Nano-Biochar Enhanced Adsorption of NO3−-N and Its Role in Mitigating N2O Emissions: Performance and Mechanisms
Abstract
1. Introduction
- RQ1—Can rice straw-derived NBC significantly enhance NO3−-N adsorption under acidic conditions, and what are the underlying adsorption mechanisms?
- RQ2—How does NBC application affect N2O emissions in simulated soils, and is this mitigation effect driven by improved microscale surface properties?
2. Experimental Materials and Methods
2.1. Fabrication and Characterization of Nano-Biochar
2.2. Batch Adsorption Experiments
- (1)
- Adsorption experiments with different biochar dosages. A 1000 mg L−1 stock solution of KNO3 was prepared with an initial pH of 5.0. A total of 50 mL of this solution was mixed with varying amounts of bulk BC and NBC (0.2, 0.4, 0.6, 0.8, 1.0, 2.0, and 3.0 g). The mixture was placed in a thermostatic water bath at 25 °C and agitated at a shaking speed of 200 rpm for 4 h. After the adsorption process, the NO3−-N concentration in the supernatant was analyzed to determine the adsorption efficiency.
- (2)
- Adsorption experiments at different initial solution pH levels. A 1000 mg L−1 KNO3 solution (50 mL) was prepared and mixed with 1.0 g of bulk BC and NBC. The pH of the solution was adjusted to 5, 6, 7, 8, 9, and 10 using NaOH and HCl. The mixture was placed in a constant-temperature water bath (25 °C) and agitated at 200 rpm for 4 h. After the reaction, the NO3−-N concentration in the supernatant was measured.
- (3)
- Adsorption experiments at different initial nitrogen concentrations. A series of KNO3 solutions with concentrations of 100, 300, 600, 900, 1200, 2000, and 3000 mg L−1 were prepared. For each concentration, 50 mL of KNO3 solution with an initial pH adjusted to 5.0 was mixed with 1.0 g of bulk BC or NBC. All mixtures were placed in a constant-temperature water bath at 25 °C and stirred at 200 rpm for 4 h. The same treatment procedure (mixing time, temperature, and stirring rate) was applied to all samples. After the adsorption process, the NO3−-N concentration in the supernatant was measured.
- (4)
- Adsorption experiments at different contact times. A total of 1.0 g of bulk BC or NBC was added to 50 mL of 1000 mg L−1 KNO3 solution (pH adjusted to 5.0). The mixture was stirred at 200 rpm in a 25 °C water bath for various durations: 10, 20, 40, 60, 90, 120, 150, 180, 210, and 240 min. The pH was maintained at 5.0 throughout the experiment. All treatments followed identical procedures. After the designated contact time, the NO3−-N concentration in the supernatant was analyzed.
2.3. N2O Emissions Experiment
2.4. Sampling and Measurement
2.4.1. N2O Emissions Measurement
2.4.2. Soil NH4+-N and Soil NO3−-N Measurements
2.5. Data Analysis
2.5.1. Nitrogen Adsorption Models
- (1)
- Adsorption isotherm models
- (2)
- Adsorption kinetic models
2.5.2. N2O Emissions Fluxes
2.5.3. Statistical Analysis
3. Results
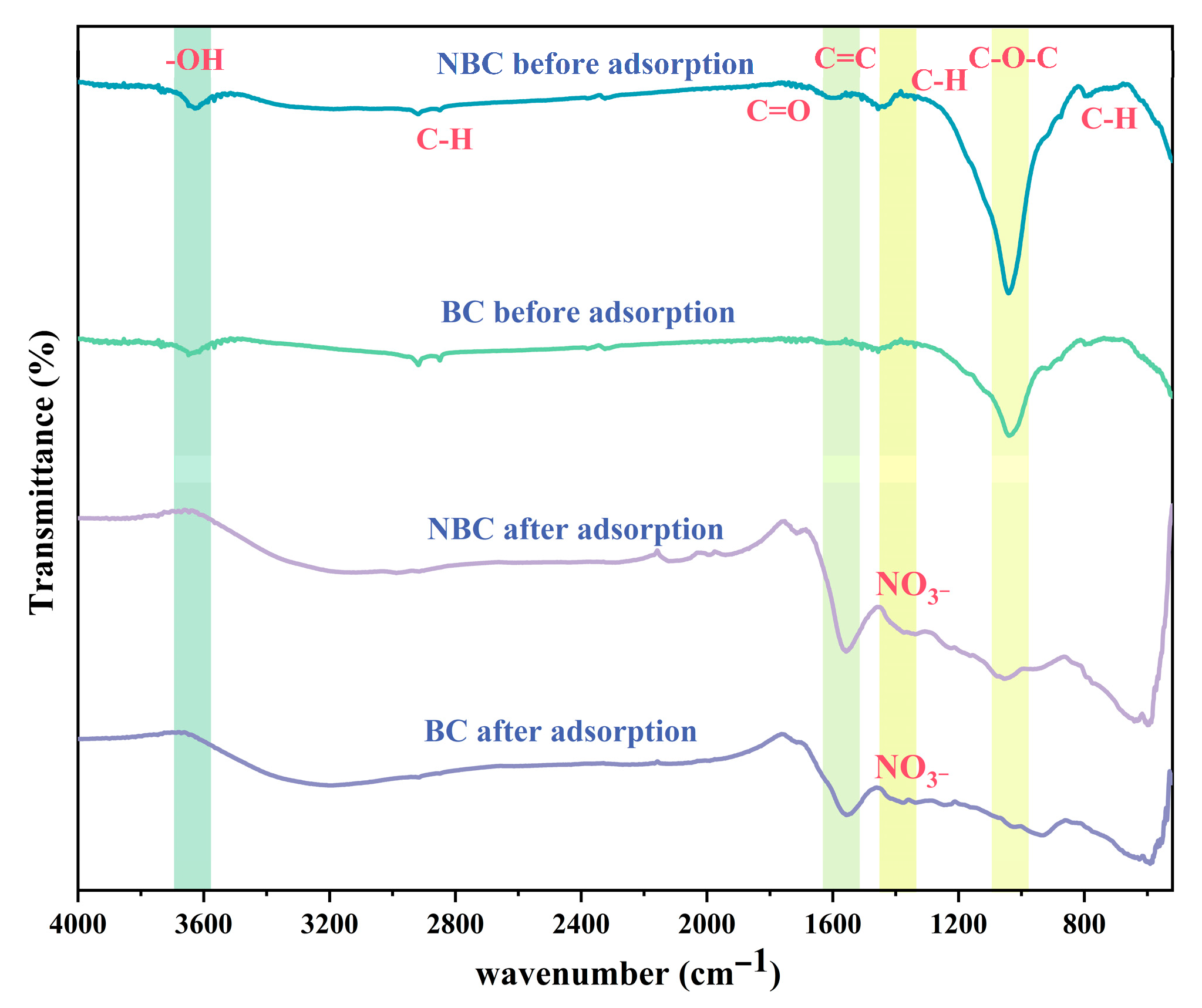
3.1. Characterization of Bulk Biochar and Nano-Biochar
