Allelopathic Effects of Moringa oleifera Lam. on Cultivated and Non-Cultivated Plants: Implications for Crop Productivity and Sustainable Agriculture
Abstract
1. Introduction
2. Review Methodology and Literature Search
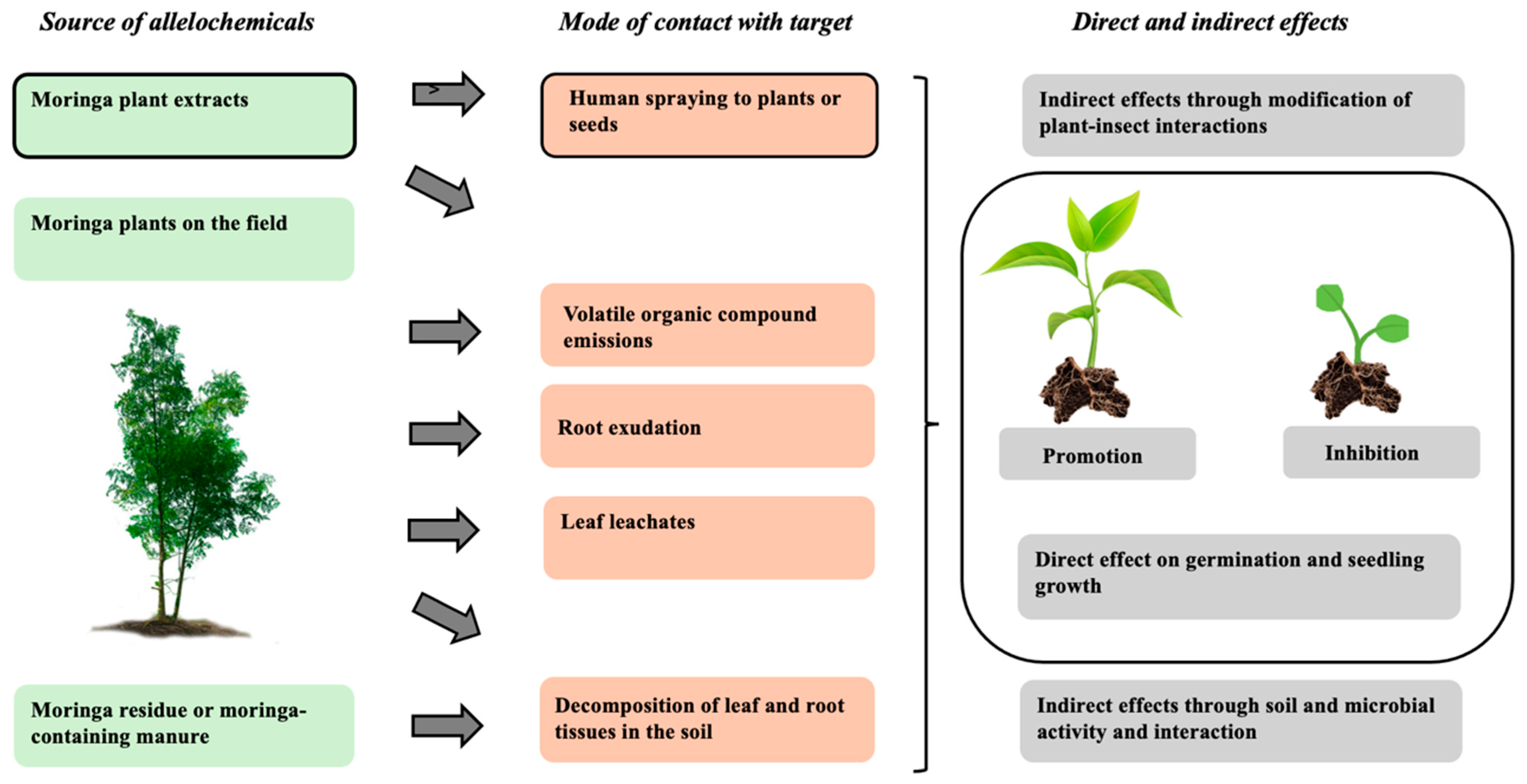
3. Mechanisms Behind Allelopathic Effects
4. Moringa Plant Extracts as Biostimulants
4.1. In Cereals
4.2. In Leguminous Plants
4.3. In Vegetable Crops
4.4. In Fruit Crops
4.5. In Other Cultivated Plant Species
5. Moringa Plant Extracts as Plant Growth Inhibitors
5.1. In Leguminous Plants
5.2. In Vegetable, Fruit and Other Crops
6. Potential Allelopathic Effect of Moringa on Weeds
7. Conclusions and Future Prospectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Csurhes, S.; Navie, S. Horseradish Tree Risk Assessment: Moringa oleifera; Queensland Government: Brisbane, Australia, 2016.
