Ozone Stress During Rice Growth Impedes Grain-Filling Capacity of Inferior Spikelets but Not That of Superior Spikelets
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Ozone Treatment
2.3. Sampling and Analyses
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Superior spikelets |
| IS | Inferior spikelets |
| RVA | Rapid visco analyzer |
References
- Gaudel, A.; Bourgeois, I.; Li, M.; Chang, K.-L.; Ziemke, J.; Sauvage, B.; Stauffer, R.M.; Thompson, A.M.; Kollonige, D.E.; Smith, N.; et al. Tropical tropospheric ozone distribution and trends from in situ and satellite data. Atmos. Chem. Phys. 2024, 24, 9975–10000. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Palmer, P.I.; Zhang, L.; Lu, K.; Li, K.; Nagashima, T.; Koo, J.-H.; Tanimoto, H.; Wang, H.; et al. Deciphering decadal urban ozone trends from historical records since 1980. Natl. Sci. Rev. 2024, 11, nwae369. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos. Environ. 2009, 43, 1510–1519. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Buse, A.; Gimeno, B.; Bermejo, V.; Holland, M.; Emberson, L.; Pleijel, H. A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos. Environ. 2007, 41, 2630–2643. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, Y.; Kobayashi, K.; Dai, L.; Zhang, T.; Agathokleous, E.; Calatayud, V.; Paoletti, E.; Mukherjee, A.; Agrawal, M.; et al. Ozone pollution threatens the production of major staple crops in East Asia. Nat. Food 2022, 3, 47–56. [Google Scholar] [CrossRef]
- Li, S.; Leakey, A.D.B.; Moller, C.A.; Montes, C.M.; Sacks, E.J.; Lee, D.; Ainsworth, E.A. Similar photosynthetic but different yield responses of C3 and C4 crops to elevated O3. Proc. Natl. Acad. Sci. USA 2023, 120, e2313591120. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Change Biol. 2008, 14, 1642–1650. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Han, Y.; Zhu, J.; Kobayashi, K.; Tang, H.; Wang, Y. The impact of elevated tropospheric ozone on grain quality of hybrid rice: A free-air gas concentration enrichment (FACE) experiment. Field Crop Res. 2012, 129, 81–89. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Q.; Frei, M.; Shao, Z.; Yang, L. Effects of elevated ozone, carbon dioxide, and the combination of both on the grain quality of Chinese hybrid rice. Environ. Pollut. 2014, 189, 9–17. [Google Scholar] [CrossRef]
- Shang, B.; Deng, T.; Chen, H.; Xu, Y.; Feng, Z. Effects of elevated ozone on physiology, growth, yield and grain quality of rice (Oryza sativa L.): An ozone gradient experiment. Agric. Ecosyst. Environ. 2024, 363, 108858. [Google Scholar] [CrossRef]
- Sawada, H.; Tsukahara, K.; Kohno, Y.; Suzuki, K.; Nagasawa, N.; Tamaoki, M. Elevated ozone deteriorates grain quality of Japonica Rice cv. Koshihikari, even if it does not cause yield reduction. Rice 2016, 9, 7. [Google Scholar] [CrossRef]
- Lyman, N.B.; Jagadish, K.S.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xia, D.; He, Y. Rice grain quality—Traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1. [Google Scholar] [CrossRef]
- Jing, L.; Dombinov, V.; Shen, S.; Wu, Y.; Yang, L.; Wang, Y.; Frei, M. Physiological and genotype-specific factors associated with grain quality changes in rice exposed to high ozone. Environ. Pollut. 2016, 210, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Frei, M. Stressed food–The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Morgan, P.B.; Ainsworth, E.A.; Long, S.P. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003, 26, 1317–1328. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Change Biol. 2008, 14, 2696–2708. [Google Scholar] [CrossRef]
