Transcriptome and Metabolome Revealed Impacts of Zn Fertilizer Application on Flavonoid Biosynthesis in Foxtail Millet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Station Description
2.2. Experimental Materials
2.3. Experimental Design
2.4. Sample Collection
2.5. Methods
2.5.1. Measurement of Grain Quality
2.5.2. Metabolome Analysis
2.5.3. Transcriptome Analysis
2.6. Statistical Analysis
3. Results
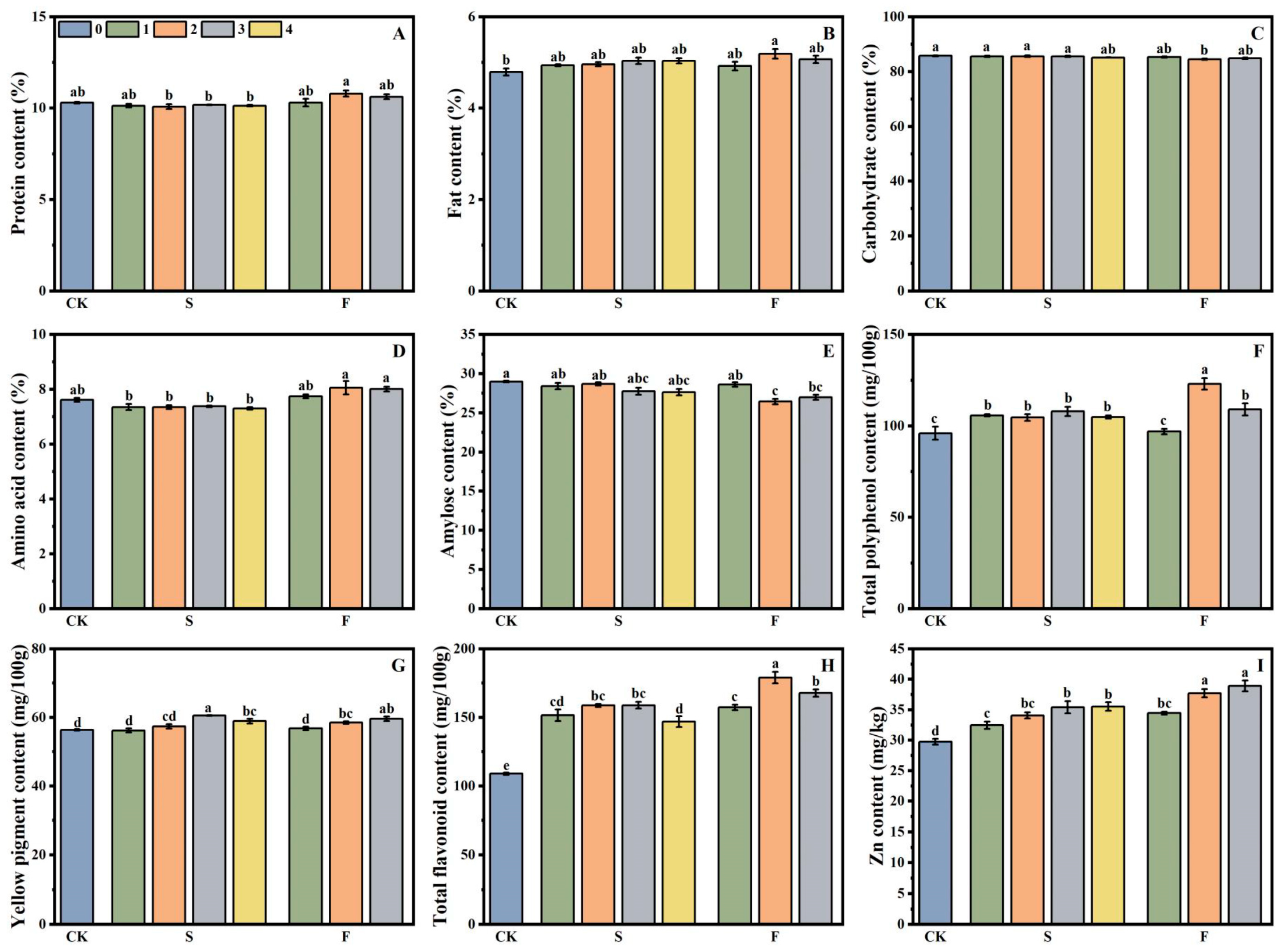
3.1. Effects of Grain Quality of Foxtail Millet Under Different Treatments
3.1.1. Nutrition Component
3.1.2. Functional Substance
3.1.3. Grain Zn Content
3.2. Effects of Foxtail Millet Grain Metabolites Under Different Treatments
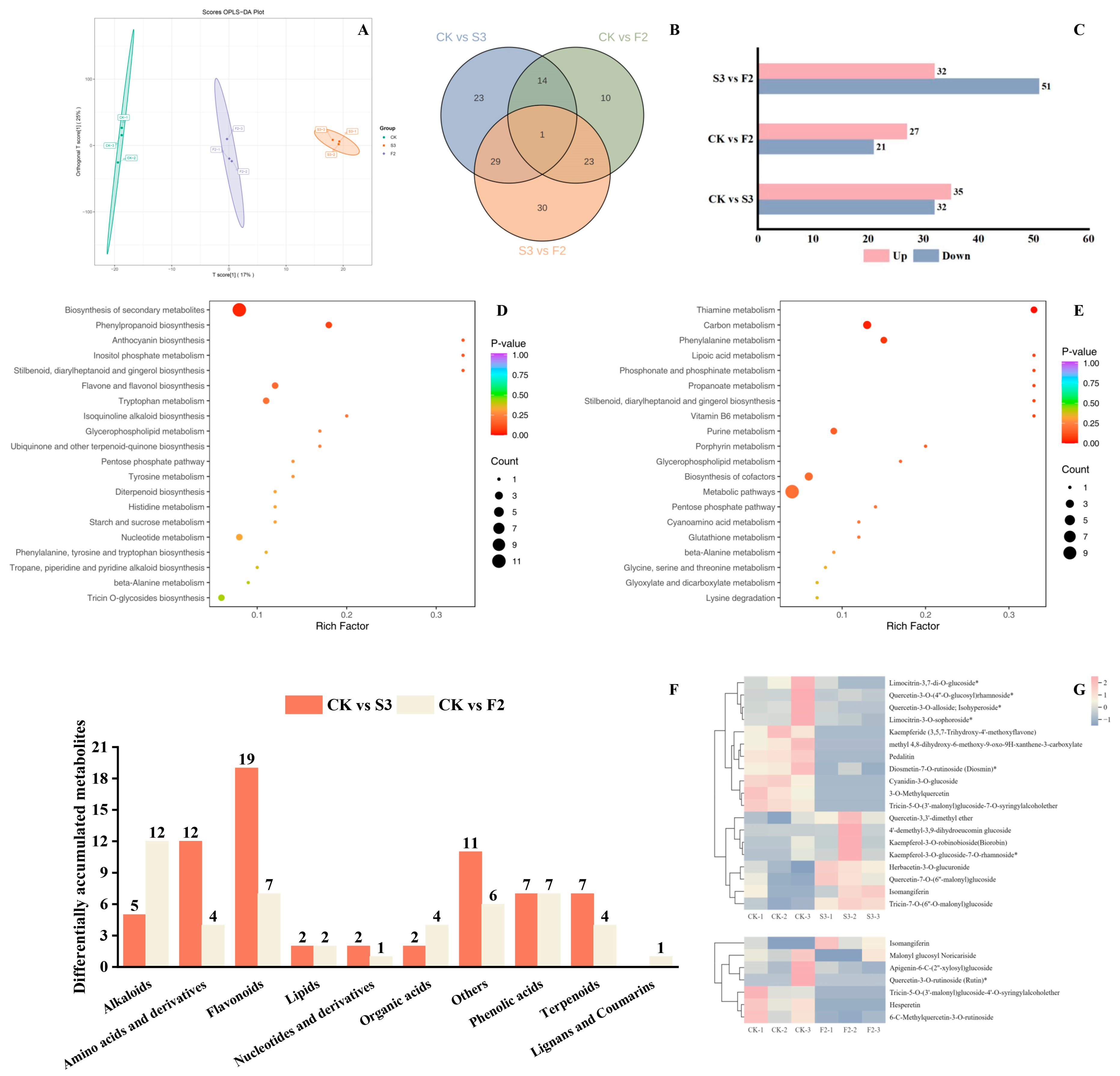
3.2.1. Overall Analysis of Metabolites
3.2.2. Identification and Analysis of DAMs
3.2.3. KEGG Pathway Analysis of DAMs
3.2.4. Flavonoid DAMs
3.3. Effects of Grain Transcriptome of Foxtail Millet Under Different Treatments
3.3.1. Overview of Transcriptome Data
3.3.2. Identification and Analysis of DEGs
3.3.3. GO Classification Analysis and KEGG Pathway Analysis of DEGs
3.3.4. Flavonoid DEGs
3.4. Flavonoid Biosynthesis Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, X.; Vitousek, P. An experiment for the world. Nature 2013, 497, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Xu, X.; Kuang, W.; Zhou, W.; Zhang, S.; Li, R.; Yan, C.; Yu, D.; Wu, S.; et al. Spatial patterns and driving forces of land use change in China during the early 21st century. J. Geogr. Sci. 2010, 20, 483–494. [Google Scholar] [CrossRef]
- Diao, X. Progresses in stress tolerance and field cultivation studies of orphan cereals in China. Sci. Agric. Sin. 2019, 52, 3943–3949. (In Chinese) [Google Scholar]
