3.2. Soil Carbon, Nitrogen, and Enzyme Dynamics
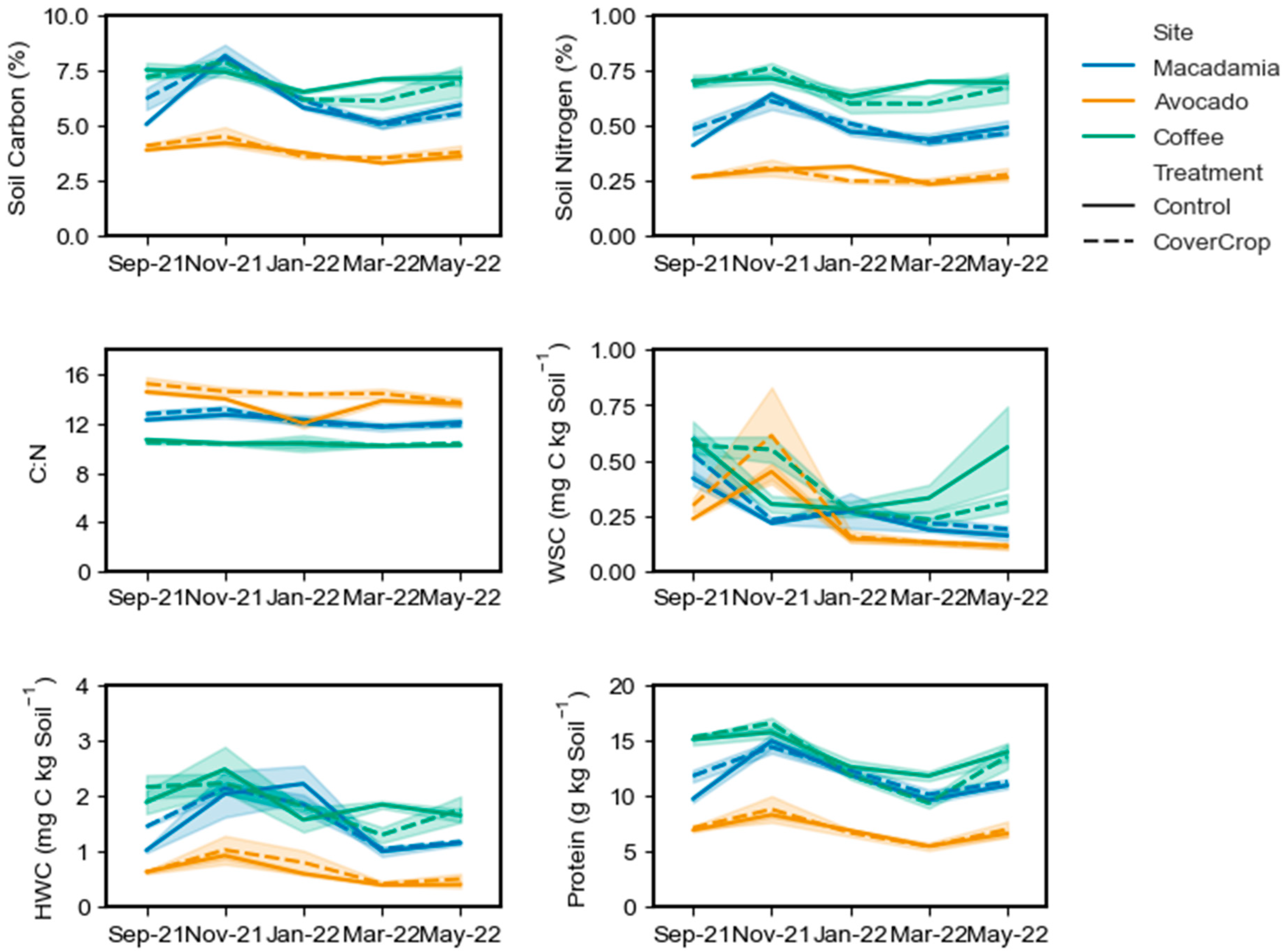
For SOC, there was no significant treatment effect at any site (
Table 6), however there was a significant effect of sample time. For the macadamia site, November 2021 had significantly higher (
p < 0.001) SOC than all other time points with a site mean of 8.12% (
Figure 2). There were no other differences between sampling time with March 2022 having the lowest value of 5.08% (
Figure 2). At the avocado site, November 2021 had significantly higher (
p < 0.02) TC (4.38%) than January 2022 (3.70%), March 2022 (3.42%), and May 2022 (3.72%) (
Figure 2). September 2021 (4.01%) had significantly higher (
p = 0.03) TC than March 2022 (
Figure 2). For the coffee site, October and September 2021 sampling returned site mean SOC values of 7.67% and 7.36%; these values were significantly higher than January 2022, where the site mean was 6.37% (
Figure 2).
