Abstract
Salinity stress is a primary abiotic constraint limiting global crop productivity, with progressive soil salinization inducing growth inhibition and physiological dysfunction in plants. Although melatonin (MT) has been extensively documented to enhance stress adaptation, the underlying mechanisms through which it mediates salt tolerance by integrating physiological processes remain unclear. This study investigated the effects of varying MT concentrations on photosynthetic performance, plant water relations, water-use efficiency, and stress-responsive physiological parameters in tomatoes, aiming to identify the key physiological pathways for MT-mediated salt stress mitigation. The results showed that salt stress significantly reduced the leaf relative water content and root hydraulic conductivity, suppressed the photosynthetic rate, and ultimately caused significant reductions in the aboveground and root biomass. MT spraying effectively improved leaf water status and root water uptake capacity, enhancing the photosynthetic rate and water-use efficiency, thereby providing material and energy support for plant growth. Furthermore, MT spraying increased the total antioxidant capacity in leaves and promoted the synthesis of phenolic and flavonoid compounds, thereby reducing oxidative damage. Simultaneously, it stimulated the accumulation of osmolytes to enhance cellular osmotic adjustment capacity and optimized ion uptake to maintain cellular ion homeostasis. Among the tested concentrations, 100 μM MT showed the most significant alleviative effects. This concentration comprehensively enhanced the salt tolerance and growth performance of tomato plants by synergistically optimizing water use, photosynthetic function, antioxidant defense, and ion balance. In conclusion, these findings provide experimental evidence for elucidating the physiological mechanisms underlying MT-mediated salt tolerance in tomatoes and offer theoretical references for the rational application of MT in crop production under saline conditions.
1. Introduction
Salt stress is a global agricultural challenge that affects approximately 20% of cultivated land and 33% of irrigated areas worldwide [1]. The annual expansion of saline soils at a rate of 2 million hectares continues to threaten a substantial proportion of arable land [2]. Soil salinity impairs plant growth and development through multifaceted disruptions of physiological processes, including photosynthesis, ion homeostasis, antioxidant defense systems, and osmotic regulation [3], ultimately leading to substantial yield losses [4].
Plants exhibit complex physiological adaptations under salt stress. Saline conditions significantly reduce chlorophyll content and PSII photochemical efficiency, thereby compromising photosynthetic apparatus integrity [5]. Elevated soil salinity restricts radial root expansion and diminishes root hydraulic conductivity through inhibited water transport [6]. Another primary threat stems from osmotic stress-induced ion toxicity, particularly due to excessive Cl− and Na+ accumulation, which disrupts Ca2+/K+ balance and impairs ionic homeostasis [5]. Furthermore, salt stress triggers an excessive overaccumulation of reactive oxygen species (ROS), damaging cellular components including membrane lipids, proteins, and nucleic acids, ultimately inducing programmed cell death [7,8]. To counteract salinity, plants activate integrated defense systems comprising both enzymatic (SOD, CAT, POD, APX) and non-enzymatic (phenols, flavonoids, ascorbic acid) antioxidants [9,10]. These components show salinity-induced upregulation, and their activity levels correlate with plant tolerance capacity. Moreover, osmolyte accumulation (proline, soluble sugars) plays an essential role in mitigating salinity damage by maintaining ROS homeostasis in chloroplasts and mitochondria [5,11]. When salt stress exceeds the adaptive thresholds, defense mechanisms become insufficient, resulting in apoptotic cell death and severe growth inhibition [12]. Although breeding salt-tolerant cultivars remains a viable strategy, the polygenic nature and pleiotropic effects of salt tolerance traits complicate genetic selection [13]. Consequently, exogenous protectant application has emerged as a promising complementary approach for enhancing plant salt tolerance.
Exogenous MT has emerged as a potent antioxidant and signaling molecule with significant potential for alleviating salt stress [14,15]. MT directly scavenges ROS and harmful oxidizing agents while concurrently enhancing key antioxidant enzyme activity through its signaling functions [16]. Studies have shown that MT-treated plants exhibit reduced ROS accumulation, ionic leakage, and cellular damage, accompanied by increased plant height and biomass, compared to untreated plants [17]. MT application plays a pivotal role in maintaining ionic homeostasis under salinity by promoting K+ uptake and inhibiting Na+ accumulation, thereby reducing salt-induced cellular damage [18]. Moreover, MT stimulates osmolyte accumulation while maintaining critical cellular functions, including growth regulation and biosynthesis, under saline conditions [19,20]. The growth-promoting effects of MT under salt stress have been validated in multiple crops, including tomatoes [21], wheat [22], and soybean [23]. However, the dose-dependent effects of melatonin on plant growth are well documented; lower concentrations generally promote growth, whereas higher concentrations may exert inhibitory effects [24]. The physiological mechanisms underlying the effects of MT on plants require further investigation.
Tomatoes have become a major global crop owing to their desirable taste and nutritional value, with extensive cultivation in China [25]. However, the development of modern protected cultivation systems has led to excessive fertilizer application and improper irrigation practices, resulting in increased soil salinity [26]. Salt stress is a primary constraint on tomato growth and yield [25]. Therefore, there is an urgent need to develop new strategies to improve the salt tolerance of tomatoes and mitigate the negative effects of climate-related salt stress. Although previous studies have reported the role of MT in mitigating abiotic stress damage, research on the regulatory mechanisms by which exogenous MT enhances tomato salt tolerance through multiple pathways remains scarce. This study aimed to systematically investigate MT-regulated salt tolerance mechanisms in tomatoes through comprehensive analyses of photosynthetic performance, water relations, antioxidant defense, osmoregulatory capacity, and ionic homeostasis, ultimately evaluating tomato salt tolerance. The findings of this study offer novel insights into the physiological basis of exogenous MT application for enhancing salt tolerance in tomatoes.
2. Materials and Methods
2.1. Plant Growth Conditions
The experiment was conducted at the Nongcui Experimental Station of Anhui Agricultural University (117.26 E 31.86 N) from July to August 2024. The main local cultivar ‘Wanza 18’ was selected as the experimental material, with seeds originating from the Anhui Academy of Agricultural Sciences. ‘Wanza 18’ is an indeterminate, pink-fruited hybrid tomato cultivar specifically bred for protected cultivation in subtropical regions. Characterized by high yield potential, disease resistance, and low-light tolerance, this cultivar is an ideal model system for investigating abiotic stress responses in tomatoes. Melatonin reagent (CAS #73-31-4; BR, 99%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China. Uniform seeds were selected and sterilized with 15% (w/v) sodium hypochlorite for 5 min, thoroughly rinsed with sterile distilled water, placed in a constant-temperature incubator for germination, and sown in seedling trays on July 10th. The physicochemical properties of the test soil were as follows: total nitrogen, 0.49 g kg−1; total phosphorus, 0.61 g kg−1; total potassium (K2O), 14.4 g kg−1; alkaline dissolved nitrogen, 31.23 mg kg−1; and effective phosphorus, 15.10 mg kg−1. When the seedlings developed two true leaves, they were transplanted into plastic pots (18 cm diameter × 25 cm height). The test soil was prepared by mixing organic fertilizer, potting soil, and sand in a 1:2:1 ratio.
2.2. Experimental Design
At the transplantation stage, all treatments were irrigated with equal volumes of water to ensure uniformity and consistent seedling growth. To minimize environmental interference, all pots were transferred to an artificial growth chamber (RTOP-B, Zhejiang Topu Yunnong Technology Co., Ltd., Hangzhou, China) during the experiment. Plants were maintained in a controlled growth chamber under the following conditions: temperature 18–25 °C, relative humidity 60%, CO2 concentration 400 µmol mol−1, and a 14-h photoperiod with 350 µmol m−2 s−1 photosynthetic photon flux density. When the seedlings developed four true leaves, tomato plants exhibiting uniform growth were selected for melatonin spraying. Based on preliminary experiments and previous studies [27], three melatonin concentrations (M1: 50 µM, M2: 100 µM, M3: 150 µM) were established. Due to the photolability of melatonin, spraying was conducted 1 h after dark adaptation. Both the adaxial and abaxial leaf surfaces were uniformly sprayed with melatonin solution until droplet runoff was observed. Melatonin-sprayed seedlings received daily applications for five consecutive days, whereas non-primed control plants were sprayed with an equivalent volume of tap water.