- Xiao, J.; Sun, Z.; Chen, G.; Liu, Z.; Xin, Z.; Kong, F. Evaluation of drought tolerance in different genotypes of foxtail millet during the entire growth period. Agron. J. 2022, 114, 340–355. [Google Scholar] [CrossRef]
- Sun, M.; Kang, X.; Wang, T.; Fan, L.; Wang, H.; Pan, H.; Yang, Q.; Liu, H.; Lou, Y.; Zhuge, Y. Genotypic diversity of quality traits in Chinese foxtail millet (Setaria italica L.) and the establishment of a quality evaluation system. Food Chem. 2021, 353, 129421. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.M.; Shen, Q. In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct. 2016, 7, 372–379. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Kalaiselvi, P.; Mahomoodally, M.F.; Zengin, G.; Abirami, A.; Srinivasan, G. Nutritional and functional roles of millets—A review. J. Food Biochem. 2019, 43, e12859. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Qie, Q.; Yang, Y.; Hou, S.; Wang, X.; Li, X.; Han, Y. Comparative analysis of flavonoid metabolites in foxtail millet (Setaria italica) with different eating quality. Life 2021, 11, 578. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Zhang, Y.; Yang, Y.; Sun, R.; Li, Y.; Gao, J.; Han, Y. Differential flavonoids and carotenoids profiles in grains of six poaceae crops. Foods 2022, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Ceasar, S.A.; Ignacimuthu, S. Zinc supply influences the root-specific traits with the expression of root architecture modulating genes in millets. J. Soil Sci. Plant Nutr. 2023, 23, 5527–5541. [Google Scholar] [CrossRef]