SN showed a similar trend, with no treatment difference, but a significant temporal response at all sites (
Table 6). At the macadamia site, SN was highest (
p < 0.01) in October with a site mean of 0.63%, compared to values of 0.43–0.48% in other months (
Figure 2). At the avocado site, the only two significantly different time points (
p = 0.01) were November 2021 (0.31%) and March 2022 (0.24%) (
Figure 2). At the coffee site, only November 2021 (0.74%) and January 2022 (0.62%) were significantly different (
p = 0.02). The CN ratio showed no treatment response at the macadamia sites but did vary significantly over time. The site mean of 13.0 in November 2021, was significantly higher (
p < 0.05) than January 2022 (12.2), March 2022 (11.8), and May 2022 (12.0) (
Figure 2). September 2021 (12.5) was also significantly higher (
p = 0.04) than March 2022. At the avocado site, there was a significant effect of treatment and time, as well as a significant interaction between the two for CN ratio (
Table 6). Overall, the control mean of 13.6 was significantly lower (
p < 0.01) than the cover crop treatment mean of 14.5. This was driven by a mean of 12 in the control treatment which was significantly lower than all other timepoints (
Figure 2). At the coffee site, there was treatment or temporal response of CN ratio with site means ranging from 10.2 to 10.6 (
Figure 2).
At the macadamia and avocado sites, there was no treatment effect on WSC but significant variation over time (
Table 6). At the macadamia site, September 2021 had a significantly higher site mean (0.47 mg C kg soil
−1;
p < 0.01) than all other sample times where mean values ranged from 0.18 to 0.27 mg C kg soil
−1 (
Figure 2). At the avocado site, WSC was highest in November 2021 with a mean of 0.53 mg C kg soil
−1 (
Figure 2). This was significantly higher (
p < 0.01) than all other time points where site mean ranged from 0.11 to 0.27 mg C kg soil
−1 (
Figure 2). At the coffee site, there was a significant response of WSC over time, but also a sampling time and treatment interaction (
Table 6). This was driven by WSC in the control treatment declining from 0.60 mg C kg soil
−1 in September 2021 to 0.30 mg C kg soil
−1 in November 2021, while WSC in the cover crop treatment was not significantly different in November 2021 (0.55 mg C kg soil
−1) to September 2021 (0.57 mg C kg soil
−1) (
Figure 2). By January 2022, there were no differences between treatments with means decreasing to 0.27 and 0.28 mg C kg soil
−1 in the control and cover crop treatments, respectively (
Figure 2). However, WSC increased to 0.56 mg C kg soil
−1 in the control in May 2022, while remaining at a mean of 0.31 mg C kg soil
−1 in the cover crop treatment (
Figure 2).
