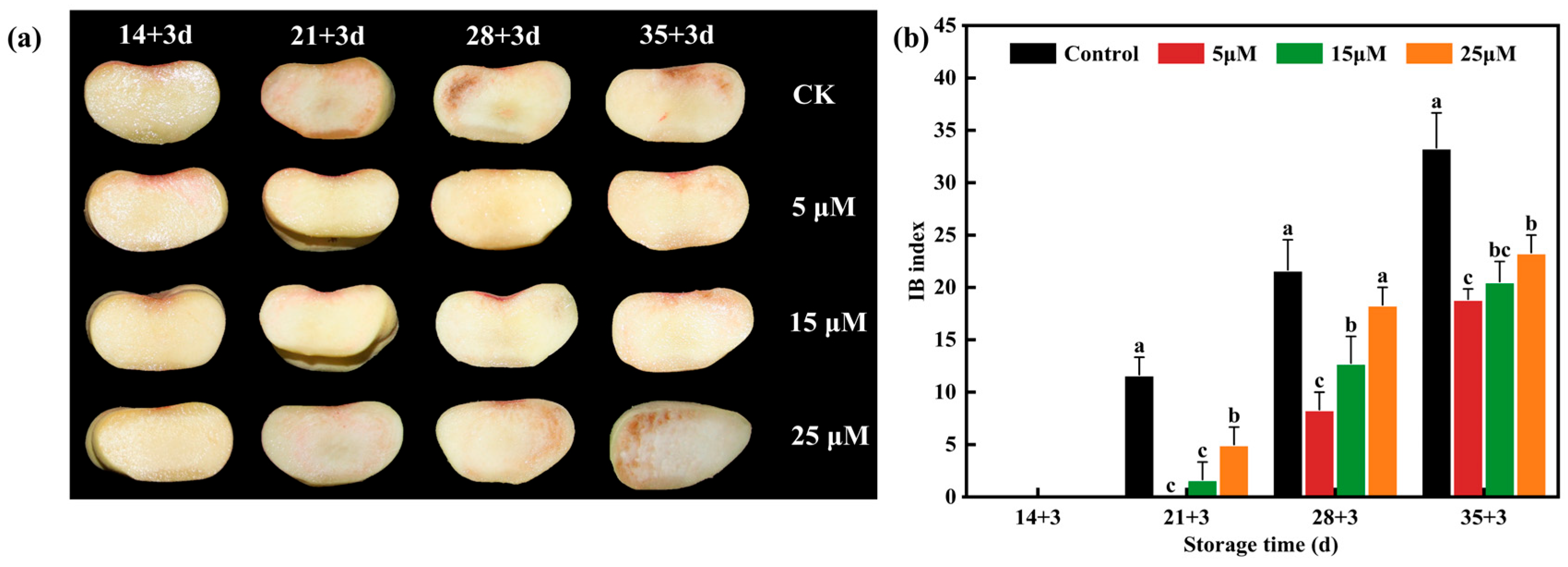
2.2. Measurement of Internal Browning Index
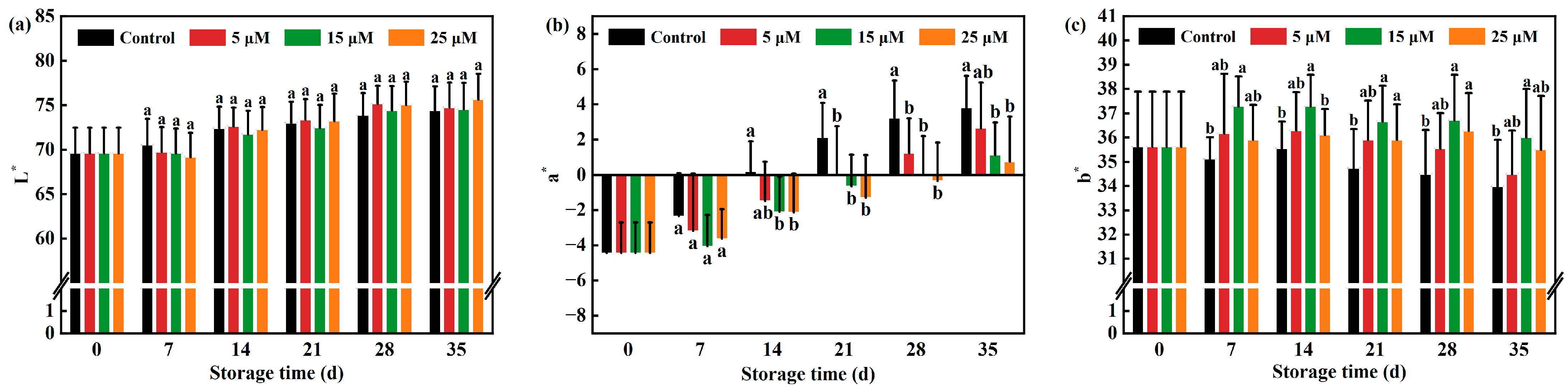
2.3. Measurement of L*, a*, b*
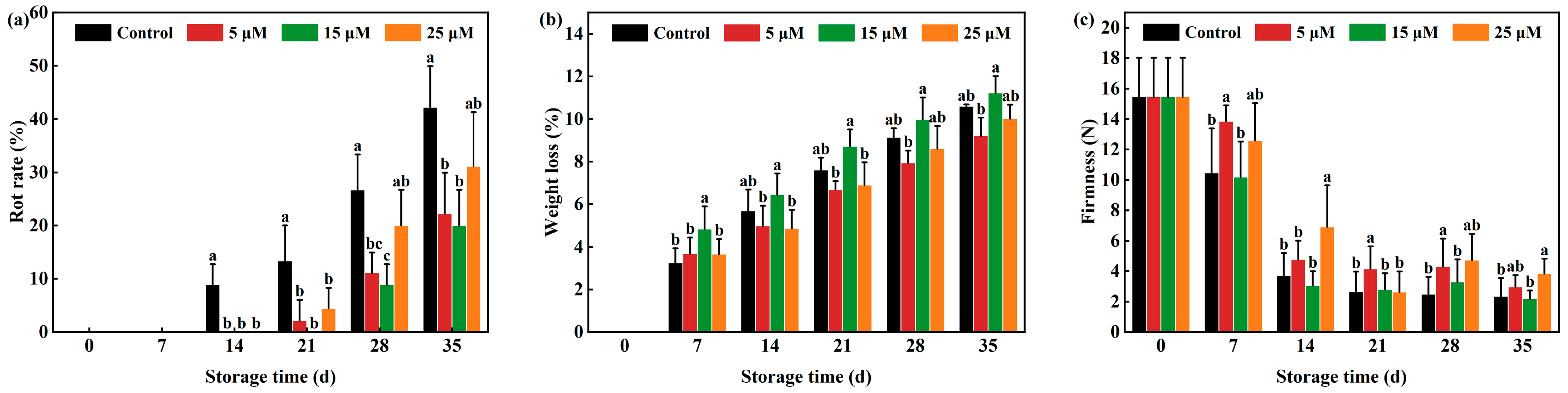
2.4. Measurement of Rot Rate, Weight Loss and Firmness
2.5. Measurement of Respiratory Rate and Ethylene Production
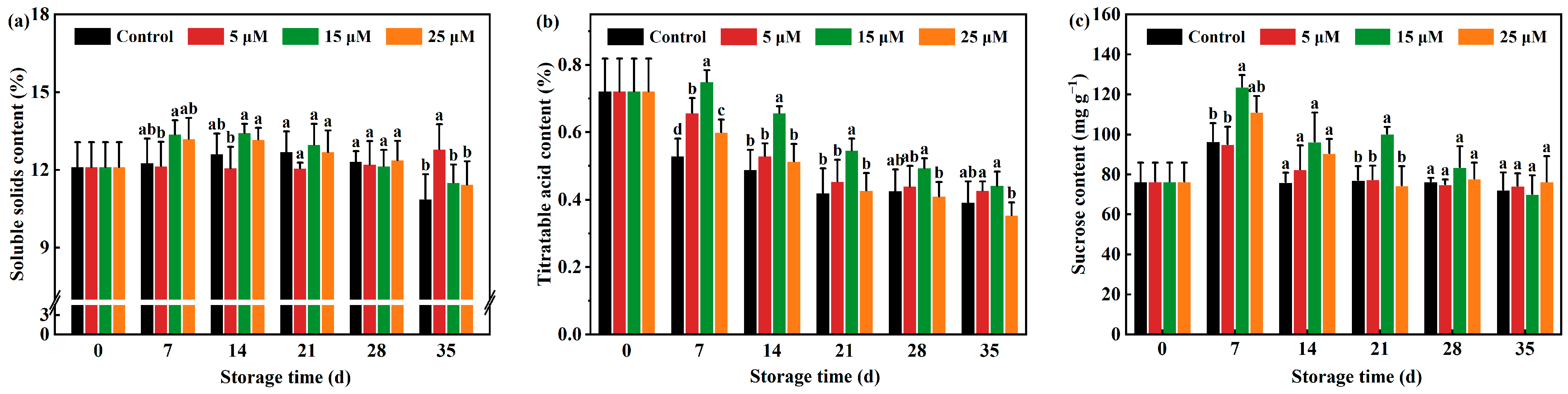
2.6. Measurement of TSS, Titratable Acid (TA) and Sucrose Content
2.7. Measurement of MDA Content and Total Phenol Content (TPC)
2.8. Measurement of O2− Production Rate and H2O2 Content
2.9. Measurement of Antioxidant Enzyme Activities
2.10. Measurement of PPO and PAL Activity
2.11. Statistical Analysis
3. Results
3.1. Effect of Different-Concentration EBR Treatments on Internal Browning Index
3.2. Effect of Different-Concentration EBR Treatments on L*, a*, b*
3.3. Effect of Different-Concentration EBR Treatments on Rot Rate, Weight Loss and Firmness
3.4. Effect of Different-Concentration EBR Treatments on TSS, Titratable Acid (TA) and Sucrose Content
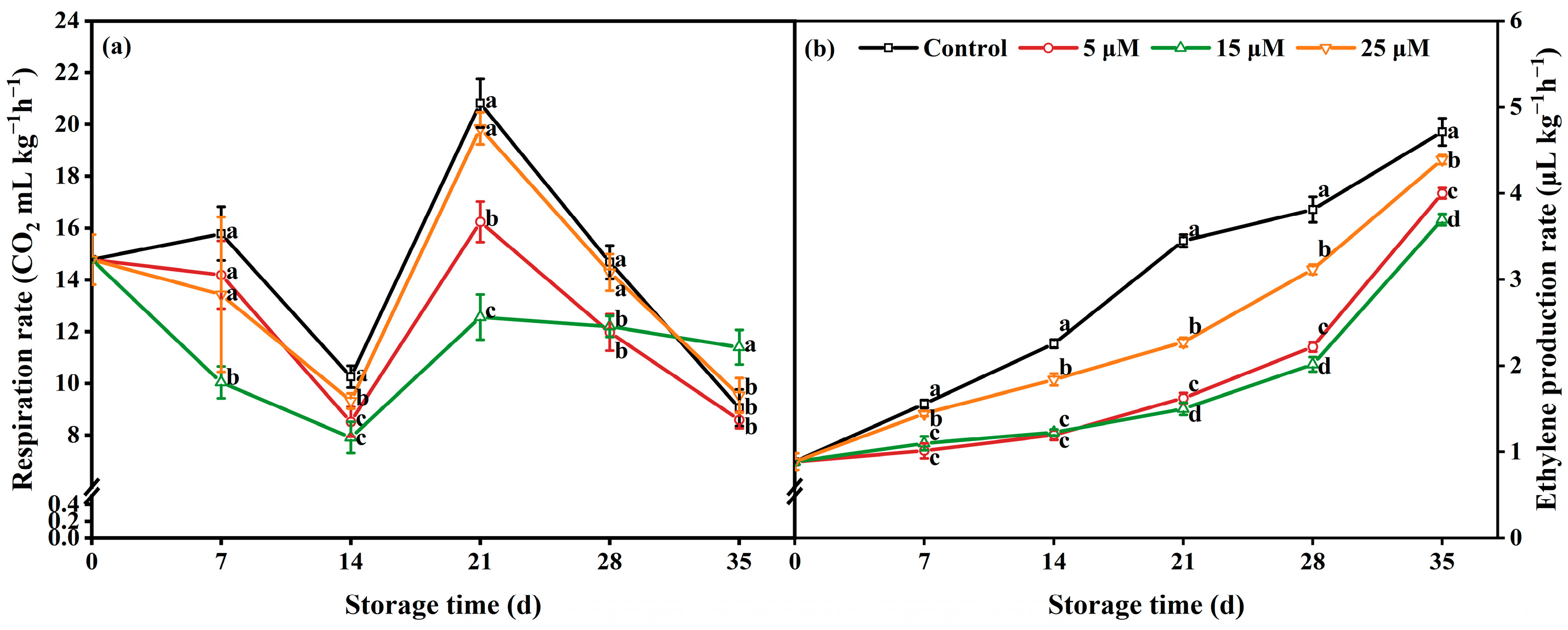
3.5. Effect of Different-Concentration EBR Treatments on Respiratory Rate and Ethylene Production
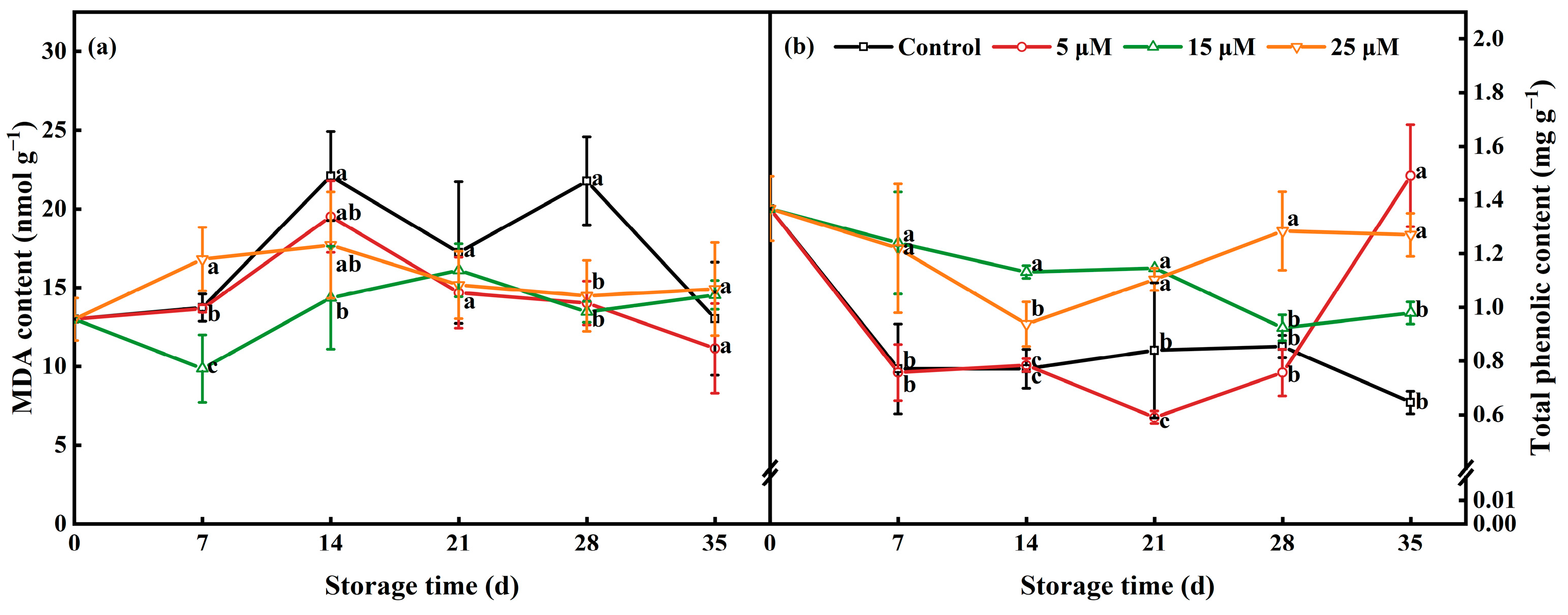
3.6. Effect of Different-Concentration EBR Treatments on MDA Content and TPC
3.7. Effect of Different-Concentration EBR Treatments on H2O2 Content and O2− Production Rate
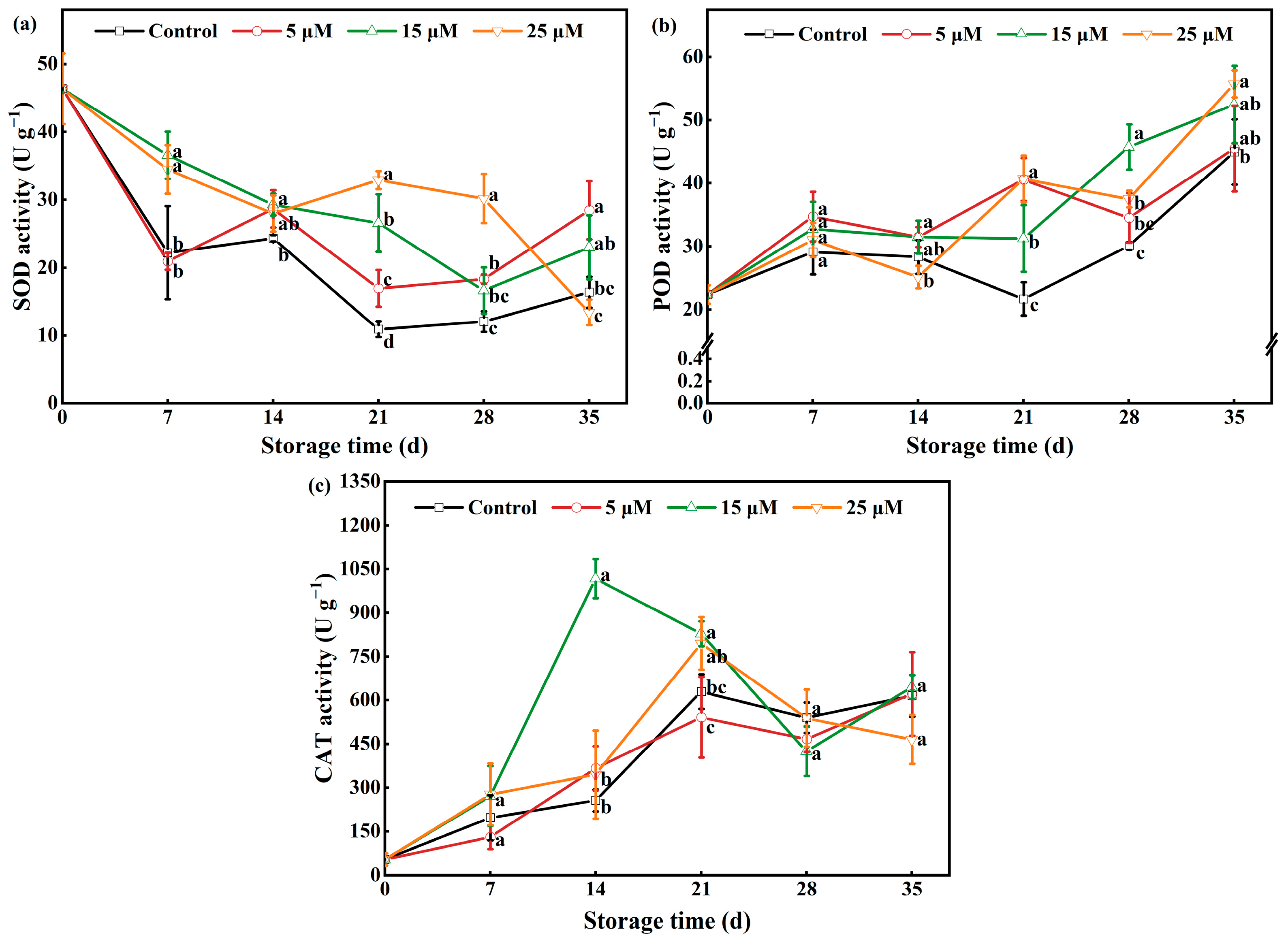
