1. Introduction
Climate change, driven by both natural variability and human activities, continues to intensify global warming and amplify the frequency of extreme weather events, posing substantial threats to agricultural productivity worldwide. Exceeding a 2 °C global temperature increase is projected to dramatically increase the frequency of extreme heat events, jeopardizing agricultural systems and global food security [
1]. Consequently, it has become imperative to re-evaluate and optimize agricultural strategies to mitigate these climate-driven risks effectively [
2]. Among strategic crops particularly suited to adapting to climate variability, sunflower (
Helianthus annuus L.) emerges as highly advantageous due to its intrinsic drought resistance, salt tolerance, and notable economic value. These traits make it especially valuable in arid and semi-arid regions of China [
3]. Sunflowers possess a deep rooting system extending 2–3 m, enabling efficient utilization of limited soil moisture, especially crucial in areas with annual precipitation below 400 mm. This characteristic renders sunflower particularly suitable for reclaiming marginal lands, such as saline-alkali regions and desert environments. In Beitun City, located within Xinjiang’s 10th Division—a critical agricultural region in China—sunflower cultivation faces considerable threats due to frequent and intense climatic variability. Local meteorological data indicate significant yield fluctuations exceeding 15% annually when temperature variability expands to ±8 °C and precipitation variability surpasses a 25% coefficient of variation (CV) [
4]. The Food and Agriculture Organization (FAO) further underscores sunflower sensitivity to climatic fluctuations, noting that even minor deviations of 1 °C from optimal temperature ranges can result in yield reductions of approximately 4–7% [
4]. Despite this vulnerability, existing cultivar selection practices in Beitun City inadequately integrate climatic variability considerations, resulting in limited resilience against extreme weather events. This gap highlights an urgent need for advanced cultivar selection frameworks explicitly tailored to address meteorological risks [
5].
To address these critical challenges, this study introduces an innovative maturity suitability model designed for sunflower cultivars, dynamically quantifying the impacts of meteorological variability on crop physiological traits and yield formation. Distinct from conventional static selection approaches, this model actively incorporates dynamic relationships between climate variability and sunflower physiological processes. It specifically evaluates how variations exceeding defined thresholds (e.g., CV > 15%) activate stress responses at physiological and molecular levels, thus informing practical cultivar selection decisions [
6,
7]. Several technical terms central to this model warrant simplified explanations. “SWEET17” is a protein transporter responsible for moving fructose into plant cell vacuoles (storage compartments), maintaining sugar stability critical for seed development and lipid production. Heat-induced reductions in SWEET17 expression hinder fructose storage, adversely affecting seed quality. “Dual-pathway blockage” refers to the concurrent disruption of two primary hormone signaling pathways—abscisic acid (ABA) and ethylene—which are crucial for regulating stress responses, including plant senescence (aging) and resource distribution. Climatic stress-induced disruptions in these pathways markedly decrease grain-filling rates by approximately 22–29% and photosynthetic transport efficiency by 34–41%, thereby significantly impairing overall yield [
8,
9,
10,
11]. By explicitly integrating these physiological parameters, meteorological thresholds, and cultivar-specific responses, our maturity suitability model fills the critical gap observed in current cultivar selection practices.
Considering the outlined challenges, our study specifically aims to: (1) Develop and validate a coupled meteorological-growth model tailored for sunflower cultivars to quantify their physiological responses to climatic variability; (2) Identify key meteorological thresholds influencing yield and quality traits across sunflower cultivars differing in maturity stages; (3) Propose practical mitigation strategies through optimized cultivar selection and sowing schedules, thereby enhancing yield stability and climatic resilience. Ultimately, this research seeks to foster climate resilience through scientifically informed cultivar selection and precision agricultural management strategies, thereby strengthening regional food security, reducing agricultural vulnerability, and aligning with global sustainability objectives such as Sustainable Development Goals 2 (Zero Hunger) and 13 (Climate Action) [
12,
13,
14,
15,
16].