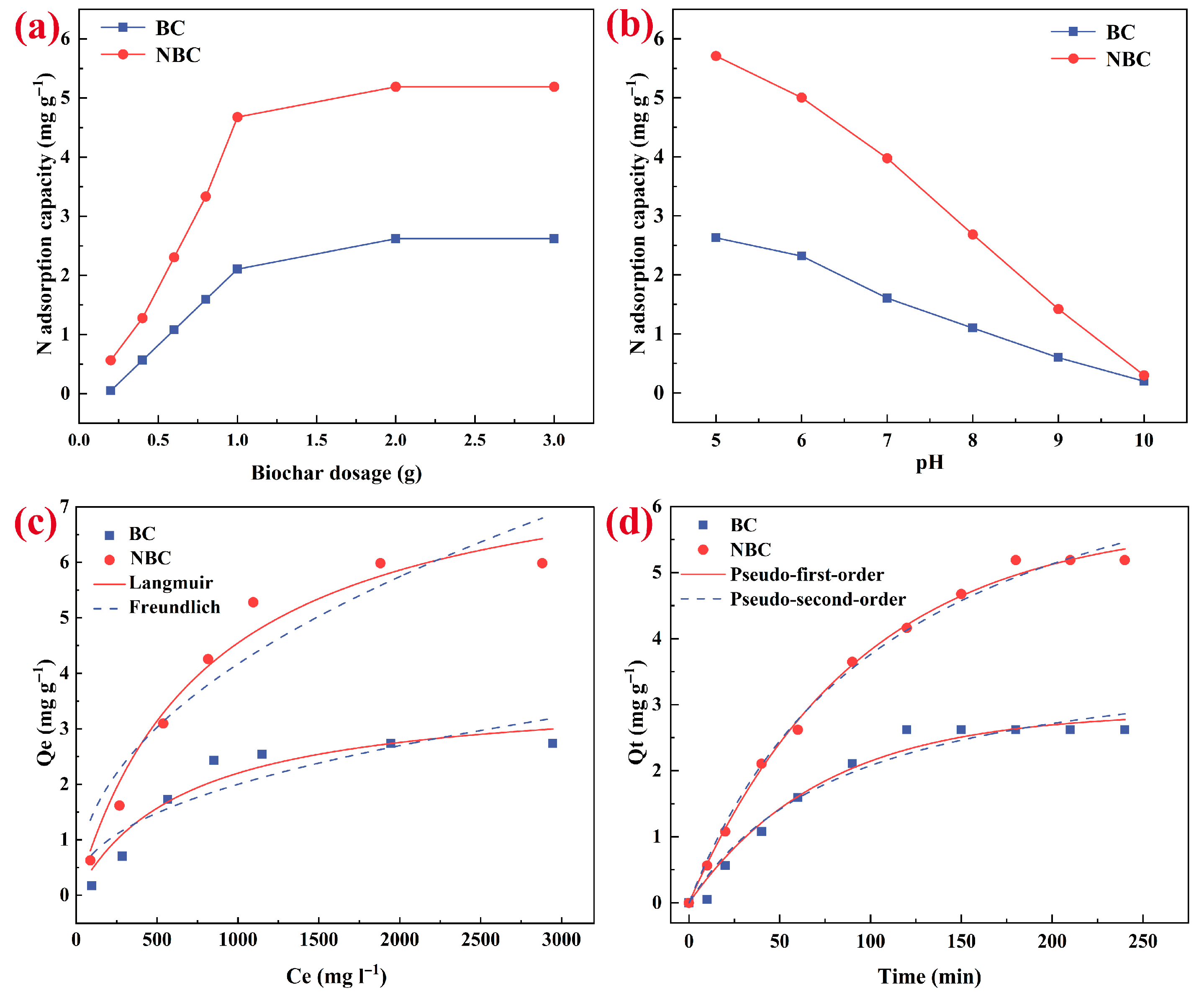
3.2. Effects of Biochar Dosage and pH of the Solution
3.3. Adsorption Isotherms
3.4. Adsorption Kinetics
3.5. Nitrous Oxide Emissions
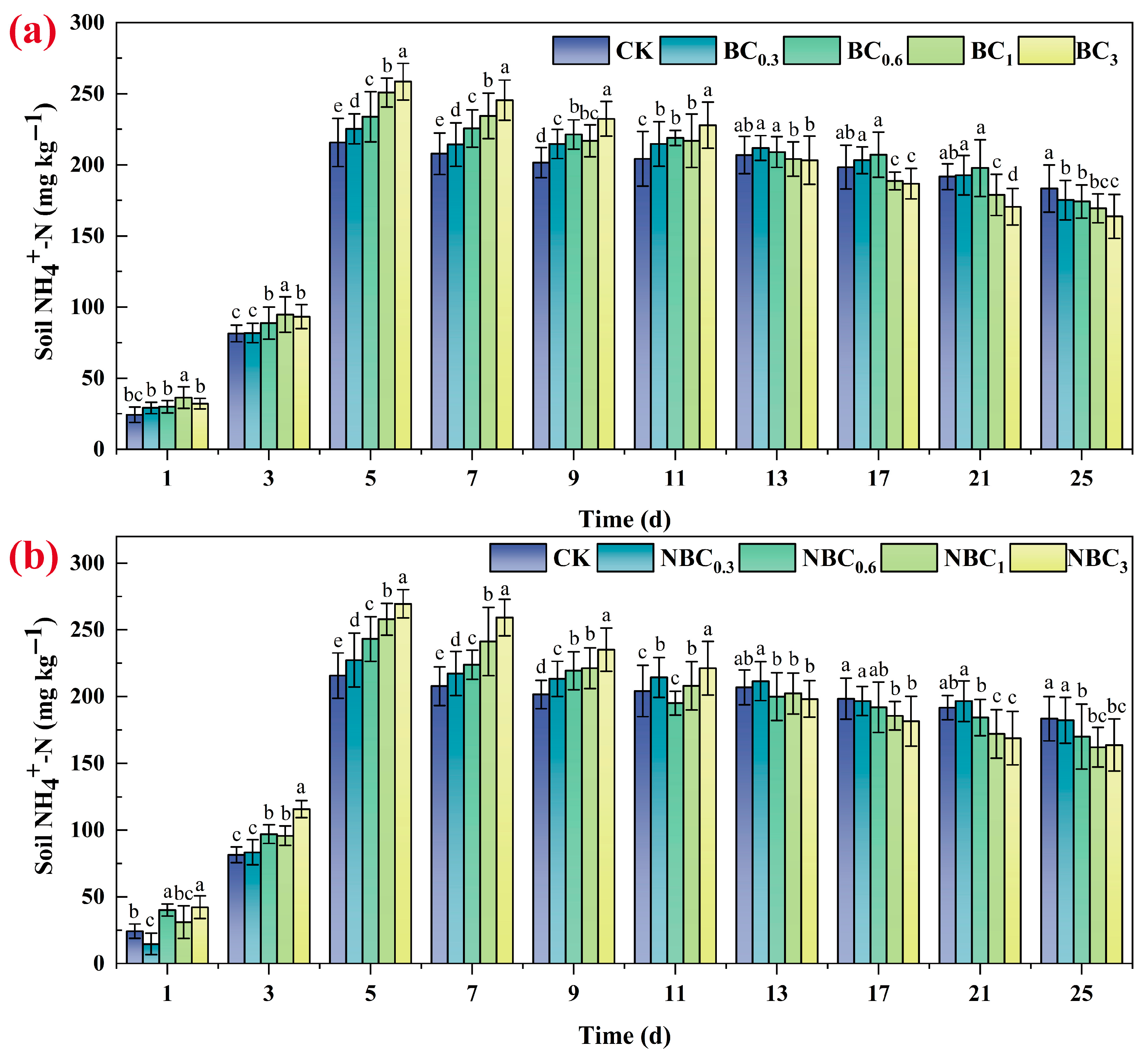
3.6. Soil NH4+-N and Soil NO3−-N
4. Discussion
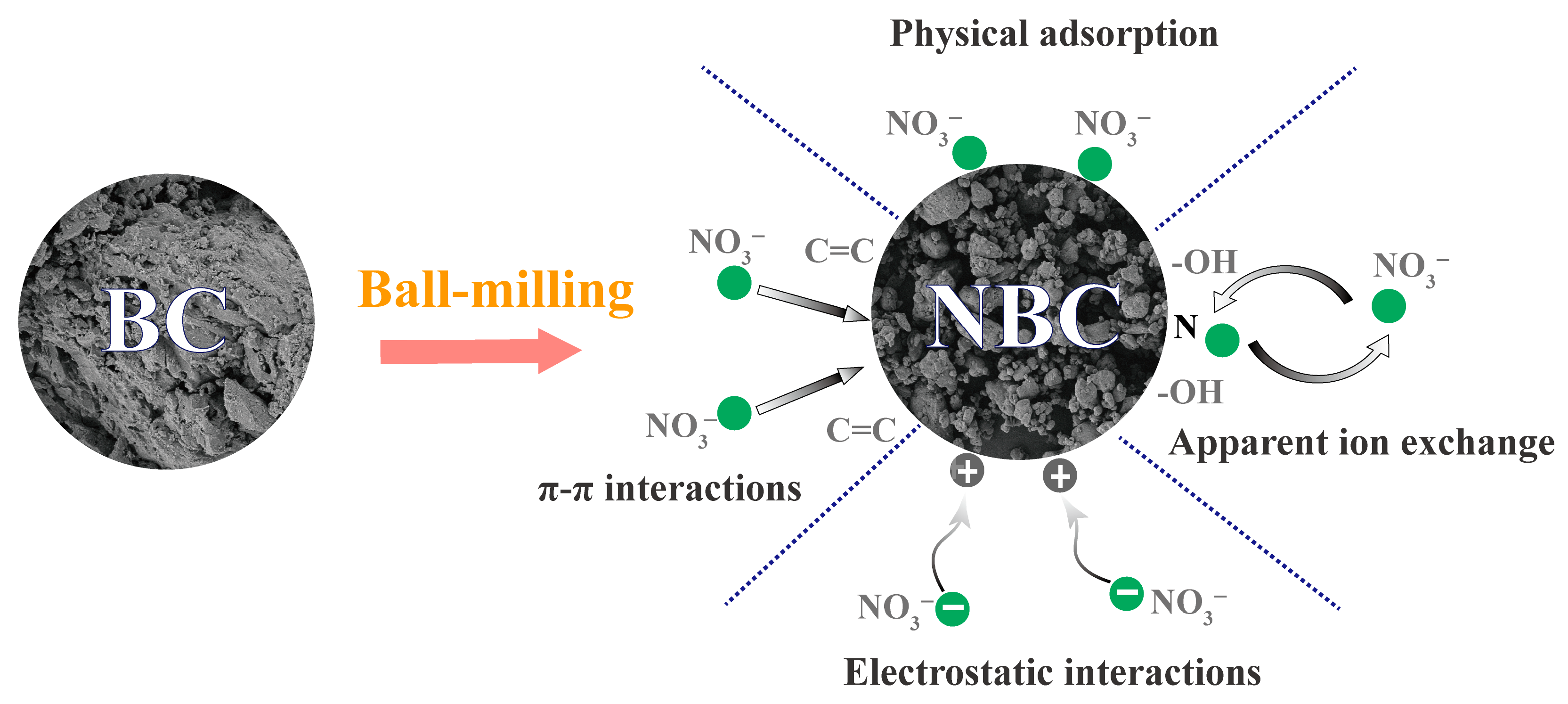
4.1. Adsorption Mechanism
4.2. Nitrous Oxide Emissions Reduction Mechanism
4.3. Application Potential of Nano-Biochar
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Wang, C.; Cao, H.; Liang, J.; Pei, S.; Li, Z. Nitrate Absorption and Desorption by Biochar. Agronomy 2023, 13, 2440. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A. Global Status of Nitrate Contamination in Groundwater: Its Occurrence, Health Impacts, and Mitigation Measures. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Bahram, M.; Espenberg, M.; Pärn, J.; Lehtovirta-Morley, L.; Anslan, S.; Kasak, K.; Kõljalg, U.; Liira, J.; Maddison, M.; Moora, M.; et al. Structure and Function of the Soil Microbiome Underlying N2O Emissions from Global Wetlands. Nat. Commun. 2022, 13, 1430. [Google Scholar] [CrossRef] [PubMed]
- Ussiri, D.; Lal, R. Soil Emission of Nitrous Oxide and Its Mitigation; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, K.; Taghizadeh-Mehrjardi, R.; Tao, J.; Fan, L.; Raza, S.; Guggenberger, G.; Kuzyakov, Y. Acidification of European Croplands by Nitrogen Fertilization: Consequences for Carbonate Losses and Soil Health. Sci. Total Environ. 2024, 924, 171631. [Google Scholar] [CrossRef] [PubMed]
- Yli-Halla, M.; Virtanen, S.; Regina, K.; Österholm, P.; Ehnvall, B.; Uusi-Kämppä, J. Nitrogen Stocks and Flows in an Acid Sulfate Soil. Environ. Monit. Assess. 2020, 192, 751. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Wang, B.; Hou, B.; Du, G.; Si, H. Analysis of the Adsorption and Fixation Process of Ammonium Nitrogen in Arable Soil by Biochar Based on Molecular Dynamics Simulation. Sci. Total Environ. 2024, 930, 172815. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Kubaczyński, A.; Szewczuk-Karpisz, K. Assessment of Agricultural Waste Biochars for Remediation of Degraded Water–Soil Environment: Dissolved Organic Carbon Release and Immobilization of Impurities in One- or Two-Adsorbate Systems. Waste Manag. 2023, 155, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Masek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Aamer, M.; Hassan, M.U.; Shaaban, M.; Rasul, F.; Tang, H.Y.; Ma, Q.; Batool, M.; Rasheed, A.; Zhong, C.; Su, Q.; et al. Rice Straw Biochar Mitigates N2O Emissions under Alternate Wetting and Drying Conditions in Paddy Soil. J. Saudi Chem. Soc. 2021, 25, 101172. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Sharma, P.C.; Dixit, B.; Jat, M.L. Soil Enzymes Activity: Effect of Climate-Smart Agriculture on Rhizosphere and Bulk Soil under Cereal-Based Systems of North-West India. Eur. J. Soil. Biol. 2021, 103, 103292. [Google Scholar] [CrossRef] [PubMed]
- Feliciano-Bruzual, C. Charcoal Injection in Blast Furnaces (Bio-PCI): CO2 Reduction Potential and Economic Prospects. J. Mater. Res. Technol. 2014, 3, 233–243. [Google Scholar] [CrossRef]
