Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production
Abstract
1. Introduction
2. Materials and Methods
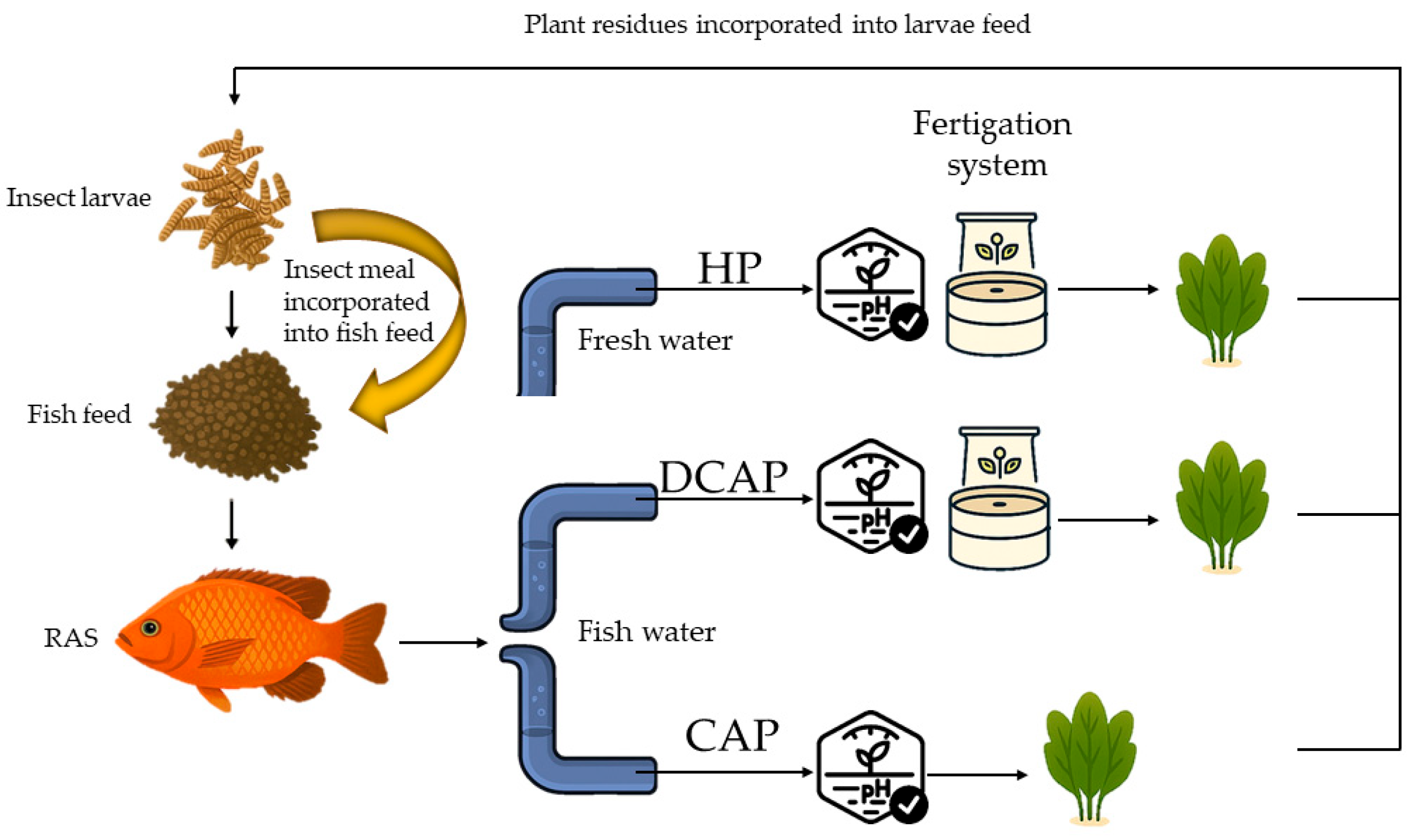
2.1. Experimental Setup
2.2. Fish Stocking
2.3. Insect Rearing and Insect Meal Preparation
2.4. Experimental Design and Plant Material
- (a)
- Hydroponic (HP) solution, which was used as the control.
- (b)
- Coupled aquaponic (DCAP) solution, consisting of RAS water, with the dissolved nutrients derived entirely from fish waste.
- (c)
- Decoupled aquaponic (DCAP) solution, which was the RAS solution enriched with additional chemical fertilizers until it reached the target HP concentration values.