This trend was not matched in HWC, with no treatment response at any site, but all sites having significant temporal variation (
Table 6). At the macadamia site, HWC was highest in November 2021 and January 2022, with means of 2.08 and 2.03 mg C kg soil
−1 respectively (
Figure 2). These values were higher than all other time points (
p < 0.01) with values ranging from 1.02 mg C kg soil
−1 (March) to 1.24 mg C kg soil
−1 (September) (
Figure 2). At the avocado site, site mean HWC in November 2021 (0.98 mg C kg soil
−1) was significantly higher (
p < 0.01) than all other time points (0.41–0.63 mg C kg soil
−1) except January 2022 (0.70 mg C kg soil
−1) (
Figure 2). HWC was not different between January 2022 and the other months (
p > 0.1) (
Figure 2). At the coffee site, there was no difference (
p = 0.54) in site mean HWC between September 2021 (2.02 mg C kg soil
−1) and November 2021 (2.35 mg C kg soil
−1). November 2021, however, was significantly higher (
p < 0.05) than all other months where means ranged from 1.57 to 1.70 mg C kg soil
−1 (
Figure 2).
Soil protein showed a treatment effect as well as significant temporal variation at the macadamia site (
Table 6). The treatment effect was driven by higher soil protein in September 2021 (11.79 mg C kg soil
−1 compared to 9.72 mg C kg soil
−1 in the control) and an overall higher mean of 12.0 mg C kg soil
−1 in the cover crop treatment compared to 11.4 mg C kg soil
−1 in the control (
Figure 2). Aside from the September time point, there were no significant differences between treatments. The temporal effect was driven by the highest values being observed in November 2021 (
p < 0.01;
Figure 2). At the avocado site, there was only a significant effect with sample time. The site mean of 8.56 mg C kg soil
−1 in November 2021 was higher than all other sample times (
p < 0.01). September 2021 (7.04 mg C kg soil
−1) January 2022 (6.81 mg C kg soil
−1) and May 2022 were not significantly different (
p > 0.9), with March 2022 (5.47 mg C kg soil