- Gustin, J.L.; Zanis, M.J.; Salt, D.E. Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol. Biol. 2011, 11, 76. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a bio-fortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.; Paknikar, K.; Rajwade, J. Nanocarrier-mediated foliar zinc fertilization in fluences expression of metal homeostasis related genes in flag leaves and enhances gluten content in durum wheat. PLoS ONE 2018, 13, e0191035. [Google Scholar] [CrossRef]
- Chen, M.; Shen, X.; Li, D.; Ma, L.; Dong, J.; Wang, T. Identification and characterization of MtMTP1, a Zn transporter of CDF family, in the Medicago truncatula. Plant Physiol. Biochem. 2009, 47, 1089–1094. [Google Scholar] [CrossRef]
- Silva, V.M.; Nardeli, A.J.; Mendes, N.A.d.C.; Rocha, M.d.M.; Wilson, L.; Young, S.D.; Broadley, M.R.; White, P.J.; dos Reis, A.R. Agronomic biofortification of cowpea with zinc: Variation in primary metabolism responses and grain nutritional quality among 29 diverse genotypes. Plant Physiol. Biochem. 2021, 162, 378–387. [Google Scholar] [CrossRef]
- Yu, B.G.; Chen, X.X.; Zhou, C.X.; Ding, T.B.; Wang, Z.H.; Zou, C.Q. Nutritional composition of maize grain associated with phosphorus and zinc fertilization. J. Food Compos. Anal. 2022, 114, 104775. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Gao, X.; Li, X.; Ren, T.; Cong, R.; Lu, J. Winter oilseed rape productivity and nutritional quality responses to zinc fertilization. Agron. J. 2014, 106, 1349–1357. [Google Scholar] [CrossRef]
- Erdem, H.; Sahin, O. Foliar Zinc Sprays Affected Yield and Bioactive Compounds of Granny Smith Apple. Int. J. Fruit Sci. 2021, 21, 670–680. [Google Scholar] [CrossRef]
- Son, Y.J.; Park, J.E.; Lee, N.; Ju, Y.W.; Pyo, S.H.; Oh, C.; Yoo, G.; Nho, C.W. Copper- or zinc-fortified nutrient solution in vertical farming system enriches copper or zinc and elevates phenolic acid and flavonoid contents in Artemisia annua L. Agronomy 2024, 14, 135. Agronomy 2024, 14, 135. [Google Scholar] [CrossRef]