3.8. Effect of Different-Concentration EBR Treatments on the Activities of SOD, POD, and CAT
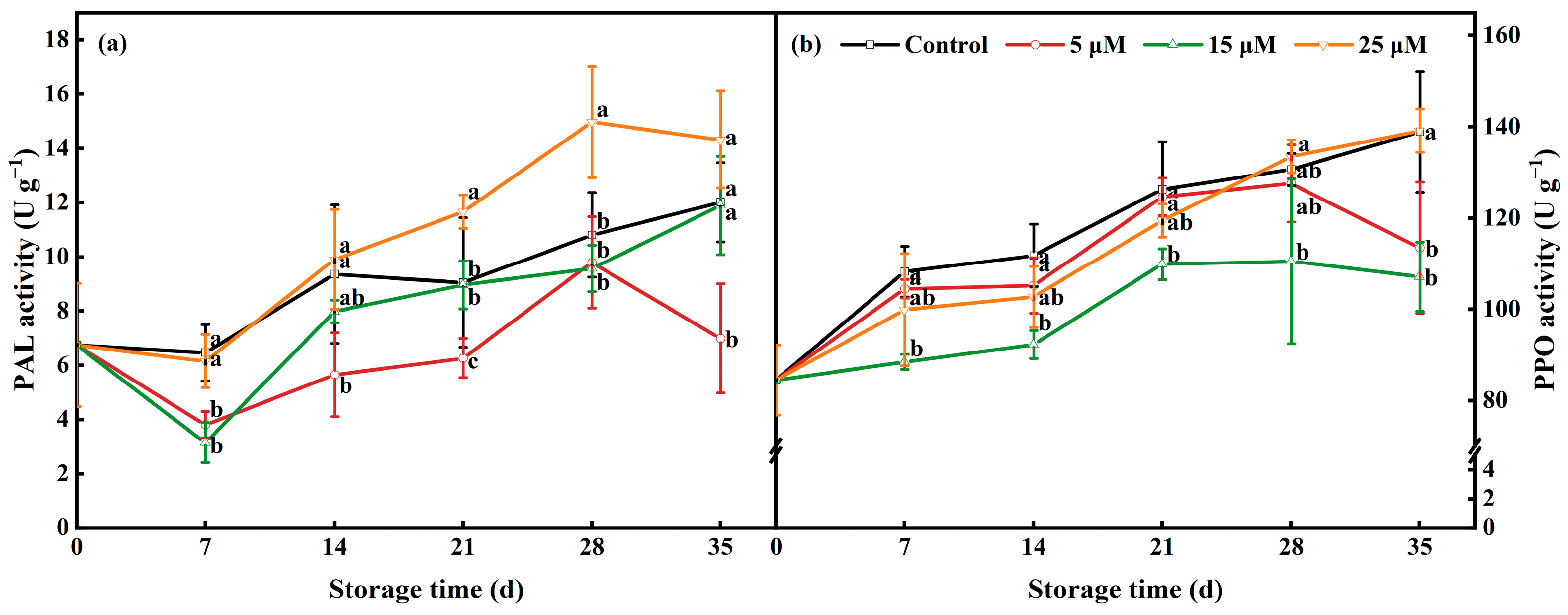
3.9. Effects of EBR Treatment on the Activities of PPO and PAL
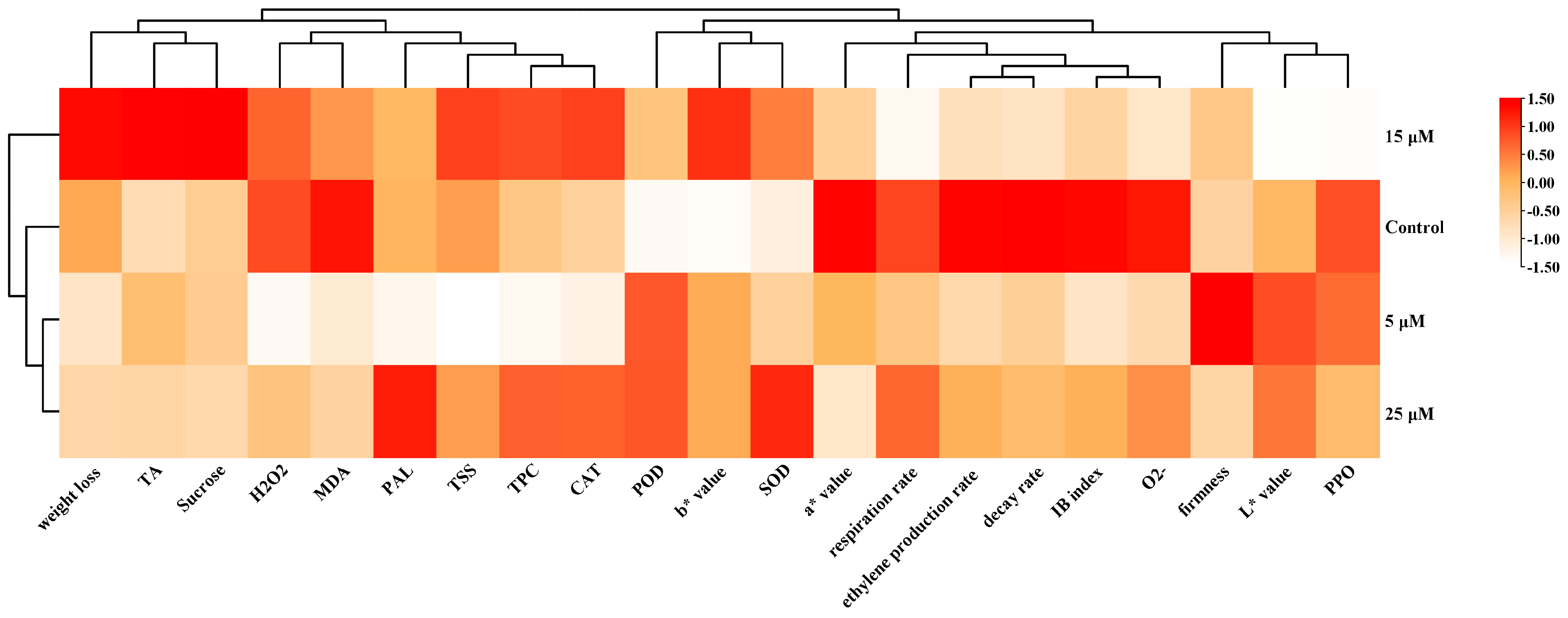
3.10. Cluster Heatmap-Based Analysis of EBR Treatment Effects on Flat Peach Fruit Quality and Cold Resistance Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of Organic Acid Accumulation and Its Related Genes in Peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Farcuh, M.; Hopfer, H. Aroma Volatiles as Predictors of Chilling Injury Development during Peach (Prunus Persica (L) Batsch) Cold Storage and Subsequent Shelf-Life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Fruk, G.; Cmelik, Z.; Jemric, T.; Hribar, J.; Vidrih, R. Pectin Role in Woolliness Development in Peaches and Nectarines: A Review. Sci. Hortic. 2014, 180, 1–5. [Google Scholar] [CrossRef]
- Lurie, S. Genomic and Transcriptomic Studies on Chilling Injury in Peach and Nectarine. Postharvest Biol. Technol. 2021, 174, 111444. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling Injury in Peach and Nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Javed, H.U.; Liu, Y.; Shi, P.; Mahreen, N.; Rastegar, S.; Hao, J.; Dai, Z.; You, G.; Ali, S. A Comprehensive Meta-Analysis Exploring Potential of GABA for Postharvest Chilling Injury Mitigation in Horticultural Produce. Sci. Hortic. 2024, 338, 113558. [Google Scholar] [CrossRef]