2.5. Measurements
2.5.1. Physicochemical Parameters of the RAS Water
2.5.2. Plant Measurements
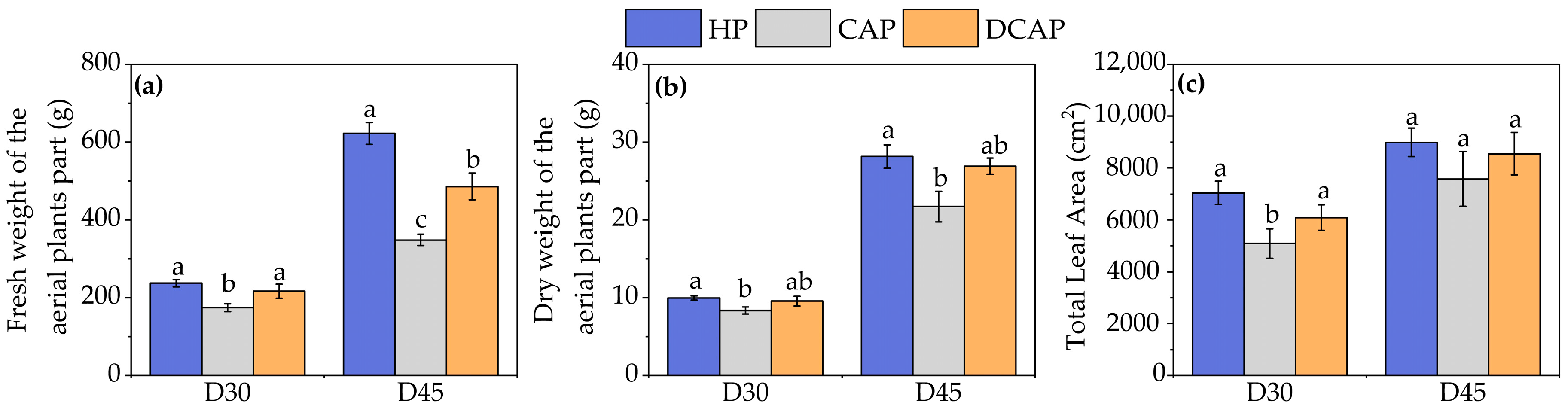
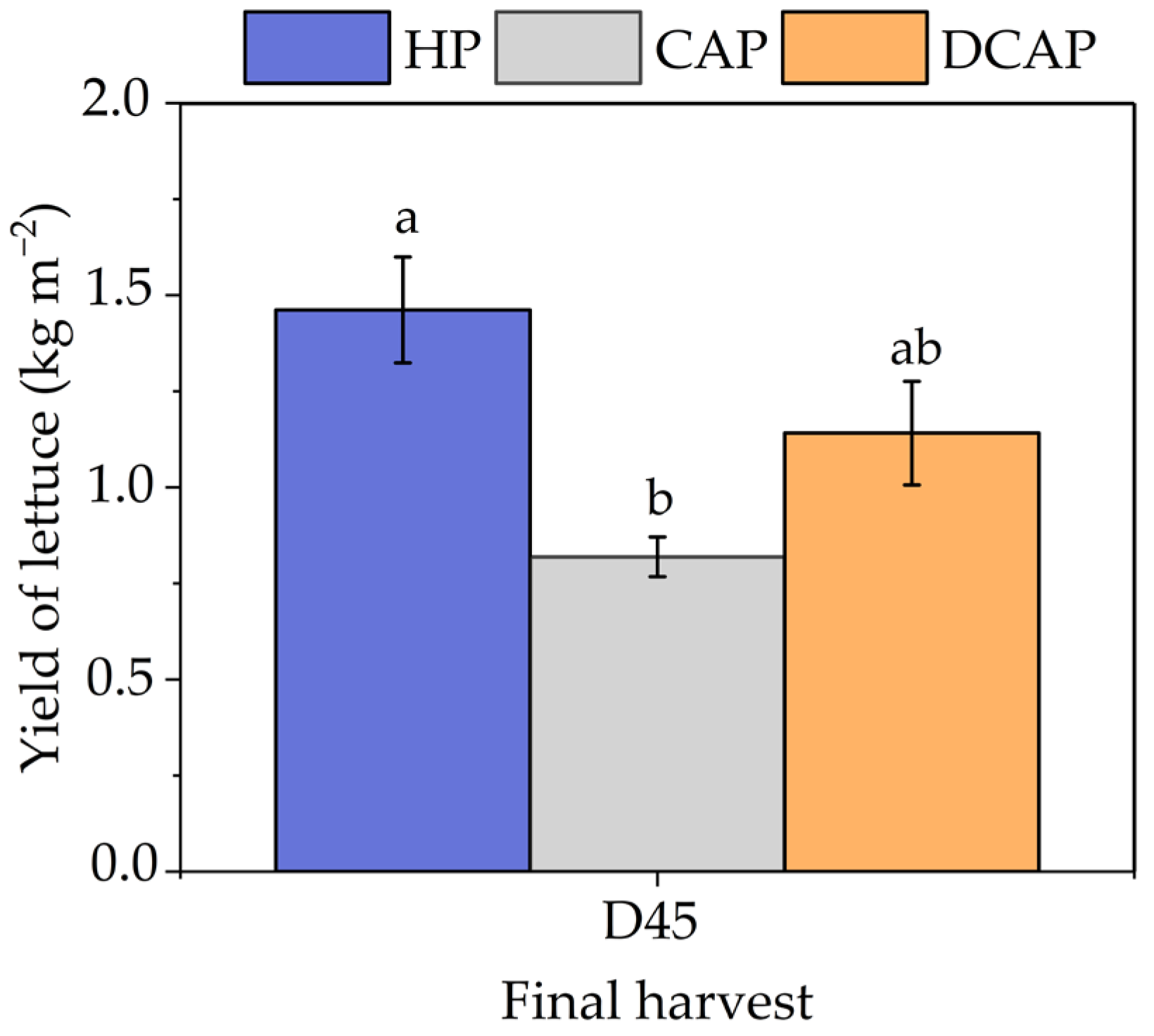
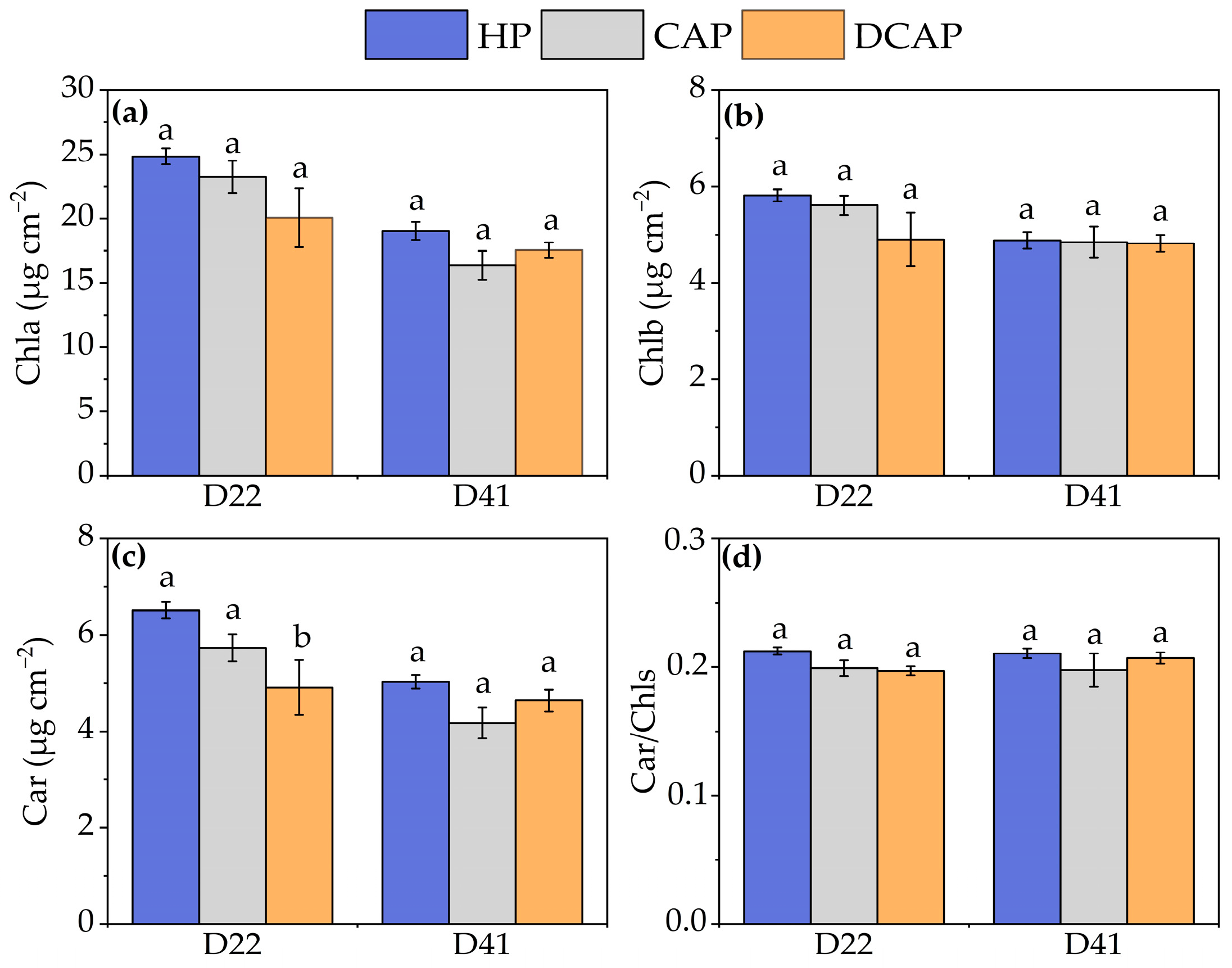
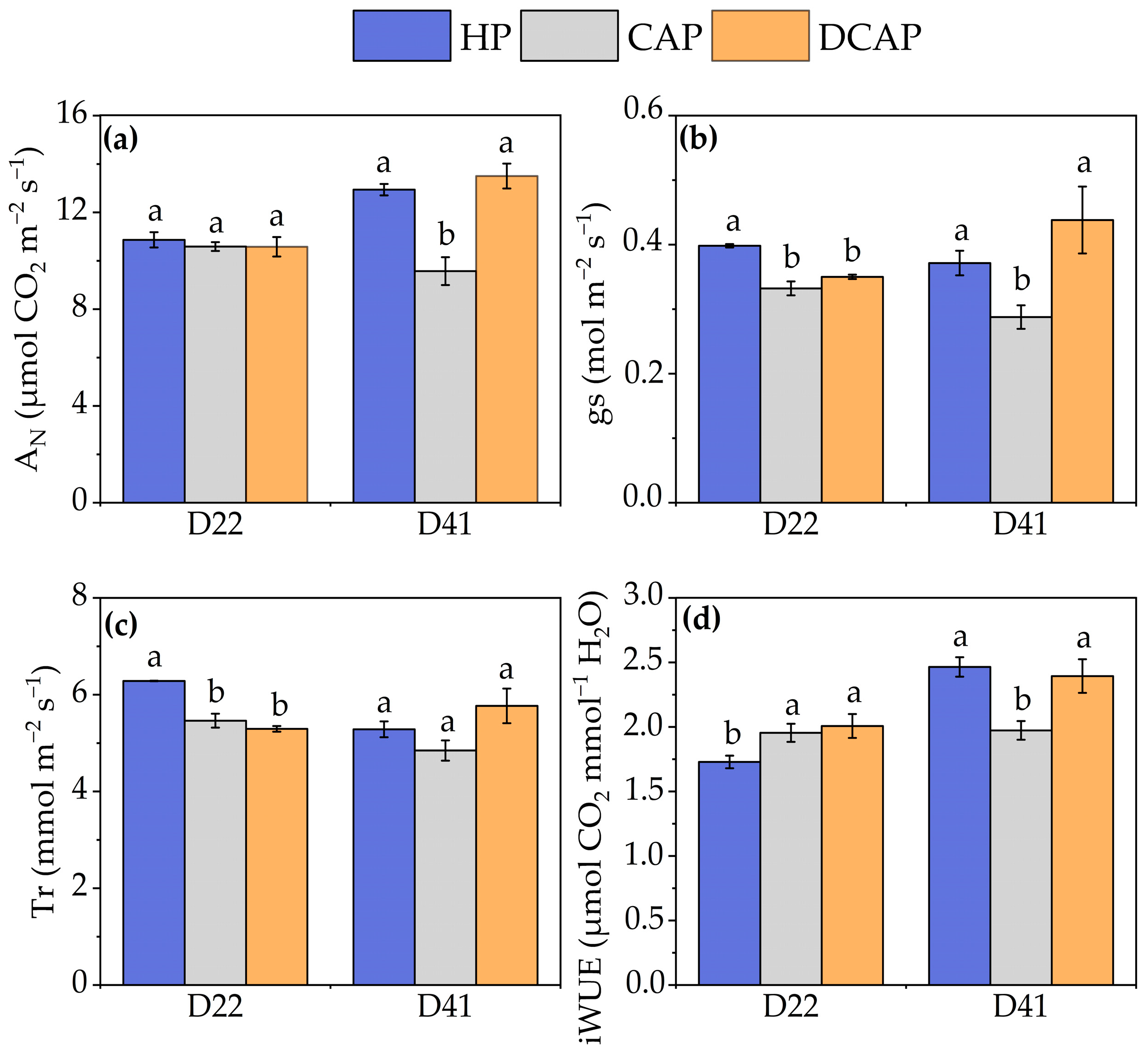
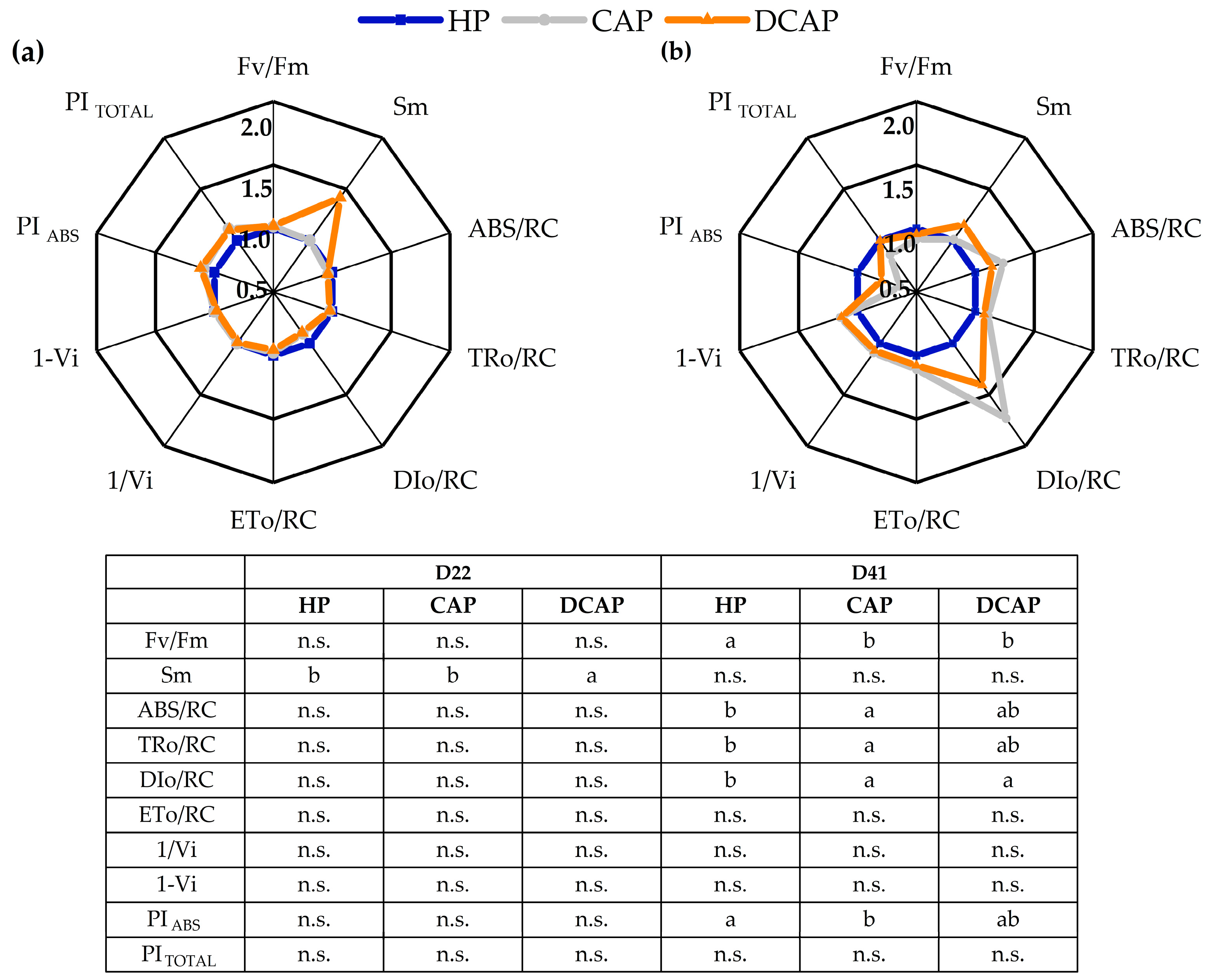
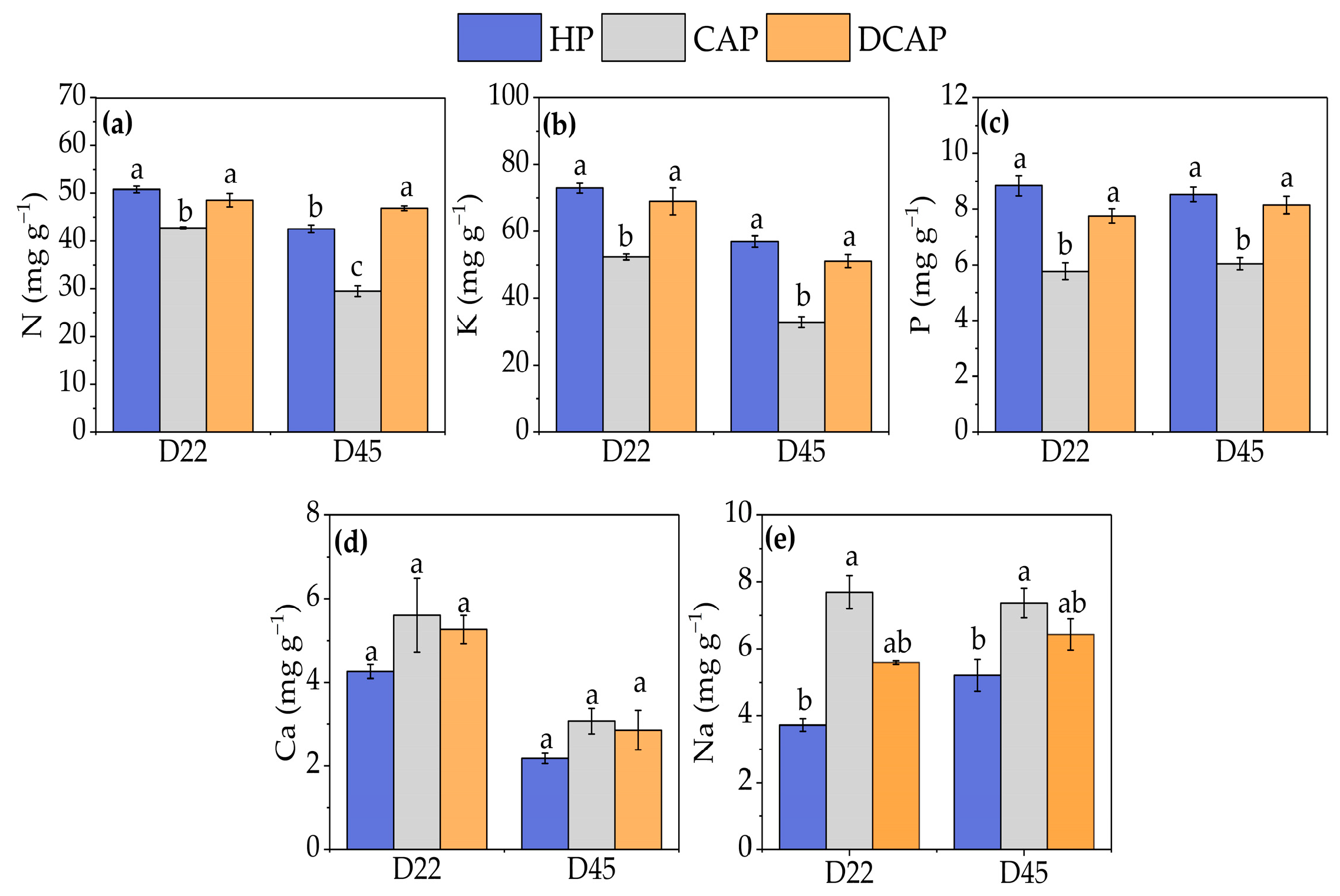
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marqués, L.; Ogle, K.; Peltier, D.M.P.; Camarero, J.J. Altered climate memory characterizes tree growth during forest dieback. Agric. For. Meteorol. 2022, 314, 108787. [Google Scholar] [CrossRef]
- Fan, Y.-T.; Lin, Z.-E.; Chiueh, P.-T.; Lin, Y.-P.; Cheng, L.-C.; Cheng, Y.-S.; Lin, S.-I.; Fan, C. Challenges and opportunities in using biowaste for sustainable hydroponic netted melon (Cucumis melo L.) cultivation. Agric. Syst. 2025, 228, 104366. [Google Scholar] [CrossRef]
- Zhu, Z.; Yogev, U.; Keesman, K.J.; Gross, A. Promoting circular economy: Comparison of novel coupled aquaponics with anaerobic digestion and conventional aquaponic systems on nutrient dynamics and sustainability. Resour. Conserv. Recycl. 2024, 208, 107716. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Mourantian, A.; Aslanidou, M.; Mente, E.; Katsoulas, N.; Levizou, E. Basil functional and growth responses when cultivated via different aquaponic and hydroponics systems. PeerJ 2023, 11, e15664. [Google Scholar] [CrossRef] [PubMed]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. (Eds.) Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- Colt, J.; Schuur, A.M. Comparison of nutrient costs from fish feeds and inorganic fertilizers for aquaponics systems. Aquac. Eng. 2021, 95, 102205. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges–A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Tsoumalakou, E.; Levizou, E.; Vanikiotis, T.; Zaoutsos, S.; Berillis, P. Iron and potassium fertilization improve rocket growth without affecting tilapia growth and histomorphology characteristics in aquaponics. Appl. Sci. 2021, 11, 5681. [Google Scholar] [CrossRef]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. [Google Scholar] [CrossRef]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Evaluation of productivity and efficiency of a large-scale coupled or decoupled aquaponic system. Sci. Hortic. 2024, 337, 113552. [Google Scholar] [CrossRef]
- Shaw, C.; Knopf, K.; Kloas, W. Fish Feeds in Aquaponics and Beyond: A Novel Concept to Evaluate Protein Sources in Diets for Circular Multitrophic Food Production Systems. Sustainability 2022, 14, 4064. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Quang Tran, H.; Van Doan, H.; Stejskal, V. Environmental consequences of using insect meal as an ingredient in aquafeeds: A systematic view. Rev. Aquac. 2022, 14, 237–251. [Google Scholar] [CrossRef]
- Pinho, S.; Leal, M.M.; Shaw, C.; Baganz, D.; Baganz, G.; Staaks, G.; Kloas, W.; Körner, O.; Monsees, H. Insect-based fish feed in decoupled aquaponic systems: Effect on lettuce production and resource use. PLoS ONE 2024, 19, e0295811. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, P.; Asimaki, A.; Berillis, P.; Vlahos, N.; Levizou, E.; Katsoulas, N.; Karapanagiotidis, I.T.; Rumbos, C.I.; Athanassiou, C.G.; Mente, E. Aqua-Ento-Ponics: Effect of Insect Meal on the Development of Sea Bass, Dicentrarchus labrax, in Co-Culture with Lettuce. Fishes 2022, 7, 397. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Ulotu, E.E.; Luvanga, S.A.; Munyi, F.M.; John, J.O.; Opiyo, M.A. Black soldier fly (Hermetia illucens, L.) larvae meal improves growth performance, feed efficiency and economic returns of Nile tilapia (Oreochromis niloticus, L.) fry. Aquac. Fish Fish. 2022, 2, 167–178. [Google Scholar] [CrossRef]