- Tahir, N.A.; Qader, K.O.; Azeez, H.A.; Rashid, J.S. Inhibitory allelopathic effects of Moringa oleifera Lamk plant extracts on wheat and Sinapis arvensis L. Allelopath. J. 2018, 44, 35–48. [Google Scholar] [CrossRef]
- Vyas, R.; Sharma, K. Phytotoxic activity of Moringa oleifera leaf extract on germination and seedling growth of tomato. Plant Arch. 2021, 21, 1231–1239. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 441–465. [Google Scholar]
- Kamanga, B.M.; McGill, C.; Halloy, S.; Bhuker, A.; Malik, A.; Clavijo McCormick, A. Combining climate models and risk assessment tools to evaluate the invasive potential of intentional plant introductions: A case study of Moringa oleifera in New Zealand. Discov. Plants 2025, 2, 195. [Google Scholar] [CrossRef]
- Ahmed, A.; El-Mahdy, A. Improving seed germination and seedling growth of maize (Zea mays, L.) seed by soaking in water and Moringa oleifera leaf extract. Curr. Chem. Lett. 2022, 11, 147–156. [Google Scholar] [CrossRef]
- Alshoaibi, A. Seed Germination, Seedling growth and photosynthetic responses to temperature in the tropical tree Moringa oleifera and Its Relative Desert, Moringa peregrina. Egypt. J. Bot. 2021, 61, 1631. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; Gaaliche, B.; Zarrelli, A.; Ghannem, M.; Mimoun, M.B. Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars. S. Afr. J. Bot. 2020, 130, 30–44. [Google Scholar] [CrossRef]
- Alshahrani, T.S.; Suansa, N.I. Application of biochar to alleviate effects of Allelopathic chemicals on seed germination and seedling growth. BioResources 2020, 15, 382–400. [Google Scholar] [CrossRef]
- Pandey, V.V.; Bhattacharya, A.; Pandey, A. Plant growth-promoting microbiomes: History and their role in agricultural crop improvement. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approache; Swapnil, P., Meena, M., Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press: Ghaziabad, India, 2023; pp. 1–44. [Google Scholar]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Chiapusio, G.; Weston, L.A. Chapter two—Allelopathy and the role of allelochemicals in plant defence. In Advances in Botanical Research; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. [Google Scholar]
- Mushtaq, W.; Siddiqui, M.B.; Hakeem, K.R. Mechanism of action of allelochemicals. In Allelopathy: Potential for Green Agriculture; Mushtaq, W., Siddiqui, M.B., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–66. [Google Scholar]
- Perveen, S.; Mushtaq, M.N.; Yousaf, M.; Sarwar, N. Allelopathic hormesis and potent allelochemicals from multipurpose tree Moringa oleifera leaf extract. Plant Biosyst. An Int. J. Deal. All Asp. Plant Biol. 2021, 155, 154–158. [Google Scholar] [CrossRef]
- Danilova, M.; Doroshenko, A.; Kudryakova, N.; Klepikova, A.; Shtratnikova, V.Y.; Kusnetsov, V. The crosstalk between cytokinin and auxin signaling pathways in the control of natural senescence of Arabidopsis thaliana leaves. Russ. J. Plant Physiol. 2020, 67, 1028–1035. [Google Scholar] [CrossRef]
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Stinson, K.; Klironomos, J. Novel weapons: Invasive plant suppresses fungal mutualists in america but not in its native europe. Ecology 2008, 89, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Clavijo McCormick, A. Invasive plants’ root extracts display stronger allelopathic activity on the germination and seedling growth of a new zealand native species than extracts of another native plant or conspecifics. J. Chem. Ecol. 2024, 50, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Buthelezi, D.; Ntuli, N.; Mugivhisa, L.; Gololo, S. Moringa oleifera Lam. seed extracts improve the growth, essential minerals, and phytochemical constituents of Lessertia frutescens L. Horticulturae 2023, 9, 886. [Google Scholar] [CrossRef]