- Xie, P.; Yang, Y.; Gong, D.; Li, Y.; Wang, Y.; Li, Y.; Prusky, D.; Bi, Y. Spraying L-Phenylalanine during Fruit Development Alleviates Chilling Injury in Harvested Muskmelons by Regulating Membrane Lipid Metabolism. Postharvest Biol. Technol. 2024, 211, 112858. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, B.; Lin, Y.; Sang, Y.; Lin, M.; Fan, Z.; Chen, Y.; Wang, H.; Lin, H. Involvements of Membrane Lipid and Phenolic Metabolism in Reducing Browning and Chilling Injury of Cold-Stored Chinese Olive by γ-Aminobutyric Acid Treatment. Postharvest Biol. Technol. 2024, 209, 112664. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and Biochemical Mechanisms Regulating Chilling Tolerance in Fruits and Vegetables under Postharvest Salicylates and Jasmonates Treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Ngaffo Mekontso, F.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of Postharvest Chilling Injury of Carambola Fruit by γ-Aminobutyric Acid: Physiological, Biochemical, and Structural Characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Palou, L.; Gohari, G. Postharvest Chitosan-Arginine Nanoparticles Application Ameliorates Chilling Injury in Plum Fruit during Cold Storage by Enhancing ROS Scavenging System Activity. BMC Plant Biol. 2022, 22, 555. [Google Scholar] [CrossRef]