2. Materials and Methods
2.1. Experimental Design and Site Characteristics
Field experiments were conducted from 2023 to 2024 at random selection of three test sites (A1: 48° N, 88° E; A2: 46° N, 85° E; A3: 47° N, 87° E) in Beitun City, Xinjiang, China, characterized by a warm temperate arid desert climate. Three sunflower hybrids with distinct maturation periods were selected: early-maturing cultivar B1 (RH3146, early-maturing varieties officially registered <100 days Seed supplier: CHS Company of the United States IGH), mid-maturing cultivar B2 (SH361, mid-maturing varieties officially registered 100–130 days Seed supplier: Sanrui Agricultural Technology Co., Ltd., Nei Monggol Autonomous Region, China), and late-maturing cultivar B3 (X3939, late-maturing varieties officially registered >130 days Seed supplier: Sanrui Agricultural Technology Co., Ltd., Nei Monggol Autonomous Region, China). The area of each test site’s plot was 1 hectare, and a completely randomized block design was adopted. Sunflowers were planted in wide and narrow rows (40 cm + 90 cm) under standardized management practices. Soil fertility conditions for each site were previously reported [
16]. Drip irrigation supplied water at 2800–3900 t·ha
−1, and planting density ranged from 1800 to 2200 plants·ha
−1. Hourly meteorological data (temperature and precipitation) were collected throughout the 2023 and 2024 growing seasons from weather stations at each experimental site and summarized according to phenological stages. Bars indicate the mean ± standard error of the mean (SEM) (n = 30 plants/site-genotype). Different lowercase letters indicate significant differences (Tukey’s HSD, α = 0.05).
2.2. Agronomic Traits and Data Collection
