Method and Optimization of Key Parameters of Soil Organic Matter Detection Based on Pyrolysis Coupled with Artificial Olfaction
Abstract
1. Introduction
2. Materials and Methods
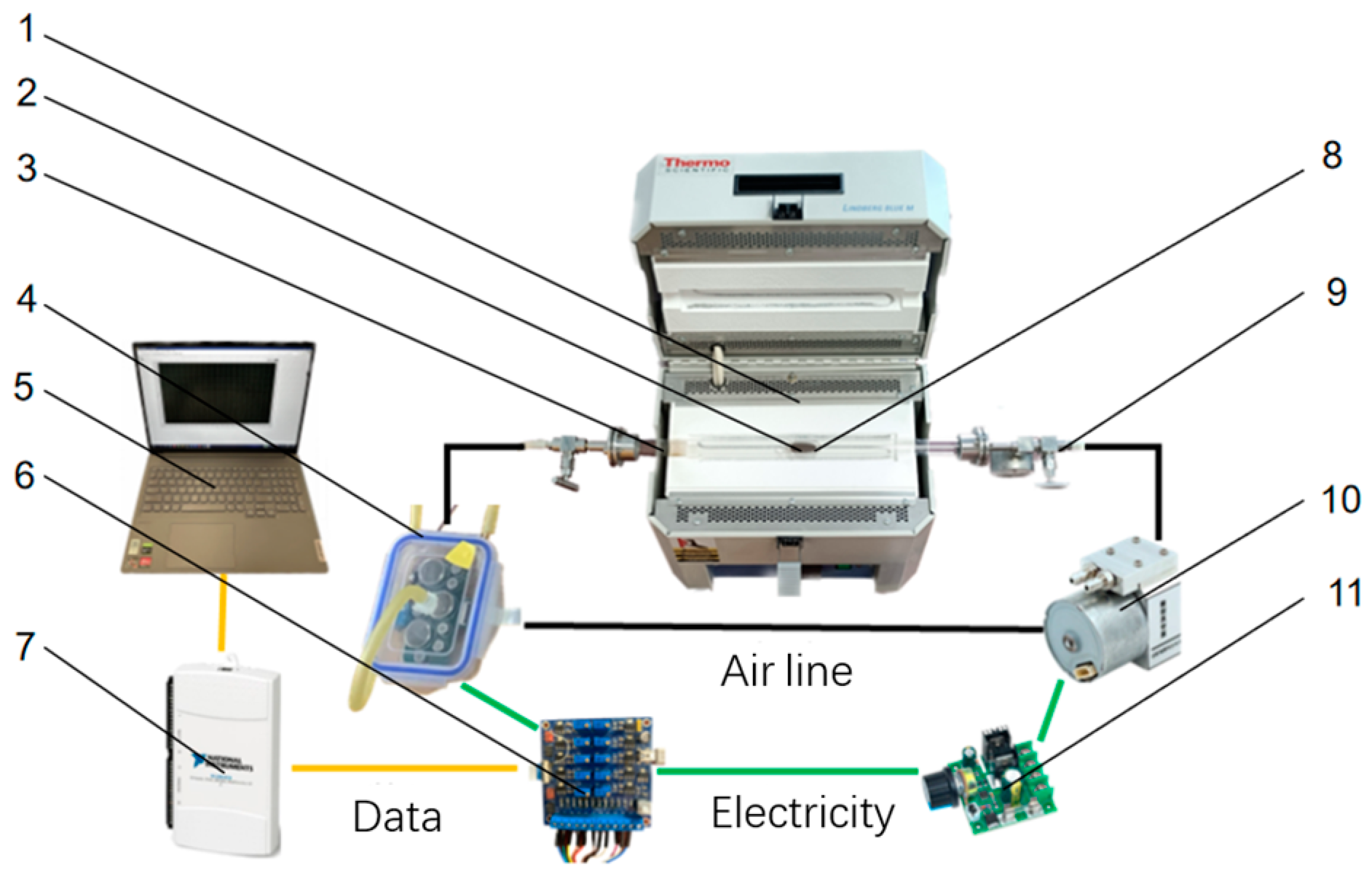
2.1. Detection System Hardware Design
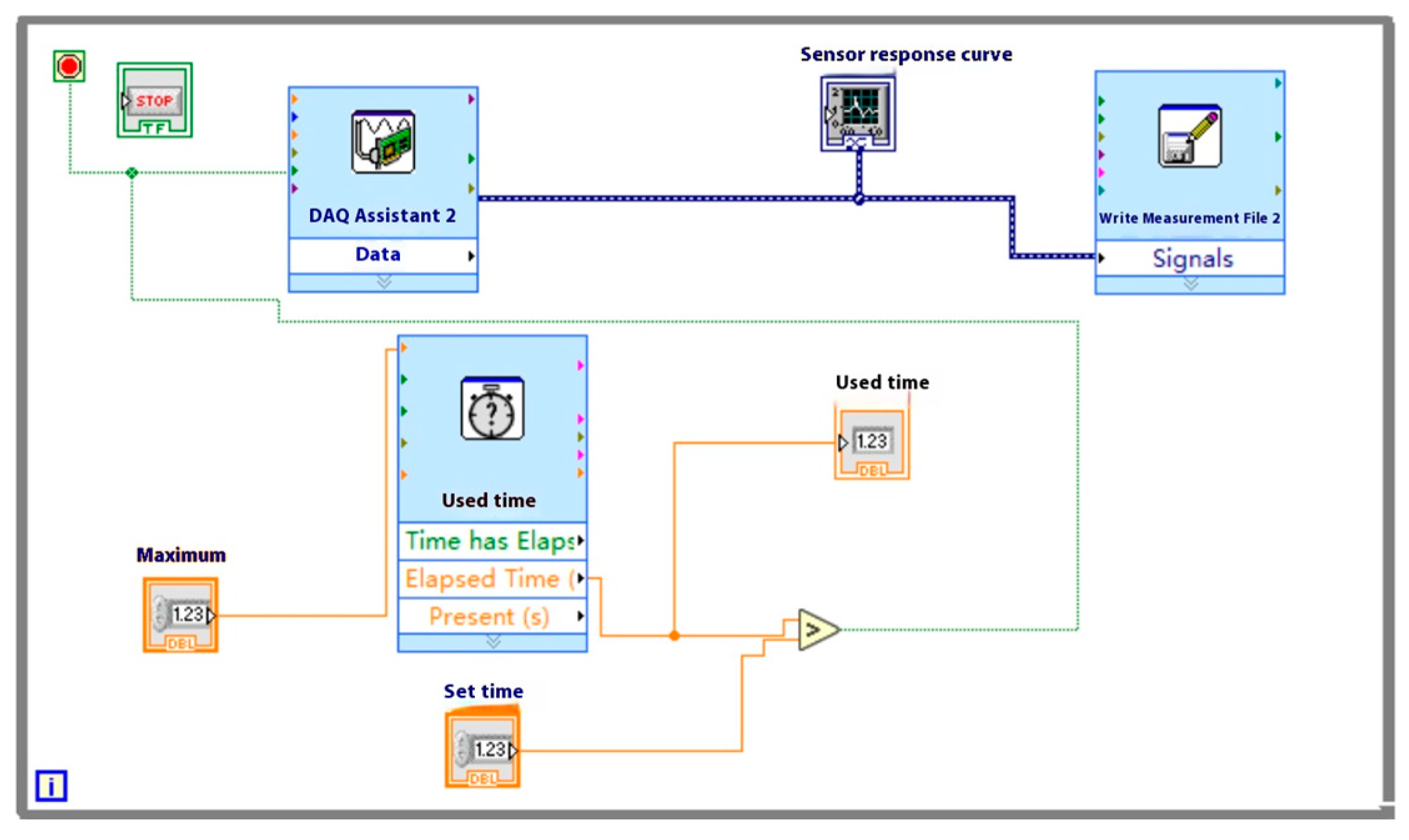
2.2. Detection System Software Design
2.3. Experiment Material
2.4. Experiment Design
| Experimental Factors | Experimental Level |
|---|---|
| Pyrolysis temperature/°C | 200, 300, 400, 500 |