- Mohapatra, P.; Patel, R.; Sahu, S. Time of flowering affects grain quality and spikelet partitioning within the rice panicle. Aust. J. Plant Physiol. 1993, 20, 231–241. [Google Scholar] [CrossRef]
- Ishimaru, T.; Matsuda, T.; Ohsugi, R.; Yamagishi, T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Funct. Plant Biol. 2003, 30, 1139–1149. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.; Cheng, F.; Liu, Y.; Zhang, G. The differences in grain weight and quality within a rice (Oryza sativa L.) panicle as affected by panicle type and source-sink relation. J. Agron. Crop Sci. 2007, 193, 63–73. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Yuan, L.; Wang, Z.; Yang, J.; Zhang, J. Post-anthesis alternate wetting and moderate soil drying enhances activities of key enzymes in sucrose-to-starch conversion in inferior spikelets of rice. J. Exp. Bot. 2012, 63, 215–227. [Google Scholar] [CrossRef]
- Liu, B.; Meng, S.; Yang, J.; Wu, J.; Peng, Y.; Zhang, J.; Ye, N. Carbohydrate flow during grain filling: Phytohormonal regulation and genetic control in rice (Oryza sativa). J. Integr. Plant Biol. 2025, 67, 1086–1104. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. Eur. J. Agron. 2010, 33, 117–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, W.; Liu, K.; Shi, W.; Peng, Y.; Zhang, C.; Shen, Y.; Liu, W.; Ding, Y.; Tang, S. Effects of structural properties of glutelin on the formation of grain quality under elevated temperatures and additional nitrogen during the grain filling period. Food Chem. 2025, 476, 143469. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Min, Z.; Zou, D.; Liu, H.; Wang, J.; Zheng, H.; Jia, Y.; Yang, L.; Xin, W.; et al. Response of rice with overlapping growth stages to water stress by assimilates accumulation and transport and starch synthesis of superior and inferior grains. Int. J. Mol. Sci. 2022, 23, 11157. [Google Scholar] [CrossRef]
- He, L.; Bao, M.; Li, Y.; Xu, Y.; Shao, Z.; Ma, Y.; Zhang, K.; Shang, B.; Feng, Z. Leaf biochemical and physiological responses to elevated atmospheric ozone concentration in eight modern rice cultivars. Ecosyst. Health Sust. 2024, 10, 0269. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Xu, Y.S.; He, L.X.; Zhang, Y.J.; Feng, Z.Z. Combined effects of elevated ozone concentration and warming on photosynthesis of rice leaves. Acta Ecol. Sin. 2025, 45, 877–888, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, G.; Sakai, H.; Tokida, T.; Usui, Y.; Zhu, C.; Nakamura, H.; Yoshimoto, M.; Fukuoka, M.; Kobayashi, K.; Hasegawa, T. The effects of free-air CO2 enrichment (FACE) on carbon and nitrogen accumulation in grains of rice (Oryza sativa L.). J. Exp. Bot. 2013, 64, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, Y.D.; Zhu, Z.; Chen, T.; Zhao, Q.Y.; Zhong, W.G.; Yang, J.; Yao, S.; Zhou, L.H.; Zhao, L.; et al. Research progress on the breeding of japonica super rice varieties in Jiangsu Province, China. J. Integr. Agric. 2017, 16, 992–999. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, Y.; Mu, H.; Wang, Y.; Wang, Y.; Yang, L. Ozone-induced reduction in rice yield is closely related to the response of spikelet density under ozone stress. Sci. Total Environ. 2020, 712, 136560. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17891-1999; High Quality Paddy. The State Bureau of Quality and Technical Supervision; National Standard of People’s Republic of China: Beijing, China, 1999.
