Effects of Pre-Emergence Application of Organic Acids on Seedling Establishment of Weeds and Crops in Controlled Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Treatment Concentrations
2.3. Growth Chamber Parameters and Experimental Design
2.3.1. Growth Chamber Parameters
2.3.2. Experimental Design for the Assessment of Radicle Emergence in Petri Dishes
2.3.3. Experimental Design for the Assessment of Shoot Emergence in Pots
2.4. Statistical Analyses
3. Results
3.1. Effect of the Organic Compounds on the Radicle Emergence Inhibition of the Three Crop and Four Weed Species in Petri Dishes
3.2. Effect of the Organic Compounds on the Shoot Emergence Inhibition of the Three Crop and Four Weed Species in Peat Pot Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| AA+EG | Acetic acid + eugenol and geraniol |
| CA | Citric acid |
| EU | European Union |
| IC50 | Inhibitory concentration at 50% |
| LA | Lactic acid |
| PA | Pelargonic acid |
References
- Vilà, M.; Beaury, E.; Blumenthal, D.; Bradley, B.; Early, R.; Laginhas, B.; Trillo, A.; Dukes, J.; Sorte, C.; Ibáñez, I. Understanding the combined impacts of weeds and climate change on crops. Environ. Res. Lett. 2021, 16, 034043. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The problem of weed infestation of agricultural plantations vs. the assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Parven, A.; Islam, M.D.; Kadiyala, V.; Mallavarapu, M. Herbicides in modern sustainable agriculture: Environmental fate, ecological implications, and human health concerns. Int. J. Environ. Sci. Technol. 2025, 22, 1181–1202. [Google Scholar] [CrossRef]
- Mohd Ghazi, R.; Nik Yusoff, N.R.; Abdul Halim, N.S.; Wahab, I.R.A.; Ab Latif, N.; Hasmoni, S.H.; Ahmad Zaini, M.A.; Zakaria, Z.A. Health effects of herbicides and its current removal strategies. Bioengineered 2023, 14, 2259526. [Google Scholar] [CrossRef]
- Pesticide Action Network (PAN) Europe. Pesticides: Play It Safe! 2023. Available online: https://www.pan-europe.info/sites/pan-europe.info/files/public/resources/reports/IPSOS%20poll%20Pesticides%20-%20Play%20it%20safe.pdf (accessed on 1 July 2025).
- Torres-Pagán, N.; Muñoz, M.; Barbero, S.; Mamone, R.; Peiró, R.; Carrubba, A.; Sánchez-Moreiras, A.M.; Gómez de Barreda, D.; Verdeguer, M. Herbicidal potential of the natural compounds carvacrol, thymol, eugenol, p-cymene, citral and pelargonic acid in field conditions: Indications for better performance. Agronomy 2024, 14, 537. [Google Scholar] [CrossRef]
- Bratovcic, A. Exploring non-chemical methods for sustainable weed management. IJAER 2025, 11, 129–153. [Google Scholar] [CrossRef]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and alternatives of herbicide-based weed management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Duke, S.; Twitty, A.; Baker, C.; Sands, D.; Boddy, L.; Travaini, M.; Sosa, G.; Polidore, A.; Jhala, A.; Kloeber, J.; et al. New approaches to herbicide and bioherbicide discovery. Weed Sci. 2024, 72, 1–21. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, M.; Sehrawat, N.; Atri, N.; Singh, R.; Upadhyay, S.K.; Yadav, M. Mycoherbicide control strategy: Concept, constraints, and advancements. Biopestic. Int. 2021, 17, 29–40. [Google Scholar]
- deNux, C.; Hou, A.; Fultz, L. Evaluation of organic and synthetic herbicide applications on weed suppression in a conventional cropping system in Louisiana. Sustainability 2024, 16, 3019. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Karim, S.M.R.; Kheya, S.A.; Yeasmin, S. Unlocking the potential of bioherbicides for sustainable and environment friendly weed management. Heliyon 2024, 10, e36088. [Google Scholar] [CrossRef]
- Brestetskiy, A. Modern approaches for the development of new herbicides based on natural compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef]
- European commission (EC). Regulation No 1107/2009. Active Substance: Pelargonic Acid (CAS 112-05-0). Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances/details/959 (accessed on 28 June 2025).