- Wang, X.; Tian, D.; Zou, C. Yield responses of the main cereal crops to the application approaches and rates of zinc fertilizer in China. J. Plant Nutr. Fertil. 2014, 20, 998–1004. (In Chinese) [Google Scholar]
- Chen, J.; Ren, X.; Shen, Q. Application of NIR transmission spectroscopy with effective wavelength selection in non-destructive determination of essential amino acid content of foxtail millet. Qual. Assur. Saf. Crops Foods 2017, 9, 237–248. [Google Scholar] [CrossRef]
- Liu, J.; Shang, B.; Xing, X.; Zhang, D.; Chang, L.; Sun, H.; Duan, X. Comparison of four methods for the determination of the amylose content in foxtail millet. Food Sci. 2023, 44, 217–224. (In Chinese) [Google Scholar]
- Ma, K.; Zhao, L.; Zhao, X.; Li, X.; Dong, S.; Zhang, L.; Guo, P.; Yuan, X.; Diao, X. The relationship between ecological factors and commercial quality of high-quality foxtail millet “Jingu 21”. Food Res. Int. 2023, 163, 112225. [Google Scholar] [CrossRef]
- Sun, T.; Sun, J.; Liu, Y.; Ren, Y.; Li, Y.; Shi, C.; Nasr, A.; Tang, Z.; Abozeid, A. Metabolome and transcriptome analyses provide new insights into the mechanisms underlying the enhancement of medicinal component content in the roots of Acanthopanax senticosus (Rupr. et Maxim.) Harms through foliar application of zinc fertilizer. Front. Genet. 2023, 14, 1259674. [Google Scholar] [CrossRef]