- Ju, Z.Y.; Hettiarachchy, N.S.; Rath, N. Extraction, denaturation and hydrophobic properties of rice flour proteins. J. Food Sci. 2001, 66, 229–232. [Google Scholar] [CrossRef]
- Vaintraub, I.A.; Lapteva, N.A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 1988, 175, 227–230. [Google Scholar] [CrossRef]
- Frei, M.; Kohno, Y.; Wissuwa, M.; Makkar, H.P.S.; Becker, K. Negative effects of tropospheric ozone on the feed value of rice straw are mitigated by an ozone tolerance QTL. Glob. Change Biol. 2011, 17, 2319–2329. [Google Scholar] [CrossRef]
- Frei, M.; Kohno, Y.; Tietze, S.; Jekle, M.; Hussein, M.A.; Becker, T.; Becker, K. The response of rice grain quality to ozone exposure during growth depends on ozone level and genotype. Environ. Pollut. 2012, 163, 199–206. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okada, M.; Nouchi, I. Effects of ozone on dry matter partitioning and yield of Japanese cultivars of rice (Oryza sativa L.). Agric. Ecosyst. Environ. 1995, 53, 109–122. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Sui, L.H.; Wang, W.; Geng, C.M.; Yin, B.H. Visible injury and nitrogen metabolism of rice leaves under ozone stress, and effect on sugar and protein contents in grain. Atmos. Environ. 2012, 62, 433–440. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, G.; Yang, L.; Yang, J.; Zhang, J.; Zhao, B. Hormones in the grains and roots in relation to post-anthesis development of inferior and superior spikelets in japonica/indica hybrid rice. Plant Physiol. Biochem. 2009, 47, 195–204. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; He, Z. Understanding the regulation of cereal grain filling: The way forward. J. Integr. Plant Biol. 2023, 65, 526–547. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yang, Y.; Tian, C.; He, F.; Wang, Y.; Wang, Y.; Yang, L. Physicochemical characteristics of lodging susceptibility of rice cultivars in response to ozone exposure. Agric. Ecosyst. Environ. 2023, 344, 108313. [Google Scholar] [CrossRef]
- Autarmat, S.; Treesubsuntorn, C.; Thiravetyan, P. Possible mechanism of inoculating Oryza sativa L. with endophytic bacteria under ambient air and ozone stress. Environ. Exp. Bot. 2022, 194, 104761. [Google Scholar] [CrossRef]
- Zhang, G.; Risalat, H.; Kobayashi, K.; Cao, R.; Hu, Q.; Pan, X.; Hu, Y.; Shang, B.; Wu, H.; Zhang, Z.; et al. Ethylenediurea reduces grain chalkiness in hybrid rice cultivars under ambient levels of surface ozone in China. Front. Plant Sci. 2022, 13, 983576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Pan, X.; Hu, Y.; Cao, R.; Hu, Q.; Fu, R.; Hamdulla, R.; Shang, B. Both short-term and long-term ozone pollution alters the chemical composition of rice grain. Bull. Environ. Contam. Toxicol. 2024, 113, 15. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.; Courtin, C.; Gebruers, K.; Delcour, J. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Baxter, G.; Zhao, J.; Blanchard, C. Albumin significantly affects pasting and textural characteristics of rice flour. Cereal Chem. 2010, 87, 250–255. [Google Scholar] [CrossRef]
