Abstract
Plant growth regulators (PGRs) enhance crop stress resistance but their roles in microbial-mediated phosphorus cycling within intercropping systems are unclear. Thus, We conducted a two-year field study using corn (Zea mays L. cv. Denghai 605) and soybean (Glycine max L. cv. Hedou 22) in fluvisols and luvisols soil according to World Reference Base for Soil Resources (WRB) standard. Under a 4-row corn and 6-row soybean strip intercropping system, three treatments were applied: a water control (CK), and two plant growth regulators—T1 (EC: ethephon [300 mg/L] + cycocel [2 g/L]) and T2 (ED: ethephon [300 mg/L] + 2-Diethyl aminoethyl hexanoate [10 mg/L]). Foliar applications were administered at the V7 stage (seventh leaf) of intercropped corn plants to assess how foliar-applied PGRs (T1/T2) modulated the soil phosphorus availability, microbial communities, and functional genes in maize intercropping systems. PGRs increased the soil organic phosphorus and available phosphorus contents, and alkaline phosphatase activity, but not total phosphorus. PGRs declined the α-diversity in fluvisols soil but increased the α-diversity in luvisols soil. The major taxa changed from Actinobacteria (CK) to Proteobacteria (T1) and Saccharibacteria (T2) in fluvisols soil, and from Actinobacteria/Gemmatimonadetes (CK) to Saccharibacteria (T1) and Acidobacteria (T2) in luvisols soil. Functional gene dynamics indicated soil-specific regulation, where fluvisols soil harbored more phoD (organic phosphorus mineralization) and relA (polyphosphate degradation) genes, whereas phnP gene dominated in luvisols soil. T1 stimulated organic phosphorus mineralization and inorganic phosphorus solubilization in fluvisols soil, upregulating regulation genes, and T2 enhanced polyphosphate synthesis and transport gene expression in luvisols soil. Proteobacteria, Nitrospirae, and Chloroflexi were positively correlated with organic phosphorus mineralization and polyphosphate cycling genes, whereas Bacteroidetes and Verrucomicrobia correlated with available potassium (AP), total phosphorus (TP), and alkaline phosphatase (ALP) activity. Thus, PGRs activated soil phosphorus by restructuring soil type-dependent microbial functional networks, connecting PGRs-induced shifts with microbial phosphorus cycling mechanisms. These findings facilitate the targeted use of PGRs to optimize microbial-driven phosphorus efficiency in strategies for sustainable phosphorus management in diverse agricultural soils.
1. Introduction
Sustainable agricultural intensification requires optimizing the efficiency of soil nutrient utilization while simultaneously minimizing environmental impacts. Phosphorus is an essential nutrient for plant growth but it is known for its low mobility within the soil matrix [1]. In certain regions, the accumulation of phosphorus in soil has reached high levels, thereby leading to environmental pressure and potentially reducing the efficiency of phosphorus utilization in agricultural systems [2]. Empirical evidence suggests that prolonged application of phosphorus fertilizers can lead to a surplus of phosphorus in the soil and increase the risk of phosphorus losses [3,4]. Various strategies have been employed in previous studies to mitigate phosphorus losses and enhance the soil available phosphorus levels, including returning straw to the field, optimized fertilizer use, biochar application, and using phosphorus-solubilizing microorganisms [5,6,7,8]. However, the inherent complexity of soil phosphorus dynamics remains a significant challenge, where they are primarily influenced by microbial activity, soil physicochemical properties, and management practices.
Intercropping, particularly cereal–legume systems such as maize–soybean, is widely recognized as enhancing the productivity of land and nutrient acquisition through complementary resource utilization [9,10]. Studies have demonstrated that intercropping significantly improves the bioavailability of soil phosphorus via rhizosphere interactions, thereby promoting crop growth and yields. The relative abundances of Proteobacteria and Sphingomonas are significantly higher in the rhizosphere soil of intercropped maize than in monoculture systems to further enhance the bioavailability of phosphorus [8]. Intercropping has been shown to significantly increase the soil organic carbon content, as well as the activities of alkaline phosphatase and phosphodiesterase, while also enhancing the abundances of specific bacterial genera, such as Microvirga and Sphingomonas, which are closely associated with soil phosphorus dynamics [11]. The intercropping system modulates the availability of soil phosphorus through microbial-mediated dissolution, mineralization, and assimilation processes. Theoretically, six functional gene categories are involved in the phosphorus cycle, as follows: organic phosphorus mineralization, inorganic phosphorus solubilization, phosphorus regulation, phosphorus transport, polyphosphate synthesis, and polyphosphate degradation [12,13]. Microorganisms encoding available phosphorus (phoA and phoD) contribute to the mineralization of organic phosphorus compounds [14]. Genes such as gcd, ppa, and ppx are involved in inorganic phosphorus solubilization [15]. In particular, inorganic pyrophosphatase encoded by ppa and exopolyphosphatase encoded by ppx catalyze the hydrolysis of inorganic polyphosphates into phosphate ions [16]. Furthermore, microorganisms harboring the pst gene exhibit a strong capacity for soil phosphorus assimilation [17]. Recent research indicates that intercropping systems enhance the expression and functionality of phosphorus-cycling genes [18]. For instance, the relative abundances of genes associated with phosphorus transformation, such as gcd, phoR, phoD, and ppx, are significantly elevated in intercropping systems [19]. The upregulation of these genes may be attributed to shifts in the microbial community composition within intercropping systems, particularly within the phyla Proteobacteria, Acidobacteria, and Cyanobacteria, which play crucial roles in phosphorus cycling [19].
Plant growth regulators (PGRs) are extensively utilized in agricultural production to enhance plant growth and increase crop yields. Research indicates that PGRs can stimulate the exudation of organic acids from the roots to alter the structure and function of the microbial community in the rhizosphere and indirectly influence the expression of phosphorus cycling genes [11,20]. These organic acids facilitate the dissolution of phosphate minerals in the soil to improve the availability of phosphorus [21]. The interactions between plants and rhizosphere microbes are crucial for nutrient acquisition and stress resistance [22,23,24,25]. However, the mechanisms that allow PGRs to affect functional microbial genes involved in phosphorus cycling and soil phosphorus availability in maize–soybean intercropping systems remain unclear. Metagenomic analysis is a robust method for evaluating both the microbial community structure and potential functions related to phosphorus cycling [26,27]. Furthermore, this method can be used to assess the correlations between microbial taxa and phosphorus cycling genes, thereby providing deeper insights into the adaptation strategies and functional contributions of these microorganisms within ecosystems [28]. Thus, in the present study, we conducted a field experiment for two years to investigate the effects of the application of PGRs in a maize–soybean intercropping system. PGRs (trinexapac-ethyl + ethephon and diethyl aminoethyl hexanoate + ethephon) were applied to the foliage exclusively in maize strips at the V7 stage of maize development. Soil samples were collected at maize maturity to determine the effects of PGRs on the soil phosphorus dynamics, microbial communities, and phosphorus cycling functional genes in the maize strips in the intercropping system. The aims of this study were (1) to investigate how plant growth regulators (PGRs) modulate soil microbial community diversity and richness in the maize rhizosphere within a maize-soybean intercropping system; and (2) to elucidate the novel mechanism by which PGRs enhance soil phosphorus (P) bioavailability through regulation of specific microbial consortia and functional gene networks. We hypothesized that: (1) PGR application will enhance microbial-mediated organic P mineralization and phosphatase activity, thereby improving soil phosphorus utilization efficiency. (2) The regulatory effects of PGRs on microbial diversity and phosphorus-cycling functional microbiota are modulated by soil type, with stronger functional resilience in luvisols soils (higher organic matter content and buffering capacity) than in alluvial soils. (3) PGRs selectively promote Proteobacteria and Acidobacteria proliferation while suppressing Actinobacteria, thereby altering the abundance of phosphorus-cycling functional genes (e.g., phoD, pstS) linked to soil P fractions. The findings obtained in this study provide novel insights to facilitate improvements in soil phosphorus bioavailability via PGR application as valuable strategies for the sustainable management of agroecosystems.
2. Materials and Methods
2.1. Experimental Site
The experiment was conducted at two locations in Shandong, China: Qihe (36°24′37″ N, 116°23′28″ E) and Laizhou (36°59′20″ N, 119°33′18″ E). Both sites are characterized by a warm, temperate monsoon climate. At the Qihe experimental site, the annual rainfall is 527 mm and primarily concentrated in summer, and the soil type is fluvisols soil according to World Reference Base for Soil Resources (WRB) standard. At the Laizhou experimental site, the annual rainfall is 809 mm and also predominantly occurs in summer, and the soil type is luvisols soil according to WRB standard. The soil fertility parameters for the 0–20 cm soil layer are shown in Table 1.

Table 1.
The soil fertility parameters for the 0–20 cm soil layer.