Salt stress treatment was initiated after melatonin spraying. Based on previous studies [21,27], a 150 mM NaCl solution was applied to the soil to establish salt stress conditions. Five treatments were implemented, as follows: M1S (50 µM melatonin + salt stress), M2S (100 µM melatonin + salt stress), M3S (150 µM melatonin + salt stress), SS (0 µM melatonin + salt stress), and CK (control, 0 µM melatonin + no salt stress), with 12 replicates (pots) per treatment. The salt stress treatment employed a pulsed application-leaching regime to simulate field salinity dynamics, consisting of (1) 100 mL of 150 mM NaCl solution applied every 2 days (6 total applications), increasing soil electrical conductivity (EC) by approximately 1.3–1.5 dS m−1 per application; and (2) 200 mL tap water flushing every 5 days to maintain target EC levels (4.5 ± 0.3 dS m−1), preventing excessive salt accumulation. Control plants received equivalent volumes of tap water with an identical leaching frequency to maintain consistent soil moisture conditions.
2.3. Sampling and Measurement
2.3.1. Plant Sampling
Leaf and root samples were collected at the 4th, 8th, and 12th days after stress (D4, D8, and D12). Samples were immediately transported to the laboratory to determine leaf relative water content, root hydraulic conductivity, and membrane stability index. Na+, K+, Mg2+, and Ca2+ contents were quantified using dried leaf and root samples. The remaining samples were stored at −80 °C for subsequent determination of antioxidant enzyme activity, phenol and flavonoid content, osmotic regulators, reactive oxygen species levels, and malondialdehyde content. All the aforementioned physiological and biochemical parameters were analyzed using three biological replicates (n = 3), with each measurement performed in triplicate.
2.3.2. Determination of Growth Traits
Growth traits were measured in tomato plants at D12. Plant height was measured using a digital caliper, and root samples were collected. Root length was determined by scanning the root systems and analyzing them using WinRhizo software, Basic version (Winrhizo, Regent Ltd., Quebec, QC, Canada). Subsequently, both aboveground and root samples were oven-dried at 80 °C to a constant weight.
2.3.3. Determination of Gas Exchange and Plant Water Relations
Gas exchange parameters were measured at D4, D8, D12, and D16 using an LI-6400XT portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA). Parameters, including net photosynthetic rate (Pn) and stomatal conductance (gs), were measured under 400 µmol mol−1 CO2 concentration at a constant flow rate of 500 mL min−1. Instantaneous water-use efficiency (WUEi) was determined as the ratio of Pn to Tr. Leaf relative water content (LRWC) was determined using the following formula:
where FW is leaf fresh weight, DW is leaf dry weight, and SW is saturated leaf weight.
LRWC (%) = [(FW − DW)/(SW − DW)] × 100
Root hydraulic conductance (Lp) was determined using excised roots placed in a pressure chamber with the cut stem exposed to the atmosphere. Increasing pressure gradients were applied to measure the volumetric flow rates until the root water potential was reached. The final Lp values were calculated according to Kang and Zhang [28].
2.3.4. Determination of Antioxidant Enzyme Activity
Superoxide dismutase (SOD) activity was determined as described by Giannopolitis and Ries [29]. The reaction mixture (3 mL) consisted of 50 mmol−1 potassium phosphate buffer (pH 7.0), 13 mM methionine, 75 μM nitroblue tetrazolium (NBT), 2 μM riboflavin, and 0.2 mL of enzyme extract. One unit of SOD activity was defined as the enzyme quantity that inhibited 50% of NBT reduction at 560 nm. Peroxidase (POD) activity was analyzed according to Cakmak and Marschner [30] by adding 16 mM guaiacol, 0.2 mL enzyme extract, and 10 mM hydrogen peroxide to 50 mM phosphate buffer (pH 7.0), and the absorbance was recorded at 470 nm. Catalase (CAT) activity was measured using the protocol described by Havir and Mchale [31], where 0.2 mL of enzyme extract was added to 50 mM phosphate buffer (pH 7.0) containing 12.5 mM H2O2, and the absorbance was monitored at 240 nm.
The total antioxidant capacity (TAC) was evaluated as described by Fatima [32]. Briefly, 100 μL of the extract was mixed with 900 μL of reagent (0.6 M sulfuric acid, 4 mM ammonium molybdate, and 28 mM sodium phosphate) and incubated at 95 °C for 90 min. Absorbance was measured at 695 nm after cooling, using ascorbic acid for standard curve calibration.
2.3.5. Determination of Phenols and Flavonoids Contents
The total phenol content was quantified using the method of Yu and Dahlgren [33]. The reaction mixture (2 mL) contained 18 µL of the extract, 70 µL of Folin–Ciocalteu reagent, 175 µL of 20% (w/v) sodium carbonate solution, and 1.75 mL of distilled water. After 30 min of incubation at 45 °C, the absorbance was measured at 750 nm. The total flavonoid content was determined according to Arvouet–Grand [34] by mixing 1 mL of the extract with 1 mL of 2% methanol-aluminum chloride solution, incubating in the dark for 20 min, and measuring the absorbance at 415 nm.
2.3.6. Determination of ROS, Malondialdehyde, and Membrane Stability Index
Hydrogen peroxide (H2O2) content was assessed following Velikova [35]. Leaf tissue was homogenized in 5 mL 0.1% (w/v) trichloroacetic acid (TCA) at 8000 rpm and 4 °C. The supernatant was mixed with 10 mM potassium iodide (KI), and the absorbance was recorded at 390 nm. The superoxide anion (O2−) content was measured using a commercial reagent kit (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China). Malondialdehyde (MDA) content was analyzed via the thiobarbituric acid reaction [36], where 1 mL extract was mixed with 4 mL 20% trichloroacetic acid containing 0.5% thiobarbituric acid, heated at 95 °C for 30 min, cooled on ice, and absorbance measured at 532, 600, and 450 nm.
The membrane stability index (MSI) was calculated according to the method of Rady [37].
where EC1 is the conductivity of the leaf sample solution heated at 40 °C for 30 min, and EC2 is the conductivity after heating at 100 °C for 20 min.
MSI (%) = [1 − (EC1/EC2)] × 100
2.3.7. Determination of Proline and Soluble Sugar Contents
The proline content was determined using the method described by Bates [38]. The reaction mixture containing 1 mL extract, 2 mL ninhydrin reagent, and 2 mL glacial acetic acid was boiled for 1 h, cooled on ice, extracted with 3.5 mL toluene, and the absorbance was measured at 520 nm. Soluble sugar content was analyzed following Yemm and Willis [39] by mixing 100 μL of the extract with 3 mL of anthrone reagent, heating at 95 °C for 10 min, cooling to room temperature, incubating for 20 min, and measuring the absorbance at 625 nm.
2.3.8. Determination of Ion Contents
The Na+, K+, Ca2+, and Mg2+ contents were quantified from dried leaf and root samples. The samples were ground into powder using a centrifugal mill (ZM 200, Retsch GmbH, Haan, Germany) at 8000 rpm. Digestion was performed with 30% hydrogen peroxide and concentrated nitric acid, followed by ion content analysis using flame atomic absorption spectrometry according to Sousa [40].