- Abouziena, H.; Omar, A.; Sharma, S.; Singh, M. Efficacy comparison of some new natural-product herbicides for weed control at two growth stages. Weed Technol. 2009, 23, 431–437. [Google Scholar] [CrossRef]
- Andert, S.; Gerowitt, B. Controlling Arable Weeds with Natural Substances as Bio-Based Herbicides. 2020. Available from CABI Databases. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20203252218 (accessed on 16 June 2025).
- Ippolito, S.J.; Jennings, K.M.; Monks, D.W.; Chaudhari, S.; Jordan, D.; Moore, L.D.; Blankenship, C.D. Response of stevia to reduced-risk synthetic and nonsynthetic herbicides applied post-transplant. Weed Technol. 2024, 38, 1–5. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Shrefler, J.W.; Spaunhorst, D.J. Impact of acetic acid concentration, application volume, and adjuvants on weed control efficacy. J. Agric. Sci. 2018, 10, 1. [Google Scholar] [CrossRef]
- Pannacci, E.; Ottavini, D.; Onofri, A.; Tei, F. Dose–response curves of pelargonic acid against summer and winter weeds in central Italy. Agronomy 2022, 12, 3229. [Google Scholar] [CrossRef]
- Johnson, E.N.; Wolf, T.M.; Caldwell, B.C. Vinegar for pre-seed and post-emergence control of broadleaf weeds in spring wheat (Triticum aestivum L.). In Proceedings of the 2003 National Meeting, Canadian Weed Science Society 57th Annual Meeting, Halifax, NS, Canada, 27 November–3 December 2003; Volume 57, p. 87. [Google Scholar]
- Kanatas, P.; Travlos, I.; Papastylianou, P.; Gazoulis, I.; Kakabouki, I.; Tsekoura, A. Yield, quality and weed control in soybean crop as affected by several cultural and weed management practices. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 329–341. [Google Scholar] [CrossRef]
- WeedScience. International Herbicide-Resistant Weed Database. 2025. Available online: https://www.weedscience.org/Home.aspx (accessed on 8 April 2025).
- Loddo, D.; Jagarapu, K.K.; Strati, E.; Trespidi, G.; Nikolić, N.; Masin, R.; Berti, A.; Otto, S. Assessing herbicide efficacy of pelargonic acid on several weed species. Agronomy 2023, 13, 1511. [Google Scholar] [CrossRef]
- Evans, G.J.; Bellinder, R.R. The potential use of vinegar and a clove oil herbicide for weed control in sweet corn, potato, and onion. Weed Technol. 2009, 23, 120–128. [Google Scholar] [CrossRef]
- Great Life. 2025. Available online: https://great-life.eu/wp-content/uploads/2022/02/GREATLIFE-CULTIVATION-GUIDELINES_EN.pdf (accessed on 4 July 2025).
- Processors and Growers Research Organization (PGRO). Alfalfa Value Chain at a Regional Scale (Province of Bologna, Italy). 2025. Available online: https://www.pgro.org/alfalfa-value-chain-at-a-regional-scale/#:~:text=The%20cultivation%20of%20alfalfa%20is,the%20Parmigiano%2DReggiano%20production%20area (accessed on 4 July 2025).