2.2. Experimental Design
The experiment was conducted over two years in wheat–maize–soybean rotation fields, spanning the 2022–2023 and 2023–2024 growing seasons. Maize and soybeans were alternately planted, with four rows of maize followed by four rows of soybeans. The maize variety was Denghai 605, which has 19–20 leaves in total, and the 13th or 14th leaf is the ear leaf, a plant height of 259 cm, an ear height of 99 cm, and a growth period of 101 days. The soybean variety was Weidou 20, which has 0.9 effective branches and 14.1 main stem nodes, a plant height of 53.14 cm, and an average growth period of 105 days. The planting densities were 67,500 plants/hm2 for maize and 120,000 plants/hm2 for soybeans. In this study, corn and soybeans were sown together (Figure 1) with fertilizer and equipped with drip irrigation machines. During sowing, 750 kg/hm2 compound fertilizer (N:P2O5:K2O = 15:15:15) was applied in the corn planting area, and 211 kg/hm2 nitrogen fertilizer (N = 46%) was applied during the large ear stage of corn; 150 kg/hm2 compound fertilizer (N:P2O5:K2O = 15:15:15) was applied in the soybean planting area. Pest and disease control after emergence was the same as that in ordinary production fields. The experiment was set up as a completely randomized block design, with each treatment having three repetitions. The control treatment (CK) was water, and the two PGRs were EC with the main active ingredients of ethephon (300 mg/L) and cycocel (2 g/L) (T1), and ED with the main active ingredients of ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) (T2). The concentration of plant growth regulators was based on the research by Ren et al. [29]. Each experimental plot had a width of 1.6 m and a length of 20 m. The PGRs were evenly sprayed onto the surfaces of maize leaves at an application dose of 450 L/hm2 during the 7th leaf stage [29], between 16:00 and 19:00. In the optimal application technique, the aim was to make the leaf surfaces moist but without dripping. The sowing date and field management practices adhered to local customs. After the wheat harvest, crop residues on the ground were crushed into fragments no larger than 10 cm. Subsequently, the soil was tilled to a depth of 0–15 cm using a rotary tiller to loosen the soil and promote the decomposition of the aboveground residues.
Figure 1.
Layout diagram of field trial planting.
2.3. Soil Sample Collection and Analysis
Soil samples were collected from the 0–20 cm soil depth using a shovel during the maize harvest season in 2024, where three replicate samples were taken from each plot. Each soil sample was then divided into two parts. After removing large rocks, roots, and other impurities, each sample was passed through a 2 mm sieve. A portion of the collected soil was immediately frozen in liquid nitrogen and stored at −80 °C (for no more than one month) for soil metagenomic analysis. The remaining soil sample was air-dried for soil nutrient analyses. The TP content of the soil was determined using the H2SO4–H2O2 and vanadium molybdenum yellow colorimetric method. Soil AP was determined using the sodium bicarbonate extraction method, followed by spectrophotometry. The pH was determined by preparing a suspension of air-dried soil sample in deionized water at a ratio of 1:5 (w/v) before measurement using a digital pH meter (Mettler FE28-Standard, Shanghai, China). The soil organic matter content was analyzed using the potassium dichromate oxidation method [30], and the total nitrogen content using the total nitrogen oxidation method [31]. Alkali nitrogen was measured using the diffusion–absorption method [32]. Soil AP was analyzed by sodium bicarbonate extraction, followed by spectrophotometry. Available potassium was measured using a flame photometer after extraction with CH3COONH4 [33]. The ALP activity was assessed using a soil enzyme activity assay kit (Solebo, Beijing, China) [34,35].
2.4. Library Preparation and Sequencing
Sequencing libraries were prepared using an Illumina TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, CA, USA). The quality of the library was assessed using a Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and an Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). Finally, the library was sequenced on the Illumina HiSeq 2500 platform.
2.5. Gene Prediction, Taxonomy, and Functional Annotation
Open reading frames were predicted in contigs based on the splicing results using Prodigal (https://github.com/hyattpd/Prodigal, accessed on 9 June 2025, Version 4.6.1) [36]. High-quality reads from each sample were compared with the non-redundant gene set (95% identity) using Bowtie2 (Version 2.3.0) [37] (https://bowtie-bio.sourceforge.net/bowtie2/index.shtml, accessed on 9 June 2025) to calculate gene abundances. Amino acid sequences in the non-redundant gene set were then aligned with the NR database using Diamond [38] (https://github.com/bbuchfink/diamond, accessed on 9 June 2025, Version 0.9.25) via BLASTP (Version 2.14.0) with an expected e-value of 1 × 10−5 to obtain species annotations. Species abundance was calculated based on the total abundance of genes corresponding to the species. Diamond was also used to align the amino acid sequences in the non-redundant gene set with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (version 94.2) (BLASTP, e-value of 1× 10−5) to obtain corresponding KEGG functions. The abundances of functional categories were calculated using the total abundances of genes linked to KEGG Orthology (KO), Pathway, EC, and Module. Functional genes involved in phosphorus cycling were classified into six categories based on the method described by Wu et al. [39], as follows: organic phosphorus mineralization, inorganic phosphorus dissolution, regulatory functions, transporters, polyphosphate synthesis, and polyphosphate degradation.
2.6. Metagenomic Analysis
Significant effects were determined by analysis of variance, and the differences between treatments were assessed with the least significant difference (LSD) test at a significance level of 0.05 using SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). Principal component analysis was conducted using v5.0.2 of Canoco5 software (www.canoco5.com) to understand the similarities and differences among treatments. The contributions of microbes to phosphorus-cycling genes were calculated according to the method described by Zhang et al. [40]. Network analysis was performed using R version 3.5.1 at Metware Cloud, a free online platform for data analysis (https://cloud.metware.cn), to elucidate the connections between soil microbial communities and phosphorus function genes [41].
3. Results
3.1. Soil Phosphorus Contents in Maize Strip in the Intercropping System
The total phosphorus contents did not differ significantly in the soil under different PGR treatments. The soil organic phosphorus contents have no significant difference at both experimental sites, and the soil available phosphorus contents followed the order of: T2 > CK > T1, with the significant level being p < 0.05 (using the LSD multiple comparison method for analysis). The alkaline phosphatase activity contents followed the order of: T1> T2 > CK, with the significant level being p < 0.05. The alkaline phosphatase activity in the soil was significantly greater under treatment with PGR spraying compared with the control treatment. Furthermore, the available phosphorus content and alkaline phosphatase activity were significantly higher at the experimental site in Qihe compared with that in Laizhou (Figure 2).
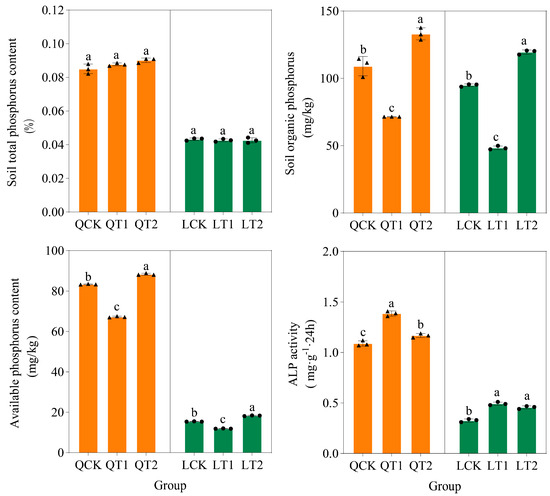
Figure 2.
Soil total phosphorus content, soil organic phosphorus content, available phosphorus content, and alkaline phosphatase (ALP) activity in crop rotation systems under three treatments. QCK: control treatment (water) in Qihe; QT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Qihe; QT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Qihe; LCK: control treatment (water) in Laizhou; LT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Laizhou; LT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Laizhou. The same letters above the bars in a subfigure indicate no significant difference according to the least significant difference test (p < 0.05).
3.2. Analysis of Soil Samples from Maize Strip
At the Qihe experimental site, the Shannon–Wiener index, Simpson index, and inverse Simpson index were significantly higher under CK than the other treatments. At the Laizhou experimental site, the α-diversity indexes, i.e., the Shannon–Wiener index, Simpson index, and inverse Simpson index, were significantly greater under T1 than the other treatments (Figure 3A). These results indicate that PGRs had different effects on microbial diversity in the different soil types, resulting in reduced microbial diversity in fluvisols soil and increased microbial diversity in luvisols soil. Principal coordinate analysis (PCoA) of microbial classifications at the genus level and functional classifications (Clusters of Orthologous Genes, COGs) showed that the soils from different experimental sites formed distinct clusters in the ordination space (Figure 3B), thereby suggesting that the PGRs altered the compositions and functionalities of the soil microbial communities. Analysis of the top 30 phyla of the most abundant microorganisms in the rhizosphere soil under different treatments detected significant differences in six phyla (LSD p < 0.05): Actinobacteria, Proteobacteria, Acidobacteria, Chloroflexi, Thaumarchaeota, and Gemmatimonadetes. At Qihe, the abundance of Actinobacteria in the rhizosphere was highest under CK, whereas Proteobacteria was most abundant in the rhizosphere under T1, and Thaumarchaeota was most abundant in the rhizosphere under T2; however, no significant differences were observed among Chloroflexi, Gemmatimonadetes, and Acidobacteria. At Laizhou, the abundances of Actinobacteria and Gemmatimonadetes in the rhizosphere were highest under CK, whereas Proteobacteria was most abundant in the rhizosphere under T1, and Acidobacteria was most abundant in the rhizosphere under T2. No significant differences were found in the abundances of Thaumarchaeota among treatments (Figure 3C,D).