- Larkin, P.D.; Mcclung, A.M.; Ayres, N.M.; Park, W.D. The effect of the Waxy locus (Granule Bound Starch Synthase) on pasting curve characteristics in specialty rices (Oryza sativa L.). Euphytica 2003, 131, 243–253. [Google Scholar] [CrossRef]
- Allahgholipour, M.; Ali, A.J.; Alinia, F.; Nagamine, T.; Kojima, Y. Relationship between rice grain amylose and pasting properties for breeding better quality rice varieties. Plant Breed. 2006, 125, 357–362. [Google Scholar] [CrossRef]
- Song, Q.L.; Qi, Y.T.; Zhao, Y.P.; Wang, Y.X.; Li, P.L.; Zhu, J.G.; Wang, Y.L.; Yang, L.X. Impact of free air ozone concentration enrichment on cooked rice (Wuyunjing 21) texture and palatability. Chin. J. Eco-Agric. 2013, 21, 566–571, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
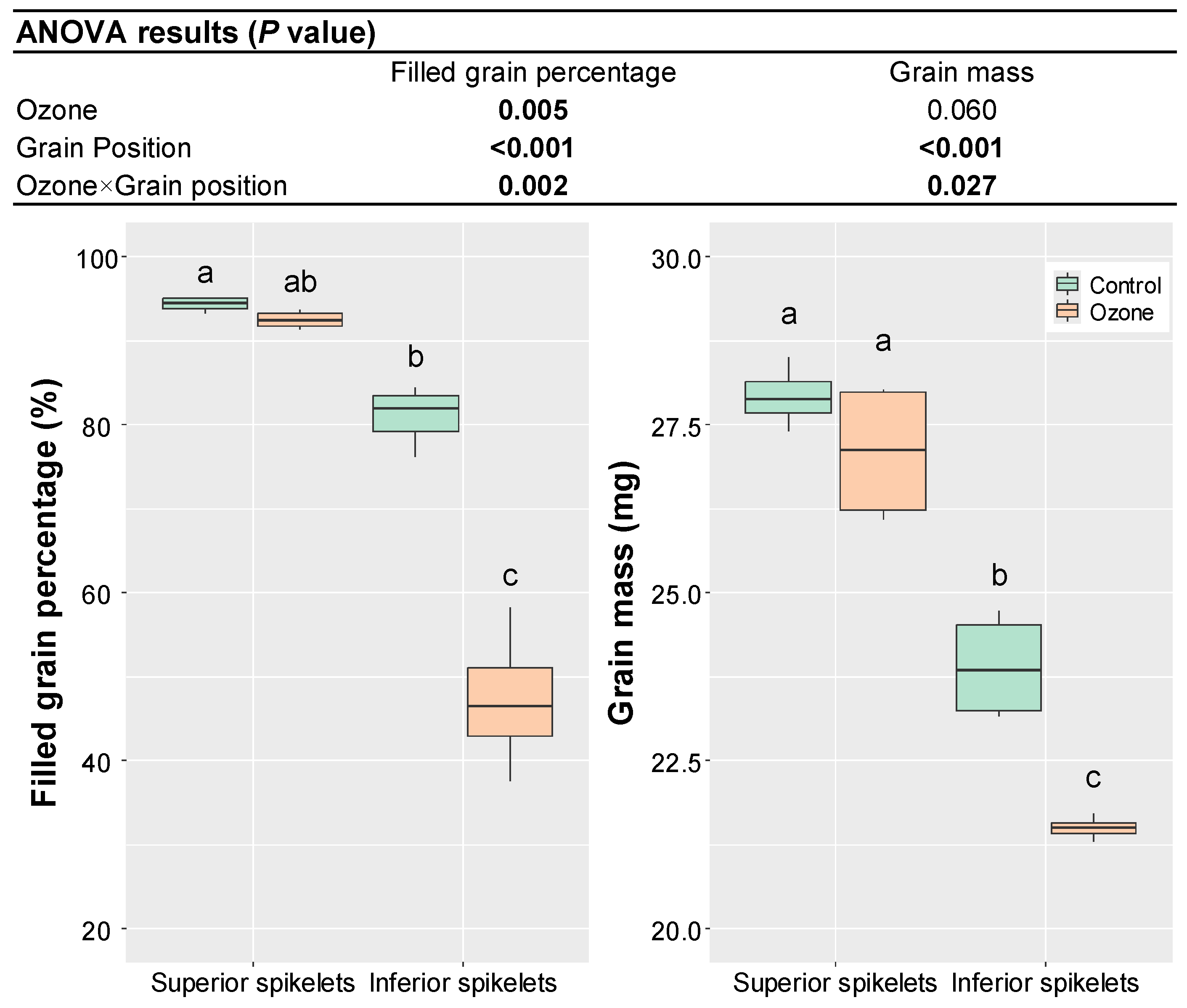
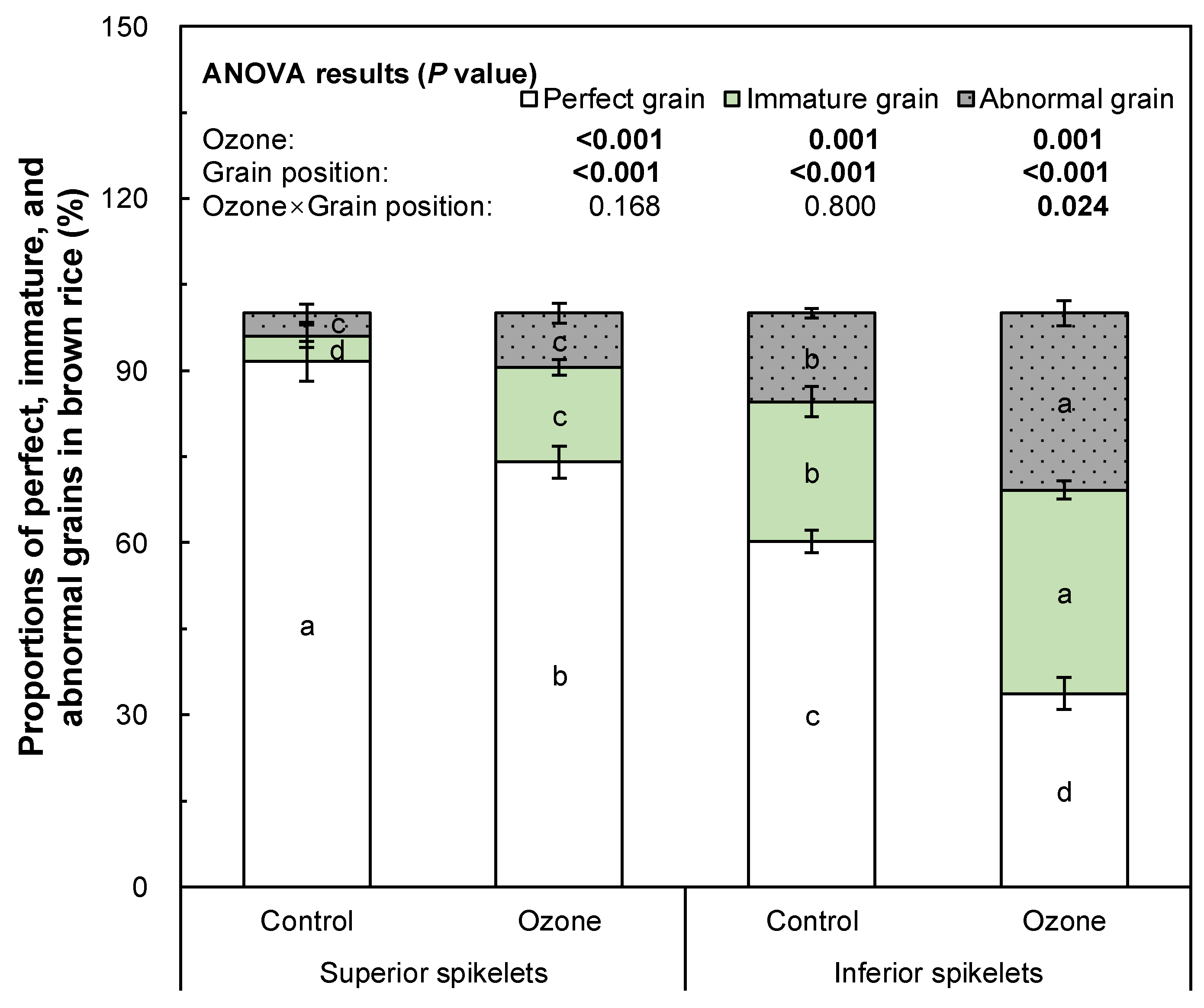
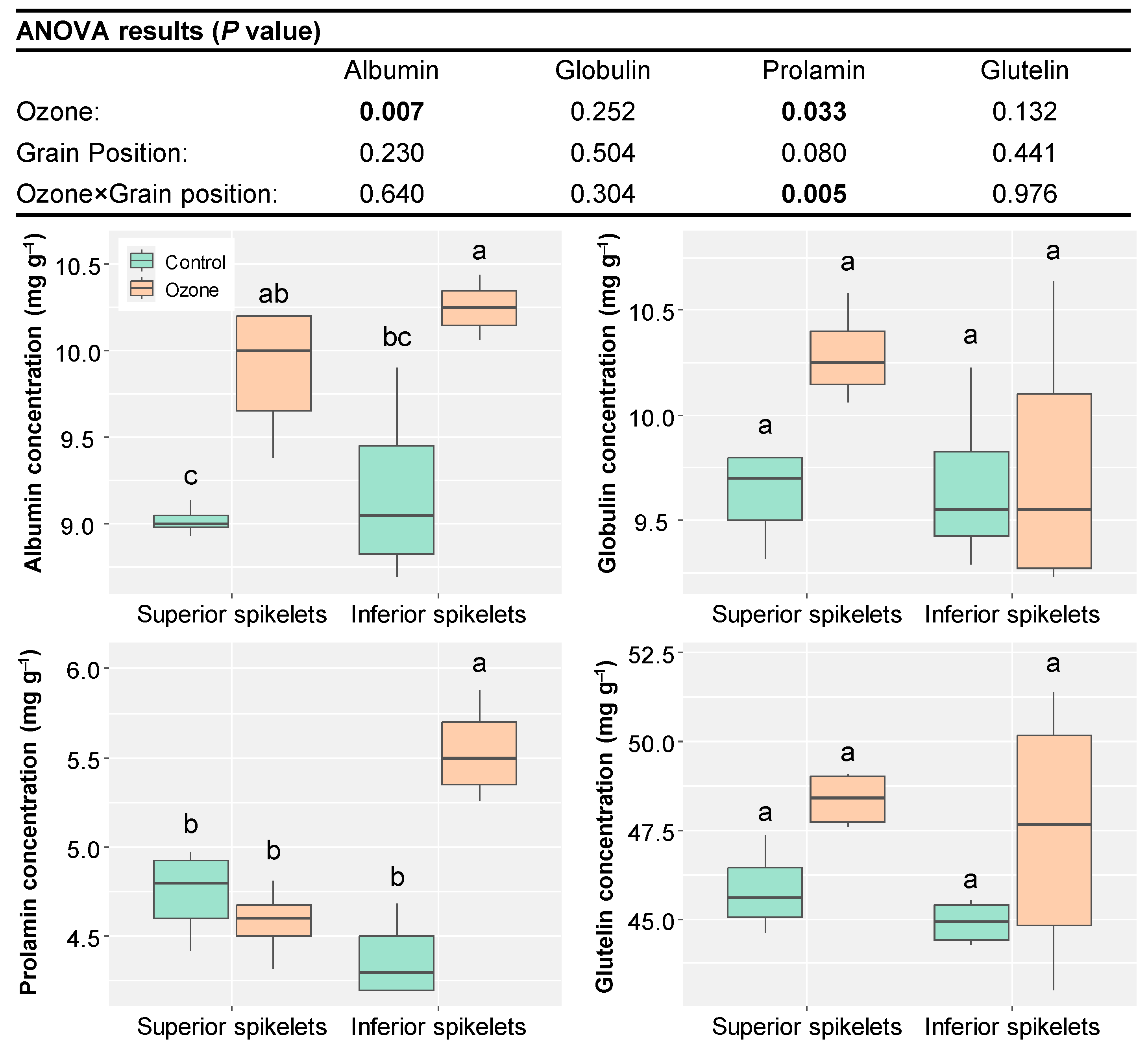
| Treatment | Temperature (°C) | Relative Humidity (%) | Ozone Concentration (nL L−1) | |||
|---|---|---|---|---|---|---|
| 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | |
| Control | 30.0 ± 0.5 | 26.0 ± 0.8 | 72.7 ± 0.6 | 69.8 ± 2.0 | 18.1 ± 1.4 | 11.3 ± 1.0 |
| Ozone | 29.9 ± 0.5 | 26.0 ± 0.8 | 72.9 ± 0.4 | 70.2 ± 1.9 | 100.3 ± 0.4 | 39.3 ± 1.2 |
| Grain Position | Treatment | Length (mm) | Width (mm) | Thickness (mm) | Volume (mm3) | Density (mg mm−3) |
|---|---|---|---|---|---|---|
| Superior spikelets | Control | 7.07 ± 0.02 a | 3.58 ± 0.03 a | 2.43 ± 0.01 a | 32.2 ± 0.3 a | 0.87 ± 0.02 ab |