2.4. Data Analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA followed by Duncan’s multiple range test (p < 0.05) using SPSS 22.0 (IBM Corp., Armonk, NY, USA). All figures were generated using Origin 2021 software (OriginLab, Northampton, MA, USA). The relationships between physiological parameters were examined using Pearson correlation analysis. Principal component analysis (PCA) was performed using R software (version 4.1.0). Data normalization was conducted using within-group maximum normalization (observed value/maximum value within the group), transforming all values to a relative 0–1 scale to eliminate unit differences and facilitate heatmap visualization and intergroup comparisons. Tomato salt tolerance was quantitatively evaluated using the membership function method described by Sun [41].
3. Results
3.1. Aboveground and Root Growth of Tomato
Exogenous melatonin (MT) significantly alleviated salt stress-induced growth inhibition in tomato plants (Figure 1A). At D12, salt stress reduced the aboveground dry mass and plant height by 26% and 19%, respectively, compared to the control. MT spraying increased the aboveground dry mass by 5%, 17%, and 16% in M1S, M2S, and M3S plants, respectively, compared to CK. M2S and M3S treatments demonstrated more pronounced growth promotion than M1S, with plant height increases of 16% and 15%, respectively, compared to salt-stressed plants. Root growth parameters were severely impaired under salt stress, showing 35% and 38% reductions in root dry mass and length, respectively. MT spraying effectively alleviated these adverse effects, increasing root dry mass by 10%, 27%, and 18% in M1S, M2S, and M3S plants, respectively (Figure 1B).
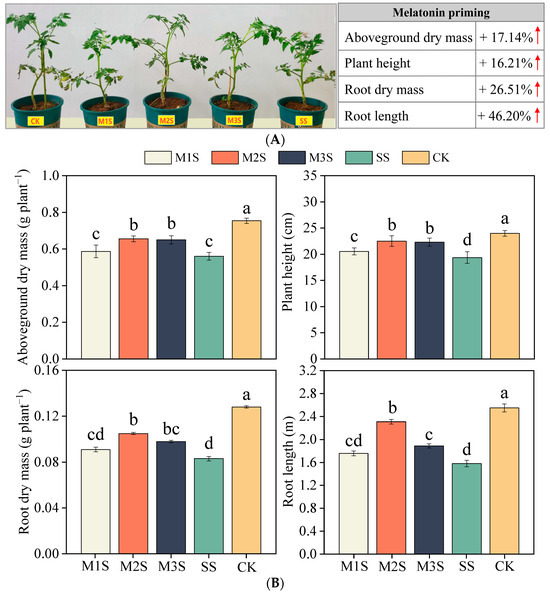
Figure 1.
Tomato growth under salt stress and melatonin spraying conditions (A). Effects of melatonin spraying on aboveground and root growth traits of tomato plants (B). The red arrow in (A) indicates the maximum increase observed with melatonin treatment compared to SS treatment. Values are expressed as mean ± SD, n = 4. M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
3.2. Plant Water Relations and Gas Exchange Parameters
The leaf relative water content (LRWC) progressively declined under prolonged salt stress (Figure 2A). At D4, MT-primed plants maintained significantly higher LRWC than salt-stressed (SS) plants. At D8, M1S, M2S, and M3S plants exhibited 2%, 5%, and 6% higher LRWC than SS plants. The M2S and M3S plants retained relatively higher LRWC than the SS plants at D12. Salt stress reduced root hydraulic conductivity (Lp), impairing water uptake capacity. At D4, Lp in SS plants decreased by 17% compared to CK, whereas M2S and M3S plants showed only 5% reduction and 2% increase, respectively, and the positive effect of M2 treatment on Lp gradually diminished with prolonged stress exposure.
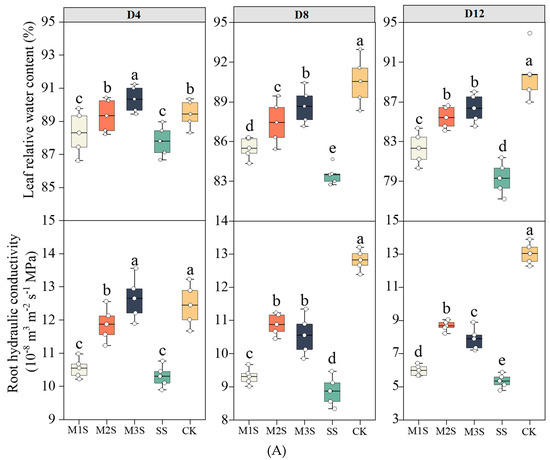
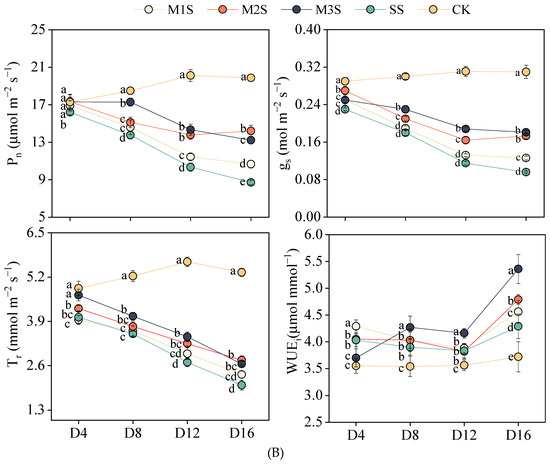
Figure 2.
Effects of melatonin spraying on leaf relative water content (LRWC), root hydraulic conductivity (Lp) (A). Effects of melatonin spraying on net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), and instantaneous water use efficiency (WUEi) of tomato plants under salt stress (B). For LRWC and Lp, values are expressed as mean ± SD, n = 5. For Pn, gs, and Tr, values are expressed as mean ± SD, n = 12 (biological replicates × technical replicates:
). M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. D4, the 4th day after stress; D8, the 8th day after stress; D12, the 12th day after stress; D16, the 16th day after stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
Pn progressively declined under salt stress (Figure 2B). At D8, M3S plants maintained Pn comparable to CK and were 26% higher than that of SS plants. Although M3S plants showed greater Pn reduction at D12, their values remained 39% higher than those of SS plants. At D16, Pn in M2S and M3S plants increased by 63% and 52% compared to SS plants, respectively. For gs, M2S, and M3S plants exhibited 1.17- and 1.28-fold higher gs than SS plants at D8 and 1.43- and 1.63-fold higher gs at D12. At D16, gs increased by 80% and 89% in M2S and M3S plants, respectively, compared with SS. MT application effectively mitigated the salt-induced reduction in Tr, particularly in the M2 and M3 treatments. At D8, D12, and D16, Tr in M2S and M3S treatments were significantly higher than in SS. M2S and M3S treatments maintained relatively high WUEi at D8, D12, and D16, with M3S showing 8.6% and 24.9% higher WUEi than SS at D12 and D16, respectively.
3.3. Phenols, Flavonoids, and Osmoregulatory Substances Contents
Salt stress significantly increased the total phenol content in leaves (Figure 3A). At D8, the total phenol content was substantially higher in SS plants than in CK, with M2S and M3S showing 6% and 23% increases compared to SS, respectively. At D12, M1S, M2S, and M3S plants maintained 16%, 42%, and 27% greater phenol content than SS plants, respectively. During early stress (D4), the total flavonoid content in M2S and M3S plants was significantly lower than that in SS plants (Figure 3B). At D8, M1S and M2S plants accumulated significantly higher flavonoid levels than SS plants. At D12, M2S and M3S plants maintained 35% and 27% higher total flavonoid content than SS plants. MT spraying progressively enhanced proline accumulation with stress duration (Figure 3C). At D8, the proline content in M3S plants increased by 35% compared to SS plants. At D12, M3S plants maintained the highest proline levels. Similarly, salt stress markedly increased the soluble sugar content (Figure 3D). At D4, the soluble sugar content of M3S plants was 24% higher than that of SS plants. M1S and M2S plants showed substantial increases in soluble sugar at D12, whereas the soluble sugar content in M3S plants declined sharply. The soluble sugar content in M1S, M2S, and M3S plants reached 2.7-, 3.2-, and 1.9-fold higher than that in SS plants, respectively.