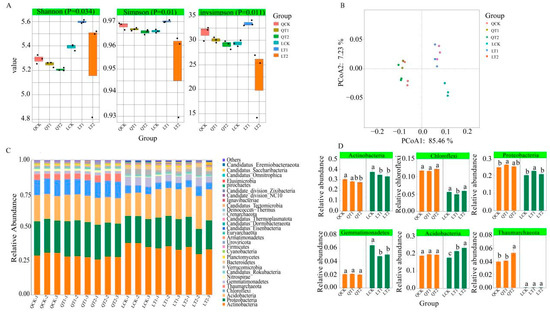
Figure 3.
Comparison of metagenomic sequencing data under different treatments in maize strips in intercropping systems. (A) Differences in Shannon index, Simpson index, inverse Simpson index under different treatments. (B) Principal coordinates analysis (PCoA) of different treatments based on Bray–Curtis distances among metagenomic profiles. (C) Relative abundances (%) of major bacterial phyla in rhizosphere microbial communities. (D) Bacterial phyla with significantly different (p < 0.05) abundances in soils among different treatments. Bars with different letters are significantly different (p < 0.05).
3.3. Functional Gene Abundances in Soil from Maize Strip
At the functional gene level, the PGRs had significant effects on inorganic phosphorus solubilization and polyphosphate synthesis gene expression at Qihe. By contrast, the PGRs significantly influenced inorganic phosphorus solubilization and polyphosphate degradation gene expression at Laizhou (Figure 4B,C, LSD, p < 0.05). In particular, compared with CK, the PGRs (T1) increased the abundances of gene associated with organic phosphorus mineralization (Figure 4A), inorganic phosphorus solubilization (Figure 4B), and regulatory (Figure 4F) at Qihe but significantly decreased the abundances of genes related to polyphosphate synthesis (Figure 4C). At Laizhou, the PGRs (T2) significantly increased the abundances of genes related to organic phosphorus mineralization (Figure 4A), transporters (Figure 4E), and regulatory (Figure 4F) compared with CK, and the abundances of genes associated with polyphosphate synthesis (Figure 4C), phosphorus transport (Figure 4E), and phosphorus regulation (Figure 4F) gene expression increased under T2, but there was no significant difference.
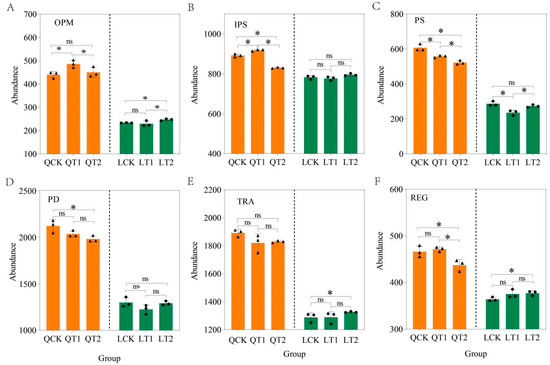
Figure 4.
Relative abundances of phosphorus cycling genes at the function level in maize strips in intercropping systems. QCK: control treatment (water) in Qihe; QT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Qihe; QT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Qihe; LCK: control treatment (water) in Laizhou; LT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Laizhou; LT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Laizhou. (A) represents: organic phosphorus mineralization (OPM); (B) represents inorganic phosphorus solubilization, (IPS); (C) represents polyphosphate synthesis, (PS); (D) represents polyphosphate degradation, (PD); (E) represents transporters, (TRA); (F) represents regulatory functions, (REG). Asterisks indicate significant differences according to the least significant difference test (p < 0.05), ns indicate no significant difference test (p < 0.05).
The variances of the gene abundance data from different phosphorus cycling pathways were decomposed, and these were plotted on a two-dimensional coordinate PCA graph. By analyzing the composition of different samples (with 97% similarity, LSD, p < 0.05), principal component analysis reveals different groups; it presents the results of statistical tests, which confirm the existence of significant differences in the principal component direction (Figure 5). At the gene level, principal component analysis based on the abundances of phosphorus-cycle genes detected differences between the effects of PGRs and test sites. Principal component 1 (PC1) primarily indicated significant variations among test sites, whereas principal component 2 (PC2) highlighted differences attributed to PGRs (Figure 5). Differences were observed in the effects of T1, T2, and CK on organic phosphorus mineralization genes, with consistent patterns across both sites. Significant differences were found between the effects of T1, T2, and CK on inorganic phosphorus solubilization genes at Qihe and of T2, CK, and T1 at Laizhou. Significant differences were also found in the effects of T1, T2, and CK on PS genes at Qihe and of T2, CK, and T1 at Laizhou. No significant differences were found in the effects on polyphosphate degradation genes between Qihe and Laizhou, but significant differences were observed among T1, T2, and CK. Significant differences were found in the effects of T1, T2, and CK on phosphorus transport genes at Qihe, but no significant differences at Laizhou. Finally, no significant differences were found in the effects on phosphorus regulation genes between Qihe and Laizhou.
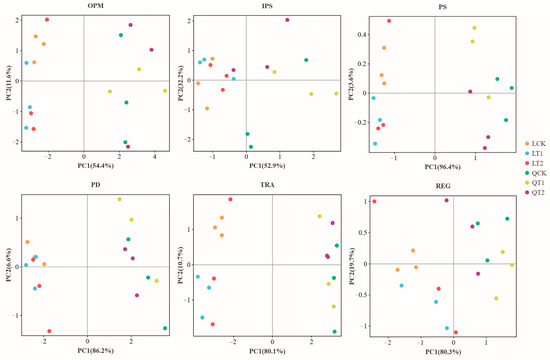
Figure 5.
Principal component analysis of phosphorus cycling genes in maize and soybean intercropping systems under treatment with different growth regulators. OPM: organic P mineralization; IPS: inorganic P solubilization; REG: regulatory; TRA: transporters; PD: polyphosphate degradation.
The abundances of organic phosphorus mineralization genes, such as phoD, PhoA, phnP, phnW, phnA, and phoN, were significantly higher in the soil at both experimental sites compared with other genes (LSD, p < 0.05). In particular, the abundance of the PhoA gene in soil was significantly higher under CK than T1 and T2. The abundances of the phoD, PhoA, phnW, and phnA genes were significantly higher at Qihe than Laizhou, and the abundance of phnP was significantly lower at Qihe compared with Laizhou (Figure 6). These results indicate that the abundances of polyphosphate degradation genes differed between Qihe and Laizhou. The abundances of relA, surE, and spoT were significantly higher at Qihe than Laizhou, whereas the abundances of ppnK, ndk, and ppgK were significantly lower at Qihe compared with Laizhou. At Qihe, the abundance of polyphosphate degradation gene ppnK was significantly higher under T2 than CK and T1. At Laizhou, the abundance of the relA gene in the soil was significantly higher under CK than T1 and T2. The abundances of polyphosphate degradation genes pstS, pstC, and ugpQ were significantly higher at Qihe than Laizhou, and the abundances of pstB and ugpB were significantly lower at Qihe compared with Laizhou. At Qihe, compared with CK, the abundance of ugpQ in the soil increased significantly under T1, whereas the abundance of ugpA decreased significantly. By contrast, no significant differences in the abundances of these genes were observed among treatments at Laizhou. Soil type drives the functional differentiation of microbial phosphorus cycling through organic matter content and available phosphorus content, as well as buffering capacity: The Qihe experimental site relies on PhoA-mediated acidic phosphatase mineralization and relA/surE emergency polyphosphate degradation to cope with phosphorus fluctuations, while the Laizhou experimental site achieves efficient phosphorus turnover through the phnP-driven phytase system and ppnK homeostatic storage.
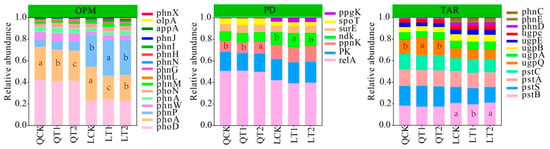
Figure 6.
Relative abundances of phosphorus cycling genes in maize strips in intercropping systems. QCK: control treatment (water) in Qihe; QT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Qihe; QT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Qihe; LCK: control treatment (water) in Laizhou; LT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Laizhou; LT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Laizhou. OPM: organic P mineralization; PD: polyphosphate degradation; TRA: transporters. Different lowercase letters indicate significant differences between treatments according to the least significant difference test (p < 0.05).