- Tullu, M.S. Writing the title and abstract for a research paper: Being concise, precise, and meticulous is the key. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Affengruber, L.; van der Maten, M.M.; Spiero, I.; Nussbaumer-Streit, B.; Mahmić-Kaknjo, M.; Ellen, M.E.; Goossen, K.; Kantorova, L.; Hooft, L.; Riva, N.; et al. An exploration of available methods and tools to improve the efficiency of systematic review production: A scoping review. BMC Med. Res. Methodol. 2024, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.d.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.d.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Sousa, C.; Alves-Silva, J.; Salgueiro, L. Plant monoterpenes and essential oils as potential anti-ageing agents: Insights from preclinical data. Biomedicines 2024, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.; Kaur, M.; Gautam, C.; Kumar, R.; Tilgam, J.; Natta, S. Phenolic biosynthesis and metabolic pathways to alleviate stresses in plants. In Plant Phenolics in Abiotic Stress Management; Lone, R., Khan, S., Mohammed Al-Sadi, A., Eds.; Springer Nature: Singapore, 2023; pp. 63–87. [Google Scholar]
- Lacerda, J.W.F.; Uliana, M.P.; Bellete, B.S.; Vasconcelos, L.G.d.; Dall’Oglio, E.L.; Brocksom, T.J.; Vieira, L.C.C.; Sampaio, O.M. Evaluation of p-benzoquinones derivatives as post-emergent plant growth inhibitor/Avaliação de derivados p-benzoquinonas como inibidores pós-emergentes do crescimento vegetal. Braz. J. Dev. 2020, 6, 32516–32530. [Google Scholar] [CrossRef]
- Bibi, A.; Ullah, F.; Mehmood, S.; Bibi, K.; Khan, S.U.; Khattak, A.; Ullah Khan, R. Moringa oleifera Lam. leaf extract as bioregulator for improving growth of maize under mercuric chloride stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 469–475. [Google Scholar] [CrossRef]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Pervez, K.; Ullah, F.; Mehmood, S.; Khattak, A. Effect of Moringa oleifera Lam. leaf aqueous extract on growth attributes and cell wall bound phenolics accumulation in maize (Zea mays L.) under drought stress. Kuwait J. Sci. 2017, 44, 110–118. [Google Scholar]
- El-Rokiek, K.G.; Shehata, A.N.; El-Din, S.A.S.; Eid, R.A. Herbicidal potential and identification of allelochemicals from Moringa oleifera. Asian J. Plant Sci. 2022, 21, 154–162. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Adekanmi, A.A.; Adekanmi, S.A.; Adekanmi, O. Qualitative and quantitative phytochemical constituents of Moringa leaf. Int. J. Eng. Inf. Syst. 2020, 4, 10–17. [Google Scholar]
- Ahmed, M.E.; Elzaawely, A.A.; Al-Ballat, I.A. Moringa leaf extract stimulates growth and yield of cucumber (Cucumis sativus L.). Menoufia J. Plant Prod 2020, 5, 63–75. [Google Scholar] [CrossRef]
- Hanafy, R. Using Moringa olifera leaf extract as a bio-fertilizer for drought stress mitigation of Glycine max L. plants. Egypt. J. Bot. 2017, 57, 281–292. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Desamparados Soriano, M. Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous applications of Moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. Plant bioactives and redox signaling: (-)-Epicatechin as a paradigm. Mol. Asp. Med. 2018, 61, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Merwad, A.-R.M.A. Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 2018, 41, 425–431. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, M.N.; Khanum, M.; Zaman, G.; Ullah, M.A.; Farooq, U.; Waqas, M.; Ahmad, N.; Hano, C.; Abbasi, B.H. Effect of wide-spectrum monochromatic lights on growth, phytochemistry, nutraceuticals, and antioxidant potential of in vitro callus cultures of Moringa oleifera. Molecules 2023, 28, 1497. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Alam, P.; Sharaf-Eldin, M.A.; Alqarni, M.H. Simultaneous identification of rutin, chlorogenic acid and gallic acid in Moringa oleifera by densitometric high-performance thin-layer chromatography method. JPC—J. Planar Chromatogr. —Mod. TLC 2020, 33, 27–32. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Abdelnaser, A.; Ahmed, M.E.; Maswada, F.H.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with Moringa leaf extract. Arch. Agron. Soil Sci. 2017, 63, 687–699. [Google Scholar] [CrossRef]
- Arif, Y.Y.; Bajguz, A.; Hayat, S. Moringa oleifera extract as a natural plant biostimulant. J. Plant Growth Regul. 2023, 42, 1291–1306. [Google Scholar] [CrossRef]
- Delvin, E.; Levy, E. Chapter 47—Trace elements: Functions and assessment of status through laboratory testing. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Marzinke, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 851–864. [Google Scholar]