- Sarker, T.R.; Ethen, D.Z.; Nanda, S. Decarbonization of Metallurgy and Steelmaking Industries Using Biochar: A Review. Chem. Eng. Technol. 2024, 47, e202400217. [Google Scholar] [CrossRef]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of Soil Physical Properties and Soil Water Retention with Biochar-Based Soil Amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, L.; Gui, D.; Xu, B.; Li, R.; Chen, X.; Sha, Z.; Pan, X. Suitable Biochar Application Practices Simultaneously Alleviate N2O and NH3 Emissions from Arable Soils: A Meta-Analysis Study. Environ. Res. 2024, 242, 117750. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhong, T.; Zhang, L.; Zhang, K.; Wu, W. Reducing Ammonia Volatilization from Paddy Field with Rice Straw-Derived Biochar. Sci. Total Environ. 2019, 660, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, J.; Hou, W.; Cui, Y.; Yu, L.; Zhang, Y.; Wang, S. Influence of Modified Biochar-Supported Sulfidation of Nano-Zero-Valent Iron on Nitrate Removal and Greenhouse Gas Emission in Constructed Wetland. J. Environ. Sci. 2023, 125, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, Q.; Yuan, J.; Fu, S.; Lan, Y.; Jiang, X.; Meng, J.; Han, X.; Chen, W. Successive Corn Stover and Biochar Applications Mitigate N2O Emissions by Altering Soil Physicochemical Properties and N-Cycling-Related Enzyme Activities: A Five-Year Field Study in Northeast China. Agric. Ecosyst. Environ. 2022, 340, 108183. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How Does Biochar Influence Soil N Cycle? A Meta-Analysis. Plant and Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Biochar Enhances the Retention Capacity of Nitrogen Fertilizer and Affects the Diversity of Nitrifying Func-tional Microbial Communities in Karst Soil of Southwest China. Ecotoxicol. Environ. Saf. 2021, 226, 112819. [Google Scholar] [CrossRef] [PubMed]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, Soil and Land-Use Interactions That Reduce Nitrate Leaching and N2O Emissions: A Meta-Analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball-Milled Biochar Effectively Removes Sulfamethoxazole and Sulfapyridine Antibiotics from Water and Wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-Milling Synthesis of Biochar and Biochar-Based Nanocomposites and Prospects for Removal of Emerging Contaminants: A Review. J. Water Process Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Hüser, D.; Bergmann, D.; Klein, T. Traceable Determination of the Size of Nanoparticles up to 500 nm by Scanning Electron Microscopy in Transmission Mode Based on Simulation. Meas. Sci. Technol. 2023, 34, 85016. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball Milling as a Mechanochemical Technology for Fabrication of Novel Biochar Nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.; Dhandapani, B.; Vaishnavi, G.; Preethi, V. Synthesis of Tri-Metallic Surface-Engineered Nanobiochar from Cynodon dactylon Residues in a Single Step: Batch and Column Studies for the Removal of Copper and Lead Ions. Chemosphere 2022, 286, 131572. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, R.; Chen, G. Micro-Nano-Engineered Nitrogenous Bone Biochar Developed with a Ball-Milling Technique for High-Efficiency Removal of Aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, Y.; Zhou, D.; Wang, L.; Liang, X.; Sun, Y. Development and Optimization of High-Performance Nano Biochar for Efficient Cd Removal in Aqueous Solution: Adsorption Performance and Interaction Mechanisms. Chem. Eng. Res. Des. 2023, 189, 516–529. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X.; et al. Effects of Ball Milling on Biochar Adsorption of Contaminants in Water: A Meta-Analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning Oxygenated Functional Groups on Biochar for Water Pollution Control: A Critical Review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and Modeling Investigations of Ball-Milled Biochar for the Removal of Aqueous Methylene Blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Wang, Q.; Tao, W.; Lin, H. Nano Biochar Reduced Soil Erosion and Nitrate Loss in Sloping Fields on the Loess Plateau of China. CATENA 2020, 187, 104346. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Nine, M.J.; Jeoun, J.; Bat-Erdene, M.; Chung, H.; Jeong, H. Influence of Dry and Wet Ball Milling on Dispersion Characteristics of Multi-Walled Carbon Nanotubes in Aqueous Solution with and without Surfactant. Powder Technol. 2013, 234, 132–140. [Google Scholar] [CrossRef]
- Sha, Z.; Li, Q.; Lv, T.; Misselbrook, T.; Liu, X. Response of Ammonia Volatilization to Biochar Addition: A Meta-Analysis. Sci. Total Environ. 2019, 655, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zong, T.; Wu, Q.; Wang, X.; Hou, H.; Xin, X.; Xie, J.; Zhou, Y.; Yang, J. Fabrication of Rice Straw Nano Biochar by Ball Milling for Efficient Adsorption of Ammonium Nitrogen and Reduction of Ammonia Volatilization: Effects and Mechanisms. Environ. Sci. Nano 2025, 12, 3122–3138. [Google Scholar] [CrossRef]
- Bhandari, G.; Gangola, S.; Dhasmana, A.; Rajput, V.; Gupta, S.; Malik, S.; Slama, P. Nano Biochar: Recent Progress, Challenges, and Opportunities for Sustainable Environmental Remediation. Front. Microbiol. 2023, 14, 1214870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; He, L.; Niazi, N.K.; Wang, H.; Gustave, W.; Vithanage, M.; Geng, K.; Shang, H.; Zhang, X.; Wang, Z. Nanobiochar for the Remediation of Contaminated Soil and Water: Challenges and Opportunities. Biochar 2023, 5, 2. [Google Scholar] [CrossRef]
- Pathak, H.K.; Seth, C.S.; Chauhan, P.K.; Dubey, G.; Singh, G.; Jain, D.; Upadhyay, S.K.; Dwivedi, P.; Khoo, K.S. ecent Advancement of Nano Biochar for the Remediation of Heavy Metals and Emerging Contaminants: Mechanism, Adsorption Kinetic Model, Plant Growth and Development. Environ. Res. 2024, 255, 119136. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.; Kalia, A.; Sikka, R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy-Metal Adsorption from Wastewaters: A Review. ACS Omega 2022, 7, 45825–45836. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, A.K.; Pratap, T.; Preetiva, B.; Patel, M.; Singsit, J.S.; Pittman, C.U., Jr.; Mohan, D. Definitive Review of Nanobiochar. ACS Omega 2024, 9, 12331–12379. [Google Scholar] [CrossRef] [PubMed]