3.4. Contributions of Microorganisms in Maize Strip to Phosphorus Cycle Genes
At Qihe, compared with CK, T1 and T2 enhanced the contributions of Proteobacteria to organic phosphorus mineralization, polyphosphate synthesis, and polyphosphate degradation, but there were no significant differences between T1 and T2 (Figure 7, LSD, p < 0.05). T1 increased the contributions of Acidobacteria to inorganic phosphorus solubilization and polyphosphate synthesis but also decreased their contributions to organic phosphorus mineralization and polyphosphate degradation. By contrast, T2 reduced the contributions of Actinobacteria to organic phosphorus mineralization, polyphosphate synthesis, polyphosphate degradation, and phosphorus transport. At Laizhou, compared with CK, T1 and T2 increased the contributions of Proteobacteria to organic phosphorus mineralization and polyphosphate synthesis, as well as increasing the contributions of Acidobacteria to polyphosphate synthesis, polyphosphate degradation, phosphorus regulation, and phosphorus transport, and reducing their contribution to inorganic phosphorus solubilization. Furthermore, T1 and T2 decreased the contributions of Actinobacteria to polyphosphate synthesis, polyphosphate degradation, phosphorus regulation, and phosphorus transport, as well as reducing the contributions of Gemmatimonadetes to inorganic phosphorus solubilization, polyphosphate synthesis, phosphorus regulation and phosphorus transport. In the alluvial soil of Qihe, the application of plant growth regulators (PGRs) (T1/T2) suppressed high-P-adapted Actinobacteria (e.g., Solirubrobacter, Nocardioides, Gaiella), resulting in reduced phosphorus immobilization. Conversely, in the luvisols soil of Laizhou, PGRs further activated Proteobacteria and diazotrophic phosphorus-solubilizing consortia (e.g., Pseudolabrys, Bradyrhizobium), promoting a synergistic activation of both organic and inorganic phosphorus pools.
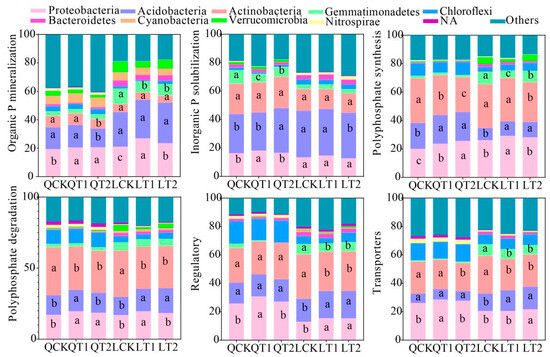
Figure 7.
Relative contributions of phyla to phosphorus cycling genes. QCK: control treatment (water) in Qihe; QT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Qihe; QT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Qihe; LCK: control treatment (water) in Laizhou; LT1: treatment with ethephon (300 mg/L) and cycocel (2 g/L) in Laizhou; LT2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L) in Laizhou. NA: not available. Different lowercase letters indicate significant differences between treatments according to the least significant difference test (p < 0.05).
3.5. Differences in Microbial Communities and Relationships with Phosphorus Cycling Functional Genes and Soil Phosphorus Availability
The microbial communities in the rhizosphere of the intercropping system were also analyzed in different field types using metagenomic sequencing, which detected significant differences in the microbial community structures in soils from different fields (LSD, p < 0.05). The numbers of operational taxonomic units (OTUs) per sample ranged from 341,325 to 813,197. The abundances of OTU differed significantly among the different rhizospheric soils. The Venn diagram in Figure 8A shows that 10,987 OTUs were identified in the rhizospheres of plants in all three field types.
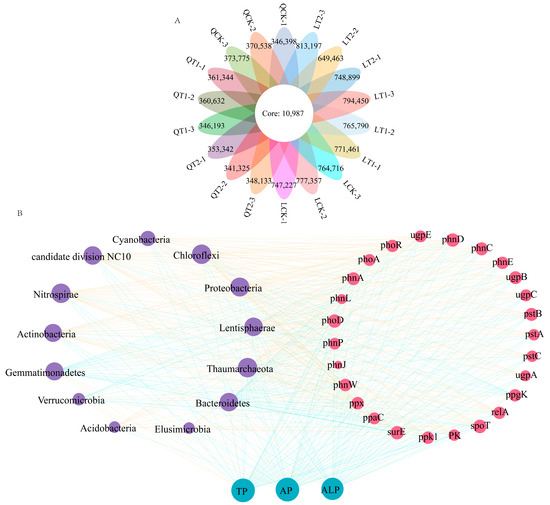
Figure 8.
Venn diagram showing rhizosphere-enriched bacterial operational taxonomic units (OTUs) in soils under three field treatments at Qihe and Laizhou (A). Network depicting relationships among crop systems, abundances of phosphorus cycling genes, soil available phosphorus content, total phosphorus content, and soil alkaline phosphatase activity (B).
We examined the correlations between the top 14 phyla, phosphorus cycle genes, total phosphorus, available phosphorus, and alkaline phosphatase in soil. Proteobacteria had significant positive correlations with organic phosphorus mineralization genes (phnA, phnW, and phoD) (R ≥ 0.98). Nitrospirae was significantly positively correlated with an organic phosphorus mineralization gene (phoD) and a polyphosphate degradation gene (relA) (R ≥ 0.98). Thaumarchaeota was significantly positively correlated with an organic phosphorus mineralization gene (phoD) and polyphosphate degradation genes (relA and spoT) (R ≥ 0.98). Chloroflexi was significantly positively correlated with an organic phosphorus mineralization gene (phoD), a polyphosphate synthesis gene (ppk1), and a polyphosphate degradation gene (relA) (R ≥ 0.98). Lentisphaerae was significantly positively correlated with a phosphorus transport gene (pstB) (R ≥ 0.98). Bacteroidetes, Gemmatimonadetes, and Verrucomicrobia were significantly positively correlated with a polyphosphate degradation gene (ppgK) (R ≥ 0.8) and negatively correlated with other phosphorus cycle genes (R ≥ 0.8). Furthermore, Bacteroidetes, Gemmatimonadetes, and Verrucomicrobia were significantly positively correlated with total phosphorus, available phosphorus, and alkaline phosphatase in soil (R ≥ 0.8), and the remaining microbial communities were also significantly positively correlated with total phosphorus, available phosphorus, and alkaline phosphatase in soil (R ≥ 0.8) (Figure 8B, Table 2 and Table 3).

Table 2.
The correlation coefficient between the microbial phylum level and phosphorus cycling genes in the rhizosphere soil.

Table 3.
The correlation coefficient between different phosphorus forms in rhizosphere soil and phosphorus cycling genes.
At Qihe, Solirubrobacter, Nocardioides, Gaiella, and Nitrospira were the four most abundant genera under CK, T1, and T2. Solirubrobacter (3.92%), Nocardioides (3.67%), Gaiella (2.41%), and Nitrospira (1.29%) were the most abundant genera under CK. The abundances of Solirubrobacter, Nocardioides, Gaiella, and Nitrospira were significantly lower in the soils under T1 and T2 compared with CK (Figure 9A and Figure 10A, Table 4, LSD, p < 0.05). Solirubrobacter, Nocardioides, and Gaiella belong to the Actinobacteria phylum. Nitrospira belongs to Nitrospirota and contains genes such as phoU, phoD, phnK, phnK, and narG. These bacteria reduce soil phosphorus availability by fixing and storing phosphorus and inhibiting phosphorus release. In the Qihai experimental site, growth regulators reduced the abundance of these bacterial populations, indicating a decrease in soil phosphorus fixation and an increase in available phosphorus.
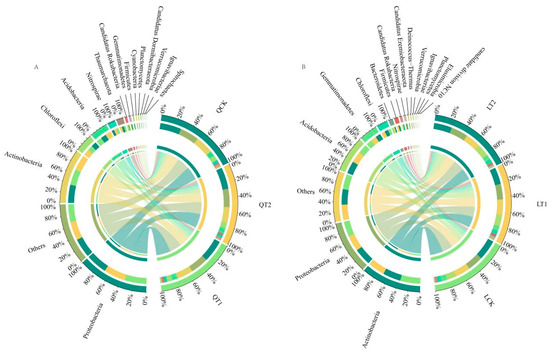
Figure 9.
Circos diagram showing microbial communities at the genus level, illustrating the taxonomic differences between soils under CK, T1, and T2 in Qihe (A) and Laizhou (B). The width of each ribbon corresponds to the relative abundance of each taxon. CK: control treatment (water); T1: treatment with ethephon (300 mg/L) and cycocel (2 g/L); T2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L).
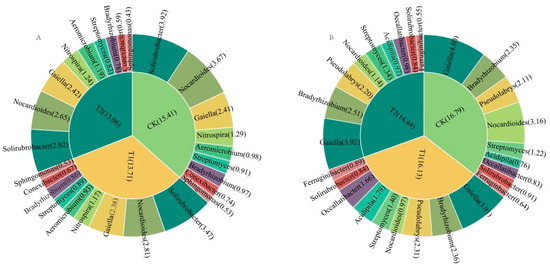
Figure 10.
Pie charts show the top nine bacterial groups with the highest relative abundances in Qihe (A) and Laizhou (B). CK: control treatment (water); T1: treatment with ethephon (300 mg/L) and cycocel (2 g/L); T2: treatment with ethephon (300 mg/L) and 2-Diethyl aminoethyl hexanoate (10 mg/L).

Table 4.
The rhizosphere soil is at the relative abundance level of soil microbial Genus in Qihe.