- Kawade, K.; Tabeta, H.; Ferjani, A.; Hirai, M.Y. The roles of functional amino acids in plant growth and development. Plant Cell Physiol. 2023, 64, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on Moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Bhuker, A.; Malik, A.; Punia, H.; McGill, C.; Sofkova-Bobcheva, S.; Mor, V.S.; Singh, N.; Ahmad, A.; Mansoor, S. Probing the phytochemical composition and antioxidant activity of Moringa oleifera under ideal germination conditions. Plants 2023, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pratibha; Pareek, S. Bioactive compounds of Moringa (Moringa Species). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, K.Y., Paek, H.N., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 1–22. [Google Scholar]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef] [PubMed]
- Zia, U.A.; Furqan, M.; Rashid, E.; Gul, S.; Dhaku, H.N.; Murtaza, G.; Haider, L.; Haider, S.Z.; Iqbal, M.; Aslam, S. Allelopathic effect of Moringa oleifera leaf extraction on growth of Maize (Zea mays). Plant Cell Biotechnol. Mol. Biol. 2021, 22, 12–23. [Google Scholar] [CrossRef]
- Pal, P.; Ansari, S.A.; Jalil, S.U.; Ansari, M.I. Chapter 1—Regulatory role of phytohormones in plant growth and development. In Plant Hormones in Crop Improvement; Khan, M.I.R., Singh, A., Poór, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–13. [Google Scholar]
- Yasmeen, A.; Basra, S.M.A.; Wahid, A.; Nouman, W.; Rehman, H. Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance. Turk. J. Bot. 2013, 37, 512–520. [Google Scholar] [CrossRef]
- Sakr, W.R.A.; El-Sayed, A.A.; Hammouda, A.M.; Deen, F.S.A.E. Effect of NPK, aloe gel and Moringa extracts on geranium plants. J. Hortic. Sci. Ornam. Plants 2018, 10, 1–16. [Google Scholar]
- Mona, H.S.; Ahlam, H.H.; Hamdah, A.; Shroug, S.A. Allelopathic effect of Moringa oleifera leaves extract on seed germination and early seedling growth of Faba Bean (Vicia faba L.). J. Agric. Technol. 2017, 13, 105–117. [Google Scholar]
- Muneeba; Khaliq, A.; Muhammad, F.; Shahzad, H.; Alharbi, S.A.; Alfarraj, S.; Arshad, M.; Akram, M.; Baoyi, Z. Hermetic effect of Moringa oleifera leaf extract mitigates salinity stress in maize by modulating photosynthetic efficiency, and antioxidant activities. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13862. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Nawaz, M.; Rehman, H.U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 2017, 24, 27601–27612. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Yasmeen, A.; Anjum, M.A.; Hussain, N. Exogenous application of growth enhancers mitigate water stress in wheat by antioxidant elevation. Front. Plant Sci. 2016, 7, 597. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Musa, D.; Mohammed, I. Exploring the potential of drumstick (Moringa oleifera) leaf extract as vegetative growth enhancer of guinea corn (Sorghum bicolor L.). Int. J. Curr. Sci. Stud. 2017, 1, 9–12. [Google Scholar]
- Khan, S.; Ibrar, D.; Bashir, S.; Rashid, N.; Hasnain, Z.; Nawaz, M.; Al-Ghamdi, A.A.; Elshikh, M.S.; Dvořáčková, H.; Dvořáček, J. Application of Moringa leaf extract as a seed priming agent enhances growth and physiological attributes of rice seedlings cultivated under water deficit regime. Plants 2022, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Maishanu, H.; Mainasara, M.; Yahaya, S.; Yunusa, A. The use of Moringa leaves extract as a plant growth hormone on cowpea (Vigna anguiculata). Path Sci. 2017, 3, 3001–3006. [Google Scholar] [CrossRef]
- Hoque, T.; Abedin, M.; Kibria, M.; Jahan, I.; Hossain, M.A. Application of Moringa leaf extract improves growth and yield of Tomato (Solanum lycopersicum) and Indian Spinach (Basella alba). Plant Sci. Today 2021, 9, 137–143. [Google Scholar] [CrossRef]
- Hoque, T.; Jahan, I.; Ferdous, G.; Abedin, M. Foliar application of Moringa leaf extract as a bio-stimulant on growth, yield and nutritional quality of brinjal. J. Agric. Food Environ. 2020, 1, 94–99. [Google Scholar] [CrossRef]
- Yaseen, A.; Madar, Á.; Vojnović, Đ.; Takacs-Hajos, M. Examining the optimal amount of moringa leaf extract to improve the morphological and inner quality of cabbage (Brassica oleracea var. capitata). J. Food Qual. 2023, 2023, 3210253. [Google Scholar] [CrossRef]