| Pyrolysis time/min | 1, 3, 5, 10 |
| Soil sample mass/g | 1, 2, 3 |
| Level | Experimental Factors | ||
|---|---|---|---|
| Pyrolysis Temperature/°C | Pyrolysis Time/min | Soil Sample Mass/g | |
| −1 | 350 | 2 | 1.5 |
| 0 | 400 | 3 | 2 |
| 1 | 450 | 4 | 2.5 |
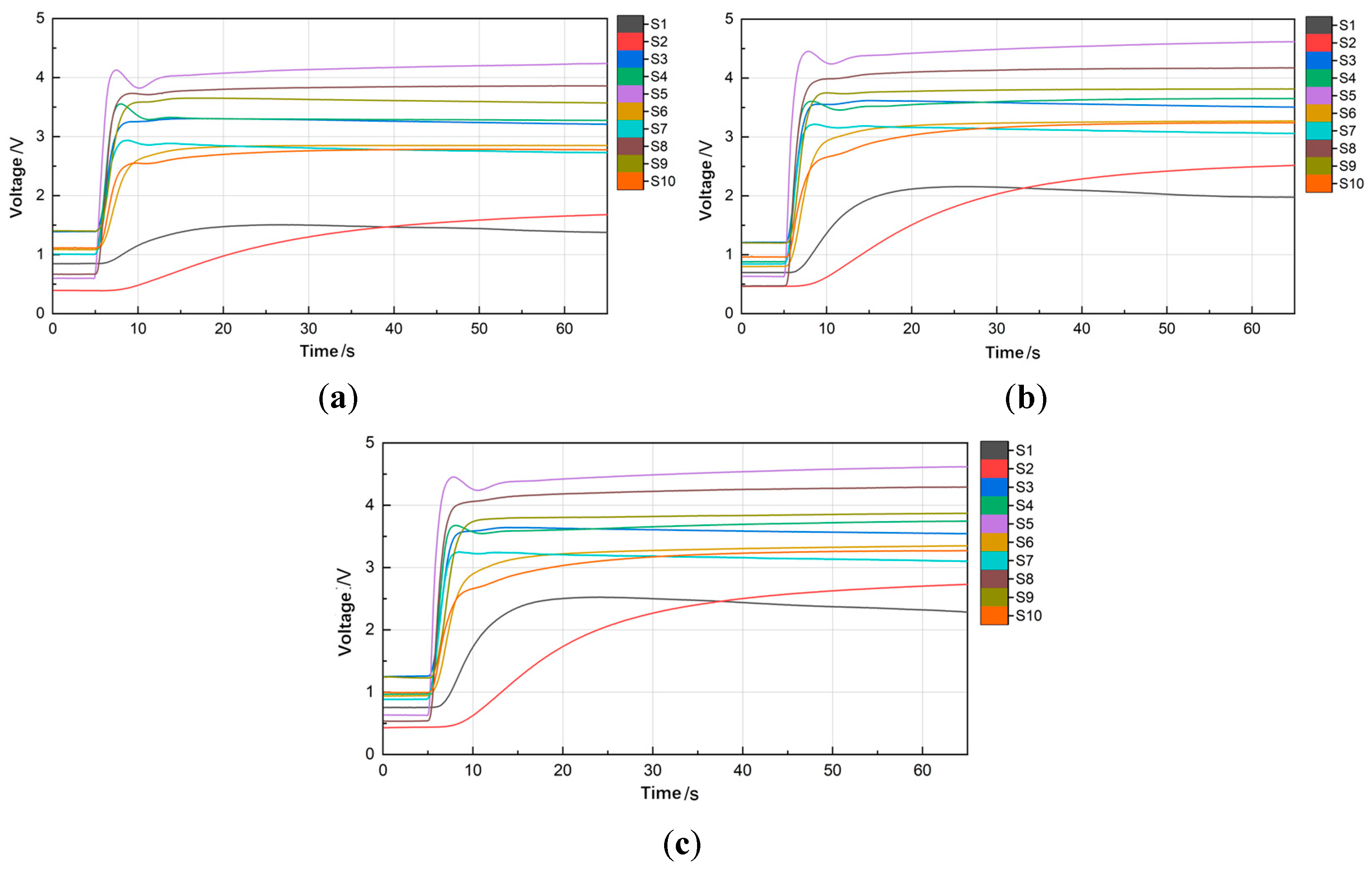
2.5. Characteristic Value and Evaluation Index
2.6. Data Processing
3. Results and Discussion
3.1. Results of Chemical Analyses of Soil Organic Matter Content