At Laizhou, Gaiella, Bradyrhizobium, Pseudolabrys, and Nocardioides were the four most abundant genera under CK, T1, and T2. Gaiella (4.80%), Nocardioides (3.16%), Bradyrhizobium (2.35%), and Pseudolabrys (2.11%) were the most abundant genera under CK. The abundances of Gaiella and Nocardioides in the soil were significantly lower under T1 compared with CK, but the abundances of Bradyrhizobium and Pseudolabrys did not differ significantly. The abundances of Gaiella and Nocardioides in the soil decreased significantly under T2, whereas the abundances of Bradyrhizobium and Pseudolabrys increased (Figure 9B and Figure 10B, Table 5, LSD, p < 0.05). Bradyrhizobium carries the complete phnCDE gene cluster, efficiently mineralizes soil organic phosphorus, and cooperates with symbiotic nitrogen fixation-phosphorus fixation to drive an increase in the effective utilization of phosphorus. Pseudolabrys is an acid-driven community that dissolves inorganic phosphorus, containing the denitrification gene (narG), which can reduce soil pH and increase acidification-induced phosphorus solubilization in the rhizosphere. In the Laizhou experimental site, the abundance of Bradyrhizobium and Pseudolabrys communities was high, which increased soil phosphorus dissolution and enhanced soil phosphorus availability.

Table 5.
The rhizosphere soil is at the relative abundance level of soil microbial Genus in Laizhou.
The two experimental sites host diverse bacterial communities, including Solirubrobacter, Nocardioides, and Gaiella, all of which belong to the phylum Actinobacteria. Pseudolabrys is classified within the phylum Proteobacteria, while Bradyrhizobium is recognized as a nitrogen-fixing bacterium. Solirubrobacter is capable of secreting phosphodiesterase, which mineralizes organic phosphorus and enhances phosphorus availability [42]. Nocardioides possesses multiple copies of the phoU gene, playing a significant role in phosphorus transformation and utilization, particularly in phosphorus mineralization and dissolution processes [43]. The Gaiella genus, characterized by phoD gene carriers, exhibits a higher dependence on phosphorus and may function more effectively in phosphorus-rich environments [44]. Nitrospira enhances phosphorus fixation by competing for NH4+ adsorption sites. Together, Solirubrobacter, Nocardioides, Gaiella, and Nitrospira form a complex microbial community. The diversity and functionality of these microbial communities are critical for plant growth and soil health [45,46]. Pseudolabrys contributes to rhizosphere acidification through NO3−-N reduction, facilitating phosphorus dissolution in the soil. Bradyrhizobium secretes carboxylates, such as oxalic acid and citric acid, to increase phosphorus availability. In the alluvial soil of Qihe, the application of plant growth regulators (PGRs) (T1/T2) suppressed high-phosphorus-adapted Actinobacteria (e.g., Solirubrobacter, Nocardioides, Gaiella), resulting in reduced phosphorus immobilization. Conversely, in the luvisols soil of Laizhou, PGRs further activated Proteobacteria and diazotrophic phosphorus-solubilizing consortia (e.g., Pseudolabrys, Bradyrhizobium), promoting a synergistic activation of both organic and inorganic phosphorus pools.
4. Discussion
4.1. Effects of Growth Regulators on Soil Phosphorus Contents in the Intercropping System
Increased crop yields and nutrient use efficiency improvements are considered advantages of intercropping systems as eco-friendly agricultural practices [47,48]. In field cultivation, the addition of fertilizers leads to the increased accumulation of phosphorus, but the phosphorus utilization rate remains low [49]. Alkaline phosphatase hydrolyzes approximately 90% of the organic phosphorus in the soil and makes it available to plants [50]. The present study was conducted at two experimental sites, and significant differences in the total phosphorus contents were observed under the same soil tillage practices. Spraying PGRs significantly increased the soil organic phosphorus and available phosphorus contents of the soil, as well as the soil alkaline phosphatase activity. Therefore, in the maize–soybean intercropping system, spraying PGRs can enhance the availability of phosphorus in soil in the maize strip, which is beneficial for phosphorus uptake by maize plants.
4.2. Effects of Growth Regulators on Soil Phosphorus Cycling Microbial Community
In this study, we analyzed the compositions of the soil microbial communities in fields over two consecutive years. At Qihe, the Shannon–Wiener index, Simpson index, and inverse Simpson index were significantly higher under CK than the other treatments, thereby suggesting that PGRs reduced the microbial diversity. By contrast, at Laizhou, the α-diversity indexes (including the Shannon–Wiener index, Simpson index, and inverse Simpson index) were significantly higher under T1 than the other treatments, indicating that PGRs may enhance the microbial diversity. This difference may be attributed to the structural and nutrient characteristics of different soil types, as well as the mechanistic responses of microorganisms to environmental factors [51,52,53]. The fluvisols soil typically has low organic matter and clay content. PGRs may exacerbate soil acidification or alter the nutrient distribution to inhibit the activities of certain microbial groups (e.g., Actinobacteria) and lead to a decline in diversity [54]. By contrast, the high buffering capacity and nutrient retention capacity of luvisols soil may mitigate the negative effects of growth regulators and even enhance diversity by promoting the proliferation of functional flora (e.g., Proteobacteria) [7].
Our results obtained by PCoA at the genus level and COG functional classification indicated that the microbial communities differed significantly between the two experimental sites. Moreover, the application of PGRs altered the compositions and functionalities of the microbial communities in both soil types. These findings suggest that PGRs may influence soil functions by altering the abundances of functional genes [7,55]. For instance, Proteobacteria are associated with soil phosphorus solubilization. The microbes involved in phosphate solubilization include phosphate-solubilizing bacteria, fungi, and actinomycetes [21]. It has been reported that Bacillus and Pseudomonas are among the most potent phosphate-solubilizing bacteria [56,57]. Our results showed that at Qihe, the abundance of Actinobacteria was highest under CK, whereas Proteobacteria dominated under T1. The phn operon and phoD gene in Proteobacteria play important roles. The presence of the phn operon (including phnW-phnA) and the phoD gene promotes the mineralization process of organic phosphorus. Studies have shown that Proteobacteria can dissolve phosphate in the soil by secreting low-molecular-weight organic acids, such as oxalic acid, thereby increasing the bioavailability of phosphorus [58]. The alkaline phosphatase encoded by the phoD gene can catalyze the hydrolysis of organic phosphorus, releasing inorganic phosphorus that can be absorbed by plants [59]. In different soil environments, the abundance and diversity of the phoD gene are closely related to the availability of soil phosphorus [60]. The interaction between Proteobacteria and nitrogen-fixing bacteria can improve the bioavailability of phosphorus in the soil, thereby promoting plant growth [61]. The Qihui experimental site showed a decrease in diversity, but the abundance of microbial communities related to the phosphorus cycle increased, and the availability of phosphorus still increased. Actinomycetes primarily participate in the decomposition of complex organic matter, whereas Proteobacteria are crucial for rapid nutrient cycling in the rhizosphere [7,55]. The increased abundance of Proteobacteria under T1 at Laizhou may be attributed to the stimulated secretion of sugars and organic acids by plant roots [7]. In Actinobacteria, the co-expression of the phoD gene and the phosphorus-binding kinase gene ppk achieves the “mineralization-storage” coupling. The phoD gene encodes alkaline phosphatase, while the ppk gene is responsible for converting inorganic phosphorus into polyphosphate for storage [62]. Additionally, the acid phosphatase encoded by the phoN gene exhibits significant activity under high pH conditions. Studies have shown that the expression of the phoN gene is regulated by the PhoPQ two-component system [63]. In different environmental conditions, Actinobacteria can effectively adapt to changes in the availability of phosphorus by regulating the expression of the phoD and phoN genes. For example, in low phosphorus environments, the expression of the phoD gene can be significantly enhanced, thereby improving the mineralization ability of organic phosphorus, while in high phosphorus environments, the expression of the phoN gene may be inhibited to avoid excessive hydrolysis of phosphate esters [64]. Moreover, it has been found that the phoD and phoX gene families in Actinobacteria exhibit diversity, which enables these bacteria to hydrolyze a wide range of organic phosphorus substrates under different nutritional conditions, thereby releasing inorganic phosphorus [65]. In agricultural soils, the diversity and abundance of the phoD gene play an important role in the mineralization process of soil phosphorus. By regulating the expression of the phoD gene, soil microorganisms can effectively promote the bioavailability of phosphorus, thereby supporting plant growth [66]. We found that eutrophic microbial communities, such as Proteobacteria and Actinobacteria, were enriched in soils treated with PGRs, possibly due to the tendency of legumes in the maize–soybean intercropping system to enhance the availability of soil nutrients, promoting the proliferation of nutrient-rich microorganisms [67]. This process probably facilitates the mineralization of organic phosphorus in soil [68,69].
4.3. Effects of PGRs on Soil Phosphorus Cycling Genes
Microorganisms can enhance the availability of phosphorus by promoting organic phosphorus mineralization and facilitating inorganic phosphorus compatibilization [70,71]. In this experiment, the dominant phyla, such as Proteobacteria, Acidobacteria, Actinobacteria, and Chloroflexi, contain the phn operon (including phnW-phnA), phoD, phoA, phoX, phoN, phnW, ugpQ, ppk, pstS, relA, surE, etc., which are genes related to the conversion of soil-impossible-to-utilize phosphorus into available phosphorus.