- Huang, T.; Liu, G.; Zhu, L.; Liu, J.; Xiang, Y.; Xu, X.; Zhang, Z. Mitigation of Chilling Injury in Mango Fruit by Methyl Jasmonate Is Associated with Regulation of Antioxidant Capacity and Energy Homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Shafi, M.; Liu, Y.; Wang, J.; Wu, J.; Wu, A. Hypobaric Treatment Effects on Chilling Injury, Mitochondrial Dysfunction, and the Ascorbate–Glutathione (AsA-GSH) Cycle in Postharvest Peach Fruit. J. Agric. Food Chem. 2016, 64, 4665–4674. [Google Scholar] [CrossRef]
- Zhao, Q.; Jin, M.; Guo, L.; Pei, H.; Nan, Y.; Rao, J. Modified Atmosphere Packaging and 1-Methylcyclopropene Alleviate Chilling Injury of ‘Youhou’ Sweet Persimmon during Cold Storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Appezzato-da-Glória, B.; Kluge, R.A. Evaluation of Storage Temperatures to Astringency ‘Giombo’ Persimmon: Storage at 1 °C Combined with 1-MCP Is Recommended to Alleviate Chilling Injury. Sci. Hortic. 2019, 257, 108675. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a Combination of Preharvest and Postharvest Treatments with Methyl Jasmonate Reduced Chilling Injury, by Maintaining Higher Unsaturated Fatty Acids, and Increased Aril Colour and Phenolics Content in Pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Islam, M.; Ali, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Shah, A.A.; Razzaq, K. Postharvest 24-Epibrassinolide Treatment Alleviates Pomegranate Fruit Chilling Injury by Regulating Proline Metabolism and Antioxidant Activities. Postharvest Biol. Technol. 