3.2. Single-Factor Experiment
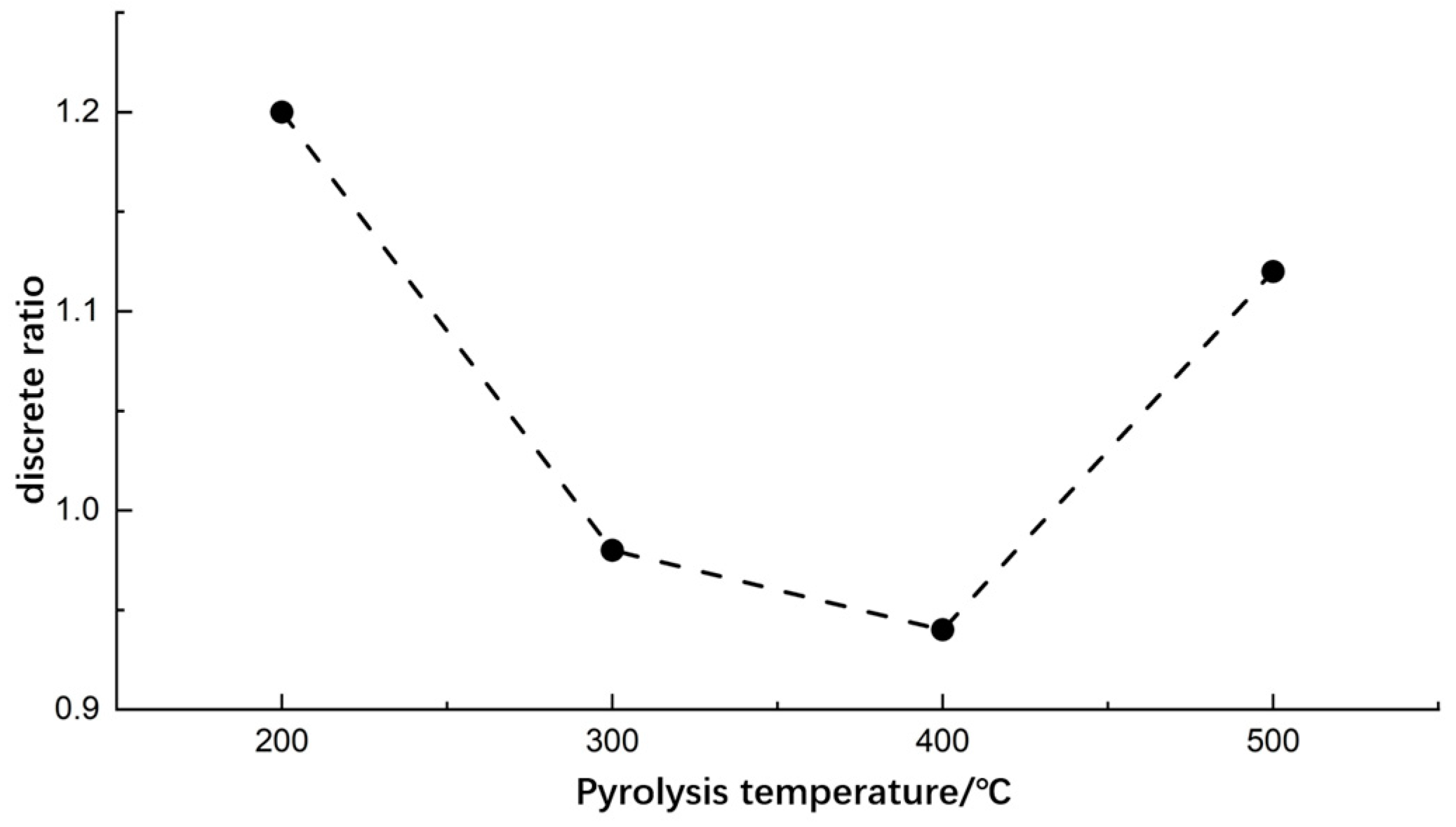
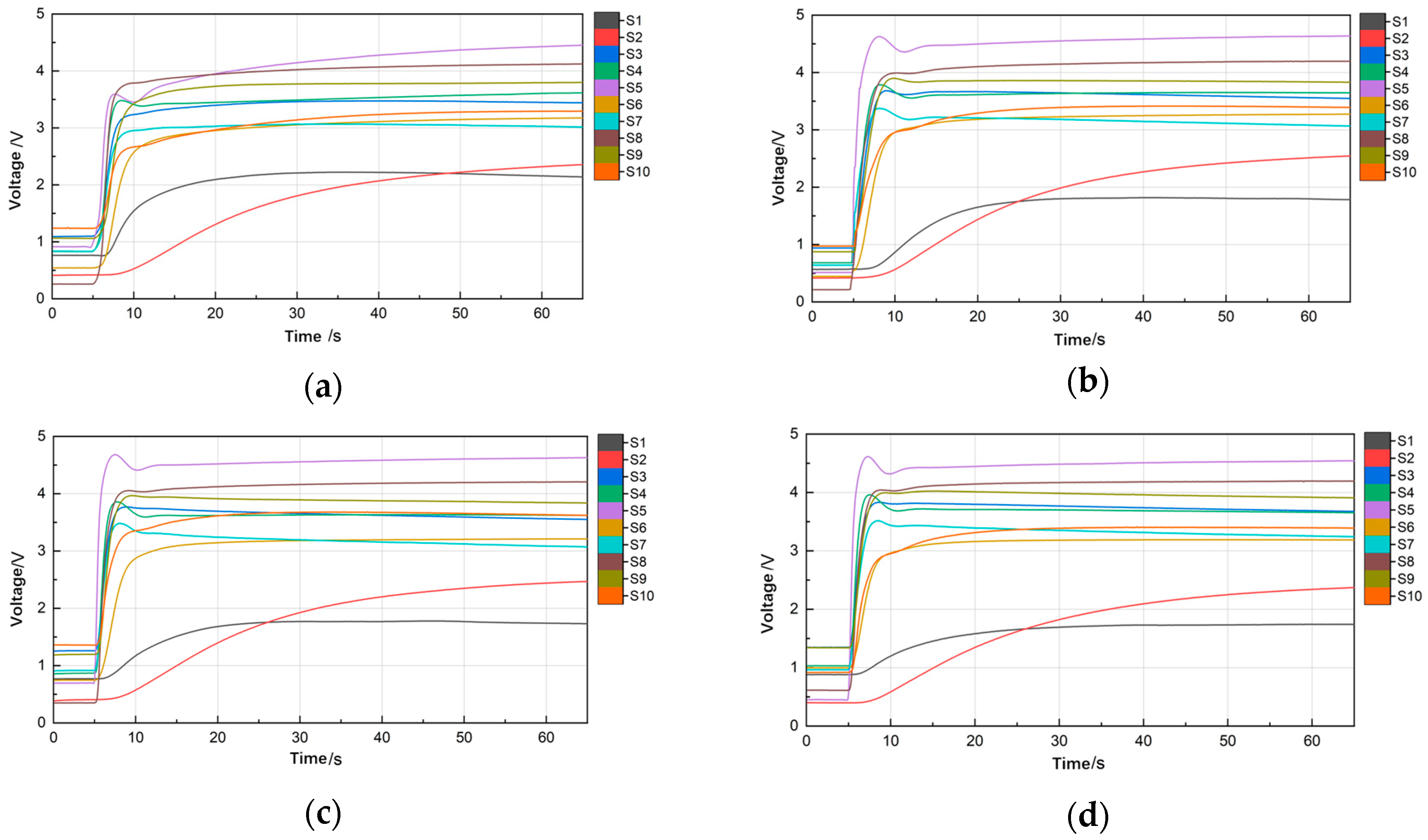
3.2.1. Pyrolysis Temperature
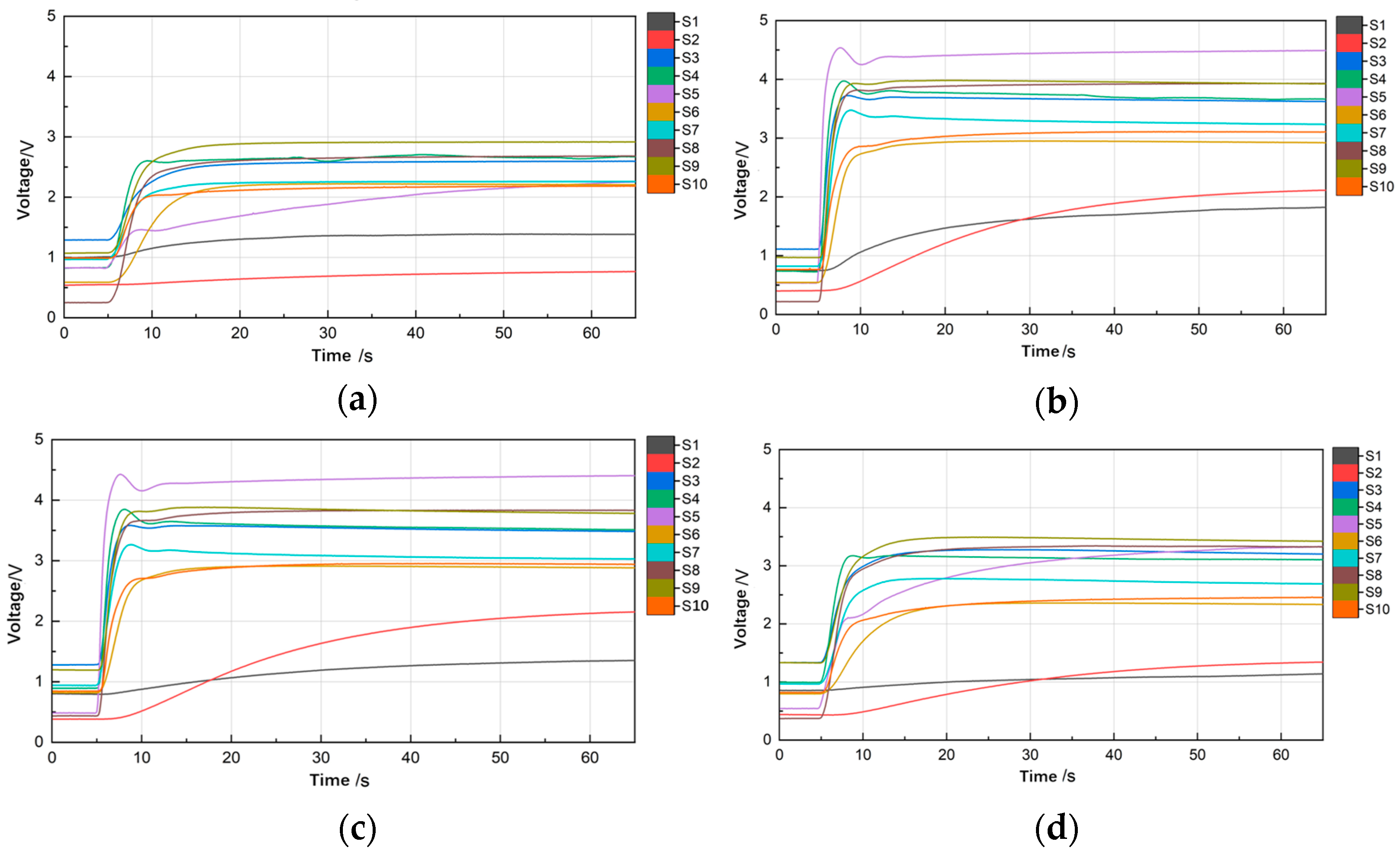
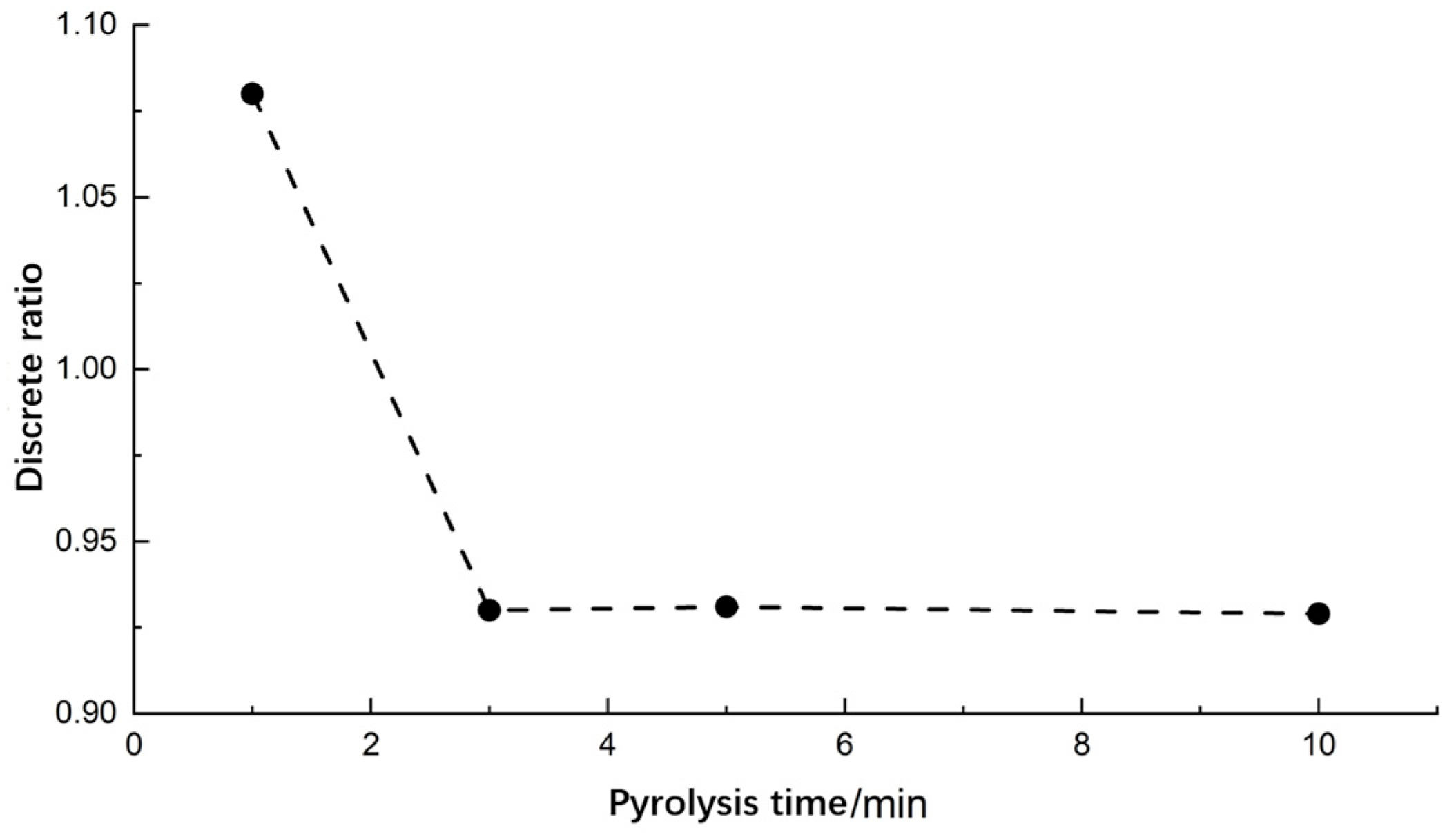
3.2.2. Pyrolysis Time
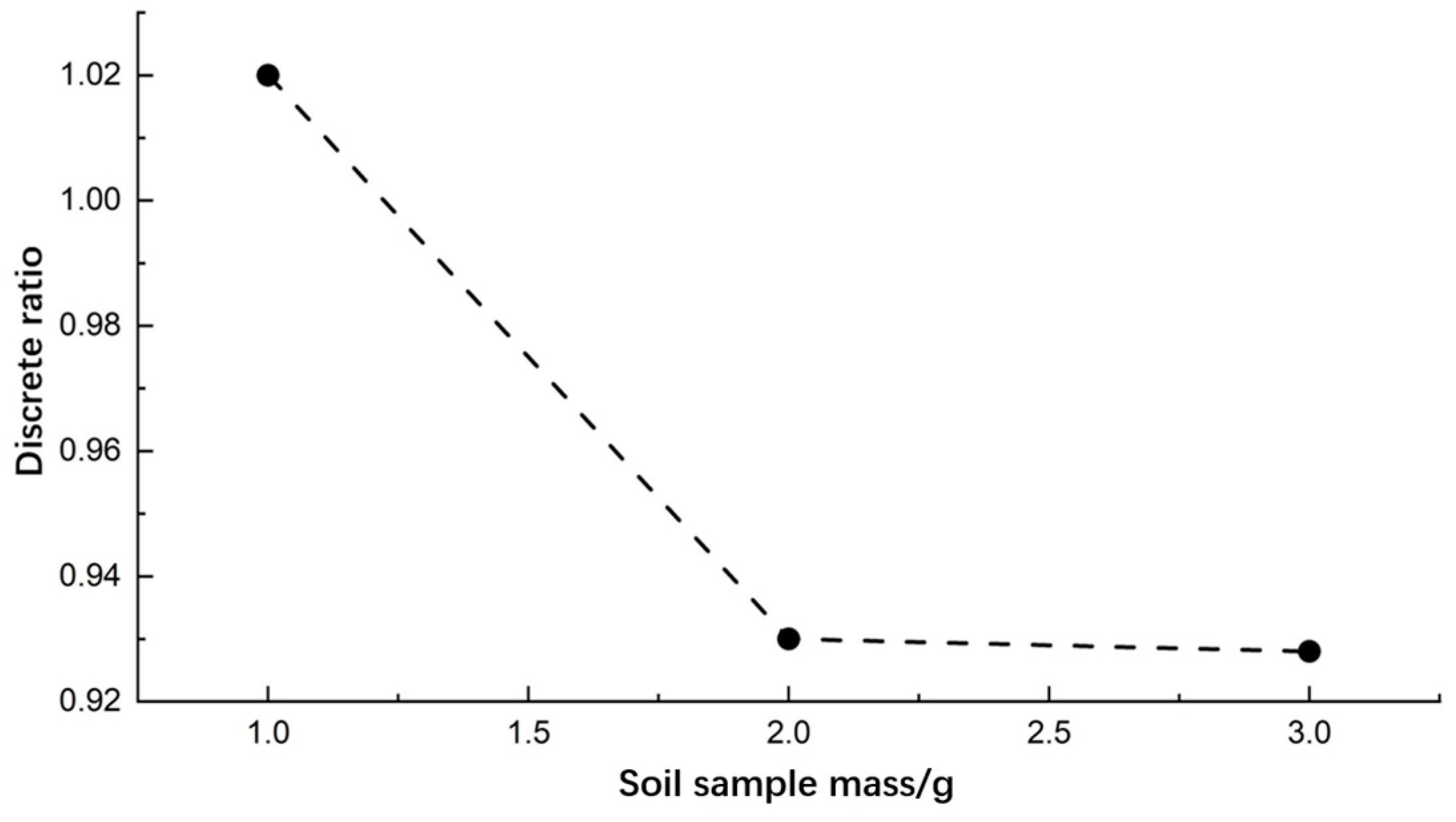
3.2.3. Soil Sample Mass
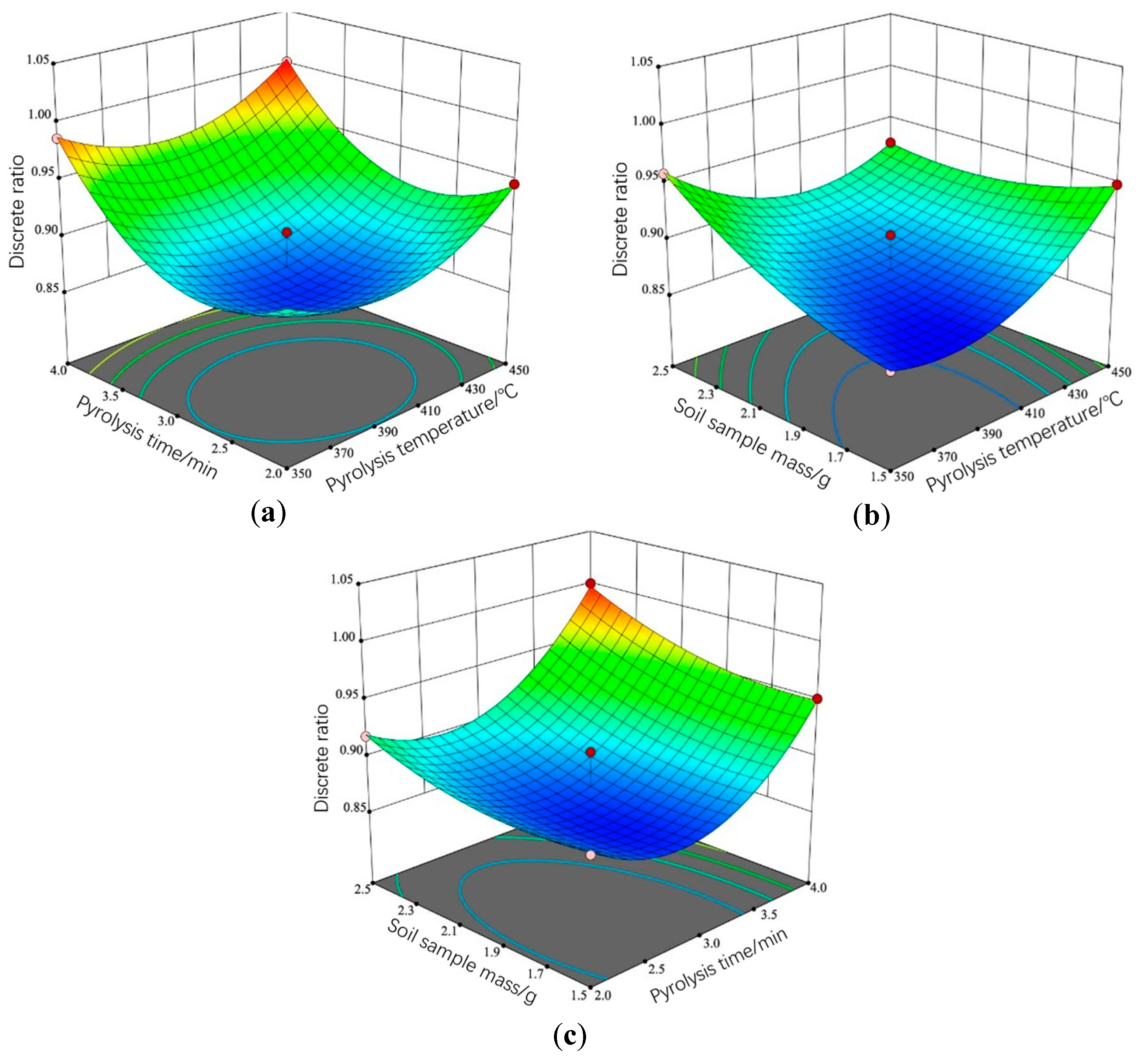
3.3. Analysis of Response Surface Results
3.3.1. Regression Analysis
3.3.2. Response Surface Analysis and Parameter Optimization