| Ozone | 7.06 ± 0.02 a | 3.51 ± 0.02 a | 2.33 ± 0.02 b | 30.3 ± 0.4 b | 0.90 ± 0.01 a | |
| % change | −0.1 | −2.0 | −4.0 | −5.9 | 3.7 | |
| Inferior spikelets | Control | 6.81 ± 0.04 b | 3.35 ± 0.03 b | 2.36 ± 0.02 b | 28.3 ± 0.5 c | 0.86 ± 0.01 ab |
| Ozone | 6.84 ± 0.06 b | 3.29 ± 0.05 b | 2.18 ± 0.01 c | 25.8 ± 0.6 d | 0.85 ± 0.02 b | |
| % change | 0.5 | −1.8 | −7.6 | −8.8 | −0.9 | |
| ANOVA results (p value) | ||||||
| Ozone | 0.767 | 0.096 | <0.001 | 0.004 | 0.633 | |
| Grain position | 0.001 | 0.001 | 0.001 | <0.001 | 0.065 | |
| Ozone × grain position | 0.629 | 0.866 | 0.046 | 0.567 | 0.199 | |
| Grain Position | Treatment | Amylose Content (%) | RVA Profile | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Maximum Viscosity (cP) | Minimum Viscosity (cP) | Breakdown (cP) | Final Viscosity (cP) | Setback (cP) | Peak Time (min) | Gelatinization Temperature (°C) | |||
| Superior spikelets | Control | 9.61 ± 0.07 a | 2060 ± 17 c | 1139 ± 16 d | 921 ± 11 a | 1767 ± 25 c | −293 ± 22 b | 5.78 ± 0.02 b | 73.9 ± 0.2 a |
| Ozone | 8.98 ± 0.21 a | 2261 ± 15 a | 1420 ± 29 a | 841 ± 38 a | 2145 ± 26 a | −117 ± 35 a | 6.13 ± 0.06 a | 73.2 ± 0.4 a | |
| % change | −6.6 | 9.8 | 24.7 | −8.7 | 21.4 | 60.2 | 6.1 | −0.8 | |
| Inferior spikelets | Control | 9.31 ± 0.32 a | 2092 ± 18 bc | 1193 ± 18 c | 899 ± 31 a | 1858 ± 17 b | −233 ± 22 b | 5.88 ± 0.05 b | 74.3 ± 0.6 a |
| Ozone | 8.36 ± 0.10 b | 2184 ± 52 ab | 1346 ± 32 b | 837 ± 32 a | 2119 ± 29 a | −65 ± 38 a | 6.13 ± 0.04 a | 73.2 ± 0.2 a | |
| % change | −10.2 | 4.4 | 12.9 | −6.8 | 14.0 | 72.2 | 4.2 | −1.4 | |