Physiological and agronomic parameters, including phenological progression, yield components (seed setting rate, 100-seed weight), and quality traits (crude fat content via Soxhlet extraction), were systematically assessed. Thermal–hydrological thresholds for stress conditions were quantified: flowering-phase heat stress (>30 °C daily) and grain-filling low-temperature inhibition (<15 °C).
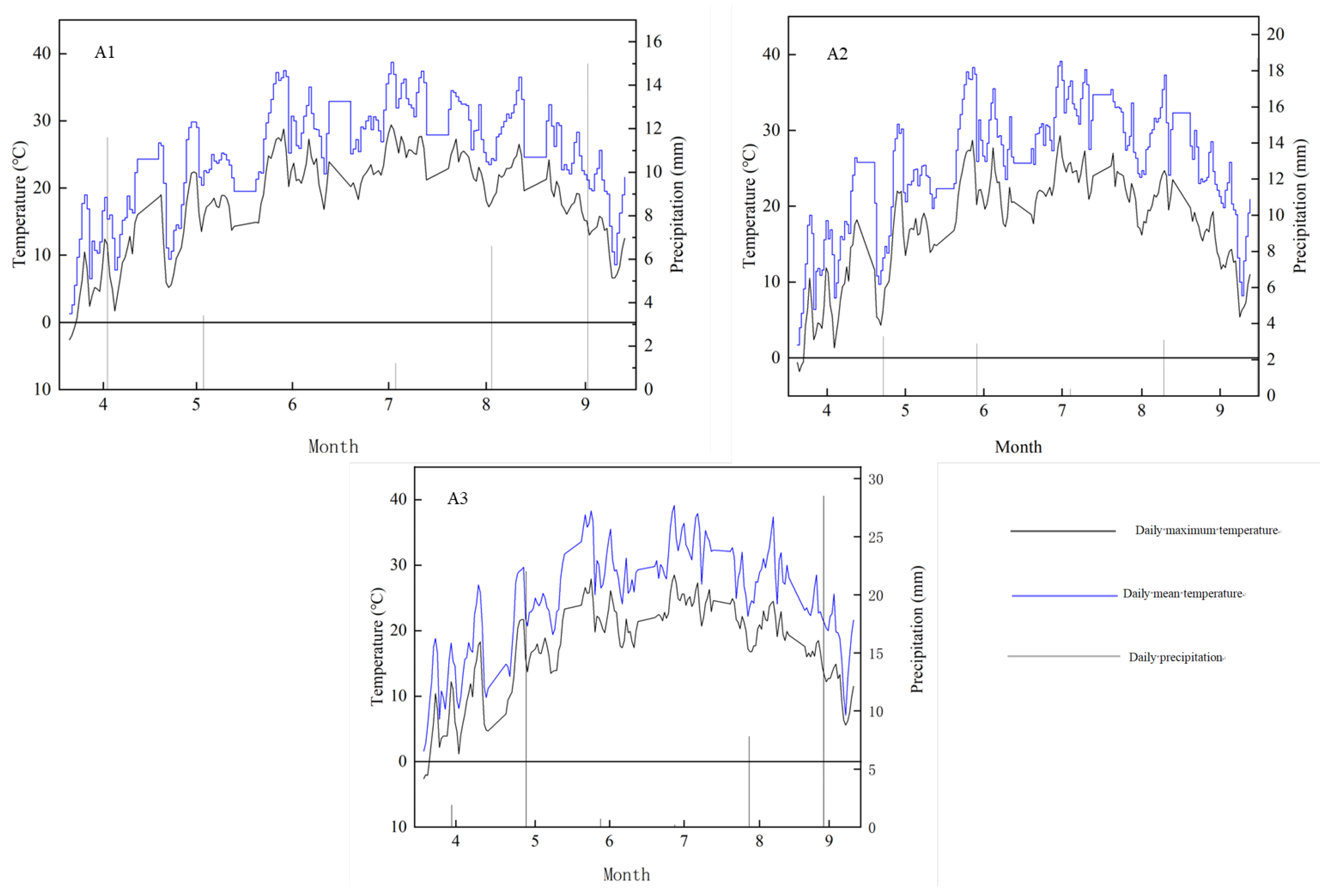
2.3. Site-Specific Hydrothermal Regimes Across Phenological Stages
Analysis of site-specific hydrothermal conditions during critical sunflower phenophases revealed three distinct patterns: (1) Hydrological divergence occurred, with 15% higher irrigation at the bud stage at site A3 (230 mm) compared to site A1 (200 mm), while site A2 (Beitun) received peak water supply (300 mm) during flowering; (2) Thermal stratification showed consistently cooler temperatures at site A1 (early growth: 13–25 °C; late growth: 18–29 °C) compared to warmer conditions at site A2 (15–32 °C) and intermediate temperatures at site A3 (13–29 °C); (3) Radiation consistency was evident across sites, with synchronized peak sunshine hours during the flowering-to-maturity period (A1: 380 h; A2: 410 h; A3: 395 h), aligning with regional solar periodicity (
Figure 1).
2.4. Seed Morphometric Profiling
Seed dimensional traits were measured using a calibrated phenotyping platform (TPKZ-3-L, Beijing LiGaotai Technology Co., Ltd., Beijing, China). Mature seeds (n = 250 per cultivar-site group) were placed ventral side down on an anti-reflective stage and imaged at 2400 dpi resolution under standardized LED illumination (5000 K ± 5% Cool Net Optoelectronics Technology (Shanghai) Co., Ltd., Shanghai, China). High-resolution images were processed using ImageJ v1.53 (Developed by the National Institutes of Health of the United States), employing a custom algorithm with edge detection (Canny operator, σ = 1.0) and morphological filtering. Length (major axis) and width (minor axis) measurements were extracted from fitted ellipses. System accuracy was validated periodically against geometric calibration standards (certified tolerance ± 0.015 mm). Morphometric outliers exceeding ± 3 standard deviations from cultivar-specific means, primarily attributed to measurement errors, were excluded to ensure data integrity (