- Mehdawe, A.; Mahadeen, A.; Al-ramamneh, E.A.-D. Foliar application of Moringa leaf extracts affects growth, yield and mineral composition of pepper (Capsicum annuum L.) Under Greenhouse Conditions. J. Ecol. Eng. 2023, 24, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hala, H.; El-Nour, A.; Ewais, N.A. Effect of Moringa oleifera leaf extract (MLE) on pepper seed germination, seedlings improvement, growth, fruit yield and its quality. Middle East J. Agric. Res. 2017, 6, 448–463. [Google Scholar]
- Nasir, M.; Khan, A.; Basra, S.; Malik, A. Improvement in growth, productivity and quality of ‘Kinnow’mandarin fruit after exogenous application of Moringa olifera leaf extract. S. Afr. J. Bot. 2020, 129, 263–271. [Google Scholar] [CrossRef]
- Ismail, S.; Kamal, S.; Shimaa, G. Efficiency of foliar spraying with Moringa leaves extract and potassium nitrate on yield and quality of strawberry in sandy soil. Int. J. Agric. Stat. Sci. 2021, 17, 383–398. [Google Scholar]
- Ali, M.A.; Harhash, M.M.; Bassiony, S.S.; Felifal, M.M.S. Effect of foliar spray of sitofex, moringa leaves extract and some nutrients on productivity and fruit quality of “Thompson seedless” grapevine. J. Adv. Agric. Res. 2020, 25, 112–129. [Google Scholar]
- Rashid, N.; Wahid, A.; Ibrar, D.; Irshad, S.; Hasnain, Z.; Al-Hashimi, A.; Elshikh, M.S.; Jacobsen, S.; Khan, S. Application of natural and synthetic growth promoters improves the productivity and quality of quinoa crop through enhanced photosynthetic and antioxidant activities. Plant Physiol. Biochem. 2022, 182, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Irshad, J.; Bashir, S.; Khan, S.; Yousaf, M.; Shah, A.N. Comparative study of water extracts of Moringa leaves and roots to improve the growth and yield of sunflower. S. Afr. J. Bot. 2020, 129, 221–224. [Google Scholar] [CrossRef]
- Ali, A.; Abbas, N.; Maqbool, M.; Haq, T.; Ahmad, M.; Mahmood, R. Influence of soil applied Moringa leaf extract on vegetative growth of Cyperus rotundus. Asian J. Agric. Biol. 2015, 3, 79–82. [Google Scholar]
- Nouman, W.; Basra, S.M.A.; Siddiqui, M.T.; Khan, R.A.; Mehmood, S. Seed priming improves the growth and nutritional quality of rangeland grasses. Int. J. Agric. Biol. 2012, 14, 751–756. [Google Scholar]
- Mvumi, C.; Tagwira, F.; Chiteka, A.Z. Effect of Moringa extract on growth and yield of maize and common beans. Greener J. Agric. Sci. 2013, 3, 59–62. [Google Scholar] [CrossRef]
- Biswas, A.; Hoque, T.; Abedin, M. Effects of Moringa leaf extract on growth and yield of maize. Progress Agric. 2016, 27, 136–143. [Google Scholar] [CrossRef][Green Version]
- Brockman, H.; Brennan, R.; van Burgel, A. The impact of phytohormone concentration in Moringa oleifera leaf extract on wheat yield and components of yield. J. Plant Nutr. 2019, 43, 396–406. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A.; Arif, M.; Hussain, N.; Malik, W.; Qadir, I. Morphological, growth and yield response of cotton to exogenous application of natural growth promoter and synthetic growth retardant. Int. J. Agric. Biol. 2016, 18, 1109–1121. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of Moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Abusuwar, A.O.; Abohassan, R.A. Effect of Moringa olifera leaf extract on growth and productivity of three cereal forages. J. Agric. Sci. 2017, 9, 236–243. [Google Scholar] [CrossRef][Green Version]
- Phiri, C.; Mbewe, D. Influence of Moringa oleifera leaf extracts on germination and seedling survival of three common legumes. Int. J. Agric. Biol. 2010, 12, 315–317. [Google Scholar]
- Kumar, N.; Singh, H.; Giri, K.; Kumar, A.; Joshi, A.; Yadav, S.; Singh, R.; Bisht, S.; Kumari, R.; Jeena, N.; et al. Physiological and molecular insights into the allelopathic effects on agroecosystems under changing environmental conditions. Physiol. Mol. Biol. Plants 2024, 30, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Minamikawa, M.F.; Pratama, B.B.; Koyama, S.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Igawa, T. Autonomous differentiation of transgenic cells requiring no external hormone application: The endogenous gene expression and phytohormone behaviors. Front. Plant Sci. 2024, 15, 1308417. [Google Scholar] [CrossRef] [PubMed]