| ANOVA results (p value) | |||||||||
| Ozone | 0.040 | 0.003 | 0.003 | 0.068 | <0.001 | 0.002 | 0.003 | 0.207 | |
| Grain position | 0.037 | 0.470 | 0.465 | 0.672 | 0.139 | 0.111 | 0.254 | 0.570 | |
| Ozone × grain position | 0.393 | 0.116 | 0.002 | 0.768 | 0.022 | 0.904 | 0.254 | 0.570 | |
| Parameter | Grain Position | Control | Ozone | % Change | ANOVA Results (p Value) | ||
|---|---|---|---|---|---|---|---|
| Ozone | Grain Position | Ozone × Grain Position | |||||
| P (mg g−1) | Superior | 3.43 ± 0.05 a | 3.41 ± 0.05 a | −0.8 | 0.225 | 0.222 | 0.323 |
| Inferior | 3.58 ± 0.08 a | 3.42 ± 0.05 a | −4.3 | ||||
| K (mg g−1) | Superior | 2.88 ± 0.04 ab | 2.69 ± 0.05 b | −6.5 | 0.060 | 0.277 | 0.761 |
| Inferior | 2.93 ± 0.08 a | 2.78 ± 0.07 ab | −5.1 | ||||
| Ca (mg g−1) | Superior | 0.18 ± 0.02 a | 0.19 ± 0.02 a | 6.4 | 0.998 | 0.874 | 0.496 |
| Inferior | 0.19 ± 0.01 a | 0.18 ± 0.01 a | −6.0 | ||||
| Mg (mg g−1) | Superior | 1.41 ± 0.02 a | 1.40 ± 0.02 a | −0.8 | 0.226 | 0.438 | 0.399 |
| Inferior | 1.46 ± 0.04 a | 1.40 ± 0.01 a | −4.0 | ||||
| S (mg g−1) | Superior | 0.96 ± 0.02 c | 1.06 ± 0.02 a | 10.7 | 0.010 | 0.979 | 0.149 |
| Inferior | 0.98 ± 0.02 bc | 1.03 ± 0.01 ab | 5.2 | ||||
| B (μg g−1) | Superior | 0.85 ± 0.12 a | 0.83 ± 0.16 a | −2.1 | 0.490 | 0.405 | 0.165 |
| Inferior | 0.63 ± 0.17 a | 0.89 ± 0.04 a | 42.1 | ||||
| Cu (μg g−1) | Superior | 4.90 ± 0.12 c | 6.69 ± 0.12 a | 36.6 | <0.001 | 0.604 | 0.019 |
| Inferior | 5.45 ± 0.25 b | 6.30 ± 0.07 a | 15.7 | ||||