- Henry, M.A.; Golomazou, E.; Asimaki, A.; Psofakis, P.; Fountoulaki, E.; Mente, E.; Rumbos, C.I.; Athanassiou, C.G.; Karapanagiotidis, I.T. Partial dietary fishmeal replacement with full-fat or defatted superworm (Zophobas morio) larvae meals modulates the innate immune system of gilthead seabream, Sparus aurata. Aquac. Rep. 2022, 27, 101347. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Neofytou, M.C.; Asimaki, A.; Daskalopoulou, E.; Psofakis, P.; Mente, E.; Rumbos, C.I.; Athanassiou, C.G. Fishmeal Replacement by Full-Fat and Defatted Hermetia illucens Prepupae Meal in the Diet of Gilthead Seabream (Sparus aurata). Sustainability 2023, 15, 786. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-H.; Puniamoorthy, N. Impact of Rearing Substrates on Black Soldier Fly Growth and Fertility: A Semi-Industrial Scale Study to Optimize Egg Collection. Insects 2025, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Matsakidou, A.; Sarivasiliou, S.-I.; Pissia, M.-A.; Rumbos, C.I.; Athanassiou, C.G.; Paraskevopoulou, A. Compositional, volatile, and structural features of Hermetia illucens (black soldier fly) flours: The effect of population and life stages. Future Foods 2024, 9, 100320. [Google Scholar] [CrossRef]
- Hirayama, K. Water control by filtration in closed culture systems. Aquaculture 1974, 4, 369–385. [Google Scholar] [CrossRef]
- FAO. Good Agricultural Pratices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; ISBN 978-92-5-107649-1. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. ISBN 978-1-4020-3217-2. [Google Scholar]
- Tsoumalakou, E.; Mente, E.; Vlahos, N.; Levizou, E. Spinach Responds to Minimal Nutrient Supplementation in Aquaponics by Up-Regulating Light Use Efficiency, Photochemistry, and Carboxylation. Horticulturae 2023, 9, 291. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Harika, N.; Verma, A.K.; Krishnani, K.K.; Hittinahalli, C.M.; Reddy, R.; Pai, M. Supplementation of potassium in aquaculture wastewater and its effect on growth performance of basil (Ocimum basilicum L) and pangasius (Pangasianodon hypophthalmus) in NFT-based aquaponics. Sci. Hortic. 