−1) significantly lower than September 2021 and January 2022 (
p < 0.05). At the coffee site, there was a significant interaction between treatment and time of sampling on soil protein (
Table 6). The temporal effect is driven by higher values across the September to January period with mean values varying from 12.60 to 15.72 mg C kg soil
−1 in the control, and 11.87 to 16.55 mg C kg soil
−1 in the cover crop treatment. There was then a general trend towards lower values in the cover crop treatment from January 2022 to May 2022; however, there were no individual treatment differences at the same time point (
p = 0.15).
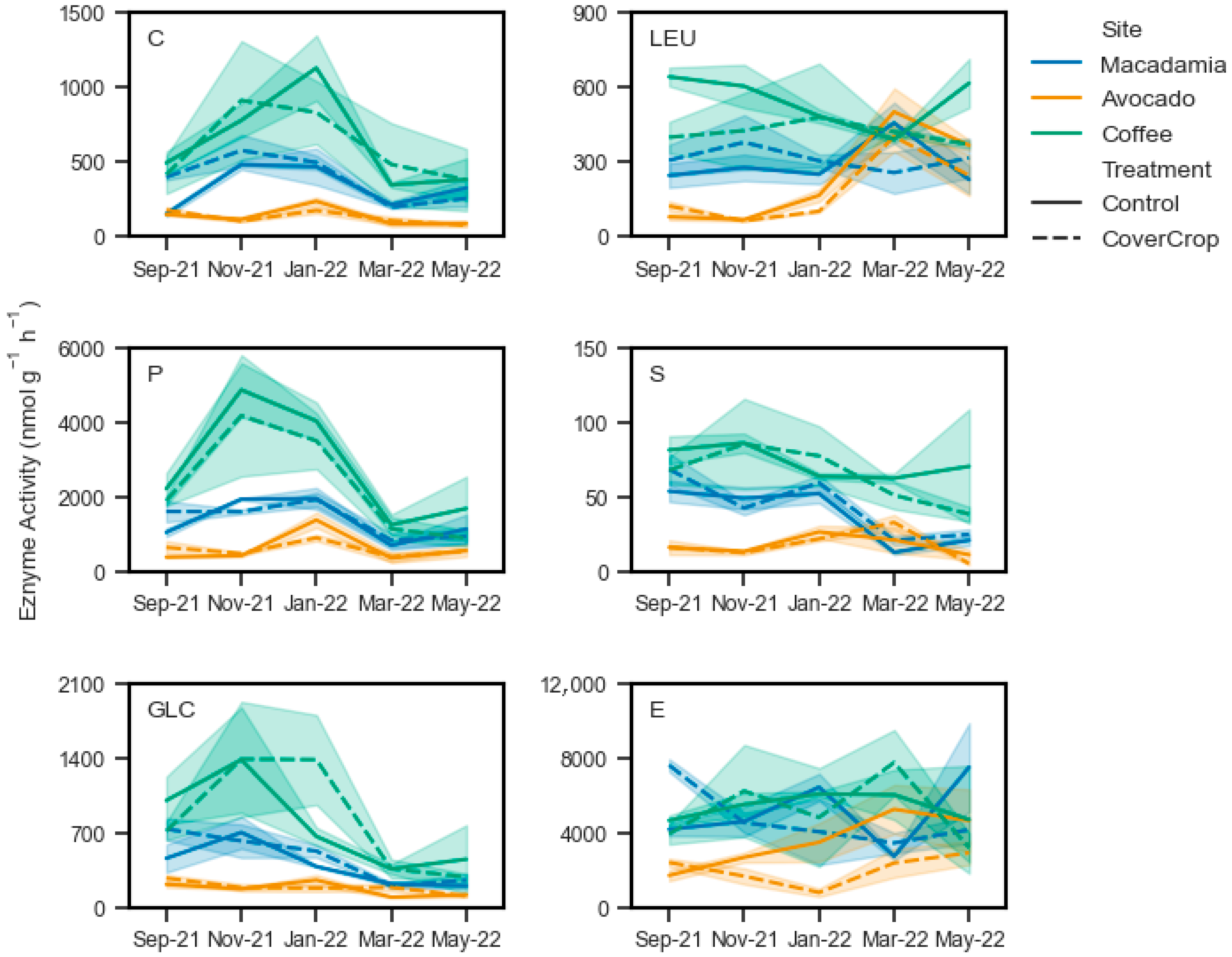
Chitinase activity did not show a treatment effect, but there was significant variation with sampling date at the macadamia and avocado sites (
Table 6). At the macadamia site, site means in November 2021 (528 nmol g
−1 h
−1) and January (481 nmol g
−1 h
−1) were significantly higher than all other sample months (
p < 0.05) (
Figure 3). At the avocado site, the site mean of 203.5 nmol g
−1 h
−1 in January 2022 was significantly higher (
p < 0.05) than means for November 2021 (107.2 nmol g
−1 h
−1), March 2022 (94 nmol g
−1 h
−1), and May (79.5 nmol g
−1 h
−1) (
Figure 3). The site mean was 159.7 nmol g
−1 h
−1 in September 2021, which was only significantly higher than March 2022 and May 2022 (
p < 0.02). At the coffee site, there were no significant differences (
Table 6), with site means ranging from 980 nmol g
−1 h
−1 in January 2022 to 379 nmol g
−1 h
−1 in May 2022 (
Figure 3). There were no differences (
Table 6) between treatments of sample dates for leucine aminopeptidase activity at the macadamia (means 271 to 356 nmol g
−1 h
−1) or coffee sites (means 404 to 520 nmol g
−1 h
−1) (
Figure 3). While at the avocado site, site means in March 2022 (450.3 nmol g
−1 h
−1) and May 2022 (305.3 nmol g
−1 h
−1) were higher (
p < 0.01) than all other months with means ranging from 64.3 to 132.2 nmol g
−1 h
−1 (
Figure 3).