| Mn (μg g−1) | Superior | 20.3 ± 0.2 c | 24.3 ± 0.8 b | 19.3 | 0.002 | 0.004 | 0.612 |
| Inferior | 23.0 ± 1.0 b | 27.6 ± 0.7 a | 20.2 | ||||
| Zn (μg g−1) | Superior | 29.9 ± 1.5 a | 32.5 ± 4.8 a | 8.6 | 0.727 | 0.296 | 0.080 |
| Inferior | 31.8 ± 1.5 a | 26.0 ± 1.7 a | −18.4 | ||||
| Phytate (mg g−1) | Superior | 12.4 ± 0.9 a | 13.6 ± 0.5 a | 9.2 | 0.285 | 0.332 | 0.597 |
| Inferior | 13.1 ± 0.3 a | 13.8 ± 0.4 a | 5.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Mu, H.; Wang, Y.; Jing, L.; Wang, Y.; Huang, J.; Yang, L. Ozone Stress During Rice Growth Impedes Grain-Filling Capacity of Inferior Spikelets but Not That of Superior Spikelets. Agronomy 2025, 15, 1809. https://doi.org/10.3390/agronomy15081809
Hu S, Mu H, Wang Y, Jing L, Wang Y, Huang J, Yang L. Ozone Stress During Rice Growth Impedes Grain-Filling Capacity of Inferior Spikelets but Not That of Superior Spikelets. Agronomy. 2025; 15(8):1809. https://doi.org/10.3390/agronomy15081809
Chicago/Turabian StyleHu, Shaowu, Hairong Mu, Yunxia Wang, Liquan Jing, Yulong Wang, Jianye Huang, and Lianxin Yang. 2025. "Ozone Stress During Rice Growth Impedes Grain-Filling Capacity of Inferior Spikelets but Not That of Superior Spikelets" Agronomy 15, no. 8: 1809. https://doi.org/10.3390/agronomy15081809
APA StyleHu, S., Mu, H., Wang, Y., Jing, L., Wang, Y., Huang, J., & Yang, L. (2025). Ozone Stress During Rice Growth Impedes Grain-Filling Capacity of Inferior Spikelets but Not That of Superior Spikelets. Agronomy, 15(8), 1809. https://doi.org/10.3390/agronomy15081809