Table 1).
Note: msample represents sample quality; mbottle represents bottle quality.
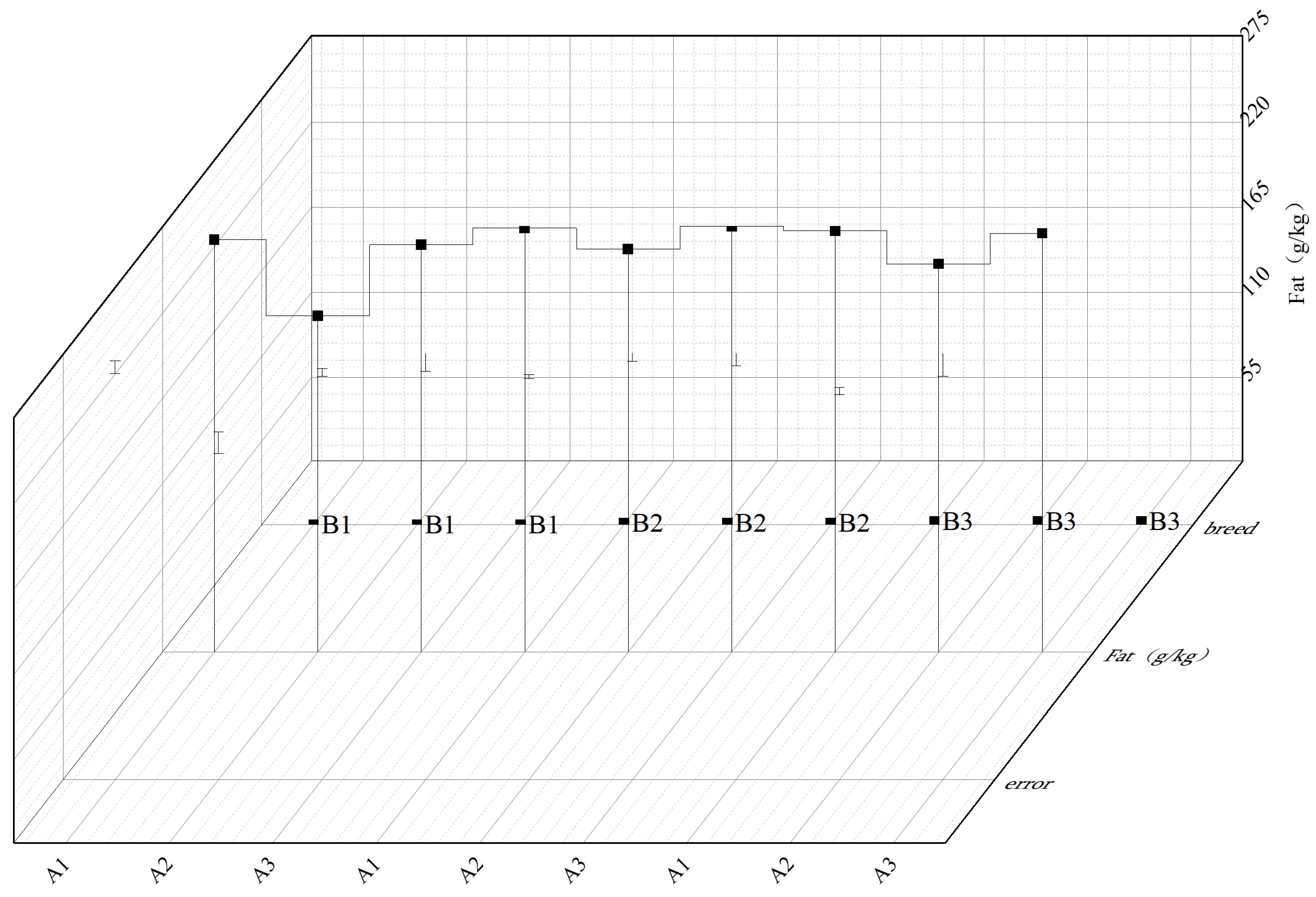
2.5. Grain Protein and Fat Content
Total nitrogen content was determined using the micro-Kjeldahl method (K9840 analyzer Shanghai Lijing Scientific Instrument Co., Ltd., Shanghai, China). Freeze-dried seed powder (0.100 ± 0.001 g) was digested with 10 mL concentrated sulfuric acid (H2SO4, 98% Changsha Rongqing Chemical Products Co., Ltd.) and 5 g catalyst mixture (K2SO4:CuSO4:SeO2 = 10:1:0.1 Changsha Rongqing Chemical Products Co., Ltd.) at 420 °C for 85 min in a block digestion system. The digested sample was distilled with 40% NaOH (Changsha Rongqing Chemical Products Co., Ltd.), and liberated ammonia was titrated with 0.1 M HCl (Changsha Rongqing Chemical Products Co., Ltd.). Protein content was calculated as nitrogen percentage × 6.25, validated against NIST SRM 3234 (soy flour reference material, recovery rate 98.2–101.5%).
Crude fat content was analyzed using Soxhlet extraction (BUCHI B-811 system Gerhardt). Dehulled seeds were dried at 105 °C for 4 h to constant weight (±0.1 mg) and ground through a 40-mesh sieve. Aliquots (2–5 g ± 0.001 g) were encapsulated in Whatman Grade 1 filter paper. Petroleum (C5H12) ether (boiling range: 40–60 °C Shanghai Shensheng Technology Co., Ltd., Shanghai, China) was refluxed at 6 cycles·h−1 for 8 h. After extraction, the solvent was recovered using rotary evaporation (40 °C, 200 mbar), and residual lipid content was quantified gravimetrically. Method precision was confirmed through triplicate analyses (CV < 1.2%), with blank corrections applied using pre-extracted control samples.