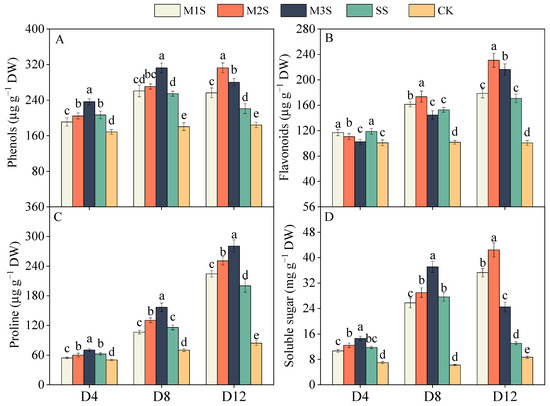
Figure 3.
Effects of melatonin spraying on total phenol (A), total flavonoid (B), proline (C), and soluble sugar (D) content of tomato plants under salt stress. Values are expressed as mean ± SD, n = 9 (biological replicates × technical replicates: ). M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. D4, the 4th day after stress; D8, the 8th day after stress; D12, the 12th day after stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
3.4. Membrane Stability and Reactive Oxygen Species Contents
Salt stress significantly reduced the membrane stability index (MSI) (Figure 4A). At D8, M2 and M3 treatments effectively alleviated this decline, exhibiting 13% and 14% higher values than SS plants, respectively. At D12, the MSIs in M1S, M2S, and M3S plants remained 14%, 30%, and 26% higher than those in SS plants, respectively. MDA content increased markedly under salt stress (Figure 4B). At D4, MDA levels in SS plants rose to 2.06-fold of control values, whereas M1S, M2S, and M3S plants showed increases of 1.86-, 1.66-, and 1.74-fold, respectively. At D12, the MDA contents in M2S and M3S plants were 41% and 44% lower than those in SS plants, respectively. Salt stress induced the accumulation of O2− and H2O2 in leaves, with progressively stronger effects over time (Figure 4C,D). At D8, the O2− levels in SS plants were 140% higher than those in the controls, whereas M3S plants exhibited only a 74% increase. The regulatory effects of MT on H2O2 were evident at D8. SS plants showed a 74% increase in H2O2 content compared to the control, whereas M2S plants displayed a mere 30% increase. At D12, M2S plants retained 22% lower H2O2 content than M1S plants.
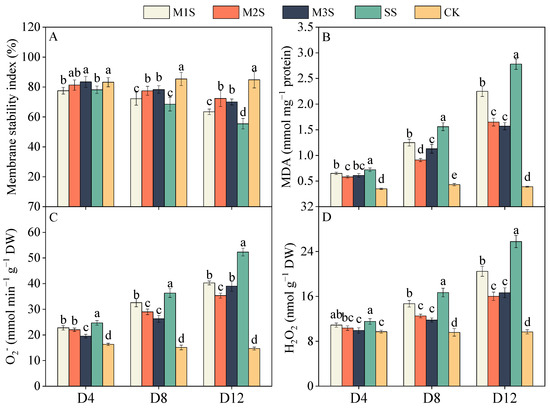
Figure 4.
Effects of melatonin spraying on membrane stability index (A), malondialdehyde (MDA, (B)), superoxide anion (O2−, (C)), and hydrogen peroxide (H2O2, (D)) contents of tomato plants under salt stress. Values are expressed as mean ± SD, n = 9 (biological replicates × technical replicates: ). M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. D4, the 4th day after stress; D8, the 8th day after stress; D12, the 12th day after stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
3.5. Antioxidant Enzyme Activity
Salt stress significantly enhanced the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) (Figure 5A). At D8, the SOD activity in M2S plants was 16% higher than that in SS plants. At D12, M3S plants exhibited substantially higher SOD activity than M1S, M2S, and SS plants. For POD, SS plants showed an 89% increase at D8 compared to CK (Figure 5B), whereas M2S and M3S plants displayed even greater increases of 127% and 115%, respectively. During the late stress stage (D12), M2S and M3S plants maintained 1.57- and 1.34-fold higher POD activity than SS plants at D12. CAT activity in M2S plants increased by 12% at D8 compared to SS plants (Figure 5C). At D12, CAT activity in M1S and M2S plants further increased to 1.96- and 2.12-fold higher levels than in SS plants, respectively. Salt stress also significantly elevated the total antioxidant capacity (TAC) of tomato plants (Figure 5D). The positive effects of MT spraying on TAC levels became more pronounced with prolonged stress exposure. At D12, TAC levels in M2S and M3S, plants were 33% and 29% higher than those in SS plants, respectively.
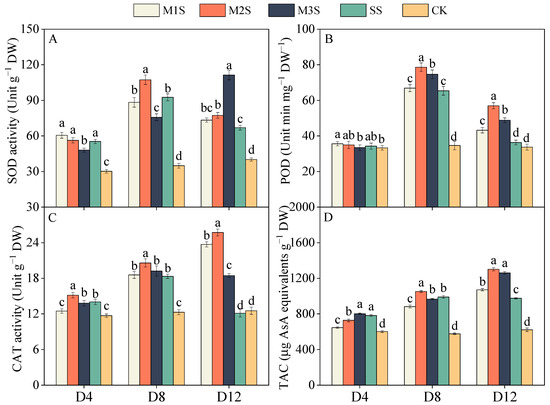
Figure 5.
Effects of melatonin spraying on superoxide dismutase (SOD, (A)), peroxidase (POD, (B)), catalase (CAT, (C)), and total antioxidant capacity (TAC, (D)) of tomato plants under salt stress. Values are expressed as mean ± SD, n = 9 (biological replicates × technical replicates: ). M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. D4, the 4th day after stress; D8, the 8th day after stress; D12, the 12th day after stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
3.6. Ion Contents of Leaves and Roots
The leaf Na+ content in M1S, M2S, and M3S plants decreased by 22%, 19%, and 44% at D4, compared to SS plants, respectively (Figure 6A). Root Na+ content in M2S and M3S plants was 15% and 28% lower than that in SS plants at D4. At D12, M3S plants showed further reductions, with leaf and root Na+ contents 29% and 46% lower than those of SS plants, respectively. Salt stress suppressed K+ uptake in both leaves and roots (Figure 6B). At D8, M2S and M3S plants displayed 14% and 13% higher leaf K+ content and 41% and 30% higher root K+ content than SS plants, respectively. At D12, the root K+ content in M2S and M3S plants was 93% and 80% higher than that in SS plants, respectively. The K+/Na+ improved markedly with melatonin treatment (Figure 6C). At D4, leaf and root K+/Na+ levels in M3S plants were 1.73- and 1.76-fold higher than those in SS plants (Figure 6C). At D8, leaf K+/Na+ in M2S and M3S plants exceeded SS levels by 57% and 84%, respectively, whereas root K+/Na+ increased by 78% and 104%, respectively. At D12, the root K+/Na+ in M2S and M3S plants was significantly higher than that in SS plants.
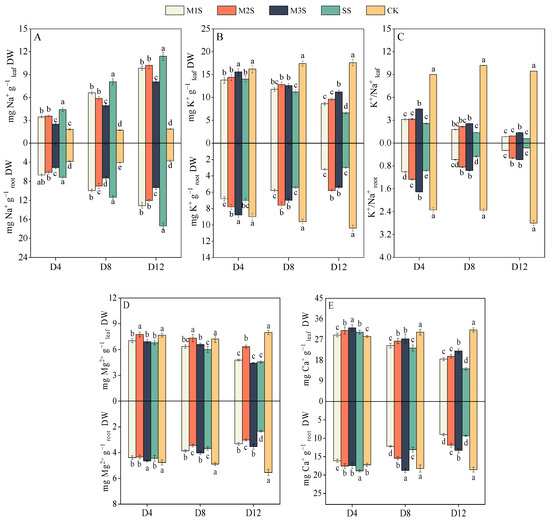
Figure 6.