Phosphomonoesterase activity did not respond to the different treatments but varied significantly with time (
Table 6). Similarly to chitinase activity at the macadamia site, activity in September 2021 (1336 nmol g
−1 h
−1), November 2021 (1777 nmol g
−1 h
−1), and January 2022 (1951 nmol g
−1 h
−1) were significantly higher (
p < 0.01) than March 2022 (774 nmol g
−1 h
−1) and May 2022 (1045 nmol g
−1 h
−1) (
Figure 3). There were no differences between January 2022, November 2021, and September 2021 (
p > 0.06) (
Figure 3). Phosphomonoesterase activity was highest at the avocado site in January 2022, with a site mean of 1147 P nmol g
−1 h
−1 (
Figure 3). This was significantly higher (
p < 0.01) than all other time points with values ranging from 381 to 562 nmol g
−1 h
−1 (
Figure 3). For the coffee site, phosphomonoesterase activity peaked in November 2021 at 4532 nmol g
−1 h
−1 (
Figure 3). This was not significantly different to the site mean of 3777 nmol g
−1 h
−1 in January 2022 (
p = 0.82). These two values were higher (
p < 0.03) than all other sample points, with values ranging from 1203 to 2066 nmol g
−1 h
−1 (
Figure 3). Arylsulfatase activity also showed no treatment response at any site, but did show significant variation over time at the macadamia and avocado sites (
Table 6). Arylsulfatase activity was highest in September 2021 at the macadamia site, with a site mean of 61.3 nmol g
−1 h
−1 (
Figure 3). This was not significantly different (
p > 0.1) to activity in November 2021 (46 nmol g
−1 h
−1) and January 2022 (56 nmol g
−1 h
−1) (
Figure 3). However, activity in these three months were all significantly higher (
p < 0.01) than in March 2022 (17 nmol g
−1 h
−1) and May 2022 (23 nmol g
−1 h
−1) (
Figure 3). At the avocado site with September 2021 (16.33 nmol g
−1 h
−1), March 2022 (27.17 nmol g
−1 h
−1) and January 2022 (24.33 nmol g
−1 h
−1) site means were significantly higher (
p < 0.02) than November 2021 (13.17 nmol g
−1 h
−1) and May 2022 (nmol g
−1 h
−1) site means for arylsulfatase activity (
Figure 3). There were no significant effects of treatment or time (
p > 0.4) on arylsulfatase activity with mean values ranging from 54.7 nmol g
−1 h
−1 to 86 nmol g
−1 h
−1 (
Figure 3).
β-glucosidase activity showed no treatment response also, but significant variation with sampling date at all sites (
Table 6). At the macadamia site this was characterised again by the site mean in September 2021 (600 nmol g
−1 h
−1), November 2021 (665 nmol g
−1 h
−1), and January 2022 (458 nmol g
−1 h
−1) being significantly higher (
p < 0.01) than March (213 nmol g
−1 h
−1) and May (230 nmol g
−1 h
−1) 2022 (
Figure 3), but not different from each other (
p > 0.5). At the avocado site, β-glucosidase activity was highest in September 2021, with a site mean of 246 nmol g
−1 h
−1 (
Figure 3). This was significantly higher (
p < 0.02) than activity measured in March (143 nmol g
−1 h
−1) and May (114 nmol g
−1 h
−1) 2022. Activity in November 2021 (182 nmol g
−1 h
−1) and January 2022 (220 nmol g
−1 h
−1) was not significantly different to any other month (
p > 0.1) (
Figure 3). At the coffee site, β-glucosidase activity peaked at 1388 nmol g
−1 h
−1, with this significantly higher (
p < 0.03) than the site means of 363 and 369 nmol g
−1 h
−1 in March and May 2022, respectively. β-glucosidase activity in September 2021 (862 nmol g
−1 h
−1) and January 2022 (1027 nmol g
−1 h
−1) was not significantly different (
p > 0.2) to any other month (
Figure 3). At the macadamia site, esterase activity did not have a significant response to treatment or time (
p > 0.05) (
Table 6). Site mean values ranged from 3103 nmol g
−1 h
−1 (March 2022) to 5907 nmol g
−1 h
−1 (September 2021) (
Figure 3). At the avocado site, there was significant treatment effect, with the overall mean in the control (3569 nmol g
−1 h
−1) being higher (
p < 0.01;
Table 6) than in the cover crop treatment (2044 nmol g
−1 h
−1). There was no significant response of esterase activity at the coffee site (
Table 6), with site means ranging from 4312 to 6904 nmol g
−1 h
−1 (
Figure 3).