- Ferree, M.A.; Shannon, R.D. Evaluation of a Second-Derivative UV/Visible Spectroscopy Technique for Nitrate and Total Nitrogen Analysis of Wastewater Samples. Water Res. 2001, 35, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of Sewage Sludge-Based Compost by FT-IR Spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Zhang, X.; Wells, M.; Niazi, N.K.; Bolan, N.; Shaheen, S.; Hou, D.; Gao, B.; Wang, H.; Rinklebe, J.; Wang, Z. Nanobiochar-Rhizosphere Interactions: Implications for the Remediation of Heavy-Metal Contaminated Soils. Environ. Pollut. 2022, 299, 118810. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Abou-Ali, S.A.A.; Elweshahy, S.M.T. Efficient and Ultrafast Removal of Cd(II) and Sm(III) from Water by Leaves of Cynara scolymus-Derived Biochar. Mater. Res. Bull. 2021, 141, 111334. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A Review of Emerging Adsorbents for Nitrate Removal from Water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of Functionalized Magnetic Biochar in Environmental Remediation: A Review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Wang, M.; Huang, J.; Liang, R.; Du, J.; Fang, C.; Shen, C.; Man, Y.B.; Wong, M.H.; Shan, S.; et al. Large Particle Size Boosting the Engineering Application Potential of Functional Biochar in Ammonia-Nitrogen and Phosphorus Removal from Biogas Slurry. J. Water Process Eng. 2024, 57, 104640. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of Ball Milling on the Physicochemical and Sorptive Properties of Biochar: Experimental Observations and Governing Mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Xia, S.; Tang, J.; Zhang, Y.; Gao, B.; Shen, B. Thiol-Modified Biochar Synthesized by a Facile Ball-Milling Method for Enhanced Sorption of Inorganic Hg2+ and Organic CH3Hg+. J. Hazard. Mater. 2020, 384, 121357. [Google Scholar] [CrossRef] [PubMed]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and Ammonium Sorption Capacity of Biochar and Hydrochar from Different Wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Kohira, Y.; Fentie, D.; Lewoyehu, M.; Wutisirirattanachai, T.; Gezahegn, A.; Addisu, S.; Sato, S. Elucidation of Ammonium and Nitrate Adsorption Mechanisms by Water Hyacinth Biochar: Effects of Pyrolysis Temperature. Environ. Sci. Pollut. Res. 2025, 32, 762–782. [Google Scholar] [CrossRef] [PubMed]