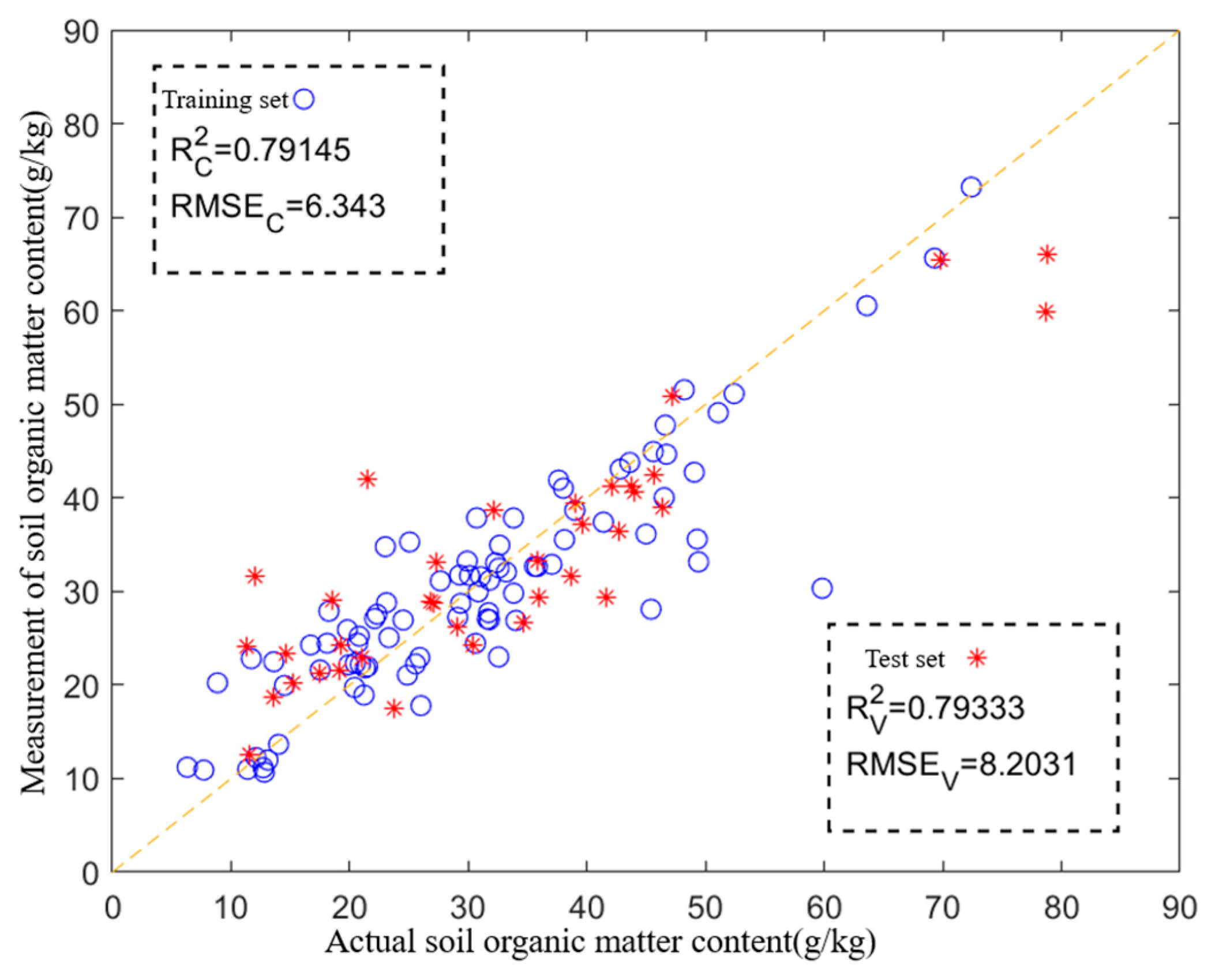
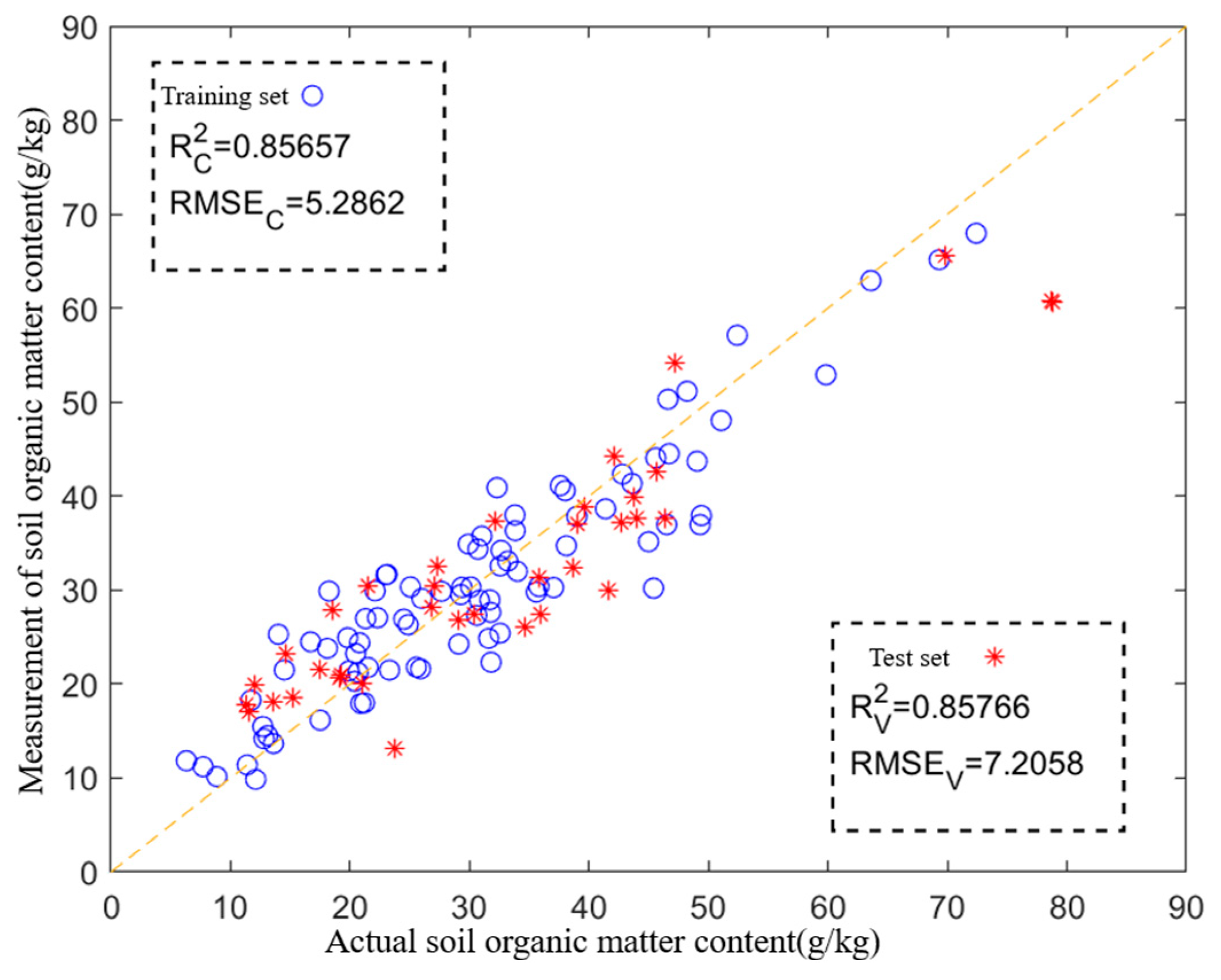
3.4. Application Under Optimal Experiment Parameters
3.5. Discussion
4. Conclusions
- (1)
- Single-factor experiments were conducted to determine the optimal values of pyrolysis process parameters. Higher differentiation and lower discrete ratio of pyrolysis gases were observed at a pyrolysis temperature of 400 °C, a pyrolysis time of 3 min, and a soil sample mass of 2 g.
- (2)
- A multi-factor experiment based on the Box–Behnken method was conducted to specify the optimal combination of parameters. The experimental results showed that the pyrolysis time had the greatest effect on the differentiation of pyrolysis gases, followed by soil sample mass and pyrolysis temperature. The pyrolysis gases exhibited the maximum differentiation and the minimum discrete ratio at a soil cracking temperature of 384 °C, a pyrolysis time of 2 min 41 s, and a soil sample mass of 1.68 g.
- (3)
- The SOM prediction model was developed. The back propagation neural network (BPNN) and partial least squares regression (PLSR) algorithms were used to establish an SOM prediction model after obtaining soil pyrolysis gases under the optimal combination of pyrolysis parameters. And the experimental results showed that the SOM prediction model constructed based on PLSR had the best prediction accuracy and the highest generalization ability (R2 > 0.85, RMSE < 7.21).