- Thanaa, S.H.M.; Kassim, N.E.; Abou-Rayya, M.S.; Abdalla, A.M. Influence of foliar application with Moringa (Moringa oleifera L.) leaf extract on yield and fruit quality of hollywood plum cultivar. J. Hortic. 2017, 4, 193. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira Junior, J.C.d.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R.d. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Bio. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Khan, S.U.; Mehmood, T.; Ahmed, W.; Rafique, M. Exploring the allelopathic potential of plant extracts for weed suppression and productivity in wheat (Triticum aestivum L.). Gesunde Pflanz. 2021, 73, 29–37. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Hossain, M.M.; Miah, G.; Ahamed, T.; Sarmin, N.S. Allelopathic effect of Moringa oleifera on the germination of Vigna radiate. Int. J. Agric. Crop Sci. 2012, 4, 114–121. [Google Scholar]
- Abou-Zeid, H.M.; El-Darier, S. Biological interactions between Moringa oleifera Lam. and two common food intercrops: Growth and some physiological attributes. Int. J. Adv. Res. 2014, 2, 823–836. [Google Scholar]
- Mangal, K.; Bhat, J.; Ajay, K.; Pragati, S. Allelopathic effect of aqueous leaves extract of Moringa oleifera L. on seedling growth of Cicer arietinum L. Afr. J. Agric. Res. 2013, 8, 1028–1032. [Google Scholar] [CrossRef][Green Version]
- Waris, M.; Khan, M.A.; Fawad, M.; Jafer, N.; Hussain, R.; Ahmad, H. Allelopathic effect of Moringa oleifera L. aqueous extract on the germination and seedling growth of okra (Abelmoschus esculentus L.). Pak. J. Weed Sci. Res. 2024, 30, 121. [Google Scholar]
- Piyatida, P.; Kato-Noguchi, H. Screening of allelopathic activity of eleven Thai medicinal plants on seedling growth of five test plant species. Asian J. Plant Sci. 2010, 9, 486–491. [Google Scholar] [CrossRef][Green Version]
- Vélez-Gavilán, J. Cabicompendium.34868, CABI Compendium, CABI International, Moringa oleifera (Horse Radish Tree), (2022). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.34868 (accessed on 13 July 2025).
- Ahmed, H.; Darier, S.E.; Migahid, M.; Belkasem, K. Biological activity of Moringa oleifera Lam. on Citrullus lanatus (Thumb) in sustainable agriculture practices. Adv. Environ. Biol. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Bio/Technol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Tahir, N.A.; Majeed, H.O.; Azeez, H.A.; Omer, D.A.; Faraj, J.M.; Palani, W.R.M. Allelopathic Plants: 27. Moringa species. Allelopath. J. 2020, 50, 35–46. [Google Scholar] [CrossRef]
- Alyssa, M.B.; Tamara, L.M. Inhibition of the general soil microbial community by allyl-isothiocyanate and benzyl-isothiocyanate. BIOS 2015, 86, 31–37. [Google Scholar] [CrossRef]
- Azam, S.; Nouman, W.; Rehman, U.-u.; Ahmed, U.; Gull, T.; Shaheen, M. Adaptability of Moringa oleifera Lam. under different water holding capacities. S. Afr. J. Bot. 2020, 129, 299–303. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Pan, C.; Gong, C.; Wang, Y.; Liu, X.; Yu, Y. Drought resistance of Camellia oleifera under drought stress: Changes in physiology and growth characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, C.; Jiang, X.; Zhu, J.; Gao, X.; Yu, C. Impact of cold stress on leaf structure, photosynthesis, and metabolites in Camellia weiningensis and C. oleifera seedlings. Horticulturae 2022, 8, 494. [Google Scholar] [CrossRef]
- El-Rokiek, K.G.; Eid, R.A.; Shehata, A.N.; El-Din, S.A.S. Evaluation of using Moringa oleifera on controlling weeds. I. Effect of leaf and seed water extracts of Moringa oleifera on broad and grassy weed associated Narcissus tazetta L. Agric. Eng. Int. CIGR J. 2017, 2017, 45–52. [Google Scholar]
- Dessalegn, E.; Rupasinghe, H.P.V. Phenolic compounds and in vitro antioxidant activity of Moringa stenopetala grown in South Ethiopia. Int. J. Food Prop. 2021, 24, 1681–1692. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, Z.; Pang, W.; Zhang, L.; Wen, Z.; Zhao, Y.; Sun, J.; Wang, Z.-Y.; Yang, C. Effects of autotoxicity and allelopathy on seed germination and seedling growth in Medicago truncatula. Front. Plant Sci. 2022, 13, 908426. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The role of membrane transporters in plant growth and development, and abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef] [PubMed]