- Divband Hafshejani, L.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of Nitrate from Aqueous Solution by Modified Sugarcane Bagasse Biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Tian, C.; Feng, C.; Wei, M.; Wu, Y. Enhanced Adsorption of Anionic Toxic Contaminant Congo Red by Activated Carbon with Electropositive Amine Modification. Chemosphere 2018, 208, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Li, Y.; Chi, D.; Wu, Q. Efficient Removal of Ammonium in Aqueous Solution by Ultrasonic Magnesium-Modified Biochar. Chem. Eng. J. 2023, 461, 142072. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of Heavy Metals from Aqueous Solution by Biochars Derived from Anaerobically Digested Biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. itrate Sorption and Desorption in Biochars from Fast Pyrolysis. Microporous Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile Ball-Milling Synthesis of CuO/Biochar Nanocomposites for Efficient Removal of Reactive Red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Saya, L.; Malik, V.; Gautam, D.; Gambhir, G.; Balendra; Singh, W.R.; Hooda, S. A Comprehensive Review on Recent Advances toward Sequestration of Levofloxacin Antibiotic from Wastewater. Sci. Total Environ. 2022, 813, 152529. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Hydrothermal Carbonization of Argan Nut Shell: Functional Mesoporous Carbon with Excellent Performance in the Adsorption of Bisphenol A and Diuron. Waste Biomass Valorization 2020, 11, 1565–1584. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic Rings in Chemical and Biological Recognition: Energetics and Structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, X.; Gong, P.; Wang, C. Nitrated Polycyclic Aromatic Compounds in the Atmospheric Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1159–1185. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of Biochar Surface Characteristics in the Adsorption of Aromatic Compounds: Pore Structure and Functional Groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, L.; Yan, B.; Zeng, H. Nanomechanics of Anion-π Interaction in Aqueous Solution. J. Am. Chem. Soc. 2020, 142, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar Application for Greenhouse-Gas Mitigation, Contaminants Immobilization and Soil Fertility Enhancement: A State-of-the-Art Review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef] [PubMed]
- Hai, W.; Lu, R.; Chen, Q.; Yi, M.; Zhang, Y. Biochar-Supported Starch/Chitosan-Stabilized Nano-Iron Sulfide Composites for the Removal of Lead Ions and Nitrogen from Aqueous Solutions. Bioresour. Technol. 2022, 347, 126700. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of Ball-Milled Biochar in Ca-Alginate Beads for the Removal of Aqueous Cd(II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Zeng, F.-X.; Jiang, H.; Zhang, X.-S. Preparation of High-Adsorption-Capacity Biochars from Waste Biomass. Bioresour. Technol. 2011, 102, 8247–8252. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A Lignosulfonate-Modified Graphene Hydrogel with Ultrahigh Adsorption Capacity for Pb(II) Removal. J. Mater. Chem. A 2016, 4, 11888–11896. [Google Scholar] [CrossRef]
- Kasera, N.; Gillikin, E.; Kolar, P.; Hall, S.G. Feasibility of Nitrate Adsorption from Aqueous Solution by Nitrogen- and Oxygen-Modified Pine Bark Biochar: Experimental and Computational Approach. Discov. Water 2023, 3, 13. [Google Scholar] [CrossRef]