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Kong, Y.; Hao, S.; Yin, X.; Xiao, X.; Jin, C. Influence of soil moisture on the inversion accuracy of near-infrared spectra of organic matter. Trans. Chin. Soc. Agric. Eng. 2024, 40, 113–123, (In Chinese with English abstract). [Google Scholar]
- Wei, L.; Yuan, Z.; Wang, Z.; Zhao, L.; Zhang, Y.; Lu, X.; Cao, L. Hyperspectral Inversion of Soil Organic Matter Content Based on a Combined Spectral Index Model. Sensors 2020, 20, 2777. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, X.; Liu, K.; Lou, Y.; Wang, Y. Particle size primarily shifts chemical composition of organic matter under long-term fertilization in paddy soil. Eur. J. Soil Sci. 2022, 73, e13170. [Google Scholar] [CrossRef]
- Dufour, L.J.P.; Wetterlind, J.; Nunan, N.; Quenea, K.; Shi, A.; Weih, M.; Herrmann, A.M. Salix species and varieties affect the molecular composition and diversity of soil organic matter. Plant Soil 2024, 508, 767–784. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, N.; Zhu, T.; Pang, R.; Sun, Y.; Zhou, X.; Xu, X. Native soil labile organic matter influences soil priming effects. Appl. Soil Ecol. 2023, 182, 104732. [Google Scholar] [CrossRef]
- Maharjan, G.R.; Prescher, A.-K.; Nendel, C.; Ewert, F.; Mboh, C.M.; Gaiser, T.; Seidel, S.J. Approaches to model the impact of tillage implements on soil physical and nutrient properties in different agro-ecosystem models. Soil Tillage Res. 2018, 180, 210–221. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Kocsis, T.; Juhos, K.; Halász, J.; Fekete, I. How Does Long-Term Organic Matter Treatment Affect the Biological Activity of a Centre European Forest Soil? Agronomy 2022, 12, 2301. [Google Scholar] [CrossRef]
- Li, X.; Cao, S.; Bai, X.; Li, H. Research Progress of Multi-Spectral Technique in the Determination of Soil Componet Cotent. Spectrosc. Spectr. Anal. 2020, 40, 2042–2047, (In Chinese with English abstract). [Google Scholar]
- Černý, J.; Balík, J.; Suran, P.; Sedlář, O.; Procházková, S.; Kulhánek, M. The Content of Soil Glomalin Concerning Selected Indicators of Soil Fertility. Agronomy 2024, 14, 1731. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Wang, H.; Li, F.; Pan, H.; Yang, Q.; Li, J.; Zhang, J. The Effects of Natural Humus Material Amendment on Soil Organic Matter and Integrated Fertility in the Black Soil of Northeast China: Preliminary Results. Agronomy 2023, 13, 794. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 2018, 423, 363–373. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Ma, J.; Wang, X.; Li, Z.; Zheng, D. Visible Near-Infrared Hyperspectral Soil Organic Matter Prediction Based on Combinatorial Modeling. Agronomy 2024, 14, 789. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Wang, D.; Li, H.; Meng, C. Soil Organic Matter Characteristic Wavelength Extraction and Prediction Model Based on Moisture and Particle Size. Trans. Chin. Soc. Agric. Mach. 2022, 5, 241–248, (In Chinese with English abstract). [Google Scholar]
- Ye, M.; Zhu, L.; Liu, X.; Huang, Y.; Chen, B.; Li, H. Hyperspectral Inversion of Soil Organic Matter Content Based on Continuous Wavelet Transform, SHAP, amd XGBoost. Environ. Sci. 2024, 45, 2280–2291, (In Chinese with English abstract). [Google Scholar]
- Wei, D.; Zheng, G.; Gao, G. Estimation of Soil Total Phosphorus Content in Coastal Areas Based on Hyperspectral Reflectance. Spectrosc. Spectr. Anal. 2022, 42, 517–523, (In Chinese with English abstract). [Google Scholar]
- Horta, A.; Azevedo, L.; Neves, J.; Soares, A.; Pozza, L. Integrating portable X-ray fluorescence (pXRF) measurement uncertainty for accurate soil contamination mapping. Geoderma 2021, 382, 114712. [Google Scholar] [CrossRef]
- Li, G.; Gao, X.; Xiao, N.; Xiao, Y. Estimation Soil Organic Matter Contents with Hyperspectra Based on sCARS and RF Algorithms. Chin. J. Lumin. 2019, 40, 1030–1039, (In Chinese with English abstract). [Google Scholar]
- Li, C.; Zhao, J.; Li, Y.; Meng, Y.; Zhang, Z. Modeling and prediction of soil organic matter content based on visible-near-Infrared spectroscopy. Forests 2021, 12, 1809. [Google Scholar] [CrossRef]
- He, D.; Chen, X. Real-time Measurement of Soil Organic Matter Content in Field. Trans. Chin. Soc. Agric. Mach. 2015, 46, 127–132, (In Chinese with English abstract). [Google Scholar]
- Jiao, C.; Zheng, G.; Xie, X.; Cui, X.; Shang, G. Prediction of Soil Organic Matter Using Visible-Short Near-Infrared Imaging Spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 3277–3281. [Google Scholar]
- Sowoidnich, K.; Vogel, S.; Maiwald, M.; Sumpf, B. Determination of Soil Constituents Using Shifted Excitation Raman Difference Spectroscopy. Appl. Spectrosc. 2022, 76, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Kania, M.; Kupka, D.; Gruba, P. The application of near infrared (NIR) spectroscopy for the quantitative assessment of soil organic matter fraction in forests. Sylwan 2022, 166, 635–646. [Google Scholar]
- Mai, M.; Wang, X. Hyperspectral Estimatioon of Soil Organic Matter Content Based on Continuous Wavelet Transformatiion. Spectrosc. Spectr. Anal. 2022, 42, 1278–1284, (In Chinese with English abstract). [Google Scholar]
- Albrecht, R.; Sebag, D.; Verrecchia, E. Organic matter decomposition: Bridging the gap between Rock-Eval pyrolysis and chemical characterization (CPMAS 13C NMR). Biogeochemistry 2015, 122, 101–111. [Google Scholar] [CrossRef]
- Biache, C.; Lorgeoux, C.; Saada, A.; Colombano, S.; Faure, P. Fast method to quantify PAHs in contaminated soils by direct thermodesorption using analytical pyrolysis. Talanta 2017, 166, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, Y.; Lei, T.; Si, G.; Zhang, G. Study on the fingerprints of soil organic components in alpine grassland based on Py-GC-MS/MS Technology. Acta Ecol. Sin. 2018, 38, 2864–2873, (In Chinese with English abstract). [Google Scholar]