- Utasee, S.; Jamjod, S.; Lordkaew, S.; Prom-U-Thai, C. Improve anthocyanin and zinc concentration in purple rice by nitrogen and zinc fertilizer application. Rice Sci. 2022, 29, 435–450. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, M.; Wang, Z.; Jiang, Z.; Zhu, Z.; Ding, J.; Wu, D.; Shu, X.J. Correction to Metabolite Profiling of a Zinc-Accumulating Rice Mutant. Agric. Food Chem. 2017, 65, 4875. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ding, Z.; Wang, H.; Song, L.; Jia, S.; Ma, D. Zinc stress affects ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 111, 318–328. [Google Scholar] [CrossRef]
- Han, S.; Huo, Y.; Li, H.; Han, H.; Hou, S.; Sun, Z.; Han, Y.; Li, H. Identification of regulatory genes related to flavonoids synthesis by weighted gene correlation network analysis in the panicle of foxtail millet. Acta Agron. Sin. 2022, 48, 1645–1657. (In Chinese) [Google Scholar]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. The effectiveness of foliar applications of synthesized zinc-amino acid chelates in comparison with zinc sulfate to increase yield and grain nutritional quality of wheat. Eur. J. Agron. 2013, 45, 68–74. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Zhou, Q.; Xu, F.; Zhang, W. Effects of exogenous zinc (ZnSO4·7H2O) on photosynthetic characteristics and grain quality of hybrid rice. Plant Physiol. Biochem 2023, 205, 108049. [Google Scholar] [CrossRef]
- Li, W.; Wen, L.; Chen, Z.; Zhang, Z.; Pang, X.; Deng, Z.; Liu, T.; Guo, Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chem. 2021, 357, 29791. [Google Scholar] [CrossRef] [PubMed]
- Mahasweta, C.; Biswapati, M.; Susmit, S.; Mrinmoy, R. Optimizing zinc fertilization technology in wheat for its sustainable production and improved human nutrition. Environ. Technol. 2024, 45, 2089–2098. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 61, 172–180. [Google Scholar] [CrossRef]

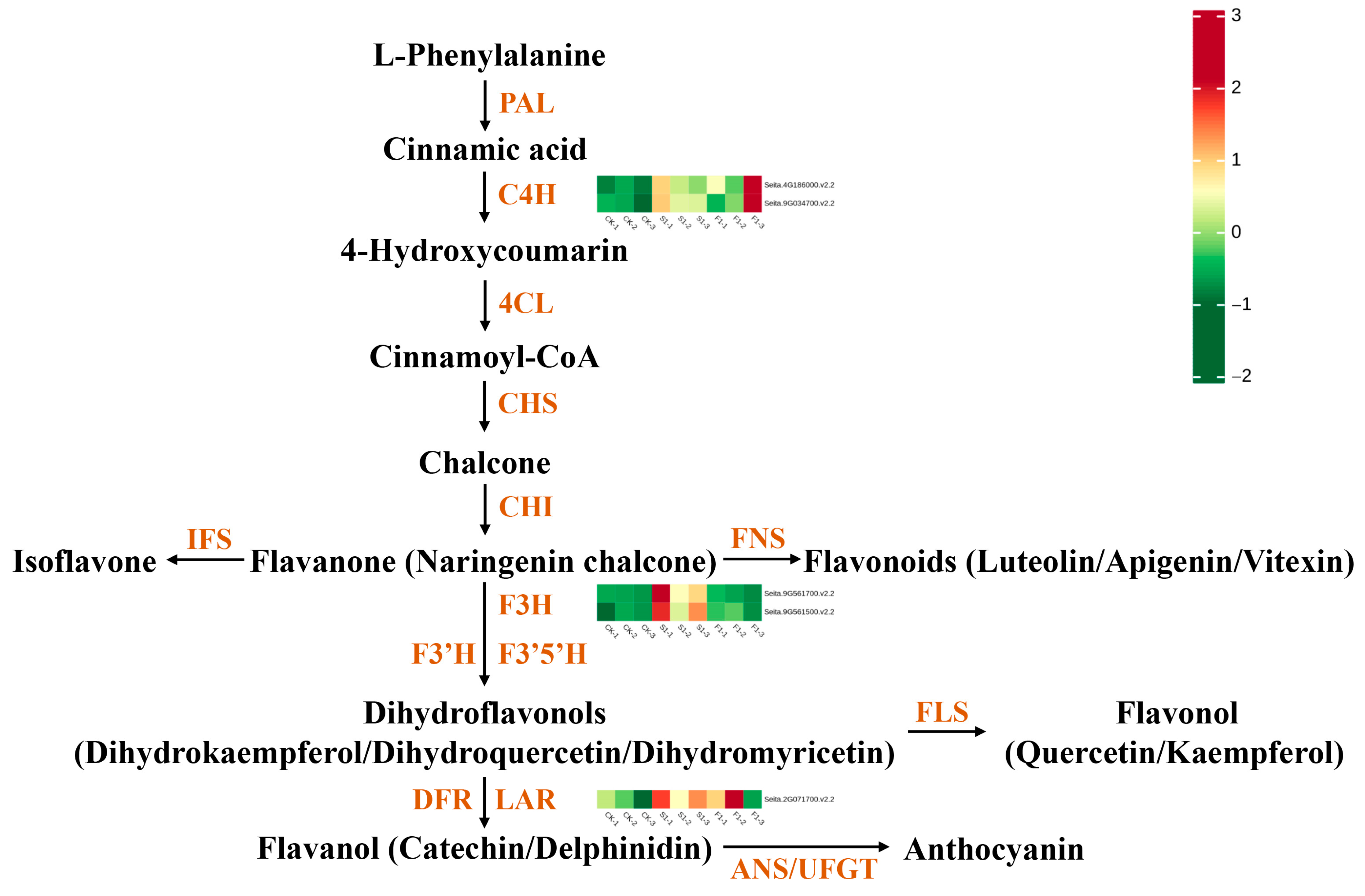