- Chandini; Kumar, R.; Kumar, R.; Prakash, O. The Impact of chemical fertilizers on our environment and ecosystem. In Research Trends in Environmental Sciences; Nova Science Pub Inc: Hauppauge, NY, USA, 2019; pp. 69–86. [Google Scholar]
- Oluwafemi, A.B. Allelopathic effects of Moringa oleifera on the germination and seedling survival of Euphorbia heterophylla L. Glob. J. Biol. Agric. Health Sci. 2014, 3, 195–198. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Secondary metabolites, ferulic acid and p-hydroxybenzoic acid induced toxic effects on photosynthetic process in Rumex acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.G.; Sahu, L.K.; Gupta, M.; Mir, B.A.; Gajbhiye, T.; Dubey, R.; Clavijo McCormick, A.; Pandey, S.K. Environmental factors affecting monoterpene emissions from terrestrial vegetation. Plants 2023, 12, 3146. [Google Scholar] [CrossRef] [PubMed]
- Clavijo McCormick, A. Can plant–natural enemy communication withstand disruption by biotic and abiotic factors? Ecol. Evol. 2016, 6, 8569–8582. [Google Scholar] [CrossRef] [PubMed]
| Compound Class | Key Compounds | Biological Role | Allelopathic Potential | Ref. |
|---|---|---|---|---|
| Phenolic acids | Chlorogenic, gallic, ferulic, tannic acids, and coumarins. | Antioxidant, allelopathic, signaling molecules. | Stimulatory at low dose (growth promoter); root induction; inhibitory at high doses (inhibit growth). | [30,31] |
| Flavonoids | Quercetin, kaempferol, rutin, naringenin. | ROS scavenging, UV protection, auxin transport molecules. | Variable; function as growth enhancers or inhibitors of competing species via ROS modulation. | [30] |
| Glucosinolates/Isothiocyanates | Benzyl glucosinolate/isothiocyanate. | Allelopathic, detoxifying enzyme induction. | Suppression of seed germination/DNA alkylation, enzyme inhibition. | [32,33] |
| Tannins | Catechin, epicatechin. | Defense compounds, metal chelators. | Inhibitory effect: interfere with nutrient uptake and enzyme activity. | [16] |
| Alkaloids | Moringine, moringinine. | Modulation of stress response, antimicrobial. | Allelopathic inhibition: affect cell division and germination in sensitive species. | [33] |
| Saponins | Triterpenoid saponin. | Antimicrobial, membrane interaction. | Inhibit growth in some plant species. | [34] |
| Amino acids | Arginine, proline, glutamic acids. | Growth regulators, hormone precursors, osmoprotectants. | Stimulatory effect: promote resilience in intercropping species via root exudation. | [35,36] |
| Minerals | Fe, Zn, Ca, Mg, K, Mn, B. | Enzyme cofactors, chlorophyll structure, osmoregulation. | Indirect allelopathy through soil nutrient alteration (enhance or inhibit growth). | [21,37] |
| Hormones (Cytokinin) | Zeatin and its derivatives, gibberellins. | Promote cell division, shoot development. | Positive allelopathy: promotes growth of plants in proximity. | [38,39] |
| Vitamins (antioxidants) | Ascorbic acid (vit. C), α-tocopherol, β-carotene. | Maintain redox balance, protect membrane and enzymes. | Stimulatory to receiving plants through oxidative stress reduction. | [40,41] |
| Source | Concentration | Observed Effects and Target Crop | Ref. |
|---|---|---|---|
| Cereal Crops | |||
| Aq. MLE | 4:1, 3, 25% (v/v) | Improve growth, yield, and stress tolerance in maize (Zea mays). | [31,56,59] |
| Aq. MLE | 3% (w/v) | Improve growth, yield, and photosynthetic activities in wheat (Triticum aestivum). | [60,61] |
| Aq. MLE | 100% (v/v) | Improved growth parameters on sorghum (Sorghum bicolor). | [62] |
| Aq. MLE | 3% (v/v) | Enhanced physiological, biochemical, and yield parameters rice (Oryza sativa). | [63] |
| Leguminous Crops | |||
| Aq. MLE | 1:30 (w/v) | Accelerated growth of cowpeas (Vigna unguiculata). | [64] |
| Aq. MLE | 1:20 (w/v) | Speeded and supported an antioxidant system and tolerance under stress in common/snap beans (Phaseolus vulgaris). | [38] |
| Aq. MLE | 1, 2, 3, and 4% | Increased yield and nutrient in pea plants (Pisum sativum) and mustard spinach (Brassica rapa var. perviridis). | [41] |