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Effects of Senna garrettiana and Identification of phytotoxic substances for the development of bioherbicides. Agriculture 2022, 12, 1338. [Google Scholar] [CrossRef]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The herbicidal potential of different pelargonic acid products and essential oils against several important weed species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Win, P.-P.; Park, H.-H.; Kuk, Y.-I. Control efficacy of natural products on broadleaf and grass weeds using various application methods. Agronomy 2023, 13, 2262. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez-Moreiras, A.M.; Verdeguer, M. Phytotoxic effects of three natural compounds: Pelargonic acid, carvacrol, and cinnamic aldehyde, against problematic weeds in Mediterranean crops. Agronomy 2020, 10, 791. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Jouini, A.; Araniti, F.; Sánchez-Moreiras, A.M.; Verdeguer, M. Control of problematic weeds in Mediterranean vineyards with the bioherbicide pelargonic acid. Agronomy 2022, 12, 2476. [Google Scholar] [CrossRef]
- Appleby, A.; Reganold, J.; Carpenter-Boggs, L. Efficacy and cost of four plant-derived, natural herbicides for certified organic agriculture. Pest Manag. Sci. 2025, 81, 3391–3402. [Google Scholar] [CrossRef]
- Charbonneau, P. What Smells Like Sour Milk and Controls Leguminous Weeds? 2010. Available online: https://archive.lib.msu.edu/tic/stnew/article/2010aut21.pdf (accessed on 11 April 2025).
- Sumalapao, D.E.; Tuppil, C.; Urtula, A.; Valdestamon, D.; Villanueva, L.; Ledesma, N. Tolerance of mung bean (Vigna radiata L. Wilczek) to lactic acid, a potential herbicide: Growth and morphology. J. Anim. Plant Sci. 2018, 28, 138–145. [Google Scholar]