2024, 323, 112521. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of Foliar Spray of K on Mint, Radish, Parsley and Coriander Plants in Aquaponic System. J. Plant Nutr. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Potassium deficiency stress tolerance in peanut (Arachis hypogaea) through ion homeostasis, activation of antioxidant defense, and metabolic dynamics: Alleviatory role of silicon supplementation. Plant Physiol. Biochem. 2022, 182, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Sieczko, L.; Kowalczyk, K.; Gajc-Wolska, J.; Kowalczyk, W.; Dąbrowski, P.; Borucki, W.; Janaszek-Mańkowska, M.; Przybył, J.L.; Mojski, J.; Kalaji, H.M. Phosphorus-deficiency stress in cucumber (Cucumis sativus L.) plants: Early detection based on chosen physiological parameters and statistical analyses. Photosynthetica 2024, 62, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, V.P.; Combot, D.; Pelissier, P.; Labbé, L.; Joyce, A. Improving Plant Health Through Nutrient Remineralization in Aquaponic Systems. Front. Plant Sci. 2021, 12, 683690. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-H.; Zhang, A.-J.; Wei, M.; Chen, X.-G.; Liu, Z.-H.; Li, H.-M.; Ding, Y.-F. Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, D.; Won, E.; Timmons, M.B.; Mattson, N. Complementary Nutrients in Decoupled Aquaponics Enhance Basil Performance. Horticulturae 2022, 8, 111. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Vlahos, N.; Levizou, E. Cultivating the Mediterranean Wild Edible Species Cichorium spinosum L. in Aquaponics: Functional and Growth Responses to Minimal Nutrient Supplementation. Sustainability 2023, 15, 5572. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Mosa, H.; Abbas, W.; Morsy, O. Nutrient supply systems and their effect on the performance of the Nile Tilapia (Oreochromis niloticus) and Lettuce (Lactuca sativa) plant integration system. Sci. Rep. 2024, 14, 4229. [Google Scholar] [CrossRef] [PubMed]