| Gene ID | Function Annotation | S3 vs. CK | F2 vs. CK |
|---|---|---|---|
| Seita.2G324600.v2.2 | Flavonoid metabolic and biosynthetic process | up | up |
| Seita.4G186000.v2.2 | Chalcone isomerase | up | |
| Seita.9G561700.v2.2 | Flavanone-3-hydroxylase | up | |
| Seita.2G289600.v2.2 | Flavonoid metabolic and biosynthetic process | up | |
| Seita.9G034700.v2.2 | Chalcone isomerase | up | |
| Seita.9G561500.v2.2 | Flavanone-3-hydroxylase | up | |
| Seita.9G142900.v2.2 | Flavonoid metabolic and biosynthetic process | up | |
| Seita.2G071700.v2.2 | Leucoanthocyanidin reductase | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Li, X.; Chen, X.; Wang, C.; Zhang, Z.; Yuan, X.; Chen, F.; Wen, X. Transcriptome and Metabolome Revealed Impacts of Zn Fertilizer Application on Flavonoid Biosynthesis in Foxtail Millet. Agronomy 2025, 15, 1767. https://doi.org/10.3390/agronomy15081767
Ma K, Li X, Chen X, Wang C, Zhang Z, Yuan X, Chen F, Wen X. Transcriptome and Metabolome Revealed Impacts of Zn Fertilizer Application on Flavonoid Biosynthesis in Foxtail Millet. Agronomy. 2025; 15(8):1767. https://doi.org/10.3390/agronomy15081767
Chicago/Turabian StyleMa, Ke, Xiangyu Li, Xiangyang Chen, Chu Wang, Zecheng Zhang, Xiangyang Yuan, Fu Chen, and Xinya Wen. 2025. "Transcriptome and Metabolome Revealed Impacts of Zn Fertilizer Application on Flavonoid Biosynthesis in Foxtail Millet" Agronomy 15, no. 8: 1767. https://doi.org/10.3390/agronomy15081767
APA StyleMa, K., Li, X., Chen, X., Wang, C., Zhang, Z., Yuan, X., Chen, F., & Wen, X. (2025). Transcriptome and Metabolome Revealed Impacts of Zn Fertilizer Application on Flavonoid Biosynthesis in Foxtail Millet. Agronomy, 15(8), 1767. https://doi.org/10.3390/agronomy15081767