Effects of melatonin spraying on Na+ (A), K+ (B), K+/Na+ (C), Mg2+ (D), and Ca2+ (E) contents in leaves and roots under salt stress. Values are expressed as mean ± SD, n = 9 (biological replicates × technical replicates: ). M1S, 50 µM melatonin + salt stress; M2S, 100 µM melatonin + salt stress; M3S, 150 µM melatonin + salt stress; SS, no spraying + salt stress; CK, no spraying + no stress. D4, the 4th day after stress; D8, the 8th day after stress; D12, the 12th day after stress. Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Duncan’s test).
Salt stress inhibited Mg2+ absorption in the leaves and roots (Figure 6D). At D4, M2S plants showed 14% higher leaf Mg2+ content than SS plants. At D8, leaf Mg2+ content in M2S and M3S plants was significantly elevated, with M1 and M3 showing stronger effects on root Mg2+ content than M2S. At D12, M2S plants achieved 39% higher leaf Mg2+ content, while M1S, M2S, and M3S plants showed 42%, 29%, and 51% increases in root Mg2+ content compared to SS plants, respectively. For Ca2+ content, M3S plants exhibited 6% and 10% higher leaf and root levels than SS plants at D4 (Figure 6E). At D8, M2S and M3S plants showed 13% and 17% increases in leaf Ca2+ content compared to SS plants, respectively. At D12, the leaf Ca2+ content in M1S, M2S, and M3S plants reached 1.30-, 1.39-, and 1.56-fold of SS levels, respectively, with similar enhancement patterns observed for root Ca2+ content in M2S and M3S plants.
3.7. Relationship Among Physiological Characteristics and Salt Tolerance Evaluation
3.7.1. Relationships Among Physiological Characteristics
Under salt stress conditions, leaf Na+ content was positively correlated with TAC, whereas leaf K+, Mg2+, and Ca2+ contents were significantly negatively correlated with TAC. Under MT spraying, the improved leaf K+, Mg2+, and Ca2+ contents contributed to a higher TAC. MSI decreased significantly with increasing leaf Na+ content, but demonstrated positive correlations with leaf K+, Mg2+, and Ca2+ contents. Lp showed a significantly negative correlation with root Na+ content and a significant positive correlation with root K+, Mg2+, and Ca2+ contents (Figure 7).
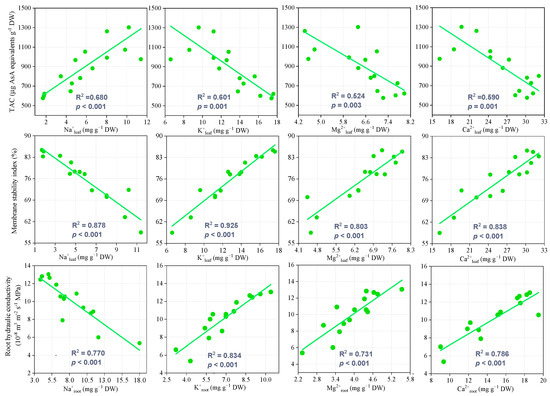
Figure 7.
Relationships of ion contents with total antioxidant capacity (TAC), membrane stability index (MSI), and root hydraulic conductivity (Lp).
3.7.2. Comprehensive Evaluation of Salt Tolerance
Principal component analysis (PCA) revealed that salt-stressed (SS) plants clustered with physiological markers (PH, TAC, O2−, FL, and Pro along the right abscissa, where Pro displayed strong positive correlations with FL, O2−, and TAC (Figure 8A). Significant positive correlations between Mg2+ and Ca2+ contents were observed in leaves and roots, whereas all measured ions showed strong associations with MSI. Under MT spraying, the data points of the M2S and M3S treatments shifted rightward relative to the SS treatment. Furthermore, the data points of the M1S, M2S, and M3S treatments were closer to the upper side of the coordinate axis than those of the SS treatment, indicating that MT spraying effectively improved multiple physiological processes to enhance salt tolerance.
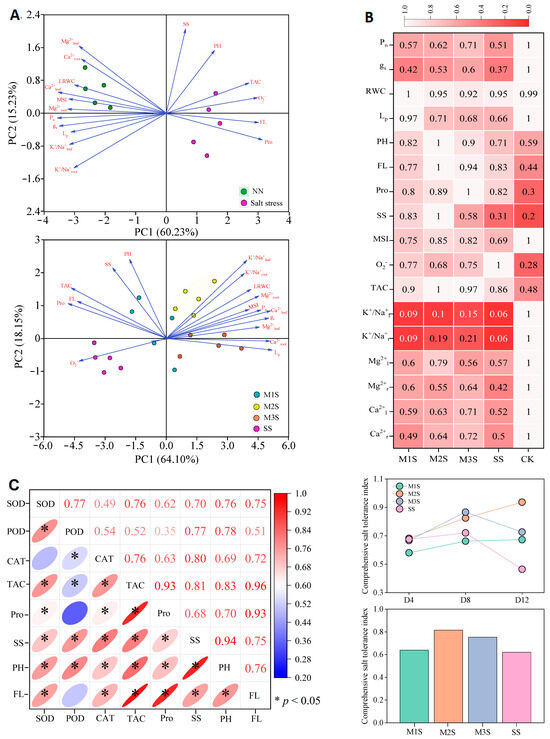
Figure 8.
Principal component analysis (PCA) of physiological indicators (A). Heatmap of physiological indicators under different melatonin spraying conditions (B) and changes in the comprehensive salt tolerance index during salt stress (C). Prior to PCA, KMO adequacy and Bartlett’s tests were conducted. The KMO value was 0.823, and the significance of Bartlett’s test was 0.004. Tests supporting intergroup separation were performed at a 95% confidence level. Ellipses represent the 95% confidence intervals for each treatment (A). The data were normalized using the within-group maximum normalization method (observed value/maximum value within the group) for heatmap analyses (B). The numbers represent the correlation coefficients among the indicators (C). RWC, leaf relative water content; Lp, root hydraulic conductivity; PH, phenols; FL, flavonoids; SS, soluble sugar; Pro, proline; TAC, total antioxidant capacity; MSI, membrane stability index; Pro, proline; SS, soluble sugar; PH, phenol; FL, flavonoid.
Heatmap analysis corroborated the PCA results (Figure 8B), demonstrating that MT spraying mitigated the decreases in Pn, water relations, and ion absorption (excluding Na+), while enhancing antioxidant enzyme activities and non-enzyme and osmoregulatory substance contents, particularly under M2 treatment. Eight key indicators of comprehensive salt tolerance were evaluated (Figure 8C), revealing a progressive improvement in M1S and M2S plants during stress, with M2S plants achieving the highest tolerance index at D12. Overall, M2 spraying provided superior salt tolerance in tomato plants, followed by M3 treatment.
4. Discussion
4.1. Exogenous Melatonin Improves Tomato Growth by Regulating Photosynthesis
Photosynthesis is a highly sensitive physiological process that is affected by salt stress. Salt stress substantially impairs plant photosynthetic capacity via stomatal closure and photosystem damage [5]. This study revealed that salt stress reduced gs in tomato leaves, which may be attributed to decreased LRWC and Lp. Melatonin (MT) treatment, particularly at 100 µM concentration, markedly enhanced gs and alleviated the stomatal limitation induced by salt stress. These findings are consistent with those reported by Ye [42], indicating that MT can improve plant gas exchange capacity by regulating stomatal aperture. Furthermore, the results demonstrated a progressive decline in the net photosynthetic rate (Pn) with prolonged salt stress exposure. Shi [17] proposed that melatonin exerts beneficial regulatory effects by upregulating the transcription of photosynthesis-related genes and protecting the photosynthetic apparatus. The current study confirmed that MT treatment significantly improved the Pn of tomato leaves subjected to salt stress. Notably, 100 and 150 μM melatonin outperformed 50 μM in sustaining photosynthetic rates. The improvement in Pn may be associated with the upregulation of genes encoding PSI-related proteins (PsaK and PsaG), the PsbO and PsbP subunits, and the oxygen-evolving complex (OEC) protein in the PSII reaction center [43].