- Delaide, B.; Teerlinck, S.; Decombel, A.; Bleyaert, P. Effect of wastewater from a pikeperch (Sander lucioperca L.) recirculated aquaculture system on hydroponic tomato production and quality. Agric. Water Manag. 2019, 226, 105814. [Google Scholar] [CrossRef]
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H.M. Microbial diversity in different compartments of an aquaponics system. Arch. Microbiol. 2017, 199, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Chandrou, E.; Faliagka, S.; Mourantian, A.; Kollaros, M.G.; Karamanoli, K.; Pechlivani, E.-M.; Katsoulas, N.; Levizou, E. Exploring the Potential of Biostimulants to Optimize Lettuce Cultivation in Coupled and Decoupled Aquaponics Systems: Growth Performance, Functional Characteristics and Metabolomic Analysis. Horticulturae 2024, 10, 514. [Google Scholar] [CrossRef]
- Lenz, G.L.; Loss, A.; Lourenzi, C.R.; Luiz De Alcantara Lopes, D.; Siebeneichler, L.D.M.; Brunetto, G. Lettuce growth in aquaponic system and in soil fertilized with fish sludge. Aquac. Res. 2021, 52, 5008–5021. [Google Scholar] [CrossRef]
- Anderson, T.; Martini, M.; De Villiers, D.; Timmons, M. Growth and Tissue Elemental Composition Response of Butterhead Lettuce (Lactuca sativa, cv. Flandria) to Hydroponic Conditions at Different pH and Alkalinity. Horticulturae 2017, 3, 41. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Chondrogiannis, C.; Grammatikopoulos, G. Photosynthesis in developing leaf of juveniles and adults of three Mediterranean species with different growth forms. Photosynth. Res. 2016, 130, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Abadía, A.; Abadía, J.; Morales, F. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynth. Res. 2006, 89, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Belkhodja, R.; Abadıa, A.; Abadıa, J. Photosystem II efficiency and mechanisms of energy dissipation in iron-deficient, field-grown pear trees (Pyrus communis L.). Photosynth. Res. 2000, 63, 9–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jardim, A.M.d.R.F.; Santos, H.R.B.; Alves, H.K.M.N.; Ferreira-Silva, S.L.; Souza, L.S.B.d.; Araújo Júnior, G.d.N.; Souza, M.d.S.; Araújo, G.G.L.d.; Souza, C.A.A.d.; Silva, T.G.F.d. Genotypic differences relative photochemical activity, inorganic and organic solutes and yield performance in clones of the forage cactus under semi-arid environment. Plant Physiol. Biochem. 2021, 162, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Co-regulation of photosynthetic processes under potassium deficiency across CO2 levels in soybean: Mechanisms of limitations and adaptations. Photosynth. Res. 2018, 137, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Javornik, T.; Poljak, M.; Carović-Stanlo, K.; Lazarević, B. Common bean (Phaseolus vulgaris L.) gas exchange capacity under nutrient deficiency. J. Cent. Eur. Agric. 2023, 24, 216–224. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [PubMed]
| Macronutrients | Concentration (mmol/L) | Micronutrients | Concentration (μmol/L) |
|---|---|---|---|
| NO3− | 16.40 | Fe | 35 |
| NH4+ | 1.30 | B | 30 |
| P | 1.40 | Cu | 0.80 |
| K | 8 | Mn | 5 |
| Ca | 4.8 | Zn | 5 |
| Mg | 1.10 | Mo | 0.50 |
| S | 1.40 |
| Fluorescence Parameters | |
|---|---|
| FM | Maximal fluorescence from a dark-adapted leaf |
| FV | Maximal variable fluorescence from a dark-adapted leaf; FV = FM−F0 |
| FV/FM | Maximum quantum efficiency of PSII photochemistry |
| Vi | Relative variable fluorescence in phase I of the fluorescence induction curve |
| 1-Vi | Measure of relative amplitude of the IP phase in the OJIP transient, related to the size of the pools of final PSI electron acceptors |
| 1/Vi | Relative measure of the pool size of final electron acceptors of PSI |