Our findings showed that PGRs significantly increased the abundances of organic phosphorus mineralization genes (such as phoD and PhoA) at both experimental sites, which were strongly related to the activities of phosphorus-solubilizing microorganisms [72]. In particular, the abundances of phoD, PhoA, and other genes were higher at Qihe, where the activities of phosphorus-solubilizing microorganisms were also greater in the rhizosphere soil, which may be attributed to the more favorable physical and chemical properties of the soil at Qihe for the metabolic activities of these microorganisms. In addition, the results showed that treatment with PGRs at Qihe inhibited the polyphosphate synthesis and polyphosphate degradation genes, whereas T2 significantly increased the abundances of related genes at Laizhou. The high abundances of relA, surE, and other polyphosphate degradation genes at Qihe may indicate that microorganisms preferentially degraded stored polyphosphate when the phosphorus supply was adequate. Furthermore, the difference in the abundance of the ppnk gene at Laizhou may have been related to the enhanced phosphorus storage capacity of specific microorganisms under PGR treatments [73]. The abundance of phosphorus transport genes was significantly higher at Qihe than Laizhou, possibly due to the low available phosphorus content of the soil (Table S1). Low available phosphorus levels encourage plants and microorganisms to absorb phosphorus more effectively through high-affinity transporters [74,75]. The abundance of the ugpq gene was significantly higher under T1 at Qihe, potentially enhancing the phosphorus utilization efficiency by promoting the transport of sugar–phosphorus complexes. However, no significant differences were observed in the abundance of the ugpq gene between the treatments at Laizhou, probably due to the high initial availability of phosphorus in the soil [76]. The abundance of phosphorus transport genes directly influences the distribution of phosphorus and utilization efficiency. Our analysis of phosphorus cycle phosphorus regulation genes indicated that these genes were influenced by PGRs at both experimental sites, but there were no significant differences between treatments, possibly due to the complexity of the regulatory mechanisms. PGRs may indirectly influence the expression of phosphorus regulation genes through hormonal signals rather than directly altering their abundances [77].
4.4. Differences in Contributions of Growth Regulators to Phosphorus Cycle Genes
In the present study, we detected specific dominant microbial communities, which agreed with the findings obtained in previous studies [8,78]. At Qihe and Laizhou, significant positive correlations were found between Proteobacteria and phosphorus cycle genes (such as phnD and pstB) under the PGR treatments, indicating that Proteobacteria contributed to phosphorus activation by regulating high-affinity phosphate transport systems (e.g., pstS, pstC, pstA, and pstB) and OPM (e.g., phnD). A significant increase in the abundance of Proteobacteria was reported previously in a maize–soybean intercropping system, and the AP content increased [8]. By contrast, Acidobacteria had significant negative correlations with genes such as phnC and phnD. The abundance of Acidobacteria has been shown to decrease as the soil available phosphorus increases in the maize–soybean intercropping system [8,78], possibly because Acidobacteria prefer low-phosphorus environments, and their activity is suppressed when available phosphorus is high. We found that Actinobacteria only had positive correlations with pstB and pstA, indicating that they participated in phosphorus absorption through phosphorus transport genes rather than direct mineralization under treatment with PGRs. These findings are consistent with studies of intercropping systems conducted by Bai et al. [79] and Zhu et al. [80]. At Qihe, the abundances of the dominant genera Solirubrobacter (Actinobacteria) and Nitrospira (Nitrospirae) were significantly lower under T1 and T2, possibly due to inhibition of their carbon metabolism-related functions by PGRs leading to a decline in the phosphorus storage capacity [80]. The competitive disadvantage of Actinobacteria in phosphorus-rich environments has been demonstrated previously in intercropping systems [8]. At Laizhou, the abundance of Bradyrhizobium (Proteobacteria) increased under T2, possibly because PGRs activated the phosphorus transport function of legume symbionts, facilitating the release of available phosphorus in the rhizosphere [8,81].
In previous studies, the phoD, phnA, and phnP genes were shown to play crucial roles in organic phosphorus mineralization [68,75,82]. Microorganisms containing these genes influence the activity of phosphatase, which then affects the soil’s available phosphorus content [39,83]. Our analysis of the correlations between phosphorus cycle genes and environmental factors showed that phosphorus cycle genes (such as phnD, pstB, phnC, phnE, ugpC, pstA, ugpB, ugpE, pstC, and ugpA) were significantly positively correlated with the soil total phosphorus and alkaline phosphatase but negatively correlated with available phosphorus. Furthermore, the expression of PhoA and phod enhances the microbial activity under phosphorus-deficient conditions [84]. The expression levels of the phoD, PhoA, phnP, and phnW genes are high during organic phosphorus mineralization. The elevated expression levels of the pstB, pstS, pstA, pstC, ugpQ, and ugpA genes during the transport process indicate that PGRs enhance the capacity of microorganisms to activate phosphorus in environments with low available phosphorus contents.
Under the conditions of this experiment, the use of plant growth regulators did reduce the biodiversity at the Qihe experimental site. However, in both Qihe and Laizhou, the microorganisms and gene expressions related to phosphorus cycling in the soil were not restricted. The content of available phosphorus and the activity of alkaline phosphatase in the soil increased under the T2 treatment. This was related to the dominant bacterial communities in the rhizosphere soil, such as Actinobacteria, Proteobacteria, Acidobacteria, Chloroflexi, Thaumarchaeota, Gemmatimonadetes, etc. The expression of genes like phoD, PhoA, phnP, phnW, phnA, phoN, relA, surE, ppnK, ndk, and ppgK in these bacterial groups increased the availability of phosphorus in the soil. In this study, we focused only on the effects of PGRs on phosphorus cycling and not related metabolic compounds, so future research should explore the relationships between phosphorus cycling, growth regulators, and changes in metabolic compounds to further understand phosphorus cycle dynamics in maize–soybean intercropping systems.
5. Conclusions
Our results highlight the changes in soil phosphorus cycling functions following the application of PGRs in a maize–soybean intercropping system over two consecutive years. T1 and T2 both significantly enhanced the abundances of organic phosphorus mineralization genes (e.g., phoD and PhoA) and phosphorus transport genes (e.g., pstS and ugpQ) in rhizosphere microorganisms, thereby promoting the conversion of organic phosphorus into available phosphorus. In particular, T2 enriched Bradyrhizobium (Proteobacteria) and other symbiotic bacteria at Laizhou, further boosting the activation and transport of phosphorus, thereby demonstrating the potential of PGRs for improving the phosphorus utilization efficiency through microbial–plant interactions. The physical and chemical properties of the soils at Qihe (fluvisols soil) and Laizhou (luvisols soil) played significant roles in shaping the differentiation of microbial functional groups. The contribution of Proteobacteria to polyphosphate metabolism was enhanced at Qihe, whereas Acidobacteria was associated with the more pronounced negative response of phosphorus regulation genes at Laizhou. The regulatory effects of PGRs depended greatly on the soil characteristics, such as the pH, phosphorus forms, and organic matter content. PGRs (especially T2) optimize rhizosphere microbial consortia to unlock soil organic phosphorus, while intercropping creates synergistic hotspots for phosphorus cycling—this soil–microbe–plant crosstalk is fine-tuned by local soil properties. The intercropping system synergistically enhanced microbial activation and plant phosphorus uptake by enriching phosphate-solubilizing bacteria such as Proteobacteria and increasing the alkaline phosphatase activity. Our findings provide a theoretical basis for optimizing phosphorus management and the targeted application of PGRs in intercropping systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071748/s1, Table S1: The soil fertility parameters for the 0–20 cm soil layer; Table S2: The correlation coefficient between the microbial phylum level and phosphorus cycling genes in the rhizosphere soil; Table S3: The correlation coefficient between different phosphorus forms in rhizosphere soil and phosphorus cycling genes; Table S4: The rhizosphere soil is at the relative abundance level of soil microbial Genus in Qihe; Table S5: The rhizosphere soil is at the relative abundance level of soil microbial Genus in Laizhou.
Author Contributions
C.G.: writing—review and editing, writing—original draft, visualization, investigation, formal analysis, data curation, conceptualization; W.K.: formal analysis; F.Z.: investigation; F.J.: investigation, methodology; P.L.: investigation, funding acquisition; Z.L.: writing—review and editing; K.L.: writing—review and editing; H.Z.: writing—review and editing, project administration, funding acquisition, formal analysis, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFD2300905) and the National Natural Science Foundation of China (32272227).