This study found that salt stress significantly inhibited the growth parameters of both aboveground parts and roots of tomato plants (Figure 1). Growth suppression can be primarily attributed to salt-induced stomatal closure, which reduces Pn and ultimately leads to diminished biomass accumulation [44]. Previous studies have confirmed that MT is a regulator of plant growth and development [21,22]. For instance, low concentrations of MT can promote root elongation, whereas higher concentrations may inhibit growth, as observed in wild mustard [45]. Consistent with previous findings, we found that 100 μM MT exhibited superior regulatory effects on tomato growth compared to 50 μM and 150 μM treatments, which aligned with the findings of Bajwa [46] in Arabidopsis. We speculated that this dose-dependent response may be associated with hormonal regulation and the activation of salt-tolerance-related genes [47]. MT may alleviate salt-induced growth inhibition in tomatoes by regulating the expression of genes encoding K+ channels and Na+/H+ antiporters [48], thereby maintaining leaf ion homeostasis. Furthermore, the growth-promoting effects of MT have been well documented in other crops, including maize [49] and rice [50].
4.2. Exogenous Melatonin Enhanced Tomato Salt Tolerance by Improving Osmoregulation
Osmolyte accumulation plays a vital role in maintaining cellular homeostasis and counteracting salt stress toxicity [20]. Our study revealed significant proline accumulation in tomatoes under salt stress, with this trend becoming more pronounced during the later stages of stress. Under salt stress conditions, plants coordinately enhance proline accumulation by upregulating key biosynthetic genes (e.g., P5CS1 and P5CR) while suppressing catabolic genes (e.g., ProDH/PDH), thereby improving salt tolerance [51]. Notably, MT spraying further strengthened this accumulation effect. This is consistent with the findings of Zhang [52], who demonstrated that MT application significantly increased betaine and proline content in plants, while upregulating the expression of related synthesis genes (BADH, P5CS, etc.), consequently enhancing osmoregulatory capacity under saline conditions. These results collectively suggest that MT application elevates proline levels in plants, and the accumulation of proline can increase leaf osmotic potential and promote water absorption by plants, thereby providing favorable conditions for photosynthesis and ultimately improving growth performance [19,53]. Notably, the regulatory effects of MT on proline synthesis exhibited significant concentration-dependent variations; 150 μM demonstrated optimal efficacy in promoting proline accumulation throughout the stress period, whereas 50 μM had the weakest effect. These results confirmed that MT regulation of proline metabolism is concentration-dependent.
In the current study, salt stress significantly increased soluble sugar content in leaves, while MT treatment exhibited a distinct concentration-dependent effect; medium MT concentration (100 μM) further enhanced sugar accumulation, whereas both low (50 μM) and high (150 μM) concentrations reduced it. These findings are consistent with previous studies demonstrating that MT-induced accumulation of osmolytes reduces cellular osmotic potential, thereby enhancing plant osmoregulatory capacity and improving water status under stress conditions [54,55]. In this study, MT-sprayed plants exhibited significantly higher LRWC and Lp than those subjected to salt stress treatment, which directly confirmed this mechanism. The improved water status in salt-stressed plants may also be attributed to melatonin-mediated promotion of root growth and aquaporin activity, which enhances water absorption and transport [56]. Additionally, higher cellular water content can induce stomatal opening in leaves, facilitating the exchange of H2O and CO2 between leaves and the external environment and providing sufficient raw materials for photosynthesis. This also explains why the MT application under salt stress increased gs and Pn in tomato plants.
4.3. Exogenous Melatonin Enhanced Tomato Salt Tolerance by Regulating the Antioxidant System
Under salt stress, excessive Na+ accumulation induces ROS overproduction, leading to lipid peroxidation and membrane disruption [7,8]. Our study showed significant increases in H2O2 and O2− levels due to persistent Na+-induced ion toxicity, which was closely related to the osmotic stress induced in the early stage of salt stress and the ionic toxicity caused by long-term Na+ accumulation [57]. Nevertheless, melatonin spraying at varying concentrations effectively reduced the levels of these oxidative markers, with 100 µM MT demonstrating significantly superior ROS-scavenging capacity compared to 50 μM and 150 μM treatments. Notably, the inhibitory effect of MT on ROS did not diminish with the prolongation of salt stress. After 8–12 days of stress, O2− and H2O2 levels remained substantially lower in MT-treated plants than in those subjected to salt stress treatment alone. This phenomenon has been observed in various crops. For instance, continuous MT application reduced ROS accumulation in tea plants under long-term arsenic stress [58], and after 15 days of salt stress, the malondialdehyde content and electrolyte leakage in the MT-treated group of alfalfa were significantly lower than those in the control group [59]. These results indicate that the ROS-scavenging capacity of MT in the short term is not affected by the extension of stress duration, which may be related to the sustained high levels of endogenous melatonin in plants after MT spraying [60]. Similarly, studies on cucumber have also found that salt stress led to an increase in H2O2 concentration, while MT spraying maintained a lower level of H2O2 throughout the experiment [61]. MT also reduces the O2− content in tomato seedlings under salt stress [62]. Our data further showed that melatonin spraying reduced MDA levels and improved the membrane stability index, which coincided with decreased ROS generation, suggesting its role in maintaining membrane integrity through oxidative damage alleviation [63]. In terms of membrane protection, both 100 and 150 µM melatonin outperformed 50 µM. This protective effect may be associated with the activation of the MAPK cascade by melatonin, a signaling pathway that not only promotes the expression of WRKY transcription factors [64] but also effectively mitigates membrane lipid peroxidation [65].
Plants have evolved complex antioxidant systems to counteract ROS accumulation during stress. Our results demonstrated that exogenous MT combined with salt stress synergistically enhanced SOD, POD, and CAT activities, indicating that MT spraying effectively upregulated antioxidant enzyme activities in tomato leaves, thereby scavenging excess ROS and protecting plants from oxidative damage. Notably, 100 μM MT treatment demonstrated significantly greater enhancement of antioxidant enzyme activity compared to 50 μM and 150 μM treatments. Zhang [66] also confirmed that MT treatment significantly upregulated the expression of POD, APX, SOD, and CAT genes in cucumbers under salt stress conditions. Similar results have been reported in naked oats [64] and rapeseeds [67]. The mechanism may involve MT enhancing antioxidant enzyme activity by upregulating the expression of antioxidant enzyme-related genes and reducing the degradation of biomacromolecules [66,68]. However, studies have shown that MT did not enhance the activities of CAT and POD under salt stress but instead reduced them [69]. These differences may be related to the MT concentration and salt stress intensity.
This study revealed that the antioxidant strategy of MT-sprayed plants was not fixed but was dynamically adjusted with prolonged stress duration. During the 0–8 days of salt stress, MT-sprayed plants primarily relied on the antioxidant enzyme system to scavenge ROS. This enzyme-dependent mechanism demonstrates both high efficiency and substrate specificity, with its activity positively correlated with plant salt tolerance [70]. The results further demonstrated that the activities of the antioxidant enzymes showed a trend of first increasing and then decreasing with the extension of stress duration. Interestingly, although MT cannot directly scavenge O2− and H2O2, it maintains cellular redox homeostasis by activating antioxidant enzymes. During the late stage of stress, MT-sprayed plants relied on small-molecule antioxidants to eliminate ROS. This is because toxic ROS, such as O2− and H2O2, are difficult to completely remove by the enzyme system, necessitating the synergistic effects of proline, flavonoids, and polyphenols [71]. Our data demonstrated that M2S plants exhibited multiplicative increases in phenol and flavonoid content compared to salt-stressed plants during later stress stages, although excessively high concentrations of MT had an inhibitory effect. Our results are consistent with the study on rubber trees [72], while uniquely revealing that the magnitude of antioxidant accumulation during late stress substantially exceeds that observed during the initial phase, indicating that MT-sprayed tomatoes primarily rely on antioxidant substances to maintain cellular homeostasis in the later stages of stress.