- Qasem, J.R. Herbicide resistant weeds: The technology and weed management. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: London, UK, 2013; pp. 445–471. [Google Scholar]
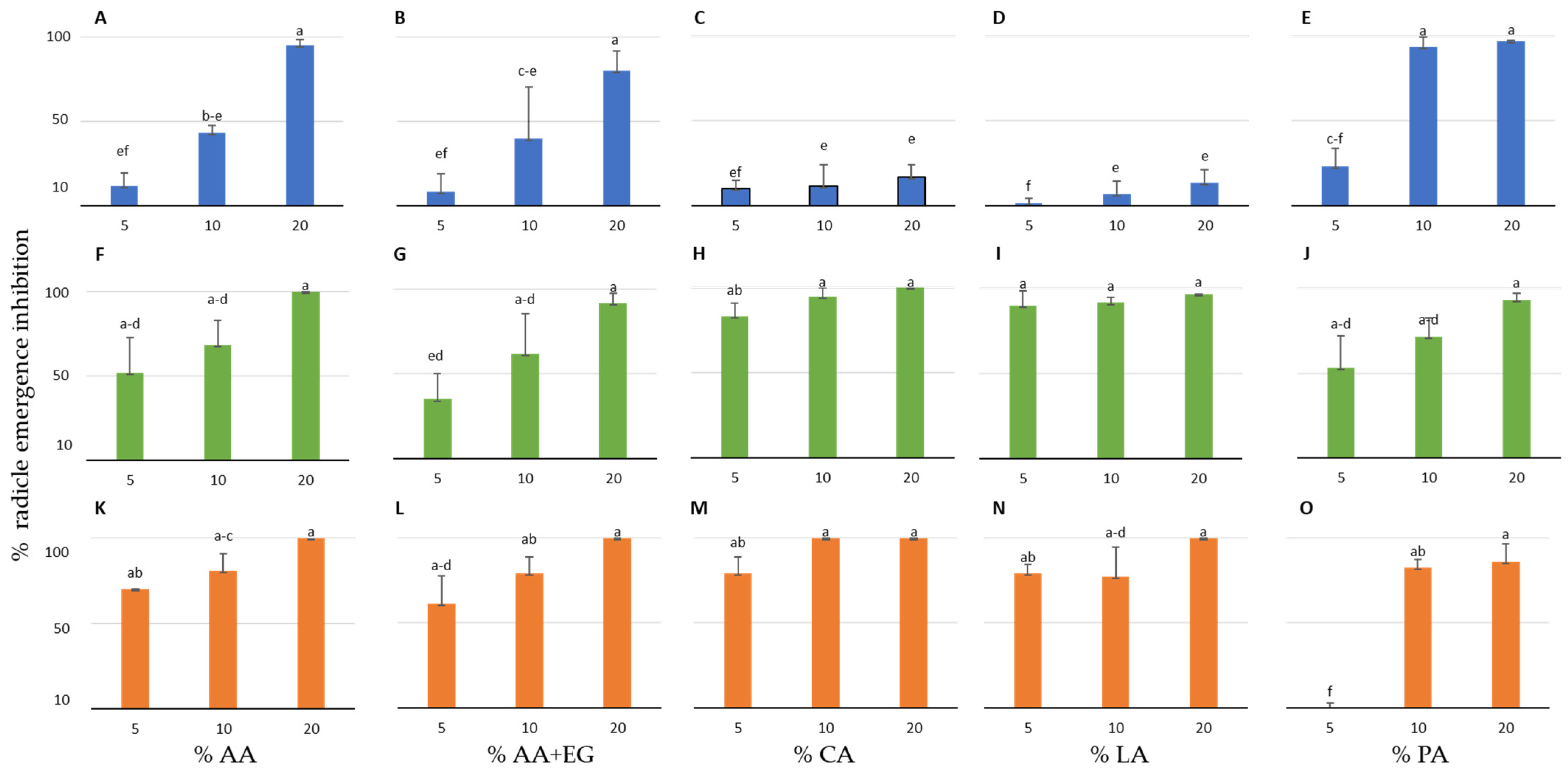
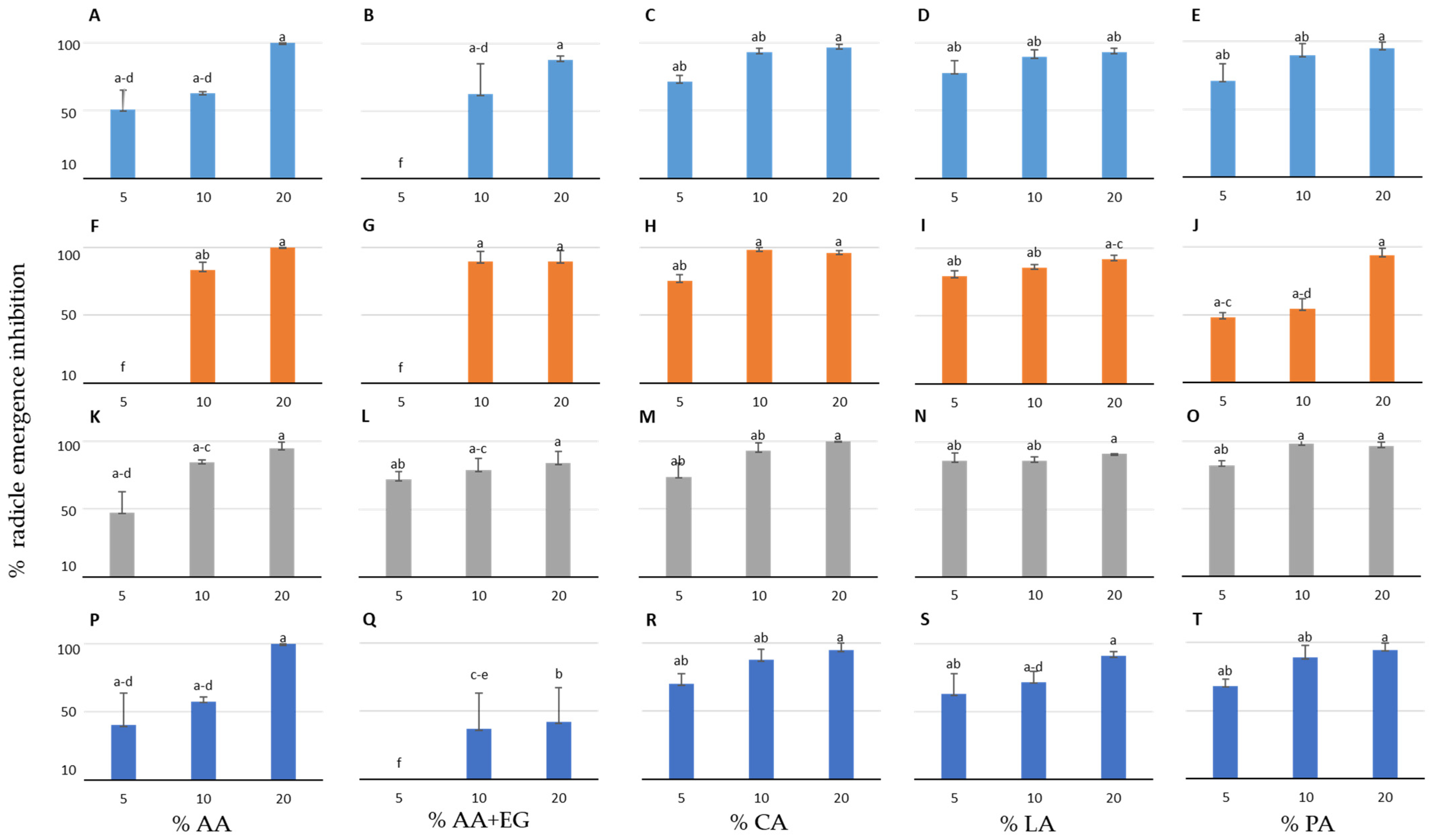
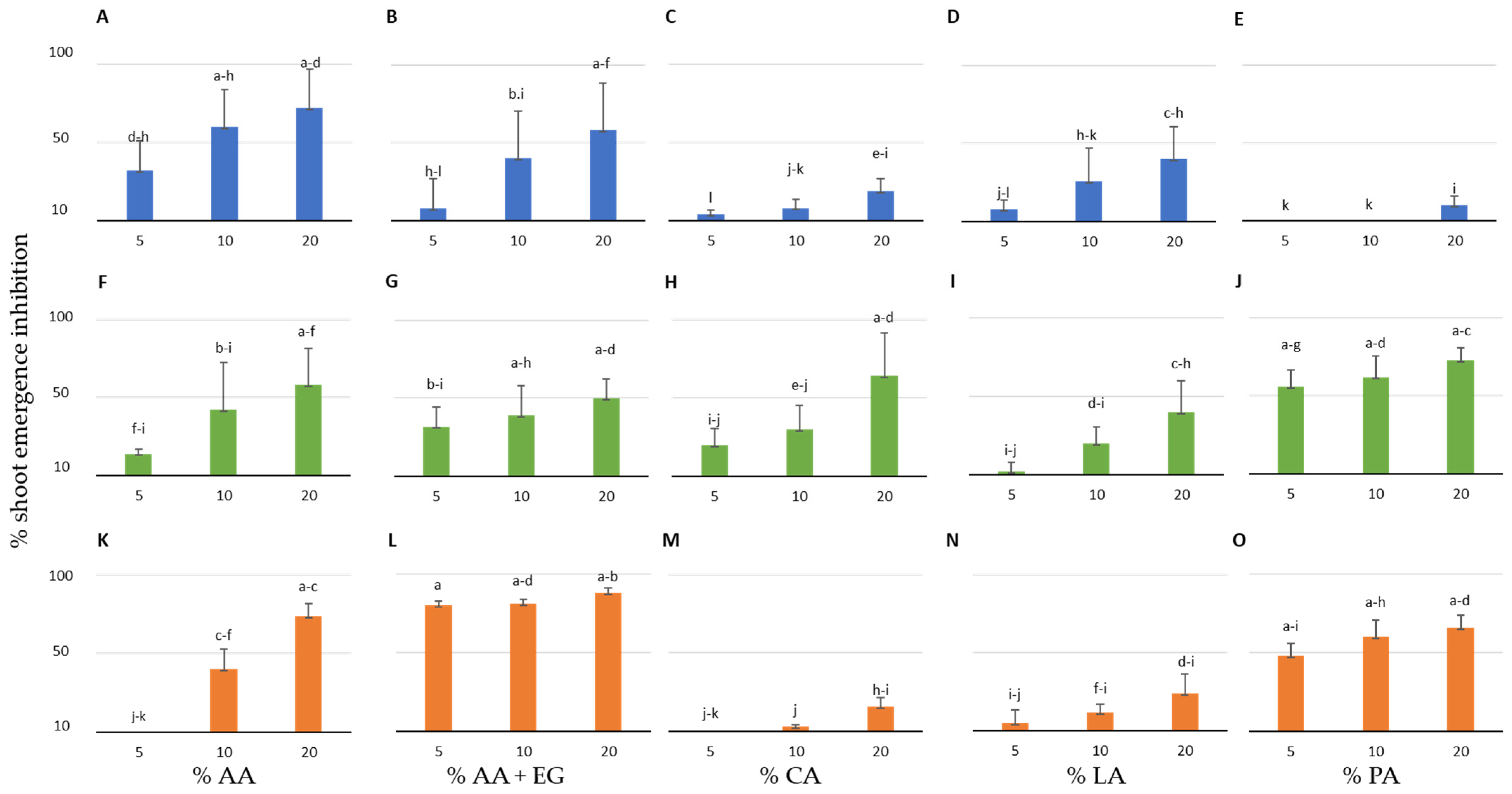
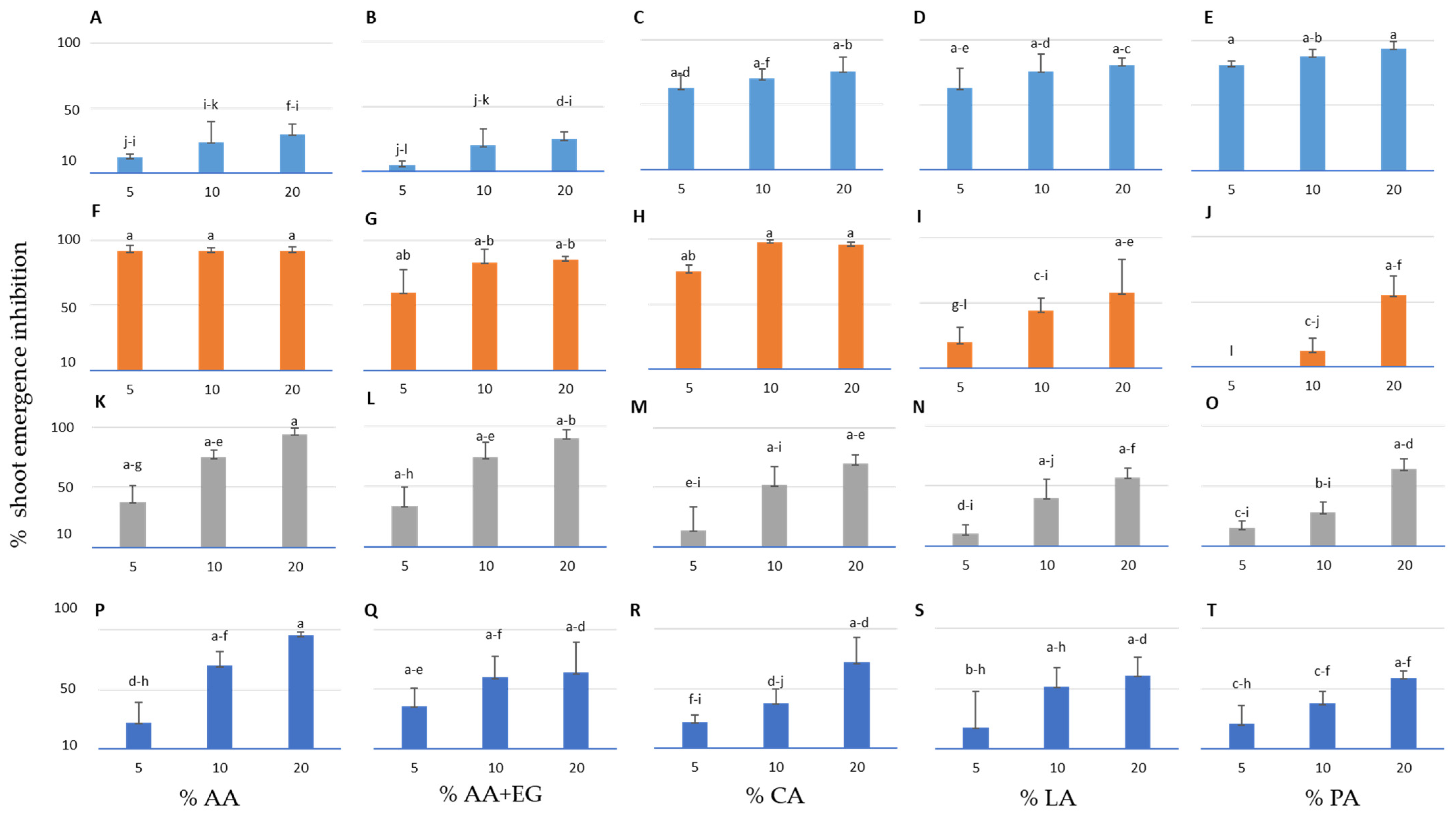
| Plant Species | IC50 AA (%) | IC50 AA+EG (%) | IC50 CA (%) | IC50 LA (%) | IC50 PA (%) |
|---|---|---|---|---|---|
| Common vetch | 8.08 bc | 8.4 c | 3.6 c | 3.8 c | 8.3 bc |
| Perennial ryegrass | 6.6 c | 12.2 b | 3.6 c | 3.6 c | 3.8 c |
| Green foxtail | 5.1 c | 4.1 c | 3.6 c | 3.34 c | 3.2 c |
| Common vetch | 8.00 bc | 22.5 b | 3.9 c | 5.0 bc | 3.9 c |
| Alfalfa | 4.5 c | 4.8 c | 3.4 c | 4.2 c | 10.3 bc |
| Millet | 6.2 c | 8.3 c | 3.3 c | 3.1 c | 7.2 bc |
| Soft wheat | 11.5 bc | 13.2 a | 71.8 a | 71.8 a | 4.1 c |