- Campo, J.; Nierop, K.; Cammeraat, E.; Andreu, V.; Rubio, J. Application of pyrolysis-gas chromatography/mass spectrometry to study changes in the organic matter of macro- and microaggregates of a Mediterranean soil upon heating. J. Chromatogr. A 2011, 1218, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart gas sensors: Recent developments and future prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Karthikeyan, M.; Johnson, I.; Raja, K.; Sekar, C.; Mary, X.A.; Basha, J.S. Ensuring food security through rapid and in-field detection of diseases in food crops using real time and portable sensors. Anal. Biochem. 2025, 705, 115925. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Su, G.; Liu, C.; Zou, Y.; Chang, Z.; Yu, H.; Wang, L.; Guo, R. Study on the application of electronic nose technology in the detection for the artificial ripening of crab apples. Horticulturae 2022, 8, 386. [Google Scholar] [CrossRef]
- Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic Noses in Medical Diagnostics. Curr. Med. Chem. 2019, 26, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Zhao, T.; Li, H.; Jiang, J.; Ye, J. Electronic nose for the detection and discrimination of volatile organic compounds: Application, challenges, and perspectives. TrAC-Trends Anal. Chem. 2024, 180, 117958. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Xia, X.; Jin, Y.; Wang, G.; Jia, H.; Huang, D. Electronic sensing combined with machine learning models for predicting soil nutrient content. Comput. Electron. Agric. 2024, 221, 108947. [Google Scholar] [CrossRef]
- Bieganowski, A.; Jaromin-Glen, K.; Guz, Ł.; Łagód, G.; Jozefaciuk, G.; Franus, W.; Suchorab, Z.; Sobczuk, H. Evaluating Soil Moisture Status Using an e-Nose. Sensors 2016, 16, 886. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, S.; Deepika, B.; Narayanan, S.; Murthy, V.K.; Uma, M.V. Indicative extent of humic and fulvic acids in soils determined by electronic nose. Comput. Electron. Agric. 2017, 139, 198–203. [Google Scholar] [CrossRef]
- Zhu, L.; Li, M.; Xia, X.; Huang, D. Soil Organic Matter Detection Method Based on Artificial Olfaction System. Trans. Chin. Soc. Agric. Mach. 2020, 51, 171–179, (In Chinese with English abstract). [Google Scholar]
- Li, M.; Zhu, Q.; Xia, X.; Liu, H.; Huang, D. Detection Method of Soil Organic Matter Based on Multi-sensor Artificial Olfactory System. Trans. Chin. Soc. Agric. Mach. 2021, 52, 109–119. [Google Scholar]
- Wang, J.; Niu, W.; Zhang, W.; Li, G.; Sun, J.; Wang, Y. Spatial variability of soil nutrients in topsoil of cultivated land. Trans. Chin. Soc. Agric. Eng. 2020, 36, 37–46, (In Chinese with English abstract). [Google Scholar]
- Xian, Y.; Song, J.; Wang, J.; Li, W.; Zhang, W.; Wang, H. Spatial Interpolation of Soil Nutrients Content Based on Environment Variables Screening and Machine Learning. Trans. Chin. Soc. Agric. Mach. 2024, 55, 379–391, (In Chinese with English abstract). [Google Scholar]
- De la Rosa, J.M.; Faria, S.R.; Varela, M.E.; Knicker, H.; González-Vila, F.J.; González-Pérez, J.A.; Keizer, J. Characterization of wildfire effects on soil organic matter using analytical pyrolysis. Geoderma 2012, 191, 24–30. [Google Scholar] [CrossRef]
| Parameter | Soil Samples |
|---|---|
| SOM content/(g·kg−1) | 32.55; 49.04; 23.30; 33.2; 16.7; 20.4; 24.86; 51.06; 32.64; 24.5; 63.59; 31.77; 12.7; 20.8; 21.3; 44.98; 23.1; 29.35; 21.2; 18.1; 48.2; 23; 14; 13.59; 42.8; 41.38; 59.83; 38.98; 45.59; 12.8; 34; 22.3; 20.47; 26; 17.5; 20.9; 19.9; 46.70; 19.8; 72.4; 21.5; 52.4; 33.8; 35.59; 33.82; 25.9; 8.85; 49.29; 31.6; 30.6; 11.39; 29.26; 31.7; 32.57; 25.55; 30.83; 27.64; 38; 37.04; 35.80; 13.1; 32.30; 20.66; 25.05; 31.03; 29.9; 7.7; 12.1; 38.1; 45.40; 46.5; 46.60; 30.1; 29.1; 6.3; 22.1; 11.7; 18.24; 14.5; 69.3; 37.6; 43.6; 31.8; 30.7; 49.4; 13.6; 45.63; 35.89; 15.2; 69.8; 42.15; 11.33; 39.60; 21.49; 27.27; 78.7; 14.6; 18.52; 44; 34.63; 23.7; 42.7; 30.42; 38.7; 20.98; 32.16; 35.86; 47.15; 46.4; 17.5; 43.8; 11.5; 19.2; 19.1; 11.97; 29.11; 41.59; 27.01; 39; 26.8; 78.8 |
| Maximum/(g·kg−1) | 78.8 |
| Minimum/(g·kg−1) | 6.3 |
| Average value/(g·kg−1) | 31.38 |
| Standard deviation/(g·kg−1) | 14.90 |
| Coefficient of variation/% | 47.47 |
| Experiment No. | Pyrolysis Temperature A/°C | Pyrolysis Time B/min | Soil Sample Mass C/g | Discrete Ratio DT |
|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 0.918189 |
| 2 | 1 | 0 | 1 | 0.926607 |
| 3 | 1 | 0 | −1 | 0.949034 |
| 4 | 1 | −1 | 0 | 0.94676 |
| 5 | 0 | 0 | 0 | 0.865316 |
| 6 | −1 | 0 | −1 | 0.866407 |
| 7 | 0 | 0 | 0 | 0.867172 |
| 8 | −1 | −1 | 0 | 0.917608 |
| 9 | 0 | 0 | 0 | 0.859884 |
| 10 | 0 | 0 | 0 | 0.868839 |
| 11 | 0 | −1 | −1 | 0.89344 |
| 12 | −1 | 1 | 0 | 0.986423 |
| 13 | 1 | 1 | 0 | 1.00188 |
| 14 | 0 | 0 | 0 | 0.904234 |
| 15 | −1 | 0 | 1 | 0.958502 |
| 16 | 0 | 1 | 1 | 1.0006 |
| 17 | 0 | 1 | −1 | 0.951452 |
| Source | Square Sum | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.0361 | 9 | 0.004 | 21.64 | 0.0003 | ** |
| A—Pyrolysis temperature | 0.0011 | 1 | 0.0011 | 6.12 | 0.0425 | * |
| B—Pyrolysis time | 0.0087 | 1 | 0.0087 | 47.08 | 0.0002 | ** |
| C—Soil sample mass | 0.0026 | 1 | 0.0026 | 13.88 | 0.0074 | ** |
| AB | <0.0001 | 1 | <0.0001 | 0.2528 | 0.6305 | |
| AC | 0.0033 | 1 | 0.0033 | 17.67 | 0.004 | ** |
| BC | 0.0001 | 1 | 0.0001 | 0.8019 | 0.4003 | |
| A2 | 0.0058 | 1 | 0.0058 | 31.32 | 0.0008 | ** |
| B2 | 0.0118 | 1 | 0.0118 | 63.58 | <0.0001 | ** |
| C2 | 0.0009 | 1 | 0.0009 | 5.04 | 0.0597 | |
| Residual | 0.0013 | 7 | 0.0002 | |||
| Lack of fit | <0.0001 | 3 | <0.0001 | 0.0434 | 0.9863 | |
| Net error | 0.0013 | 4 | 0.0003 | |||
| Total variation | 0.0374 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, X.; Li, X.; Wang, W.; Chen, Y.; Zhou, L.; Xia, X. Method and Optimization of Key Parameters of Soil Organic Matter Detection Based on Pyrolysis Coupled with Artificial Olfaction. Agronomy 2025, 15, 1740. https://doi.org/10.3390/agronomy15071740
Li M, Li X, Li X, Wang W, Chen Y, Zhou L, Xia X. Method and Optimization of Key Parameters of Soil Organic Matter Detection Based on Pyrolysis Coupled with Artificial Olfaction. Agronomy. 2025; 15(7):1740. https://doi.org/10.3390/agronomy15071740
Chicago/Turabian StyleLi, Mingwei, Xiao Li, Xuexun Li, Wenjun Wang, Yulong Chen, Long Zhou, and Xiaomeng Xia. 2025. "Method and Optimization of Key Parameters of Soil Organic Matter Detection Based on Pyrolysis Coupled with Artificial Olfaction" Agronomy 15, no. 7: 1740. https://doi.org/10.3390/agronomy15071740
APA StyleLi, M., Li, X., Li, X., Wang, W., Chen, Y., Zhou, L., & Xia, X. (2025). Method and Optimization of Key Parameters of Soil Organic Matter Detection Based on Pyrolysis Coupled with Artificial Olfaction. Agronomy, 15(7), 1740. https://doi.org/10.3390/agronomy15071740