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA023500), and they are publicly accessible at https://ngdc.cncb.ac.cn/gsa (accessed on 17 July 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Wang, K.Y.; Onodera, S.I.; Saito, M.; Ishida, T. Assessment of long-term phosphorus budget changes influenced by anthropogenic factors in a coastal catchment of Osaka Bay. Sci. Total Environ. 2022, 843, 156833. [Google Scholar] [CrossRef] [PubMed]
- An, X.X.; Liu, J.; Liu, X.S.; Ma, C.; Zhang, Q. Optimizing phosphorus application rate and the mixed inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria can improve the phosphatase activity and organic acid content in Alfalfa Soil. Sustainability 2022, 14, 11342. [Google Scholar] [CrossRef]
- Demay, J.; Ringeval, B.; Pellerin, S.; Nesme, T. Half of global agricultural soil phosphorus fertility derived from anthropogenic sources. Nat. Geosci. 2023, 16, 69–74. [Google Scholar] [CrossRef]
- Zhu, X.H.; Tan, J.L.; Zhou, H.Y.; Wang, T.Q.; Zhang, B.B.; Lu, X.; Tian, J.H.; Liang, C.Y.; Tian, J. Effects of different genotypes soybean and maize intercropping on soil phosphorus fractions and crop phosphorus uptake. J. Appl. Ecol. 2024, 35, 1583–1589. [Google Scholar]
- Nasar, J.; Ahmad, M.; Gitari, H.; Tang, L.; Chen, Y.; Zhou, X.B. Maize/soybean intercropping increases nutrient uptake, crop yield and modifies soil physio-chemical characteristics and enzymatic activities in the subtropical humid region based in Southwest China. BMC Plant Biol. 2024, 24, 434. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Y.; He, J.; Zhou, Z.H.; Xia, L.L.; Hu, Y.F.; Zhang, Y.L.; Zhang, Y.Y.; Luo, Y.Q.; Chu, H.Y.; Liu, W.J.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.C.; Wang, W.M.; Wang, Z.; Li, L.H.; Jiang, G.J.; Wang, J.P.; Cheng, Z.B. Effects of maize and soybean intercropping on soil phosphorus bioavailability and microbial community structure in rhizosphere. J. Appl. Ecol. 2023, 34, 3030–3038. [Google Scholar]
- Raseduzzaman, M.; Jensen, E.S. Does intercropping enhance yield stability in arable crop production? A meta-analysis(Article). Eur. J. Agron. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Han, C.Q.; Yang, D.L.; Yang, J.J.; Cade-Menun, B.J.; Chen, Y.Q.; Sui, P. Maize-soybean intercropping facilitates chemical and microbial transformations of phosphorus fractions in a calcareous soil. Front. Microbiol. 2022, 13, 1028969. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Tanaka, M.; Sakai, M.; Toyama, H.; Matsushita, K.; Adachi, O.; Yamada, M. C-terminal periplasmic domain of escherichia coli quinoprotein glucose dehydrogenase transfers electrons to ubiquinone. J. Biol. Chem. 2001, 276, 48356–48361. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Li, H.; Lakshmanan, P.; Chen, Y.; Chen, X. phoD-harboring bacterial community composition dominates organic P mineralization under long-term P fertilization in acid purple soil. Front. Microbiol. 2022, 13, 1045919. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Rensing, C.; Han, D.F.; Xiao, K.Q.; Dai, Y.X.; Tang, Z.X.; Liesack, W.; Peng, J.J.; Cui, Z.L.; Zhang, F.S. Genome-resolved metagenomics reveals distinct phosphorus acquisition strategies between soil microbiomes. mSystems 2022, 7, e0110721. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The influence of soil fertilization on the distribution and diversity of phosphorus cycling genes and microbes community of maize rhizosphere using shotgun metagenomics. Genes 2021, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, F.; Shao, M.; Huang, L.; Xie, Y.; Xu, Y.; Kong, L. Effects of rotations with legume on soil functional microbial communities involved in phosphorus transformation. Front. Microbiol. 2021, 12, 661100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, S.; Meng, L.; Liu, X.; Zhang, Y.; Zhao, S.; Zhao, H. Root exudation under maize/soybean intercropping system mediates the arbuscular mycorrhizal fungi diversity and improves the plant growth. Front. Plant Sci. 2024, 15, 1375194. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, W.; Gan, Y.; Wang, L.; Chang, X.; Wang, Y.; Yang, W. Growth promotion ability of phosphate-solubilizing bacteria from the soybean rhizosphere under maize-soybean intercropping systems. JSFA Rep. 2022, 102, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Mir, R.A.; Hussain, S.J.; Prasad, B.; Kumar, P.; Aloo, B.N.; Sharma, C.M.; Dubey, R.C. Prospects of phosphate solubilizing microorganisms in sustainable agriculture. World J. Microbiol. Biotechnol. 2024, 40, 291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Q.; Liu, W.; Li, R.; Zhang, D.; Zhang, G.; Xu, Y. Plant secretions and volatiles contribute to the evolution of bacterial antibiotic resistance in soil-crop system. J. Environ. Sci. 2025, 152, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Alzate Zuluaga, M.Y.; Fattorini, R.; Cesco, S.; Pii, Y. Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: The case of PGPR-based biofertilizers. Front. Microbiol. 2024, 15, 1440978. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Pant, P.; Pant, B.D.; Krom, N.; Allen, R.D.; Mysore, K.S. Elucidating the unknown transcriptional responses and PHR1-mediated biotic and abiotic stress tolerance during phosphorus limitation. J. Exp. Bot. 2023, 74, 2083–2111. [Google Scholar] [CrossRef] [PubMed]
- Akond, Z.; Hasan, M.N.; Alam, M.J.; Alam, M.; Mollah, M.N.H. Classification of functional metagenomes recovered from different environmental samples. Bioinformation 2019, 15, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Kariya, S.; Kurokawa, J. Efficient PCR-Based amplification of diverse alcohol dehydrogenase genes from metagenomes for improving biocatalysis: Screening of gene-specific amplicons from metagenomes. Appl. Environ. Microbiol. 2014, 80, 6280–6289. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.N.S.; Sharma, P.; Maurya, S.; Yadav, R.K. Metagenomics and metatranscriptomics as potential driving forces for the exploration of diversity and functions of micro-eukaryotes in soil. 3 Biotech 2023, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.H.; Jiang, Y.H.; Han, W.W.; Shi, L.G.; Zhang, Y.R.; Liu, G.Z.; Cui, Y.H.; Du, X.; Gao, Z.; Liang, X.G. Simultaneous enhancement of maize yield and lodging resistance via delaying plant growth retardant application. Field Crops Res. 2024, 317, 109530. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Wiley: New York, NY, USA, 1996; Volume 5.3, pp. 961–1010. [Google Scholar]
- Chen, M.M.; Zhang, S.R.; Liu, L.; Wu, L.P.; Ding, X.D. Combined organic amendments and mineral fertilizer application increase rice yield by improving soil structure, P availability and root growth in saline-alkaline soil. Soil Tillage Res. 2021, 212, 105060. [Google Scholar] [CrossRef]
- Roberts, T.; Ross, W.; Norman, R.; Slaton, N.; Wilson, C. Predicting nitrogen fertilizer needs for Rice in Arkansas using alkaline hydrolyzable-nitrogen. Soil Sci. Soc. Am. J. 2011, 75, 1161–1171. [Google Scholar] [CrossRef]
- Heinen, B.J.; Vaz, J.E.; Benzo, Z.; Mejias, C. A comparison of extraction and suspension methods for determining exchangeable potassium in soils. Appl. Clay Sci. 1999, 14, 245–255. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 79–80. [Google Scholar]
- Wang, H.; Chen, J.P.; Ruan, Y.H.; Sun, W.; Wang, S.L.; Wang, H.T.; Zhang, Y.L.; Guo, J.M.; Wang, Y.C.; Guo, H.Y.; et al. Metagenomes reveal the effect of crop rotation systems on phosphorus cycling functional genes and soil phosphorus avail–ability. Agric. Ecosyst. Environ. 2024, 364, 108886. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Peng, J.; Liu, P.; Bei, Q.; Rensing, C.; Li, Y.; Yuan, H.; Liesack, W.; Zhang, F.; Cui, Z. Metagenomic insights into nitrogen and phosphorus cycling at the soil aggregate scale driven by organic material amendments. Sci. Total Environ. 2021, 785, 147329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Xu, J.; Nadia, R.; Jin, T.; Li, J.Y.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, C.; Halitschke, R.; Paetz, C.; Kessler, D.; Burkard, K.; Gaquerel, E.; Baldwin, I.T.; Li, D. Natural history-guided omics reveals plant defensive chemistry against leafhopper pests. Science 2022, 375, eabm2948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Mou, T.; Sun, Y.; Su, J.; Yu, L.; Zhang, Y. Environmental distribution and genomic characteristics of Solirubrobacter, with proposal of two novel species. Front. Microbiol. 2023, 14, 1267771. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Search for natural products from actinomycetes of the genus Nocardia. J. Nat. Med. 2024, 78, 828–837. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Peng, S.; Zhang, J.H.; Wang, Y.M.; Hua, Q.Q.; Cheng, S.Q. The type and degree of salinized soils together shape the composition of phoD-harboring bacterial communities, thereby altering the effectiveness of soil phosphorus cycling. J. Environ. Manag. 2025, 385, 125621. [Google Scholar] [CrossRef] [PubMed]