| Plant Species | IC50 AA (%) | IC50 AA+EG (%) | IC50 CA (%) | IC50 LA (%) | IC50 PA (%) |
|---|---|---|---|---|---|
| Common vetch | 3.7 f | 14.4 d–f | 19.3 d–f | 4.6 f | 13.1 d–f |
| Perennial ryegrass | 35.9 c–e | 38.1 cd | 5.1 f | 4.7 f | 3.8 f |
| Green foxtail | 5.1 f | 4.1 f | 3.4 f | 3.4 f | 3.2 f |
| Common vetch | 8.3 f | 7.6 f | 14.3 d–f | 8.8 f | 14.3 d–f |
| Alfalfa | 16.1 d–f | 14.9 d–f | 57.8 b | 44.1 c | 19.1 d–f |
| Millet | 6.4 f | 15.5 d–f | 12.8 d–f | 6.9 f | 11.5 ef |
| Soft wheat | 10.1 ef | 15.8 d–f | 62.5 b | 35.9 c–e | 89.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpi, M.; Whittaker, A.; Frassineti, E.; Toschi, E.; Dinelli, G.; Marotti, I. Effects of Pre-Emergence Application of Organic Acids on Seedling Establishment of Weeds and Crops in Controlled Environments. Agronomy 2025, 15, 1820. https://doi.org/10.3390/agronomy15081820
Alpi M, Whittaker A, Frassineti E, Toschi E, Dinelli G, Marotti I. Effects of Pre-Emergence Application of Organic Acids on Seedling Establishment of Weeds and Crops in Controlled Environments. Agronomy. 2025; 15(8):1820. https://doi.org/10.3390/agronomy15081820
Chicago/Turabian StyleAlpi, Mattia, Anne Whittaker, Elettra Frassineti, Enrico Toschi, Giovanni Dinelli, and Ilaria Marotti. 2025. "Effects of Pre-Emergence Application of Organic Acids on Seedling Establishment of Weeds and Crops in Controlled Environments" Agronomy 15, no. 8: 1820. https://doi.org/10.3390/agronomy15081820
APA StyleAlpi, M., Whittaker, A., Frassineti, E., Toschi, E., Dinelli, G., & Marotti, I. (2025). Effects of Pre-Emergence Application of Organic Acids on Seedling Establishment of Weeds and Crops in Controlled Environments. Agronomy, 15(8), 1820. https://doi.org/10.3390/agronomy15081820