- Shang, H.; Li, Y.; Liu, J.; Wan, Y.; Feng, Y.; Yu, Y. Preparation of Nitrogen-Doped Magnesium-Oxide-Modified Biochar and Its Sorption Efficiency of Lead Ions in Aqueous Solution. Bioresour. Technol. 2020, 314, 123708. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, S.; Wang, Q.; Liang, Y.; Liu, C.; Xu, H.; Ye, Q. Enhanced Nitrogen and Phosphorus Adsorption Performance and Stabilization by Panda Manure Biochar Modified with CMC-Stabilized nZVZ Composite in Aqueous Solution J. Clean. Prod. 2021, 291, 125221. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Chen, T.; Liu, C.; Zhang, F.; Han, H.; Wang, Z.; Yi, B.; Tang, L.; Meng, J.; Chi, D.; Wilson, L.T.; et al. Acid-Modified Biochar Increases Grain Yield and Reduces Reactive Gaseous-N Losses and N-Related Global Warming Potential in an Alternate Wetting and Drying Paddy System. J. Clean. Prod. 2022, 377, 134451. [Google Scholar] [CrossRef]
- Dawar, K.; Fahad, S.; Jahangir, M.M.R.; Munir, I.; Alam, S.S.; Khan, S.A.; Mian, I.A.; Datta, R.; Saud, S.; Banout, J.; et al. Biochar and Urease Inhibitor Mitigate NH3 and N2O Emissions and Improve Wheat Yield in Urea-Fertilized Alkaline Soil. Sci. Rep. 2021, 11, 17413. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yuan, J.; Xiang, J.; Lin, Y.; Luo, J.; Lindsey, S.; Liao, X.; Liu, D.; Ding, W. Combined Biochar and Double-Inhibitor Application Offsets NH3 and N2O Emissions and Mitigates N Leaching in Paddy Fields. Environ. Pollut. 2022, 292, 118344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Richter, D.V.; Mayes, J.; Wang, J.; Zhang, X. Soil pH as the Chief Modifier for Regional Nitrous Oxide Emissions: New Evidence and Implications for Global Estimates and Mitigation. Glob. Change Biol. 2018, 24, e617–e626. [Google Scholar] [CrossRef] [PubMed]
- Aamer, M.; Shaaban, M.; Hassan, M.U.; Huang, G.; Liu, Y.; Tang, H.Y.; Rasul, F.; Ma, Q.; Li, Z.; Rasheed, A.; et al. Biochar Mitigates N2O Emissions from Acidic Soil by Increasing nosZ and nirK Gene Abundance and Soil pH. J. Environ. Manag. 2020, 255, 109891. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, M.; Zhou, B.; Cui, L. Effects of Biochar Nanoparticles as a Soil Amendment on the Structure and Hydraulic Characteristics of a Sandy Loam Soil. Soil Use Manag. 2022, 38, 836–849. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Xiong, Z.; Chen, C.; Xu, X.; Zhou, Q.; Kuzyakov, Y. Effects of Biochar Amendment on Greenhouse-Gas Emissions, Net Ecosystem Carbon Budget and Properties of an Acidic Soil under Intensive Vegetable Production. Soil Use Manag. 2015, 31, 375–383. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wu, Q.; Zong, T.; Xin, X.; Xie, J.; Yang, J. Use of Rice Straw Nano Biochar to Slow Down Water Infiltration and Reduce Nitrogen Leaching in a Clayey Soil. Sci. Total Environ. 2024, 948, 174956. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Shah, G.A.; Iqbal, Z.; Shahzad, K.; Ali, N.; Rehan, M.; Alhakamy, N.A.A.; Klemes, J.J. Nanobiochar Reduces Ammonia Emission, Increases Nutrient Mineralization from Vermicompost, and Im-proves Maize Productivity. J. Clean. Prod. 2023, 414, 137694. [Google Scholar] [CrossRef]