4.4. Exogenous Melatonin Enhanced Tomato Salt Tolerance by Regulating Ion Homeostasis
Salt stress-induced plasma membrane depolarization activates non-selective cation channels and K+ outward-rectifying channels, leading to excessive Na+ influx into cells and subsequent Na+ toxicity [57]. This study also observed similar phenomena, where salt stress significantly increased the Na+ content in tomatoes while reducing the K+ content. Accumulating evidence indicates that MT enhances salt tolerance by regulating key ion transporter genes to maintain cellular ion homeostasis. For instance, Li [73] found that MT alleviated plant damage in high-salt conditions by upregulating the expression of the MdNHX1 gene (Na+/H+ antiporter). In sugar beets, MT application under salt stress not only enhanced root plasma membrane H+-ATPase activity and H+ efflux capacity but also upregulated the relative expression of HKT1 and NHX1 in roots [74], further confirming the key role of MT in maintaining ion homeostasis under salt stress. The results demonstrated that MT spraying significantly enhanced K+ accumulation while reducing Na+ content in both leaves and roots of tomato plants, corroborating the findings of Zahedi [75] in tomatoes and confirming that MT can improve plant salt tolerance by regulating ion transport systems. Similarly, in salt-stressed cucumber roots, MT upregulated 77 differentially expressed genes, including important transcription factors such as MYB, WRKY, NAC, and ERF, whose expression is closely related to cucumber salt tolerance [76]. Moreover, we observed that salt stress-induced K+/Na+ imbalance compromised cell membrane integrity, whereas exogenous MT enhanced salt tolerance by upregulating this ratio. This may be attributed to MT promoting the expression of SOS1, NHX1, and/or AKT1, thereby maintaining K+/Na+ homeostasis [77]. Maintaining a relatively high K+/Na+ ratio helps alleviate ion toxicity, protect cell membrane integrity [78], and ensure normal photosynthesis [79].
At the initial stage of salt stress, the Ca2+ content in leaves and roots exhibited a transient increase. This phenomenon is often coordinated with reactive oxygen species (ROS) generation, both as a stress signaling mechanism and to regulate Na+ homeostasis through the activation of the membrane-bound Na+/H+ antiporter SOS1 [80,81]. With prolonged stress exposure, a progressive decline in Mg2+ and Ca2+ contents was observed in both leaves and roots. Nevertheless, MT effectively alleviated the inhibitory effect of Na+ on the absorption of Mg2+ and Ca2+ by suppressing Na+ accumulation and promoting K+ retention. Under salt stress conditions, MT stimulates Ca2+ release by upregulating the expression of genes involved in InsP3 and InsP6 synthesis [52]. Notably, leaf Na+ accumulation was positively correlated with TAC, which may represent an adaptive response of plants to oxidative stress. However, this Na+-induced enhancement of antioxidant capacity exhibited inherent limitations, as evidenced by a significant decline in the membrane stability index with increasing Na+ content, indicating that ROS scavenging alone cannot fully alleviate Na+-mediated membrane damage. MT treatment altered this relationship by simultaneously promoting the uptake and retention of K+, Mg2+, and Ca2+, thereby providing additional reinforcement to the antioxidant defense systems. Particularly noteworthy is the strong positive correlation between increased in K+, Mg2+, and Ca2+ content and membrane stability index, suggesting that MT protects membrane integrity through a dual mechanism; on one hand, by reducing Na+-induced membrane lipid peroxidation, and on the other hand, by enhancing the stabilizing effect of essential cations on membrane structure [82]. At the root level, the negative correlation between Na+ content and Lp revealed the inhibitory effect of salt stress on water transport in roots. In contrast, melatonin-sprayed plants exhibited highly significant positive correlations between K+, Mg2+, and Ca2+ content and Lp, confirming that melatonin maintained root water uptake efficiency by regulating ion homeostasis [83].
4.5. Comprehensive Evaluation of Tomato Drought Tolerance
To overcome the limitations of single-indicator evaluations, this study employed the membership function method to identify the optimal melatonin concentration for alleviating salt stress. The results showed that 50 and 100 μM MT-sprayed plants showed sustained increases in the comprehensive salt tolerance index, whereas 150 μM MT-sprayed plants exhibited an increasing and then decreasing trend. Prolonged stress exposure (12 days) resulted in a dramatic decline in the comprehensive salt tolerance index of non-primed plants, indicating that severe salt stress exceeded the tolerance threshold of non-primed plants, ultimately causing substantial growth suppression. Notably, the beneficial effects of 150 μM melatonin on tomato plants were markedly diminished at D12 compared to those at D8. Under prolonged salt stress, high concentrations of melatonin may reduce tomato salt tolerance by disrupting ion homeostasis and damaging the antioxidant defense system. Moreover, long-term treatment with high concentrations of MT inhibits the expression of key genes in the SOS signaling pathway, thereby affecting the regulation of Na+/K+ homeostasis [82]. These findings collectively indicate that melatonin’s protective effects exhibit a distinct ‘optimal concentration window’ in plants, and excessively high concentrations or prolonged treatment durations may lead to negative effects.
While this study delineated the physiological responses of tomato plants to exogenous melatonin under salt stress, the underlying mechanisms require further elucidation. Future investigations should focus on (1) transcriptomic sequencing and targeted gene quantification to decipher melatonin’s regulation of ion homeostasis and antioxidant-related genes; (2) multi-season field validation of the optimal 100 μmol/L concentration coupled with the development of cost-effective delivery systems (e.g., drip irrigation); and (3) concentration gradient refinement (75–125 μmol/L) and extension to tomato cultivars with varying salt tolerance and other vegetable crops. These approaches bridge the gap between controlled-environment findings and agricultural applications, offering practical solutions for saline-affected regions.
5. Conclusions
This study investigated the effects of exogenous melatonin on tomato plants subjected to salt stress (Figure 9). The results demonstrated that 100 μM melatonin (M2) exhibited the most pronounced alleviative effects, particularly during 8–12 days of treatment, showing superior performance over both 50 μM and 150 μM treatments in enhancing leaf relative water content, root hydraulic conductivity, net photosynthetic rate, and biomass accumulation, whereas higher concentrations displayed inhibitory effects. The regulatory efficacy of M2 treatment became increasingly prominent with prolonged stress duration, operating through multiple integrated physiological pathways, activating the antioxidant system and promoting phenolic and flavonoid accumulation to mitigate oxidative damage; elevating osmolyte content and optimizing ion homeostasis to enhance osmotic adjustment capacity; and improving root architecture to facilitate water uptake. A comprehensive evaluation of salt tolerance confirmed that 100 μM melatonin enabled tomato plants to achieve optimal salt tolerance. These findings elucidate the physiological mechanisms through which melatonin alleviates salt stress via a coordinated ‘water-photosynthesis-antioxidant-ion’ network, providing a scientific basis for the precise application of melatonin in tomato production under saline conditions.
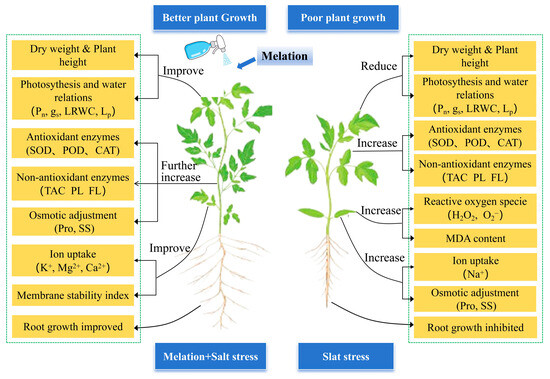
Figure 9.