- Namuli, A.; Patrick, E.O.; John, B.T.; Muwanika, V.B.; Johnson, M.; Bazira, J. Diversity of bacterial community in the rhizosphere and bulk soil of Artemisia annua grown in highlands of Uganda. PLoS ONE 2023, 18, e0269662. [Google Scholar]
- Tian, J.H.; Kuang, X.Z.; Tang, M.T.; Chen, X.D.; Huang, F.; Cai, Y.X.; Cai, K.Z. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.C.; Zhou, Y.; Chen, P.; Zhang, X.N.; Du, Q.; Yang, H.; Wang, X.C.; Yang, F.; Xiao, T.; Li, L.; et al. Maize–legume intercropping promote N uptake through changing the root spatial distribution, legume nodulation capacity, and soil N availability. J. Integr. Agric. 2022, 21, 1755–1771. [Google Scholar] [CrossRef]
- Cuartero, J.; Pascual, J.A.; Vivo, J.M.; Özbolat, O.; Sánchez-Navarro, V.; Egea-Cortines, M.; Zornoza, R.; Mena, M.M.; Garcia, E.; Ros, M. A first-year melon/cowpea intercropping system improves soil nutrients and changes the soil microbial community. Agric. Ecosyst. Environ. 2022, 328, 107856. [Google Scholar] [CrossRef]
- Ma, W.Q.; Ma, L.; Li, J.H.; Wang, F.H.; Sisák, I.; Zhang, F.S. Phosphorus flows and use efficiencies in production and consumption of wheat, rice, and maize in China. Chemosphere 2011, 84, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.G. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Bohannan, B.J. Spatial scaling of microbial biodiversity. Trends Ecol. Evol. 2006, 21, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jason, A.P.; Aymé, S.; Omry, K.; Zhao, J.; Susannah, G.T.; Jeffery, L.D.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar]
- Song, J.S.; Zhang, X.L.; Kong, F.L.; Liu, X.L.; An, W.J.; Li, Y.Y. Effects of biomass conditioner on soil nutrient and microbial community characteristics of alpine desertified grassland in northwest Sichuan, China. J. Appl. Ecol. 2021, 6, 2217–2226. [Google Scholar]
- Zhao, M.L.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.W.; Vivanco, J.M.; Zhou, J.Z.; Kowalchuk, G.A.; Shen, Q.R. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.P.; Zhu, X.L.; Zhu, F.; Ke, W.S.; Huang, Y.Y.; Wu, C.; Xu, X.G.; Guo, J.K.; Xue, S.G. Organic acid release and microbial community assembly driven by phosphate-solubilizing bacteria enhance Pb, Cd, and As immobilization in soils remediated with iron-doped hydroxyapatite. J. Hazard. Mater. 2025, 488, 137340. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Feng, L.L.; Huang, Y.; Liang, Y.M.; Pan, F.J.; Zhang, W.; Zhao, Y.; Xiao, Y.X. Planted citrus regulates the community and networks of phoD-harboring bacteria to drive phosphorus availability between Karst and Non-Karst Soils. Microorganisms 2024, 12, 2582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Z.; Yuan, Q.H.; Guan, T.; Cai, Y.W.; Liu, E.F.; Li, B.; Wang, Y. Biotic regulation of phoD-encoding gene bacteria on organic phosphorus mineralization in lacustrine sediments with distinct trophic levels. Water Res. 2024, 260, 121980. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Bisht, N.; Ansari, M.M.; Chauhan, P.S. Pseudomonas putida triggers phosphorus bioavailability and P-transporters under different phosphate regimes to enhance maize growth. Plant Physiol. Biochem. 2024, 217, 109279. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Molecular mechanisms of phosphate sensing, transport and signalling in streptomyces and related actinobacteria. Int. J. Mol. Sci. 2021, 22, 1129. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.N.; Downs, D.M. An unexpected role for the periplasmic phosphatase phoN in the salvage of B6 vitamers in salmonella enterica. Appl. Environ. Microbiol. 2021, 87, e02300-20. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, I.D.E.A.; Fraser, T.; Murphy, A.R.J.; Scanlan, D.J.; Bending, G.D.; Jones, A.M.E.; Moore, J.D.; Goodall, A.; Tibbett, M.; Hammond, J.P.; et al. The ‘known’ genetic potential for microbial communities to degrade organic phosphorus is reduced in low-pH soils. MicrobiologyOpen 2017, 6, e00474. [Google Scholar] [CrossRef] [PubMed]
- Skouri-Panet, F.; Benzerara, K.; Cosmidis, J.; Férard, C.; Caumes, G.; De, L.G.; Heulin, T.; Duprat, E. In vitro and in silico evidence of phosphatase diversity in the biomineralizing bacterium ramlibacter tataouinensis. Front. Microbiol. 2018, 8, 2592. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Xia, Y.H.; Sun, Q.; Liu, K.P.; Chen, X.B.; Ge, T.D.; Zhu, B.L.; Zhu, Z.K.; Zhang, Z.H.; Su, Y.R. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Yu, Z.H.; Yao, Q.; Hu, X.J.; Zhang, W.; Mi, G.; Chen, X.L.; Wang, G.H. Distinct soil bacterial communities in response to the cropping system in a mollisol of northeast China. Appl. Soil Ecol. 2017, 119, 407–416. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Chen, M.; Fei, C.; Zhang, W.; Li, Y.; Ding, X. Fe-modified biochar combined with mineral fertilization promotes soil organic phosphorus mineralization by shifting the diversity of phoD-harboring bacteria within soil aggregates in saline-alkaline paddy soil. J. Soils Sediments 2023, 23, 619–633. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, J.; Lu, X.; Zhao, Z.; Li, K.; Wang, F.; Zhang, C.; Sun, R. Dosage effects of organic manure on bacterial community assemblage and phosphorus transformation profiles in greenhouse soil. Front. Microbiol. 2023, 14, 1188167. [Google Scholar] [CrossRef] [PubMed]
- Buenemann, E.K. Assessment of gross and net mineralization rates of soil organic phosphorus—A review. Soil Biol. Biochem. 2015, 89, 82–98. [Google Scholar] [CrossRef]
- Pistocchi, C.; Meszaros, E.; Tamburini, F.; Frossard, E.; Bunemann, E.K. Biological processes dominate phosphorus dynamics under low phosphorus availability in organic horizons of temperate forest soils. Soil Biol. Biochem. 2018, 126, 64–75. [Google Scholar] [CrossRef]
- Fatima, F.; Ahmad, M.M.; Verma, S.R.; Pathak, N. Relevance of phosphate solubilizing microbes in sustainable crop production: A review. Int. J. Environ. Sci. Technol. 2021, 19, 9283–9296. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, L.H.; Zhang, H.; Lan, B.; Lv, J.T.; Chen, G.; Wang, L.S.; Liu, Z.G. Role of phosphate solubilizing microorganisms in soil phosphorus cycle: A review. Microbiol. China 2023, 50, 3671–3687. [Google Scholar]
- Ai, P.H.; Sun, S.B.; Zhao, J.N.; Fan, X.R.; Xin, W.J.; Guo, Q.; Yu, L.; Shen, Q.R.; Wu, P.; Miller, A.J.; et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wen, Y.; Ma, J.; Macdonald, A.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Long-term farmyard manure application affects soil organic phosphorus cycling: A combined metagenomic and 33P/14C labelling study. Soil Biol. Biochem. 2020, 149, 107959. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, Y.; Fan, Y.F.; Zhang, L.; Li, X.Y.; Zhang, Q.Q.; Shu, Q.Y.; Huang, J.R.; Chen, G.Y.; Li, Q.; et al. Genetic improvement of phosphate-limited photosynthesis for high yield in rice. Proc. Natl. Acad. Sci. USA 2024, 121, e2404199121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X.; Wang, H.R.; Ren, X.L.; Xiao, Y.H.; Liu, D.P.; Meng, W.J.; Qiu, Y.H.; Hu, B.; Xie, Q.J.; Chu, C.C.; et al. Brassinosteroid-dependent phosphorylation of PHOSPHATE STARVATION RESPONSE2 reduces its DNA-binding ability in rice. Plant Cell 2024, 36, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lai, H.L.; Gao, F.Y.; Zhang, R.P.; Wu, S.X.; Ge, F.R.; Li, Y.Y.; Yao, H.Y. The proliferation of beneficial bacteria influences the soil C, N, and P cycling in the soybean–maize intercropping system. Environ. Sci. Pollut. Res. 2024, 31, 25688–25705. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.L.; Zheng, Y.; Xiao, J.X. Below-ground biotic mechanisms of phosphorus uptake and utilization improved by cereal and legume intercropping-A review. Crops 2018, 4, 20–27. [Google Scholar]
- Zhu, S.G.; Cheng, Z.G.; Wang, J.; Gong, D.S.; Fazal, U.; Tao, H.Y.; Zhu, H.; Duan, H.X.; Yang, Y.M.; Xiong, Y.C. Soil phosphorus availability and utilization are mediated by plant facilitation via rhizosphere interactions in an intercropping system. Eur. J. Agron. 2023, 142 (Suppl. C), 126679. [Google Scholar] [CrossRef]
- Zhou, J.C.; Zhang, L.; Feng, G.; George, T.S. Arbuscular mycorrhizal fungi have a greater role than root hairs of maize for priming the rhizosphere microbial community and enhancing rhizosphere organic P mineralization. Soil Biol. Biochem. 2022, 171, 108713. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Zhang, T.X.; Jin, S.Y.; Chen, S.; Zhang, Y.L. Effects of escherichia coli alkaline phosphatase PhoA on the mineralization of dissolved organic phosphorus. Water 2021, 13, 3315. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Bissett, A.; McGuire, K.; Saltonstall, K.; Turner, B.L.; Fierer, N. The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. mBio 2020, 11, e01718–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).