2022, 188, 111906. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Shao, Z.; Meng, F.; Chen, H.; Wang, Q.; Zheng, J.; Liu, L. Brassinosteroid Biosynthetic Gene SlCYP90B3 Alleviates Chilling Injury of Tomato (Solanum Lycopersicum) Fruits during Cold Storage. Antioxidants 2022, 11, 115. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Gao, L.; Pang, J.; Yang, N. Effect of Brassinolide on Chilling Injury of Green Bell Pepper in Storage. Sci. Hortic. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Chen, G.; Hou, Y.; Zheng, Y.; Jin, P. 2,4-Epibrassinolide Enhance Chilling Tolerance of Loquat Fruit by Regulating Cell Wall and Membrane Fatty Acid Metabolism. Sci. Hortic. 2022, 295, 110813. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-Epibrassinolide on Chilling Injury of Peach Fruit in Relation to Phenolic and Proline Metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Kou, X.; Li, J.; Luo, D.; Huang, T.; Wang, X.; Cao, S. Salicylic Acid Mitigates Chilling Injury to Peaches by Improving Antioxidant Capacity and Energy Metabolism. Sci. Hortic. 2024, 338, 113841. [Google Scholar] [CrossRef]
- Algarni, E.H.A.; Elnaggar, I.A.; Abd El-wahed, A.E.N.; Taha, I.M.; AL-Jumayi, H.A.; Elhamamsy, S.M.; Mahmoud, S.F.; Fahmy, A. Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers 2022, 14, 2227. [Google Scholar] [CrossRef]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P.R.; Dixit, G.B. Total Phenolics, Flavonoids and Antioxidant Properties of Three Ceropegia Species from Western Ghats of India. South Afr. J. Bot. 2013, 88, 273–277. [Google Scholar] [CrossRef]
- Navarro, A.; Giménez, R.; Val, J.; Moreno, M.Á. The Impact of Rootstock on “Big Top” Nectarine Postharvest Concerning Chilling Injury, Biochemical and Molecular Parameters. Plants 2024, 13, 677. [Google Scholar] [CrossRef]
- Li, D.; Wu, X.; Li, L.; Wang, Y.; Xu, Y.; Luo, Z. Epibrassinolide Enhanced Chilling Tolerance of Postharvest Banana Fruit by Regulating Energy Status and Pyridine Nucleotide Homeostasis. Food Chem. 2022, 382, 132273. [Google Scholar] [CrossRef]
- Gutiérrez-Villamil, D.A.; Balaguera-López, H.E.; Álvarez-Herrera, J.G. Brassinosteroids Improve Postharvest Quality, Antioxidant Compounds, and Reduce Chilling Injury in ‘Arrayana’ Mandarin Fruits under Cold Storage. Horticulturae 2023, 9, 622. [Google Scholar] [CrossRef]
- Di, H.; Liu, R.; Zhang, Y.; Chen, Z.; Ma, J.; Escalona, V.H.; Liu, D.; Huang, H.; Huang, Z.; Tang, Y.; et al. Individual and Combined Treatments of 2, 4-Epibrassinolide (EBR) and Calcium Chloride (CaCl2) Maintain the Postharvest Quality of Baby Mustard. Postharvest Biol. Technol. 2024, 212, 112901. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food Processing Strategies to Enhance Phenolic Compounds Bioaccessibility and Bioavailability in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic Browning: The Role of Substrates in Polyphenol Oxidase Mediated Browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef]
- Moreno-Hernández, C.L.; Sáyago-Ayerdi, S.G.; García-Galindo, H.S.; Mata-Montes De Oca, M.; Montalvo-González, E. Effect of the Application of 1-Methylcyclopropene and Wax Emulsions on Proximate Analysis and Some Antioxidants of Soursop (Annona muricata L.). Sci. World J. 2014, 2014, 896853. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Yuan, Y.; Wang, Y.; Cheng, C.; Yang, S. PpWRKY65 Mediates Peach (Prunus Persica) Fruit Lignification in Both Ambient Temperature Storage and Chilling Injury Condition. Postharvest Biol. Technol. 2024, 216, 113043. [Google Scholar] [CrossRef]
- Fukasawa-Akada, T.; Kung, S.; Watson, J.C. Phenylalanine Ammonia-Lyase Gene Structure, Expression, and Evolution in Nicotiana. Plant Mol. Biol. 1996, 30, 711–722. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Leonardi, C.; Romano, D. PAL Activities in Asparagus Spears during Storage after Ammonium Sulfate Treatments. Postharvest Biol. Technol. 2018, 140, 34–41. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, J.; Guo, Z.; Chen, P.; Lei, F.; Lu, W.; Jiang, C.; Li, Y.; Li, M.; Zheng, Y. 2,4-Epibrassinolide Delays Lignification, Softening, and Quality Deterioration during Asparagus Storage. Postharvest Biol. Technol. 2025, 219, 113270. [Google Scholar] [CrossRef]