Mechanisms underlying the effects of exogenous melatonin on tomato plants under salt stress. LRWC, leaf relative water content; Lp, root hydraulic conductivity; TAC, total antioxidant capacity; PL, phenols; FL, flavonoids; Pro, proline; SS, soluble sugar.
Author Contributions
C.R.: writing—original draft, funding acquisition. Y.L.: reviewing and editing. X.Y.: investigation. C.X.: resources, funding acquisition. X.H.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Anhui Provincial Natural Science Foundation (2024AH050474), Anhui Provincial Science and Technology Commissioner Agricultural Material and Technical Equipment ‘Unveiling and Leading’ Project (2022296906020004), Anhui Provincial Science and Technology Commissioner Agricultural Material and Technical Equipment ‘Unveiling and Leading’ and Agricultural Machinery Weakness-Complementing Project (Smart Agricultural Water Conservancy Irrigation Platform S202413a10020336).
Data Availability Statement
All data generated or used in the submitted article are available from the corresponding authors upon request.
Acknowledgments
The authors would like to acknowledge all the team members of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Karim, S.; Hussain, E.; Khan, J.A.; Hameed, A.; Ougahi, J.H.; Iqbal, F. Spatiotemporal investigation of soil salinity using geospatial techniques: A case study of tehsil toba tek singh. Commun. Soil Sci. Plant Anal. 2022, 53, 1960–1978. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Ladewig, P.; Trejo-Téllez, L.I.; Servín-Juárez, R.; Contreras-Oliva, A.; Gómez-Merino, F.C. Growth, yield and fruit quality of mexican tomato landraces in response to salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12005. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zelm, E.V.; Zhang, Y.X. Testerink, Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Shah, W.H.; Rasool, A.; Tahir, I.; Rehman, R.U. Exogenously applied selenium (Se) mitigates the impact of salt stress in Setaria italica L. and Panicum miliaceum L. Nucleus 2020, 63, 327–339. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Soares, C.; Azenha, M.; Martins, V.; Fidalgo, F. A look into osmotic, ionic, and redox adjustments in wild tomato species under combined salt and water stress. Plant Stress 2024, 13, 100510. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant 2021, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.F.; Huda, S.; Yong, M.; Li, L.; Li, L.; Chen, Z.-H.; Ahmed, T. Alleviation of drought and salt stress in vegetables: Crop responses and mitigation strategies. Plant Growth Regul. 2022, 99, 177–194. [Google Scholar] [CrossRef]
- Alharby, H.F.; Fahad, S. Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: Modifications in physio-biochemical machinery. Agron. J. 2020, 112, 2826–2847. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Tan, D.; Reiter, R.J.; Chan, Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J. Pineal Res. 2015, 59, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- El-Yazied, A.A.; Ibrahim, M.F.M.; Ibrahim, M.A.R.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M.; et al. Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2022, 42, 2408–2432. [Google Scholar] [CrossRef]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial effect of melatonin on growth and chlorophyll content in wheat (Triticum aestivum L.) grown under salt stress conditions. Gesunde Pflanz. 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Chaurasia, S.; Sapna, S.; Padhy, A.K.; Bhatia, S. Emerging roles of melatonin in mitigating salinity stress of legumes. S. Afr. J. Bot. 2023, 163, 181–190. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato responses to salinity stress: From morphological traits to genetic changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Sun, H.; Xue, J.; Yan, D.; Liu, Y.; Gui, D.; Wang, X.; Yang, J. Acceleration of soil salinity accumulation and soil degradation due to greenhouse cultivation: A survey of farmers’ practices in China. Environ. Monit. Assess. 2020, 192, 399. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 2021, 285, 110145. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J. Hydraulic conductivities in soil-root system and relative importance at different soil water potential and temperature. Trans. Chin. Soc. Agric. Eng. 1997, 13, 76–81. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1993, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.-U. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement. Altern. Med. 2015, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dahlgren, R.A. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a propolis extract and identification of the main constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2020, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of combined heat and salt stresses on tomato plants—Insights into nutrient uptake and redox homeostasis. Antioxidants 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of key drought tolerance indices for cotton at the flowering and boll setting stage using the dimension reduction method. Front. Plant Sci. 2021, 12, 619926. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.-B.; Reiter, R.J.; Wei, W.; Wang, B.-M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, H.; Wu, L.; Huang, Z.; Niu, Y.; Qi, B.; Zhang, L.; Fan, S.; Ding, Y.; Li, G.; et al. Basic cognition of melatonin regulation of plant growth under salt stress: A meta-analysis. Antioxidants 2022, 11, 1610. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, D.; Zhang, X.; Song, L.; Dong, J.; Xu, Q.; Hu, M.; Cheng, Y.; Shen, F.; Wang, W. Mitigation of salt stress response in upland cotton (Gossypium hirsutum) by exogenous melatonin. J. Plant Res. 2021, 134, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Malik, Z.; Abbasi, G.H.; Irfan, M.; Ahmad, S.; Ameen, M.; Ali, A.; Sohaib, M.; Rizwan, M.; Ali, S. Potential of melatonin in enhancing antioxidant defense system and yield of maize (Zea mays L.) hybrids under saline condition. Sci. Hortic. 2024, 325, 112665. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of melatonin on antioxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R.; Wicke, S. a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2021, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Y.; Rui, C.; Zhang, H.; Xu, N.; Dai, M.; Chen, X.; Lu, X.; Wang, D.; Wang, J.; et al. Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front. Plant Sci. 2021, 12, 693690. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Ding, F.; Liu, B.; Zhang, S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ahammed, G.J.; Zhang, X.-N.; Zhang, L.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Wang, T.; Liu, H.; Tian, D.; Zhang, Y. Melatonin application Improves salt tolerance of Alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants 2020, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K and Na homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Chen, L.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 2020, 8, e10486. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Zeng, L.; Cai, J.-S.; Li, J.-J.; Lu, G.-Y.; Li, C.-S.; Fu, G.-P.; Zhang, X.-K.; Ma, H.-Q.; Liu, Q.-Y.; Zou, X.-L.; et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Yang, R.; Wang, L.; Sun, Q.; Li, D.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA(4) interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ros-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dai, L.; Wei, Y.; Deng, Z.; Li, D. Melatonin enhances salt stress tolerance in rubber tree (Hevea brasiliensis) seedlings. Ind. Crops Prod. 2020, 145, 111990. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, Y.Z.; Feng, F.J.; Liang, D.; Cheng, L.L.; Ma, F.W.; Shi, S.G. Overexpression of a Malus vacuolar Na/H antiporter gene (MdNHX1) in apple rootstock M.26 and its influence on salt tolerance. Plant Cell Tissue Org. Cult. 2010, 102, 337–345. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, G.; Chang, Y.; Lin, D.; Reiter, R.J.; He, C.; Shi, H. Melatonin biosynthesis enzymes recruit WRKY transcription factors to regulate melatonin accumulation and transcriptional activity on W-box in cassava. J. Pineal Res. 2018, 65, e12487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, R.K.; Ge, J.F.; Liu, J.G.; Wang, W.B.; Xu, M.F.; Zhang, R.; Hussain, S.; Wei, H.H.; Dai, Q.G. Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ. Exp. Bot. 2021, 189, 104530. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhawat, N.; Alshaal, T. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Ahmad, R.; Afzal, M.; Tahir, M.A.; Kanwal, S.; Maqsood, M.A. Potassium and silicon improve yield and juice quality in sugarcane (Saccharum officinarum L.) under salt stress. J. Agron. Crop Sci. 2009, 195, 284–291. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Tahjib, M.; Zahan, I.; Hossain, M.S. Melatonin-mediated ionic homeostasis in plants: Mitigating nutrient deficiency and salinity stress. Discov. Plants 2025, 2, 143. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.; Si, Z.; Ma, S.; Gao, Y. Seeds priming with melatonin improves root hydraulic conductivity of wheat varieties under drought, salinity, and combined stress. Int. J. Mol. Sci. 2024, 25, 5055. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).