2.6. Data Analysis
Data were organized in Microsoft Excel 2016 (Microsoft Corporation Redmond, Washington, USA) and statistically analyzed using SPSS 26.0 (IBM Lake Michigan, USA). Correlation analyses between meteorological parameters and sunflower traits were conducted in OriginPro 2021 (OriginLab Northampton, Massachusetts, USA)(Correlation Plot module, Pearson method). One-way analysis of variance (ANOVA) with post hoc Least Significant Difference (LSD) testing assessed treatment effects. Statistical significance was set at α = 0.05.
4. Discussion
Sunflower (
Helianthus annuus L.) yield and quality are determined by complex interactions involving genetic traits, agronomic practices, and environmental factors, especially temperature and moisture regimes [
17]. Our findings indicate that differences in maturation stage significantly modulate varietal responses to meteorological stresses, highlighting critical considerations for climate-resilient cultivation.
Late-maturing varieties (B2/B3) exhibited superior thermotolerance, maintaining pollen viability and seed set at 28–30 °C—conditions that reduced seed set in early-maturing B1 by 12–18%. These results align with [
18], where optimal higher temperatures enhanced the transport of photoassimilates to reproductive sinks. Notably, B3 showed resilience due to sustained sucrose synthase activity during grain filling [
19], resulting in an 8–10% greater 100-seed weight under heat stress. In contrast, precipitation dependence increased with maturity duration; capitulum diameter and yield correlated positively with rainfall in late cultivars. These findings extend previous work [
20,
21] by quantifying water requirements across developmental stages.
We precisely identified temperature thresholds influencing oil metabolism: at 25–30 °C, each 1 °C increase reduced seed fat content by 0.5–1.2% and 100-seed weight by 2.4–3.6% [
22,
23]. Observed yield-quality trade-offs under stress reflect metabolic reprogramming [
24,
25]. High temperatures shifted metabolism from glucose-6-phosphate dehydrogenase to the pentose phosphate pathway [
26,
27], increasing soluble sugar accumulation by 40% while decreasing oleic acid synthesis [
28,
29]. Concurrently, osmoprotectants (proline, HSP70) increased 3.5-fold under combined heat–drought stress [
30,
31], explaining varietal differences in resource allocation. These mechanisms informed our cultivar-site optimization protocol: in cool regions (A1), early-maturing B1 minimizes frost risk; in warmer zones (A2), mid-to-late maturing B2 balances heat tolerance and water efficiency; in hot, arid zones (A3), mid-maturing B3 benefits from enhanced ABA sensitivity.
This study confirms that thermal–hydrological interactions influence sunflower productivity through genotype-specific physiological mechanisms. By quantifying response thresholds (e.g., 1 °C increase = 0.8% fat loss) and connecting them to molecular mechanisms (e.g., DGAT suppression), we enable precision breeding for heat-adapted varieties. Future studies should integrate real-time meteorological forecasting with dynamic sowing windows, utilizing maturity-dependent stress-response curves identified here [
32].
In the Sunflower Marker-Assisted Selection (MAS) method, molecular markers such as SNPs and SSRs related to fat content can be developed through QTL mapping, GWAS analysis, etc., and combined with multi-gene aggregation and genome-wide selection to optimize oil quality. For ABA and ethylene, stress response markers can be developed based on genes related to their synthesis and signal transduction (such as NCED, ACS, ACO, etc.), and combined with gene expression regulation markers for stress resistance screening. For the SWEET17 gene, SNP markers near it related to sugar transport and oil synthesis can be located, and after functional verification, they can be used for the selection of high-oil content genotypes. At the same time, multi-trait markers can be integrated to conduct backcross assistance and genome-wide selection to optimize the breeding process, in order to accelerate the cultivation of high-quality and stress-resistant varieties. However, challenges such as complex trait genetic analysis, the universality of markers across populations, and cost control of technologies need to be noted.
The relationship between linoleic acid content and sunflower yield under weather stress is complex, with temperature acting as the primary regulator. Low temperatures increase linoleic acid content but limit yield, whereas high temperatures produce the opposite effect. Coordinated optimization can be achieved through regional strategies (quality-oriented cultivation in cold areas, yield-focused cultivation in warmer areas) and precise management of flowering timing (optimal sowing dates to avoid disasters, bee-assisted pollination) [
33]. Future research should integrate molecular resilience modules and dynamic modeling to effectively manage climate-fluctuation risks.