| ABS/RC | Absorption flux (for PSII antenna chls) per reaction center (RC) |
| TR0/RC | Trapped energy flux per RC (at t = 0) |
| DI0/RC | Dissipated energy flux per RC (at t = 0) |
| PΙTOTAL | Performance index total for energy conservation from photons absorbed by PSII to the reduction in PSI end acceptors |
| PΙABS | Performance index for energy conservation from photons absorbed by PSII antenna |
| Sm | Normalized area above the OJIP curve |
| pH | EC | NO3− | PO43− | K+ | Ca2+ | Na+ | |
|---|---|---|---|---|---|---|---|
| HP | 6.61 ± 0.09 | 1.95 ± 0.05 a | 9.91 ± 0.54 a | 1.18 ± 0.48 a | 5.04 ± 0.34 a | 3.04 ± 0.21 a | 1.63 ± 0.05 |
| CAP | 6.78 ± 0.06 | 1.21 ± 0.04 b | 5.33 ± 0.37 b | 0.64 ± 0.14 b | 1.53 ± 0.17 b | 1.79 ± 0.23 b | 1.65 ± 0.05 |
| DCAP | 6.59 ± 0.07 | 2.06 ± 0.01 a | 9.97 ± 0.68 a | 1.24 ± 0.30 a | 5.05 ± 0.24 a | 3.28 ± 0.33 a | 1.74 ± 0.03 |
| WUE (kg Lettuce m−3 Water Used) | FUE (kg Lettuce kg−1 Fertilizers Used) | |
|---|---|---|
| HP | 75.83 | 9.31 |
| CAP | 49.01 | |
| DCAP | 57.34 | 26.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzinikolaou, M.; Mourantian, A.; Feka, M.; Levizou, E. Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production. Agronomy 2025, 15, 1782. https://doi.org/10.3390/agronomy15081782
Chatzinikolaou M, Mourantian A, Feka M, Levizou E. Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production. Agronomy. 2025; 15(8):1782. https://doi.org/10.3390/agronomy15081782
Chicago/Turabian StyleChatzinikolaou, Michalis, Anastasia Mourantian, Maria Feka, and Efi Levizou. 2025. "Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production" Agronomy 15, no. 8: 1782. https://doi.org/10.3390/agronomy15081782
APA StyleChatzinikolaou, M., Mourantian, A., Feka, M., & Levizou, E. (2025). Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production. Agronomy, 15(8), 1782. https://doi.org/10.3390/agronomy15081782