| Vegetable Crops | |||
| Aq. WPE | 10% (w/v) | Improved growth parameters and yield of spinach (Basella alba) and tomatoes (Solanum lycopersicum). | [65] |
| Aq. MLE | 1:30 (w/v) | Enhanced growth, fruit weight, and nutrient contents of eggplant/brinjal (Solanum melongen). | [66] |
| Aq. MLE | 10% (v/v) | Influenced growth, photosynthetic activity, and nutrient absorption in cabbage (Brassica oleracea var. capitata). | [67] |
| Aq./Ethanol MLE | 1:10 (v/v) | Influenced vegetative growth and increased yield components in sweet pepper (Capsicum annum var. annum). | [68,69] |
| Aq. MLE | 1:20 (w/v) | Enhanced the growth and development of cucumber (Cucumis sativum). | [35] |
| Fruit Crops | |||
| Aq. MLE | 3% (w/v) | Improved yield and nutrient contents of kinnow mandarin (Citrus nobilis × Citrus deliciosa). | [70] |
| Aq. MLE | 6% (w/v) | Enhanced fruit weight and anthocyanin strawberry fruits (Fragaria × ananassa) and plums (Prunus domestica). | [71] |
| Aq. MLE | 3.5% (v/v) | Improved quality and nutritional contents of grapevine (Vitis vinifera). | [72] |
| Other Cultivated Plants | |||
| Aq. MLE | 3% (w/v) | Improved growth, biochemical attributes of quinoa (Chenopodium quinoa). | [73] |
| Aq. MLE | 50% (w/v) | Increased the yield and the yield components sunflower (Helianthus annuus). | [74] |
| Aq. MLE | 100% (v/v) | Promoted growth of nut sedge (Cyperus rotundus). | [75] |
| Aq. MLE | 30% (w/v) | Enhancing seed germination and seedling vigour of barnyard grass (Echinochloa crusgalli), buffel-grass (Cenchrus ciliaris), and blue panic grass (Panicum antidotale). | [76] |
| Ethanol MSE | 2,4, 6, 8% (v/v) | Improved growth and nutrient contents in cancer bush (Lessertia frutescens). | [21] |
| Source | Concentration | Target Crop and Observed Effects | Ref |
|---|---|---|---|
| Leguminous Crops | |||
| Aq. MRE | 10% (v/v) | Reduced growth parameters of mung beans (Vigna radiata). | [93] |
| Aq. MLE | 5–10% (w/v) | Inhibitory effects broad beans (Vicia faba) and chickpea (Cicer arietinum). | [60,94,95] |
| Aq. MLE | 1:10 (w/v) | Reduced germination and survival of cowpea (Vigna unguiculata) and groundnuts (Arachis hypogaea). | [84] |
| Vegetable, Fruit and Other Crops | |||
| Aq. MLE | 0.45–0.90 mg·mL−1 | Reduced germination and seedling growth of wild mustard (Synapsis arvensis). | [2] |
| Aq. MPE | >10% (v/v) | Inhibited shoot and root length of curly/garden cress (Lepidium sativum). | [17] |
| Aq. MPE | 100 (v/v) | Inhibited germination of okra (Abelmoschus esculentus). | [96] |
| Methanol MPE | 0.03, 0.01, and 0.1 g·mL−1 | Inhibited the growth of lettuce (Lactuca sativa) and curly cress (Lepidium sativum). | [97] |
| Aq. MLE | 25 mg·mL−1 | Reduce yield of eggplant (Solanum melongena) and sweet corn (Zea mays). | [98] |
| Aq. MLE | 2.5–10% (w/v) | Suppression of germination and growth of watermelon (Citrullus lanatus). | [99] |
| Ethanol MLE | 20–25 g·mL−1 | Inhibited germination and growth of tomatoes (Solanum lycopersicum). | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamanga, B.M.; Cartmill, D.L.; McGill, C.; Clavijo McCormick, A. Allelopathic Effects of Moringa oleifera Lam. on Cultivated and Non-Cultivated Plants: Implications for Crop Productivity and Sustainable Agriculture. Agronomy 2025, 15, 1766. https://doi.org/10.3390/agronomy15081766
Kamanga BM, Cartmill DL, McGill C, Clavijo McCormick A. Allelopathic Effects of Moringa oleifera Lam. on Cultivated and Non-Cultivated Plants: Implications for Crop Productivity and Sustainable Agriculture. Agronomy. 2025; 15(8):1766. https://doi.org/10.3390/agronomy15081766
Chicago/Turabian StyleKamanga, Blair Moses, Donita L. Cartmill, Craig McGill, and Andrea Clavijo McCormick. 2025. "Allelopathic Effects of Moringa oleifera Lam. on Cultivated and Non-Cultivated Plants: Implications for Crop Productivity and Sustainable Agriculture" Agronomy 15, no. 8: 1766. https://doi.org/10.3390/agronomy15081766
APA StyleKamanga, B. M., Cartmill, D. L., McGill, C., & Clavijo McCormick, A. (2025). Allelopathic Effects of Moringa oleifera Lam. on Cultivated and Non-Cultivated Plants: Implications for Crop Productivity and Sustainable Agriculture. Agronomy, 15(8), 1766. https://doi.org/10.3390/agronomy15081766