- Xin, X.; Farid, G.; Nepal, J.; He, S.; Yang, X.; He, Z. Comparative Effectiveness of Carbon Nanoparticles and Biochar in Alleviating Copper Stress in Corn (Zea mays L.). Chemosphere 2024, 355, 141745. [Google Scholar] [CrossRef] [PubMed]
- Surup, G.R.; Trubetskaya, A.; Tangstad, M. Life-cycle assessment of renewable reductants in the ferromanganese alloy production: A review. Processes 2021, 9, 185. [Google Scholar] [CrossRef]
- Sahoo, K.; Upadhyay, A.; Runge, T.; Bergman, R.; Puettmann, M.; Bilek, E. Life-cycle assessment and techno-economic analysis of biochar produced from forest residues using portable systems. Int. J. Life Cycle Assess. 2021, 26, 189–213. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A Green Method for Production of Nanobiochar by Ball Milling: Optimization and Characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
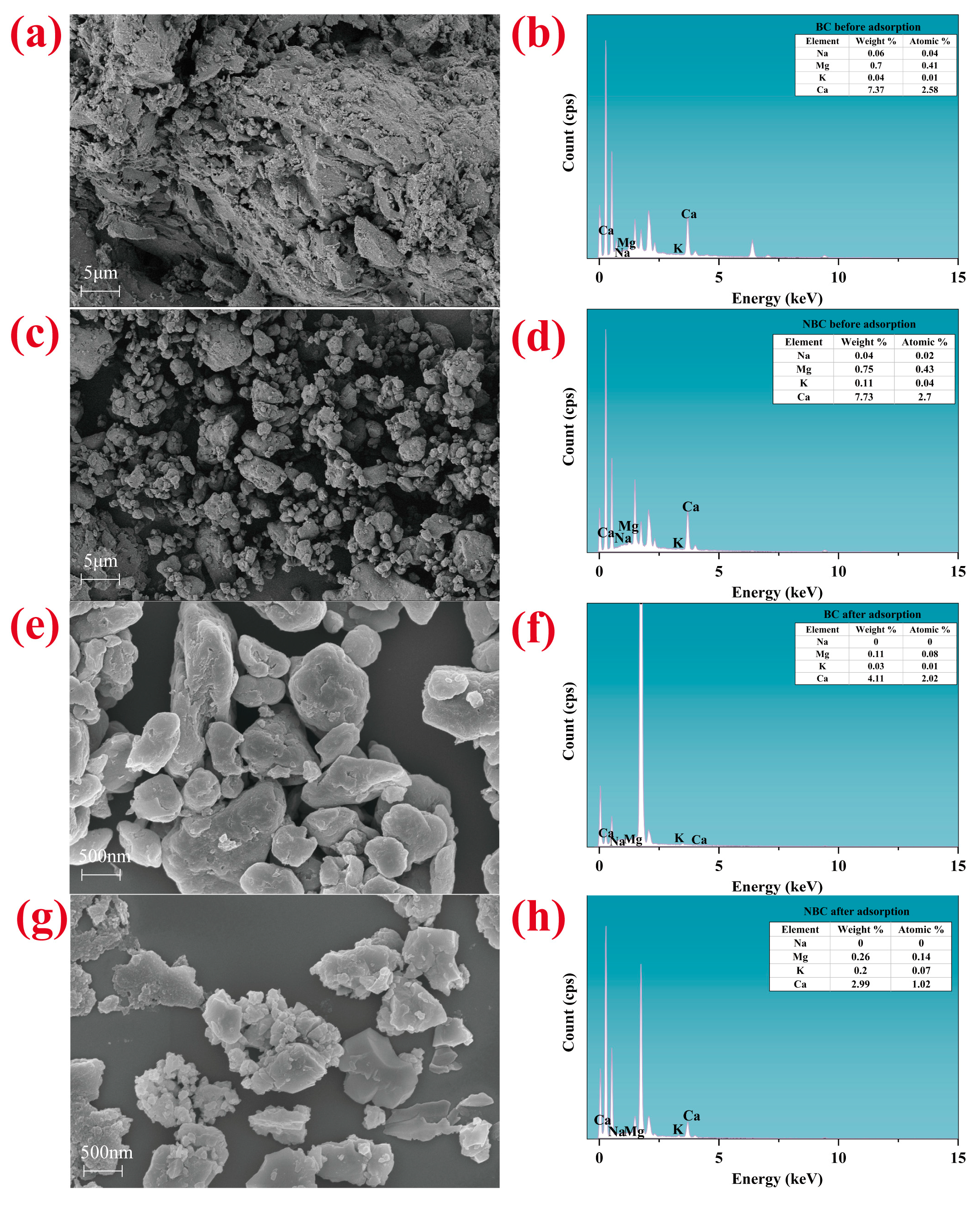



| Samples | SBET (m2 g−1) | Smicro (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) | Acidic Functional Group (mmoL g−1) | Alkaline Functional Groups (mmoL g−1) | pH | pHzpc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Carboxyl | Lactone Group | Phenolic Hydroxyl Group | ||||||||
| Bulk biochar | 19.060 | 8.200 | 0.025 | 0.003 | 0.187 | 0.269 | 0.592 | 0.931 | 10.090 | 6.730 |
| Nano-biochar | 28.450 | 23.670 | 0.034 | 0.010 | 0.226 | 0.315 | 0.719 | 0.851 | 9.550 | 6.440 |
| Samples | Langmuir | Freundlich | Pseudo-First Order | Pseudo-Second Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | n | R2 | qe | K1 | R2 | qe | K2 | R2 | |
| Bulk biochar | 3.691 | 0.001 | 0.909 | 0.101 | 2.317 | 0.770 | 2.883 | 0.014 | 0.974 | 3.915 | 0.003 | 0.960 |
| Nano-biochar | 8.235 | 0.001 | 0.964 | 0.172 | 2.165 | 0.874 | 5.789 | 0.011 | 0.997 | 8.067 | 0.001 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Zong, T.; Sun, Y.; Fang, W.; Shen, T.; Zhou, Y. Nano-Biochar Enhanced Adsorption of NO3−-N and Its Role in Mitigating N2O Emissions: Performance and Mechanisms. Agronomy 2025, 15, 1723. https://doi.org/10.3390/agronomy15071723
Xing W, Zong T, Sun Y, Fang W, Shen T, Zhou Y. Nano-Biochar Enhanced Adsorption of NO3−-N and Its Role in Mitigating N2O Emissions: Performance and Mechanisms. Agronomy. 2025; 15(7):1723. https://doi.org/10.3390/agronomy15071723
Chicago/Turabian StyleXing, Weimin, Tao Zong, Yidi Sun, Wenhao Fang, Tong Shen, and Yuhao Zhou. 2025. "Nano-Biochar Enhanced Adsorption of NO3−-N and Its Role in Mitigating N2O Emissions: Performance and Mechanisms" Agronomy 15, no. 7: 1723. https://doi.org/10.3390/agronomy15071723
APA StyleXing, W., Zong, T., Sun, Y., Fang, W., Shen, T., & Zhou, Y. (2025). Nano-Biochar Enhanced Adsorption of NO3−-N and Its Role in Mitigating N2O Emissions: Performance and Mechanisms. Agronomy, 15(7), 1723. https://doi.org/10.3390/agronomy15071723