- Duan, W.; Ngaffo Mekontso, F.; Li, W.; Tian, J.; Li, J.; Wang, Q.; Xu, X. Alleviation of Postharvest Rib-Edge Darkening and Chilling Injury of Carambola Fruit by Brassinolide under Low Temperature Storage. Sci. Hortic. 2022, 299, 111015. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Sun, H.; Rang, X.; Ren, Y.; Zhang, T.; Zhao, Y.; Duan, Y. Postharvest 2,4-Epibrassinolide Treatment Delays Senescence and Increases Chilling Tolerance in Flat Peach. Agronomy 2025, 15, 1835. https://doi.org/10.3390/agronomy15081835
Xu B, Sun H, Rang X, Ren Y, Zhang T, Zhao Y, Duan Y. Postharvest 2,4-Epibrassinolide Treatment Delays Senescence and Increases Chilling Tolerance in Flat Peach. Agronomy. 2025; 15(8):1835. https://doi.org/10.3390/agronomy15081835
Chicago/Turabian StyleXu, Bin, Haixin Sun, Xuena Rang, Yanan Ren, Ting Zhang, Yaoyao Zhao, and Yuquan Duan. 2025. "Postharvest 2,4-Epibrassinolide Treatment Delays Senescence and Increases Chilling Tolerance in Flat Peach" Agronomy 15, no. 8: 1835. https://doi.org/10.3390/agronomy15081835
APA StyleXu, B., Sun, H., Rang, X., Ren, Y., Zhang, T., Zhao, Y., & Duan, Y. (2025). Postharvest 2,4-Epibrassinolide Treatment Delays Senescence and Increases Chilling Tolerance in Flat Peach. Agronomy, 15(8), 1835. https://doi.org/10.3390/agronomy15081835




