QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4×Thatcher RIL Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pathogens
2.2. Field Experiments
2.3. Molecular Genotyping
2.4. Construction of Linkage Maps and QTL Analysis
2.5. Wheat Primers and PCR Programs
3. Results
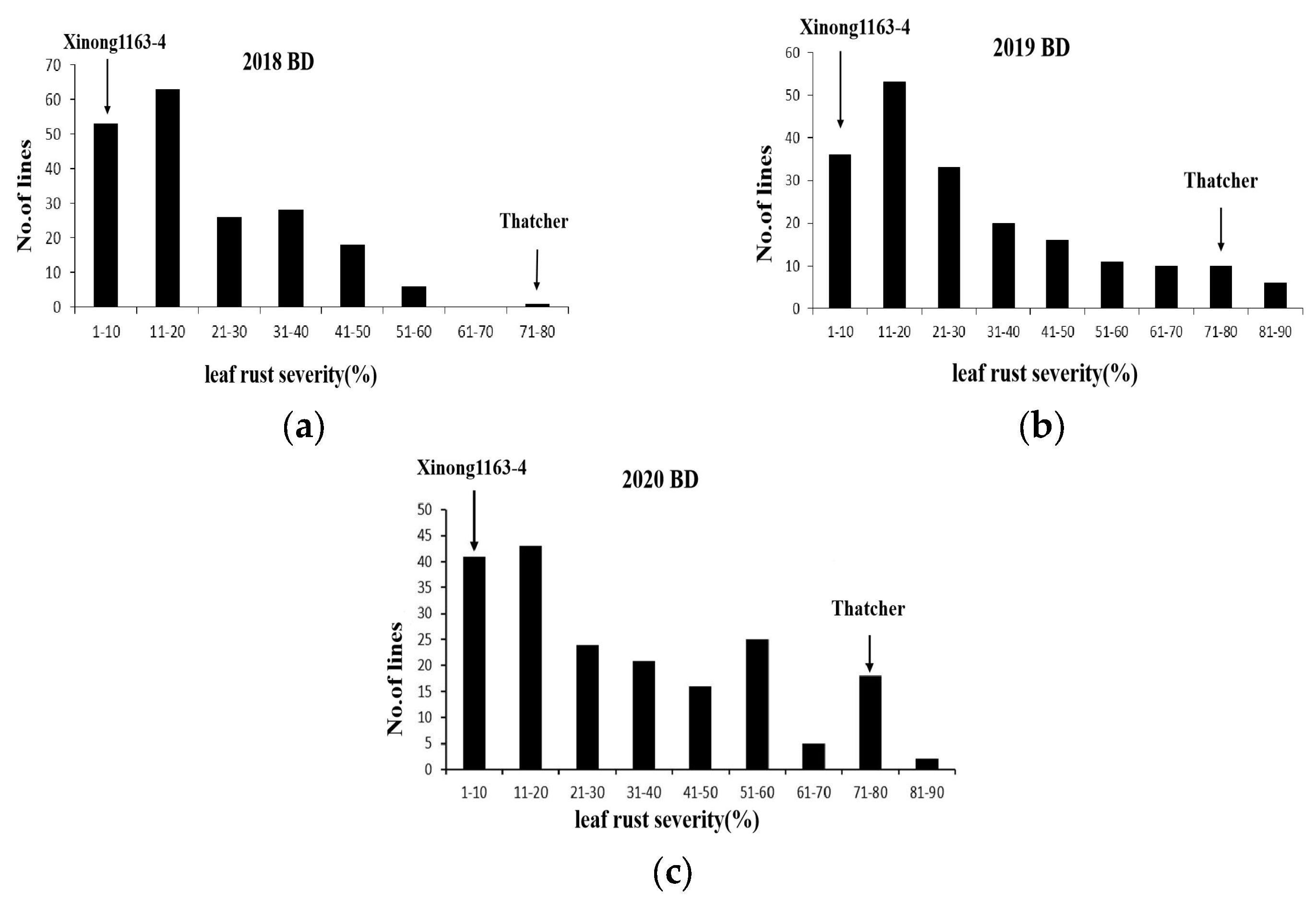
3.1. Field Testing
3.2. Genetic Analysis of Markers
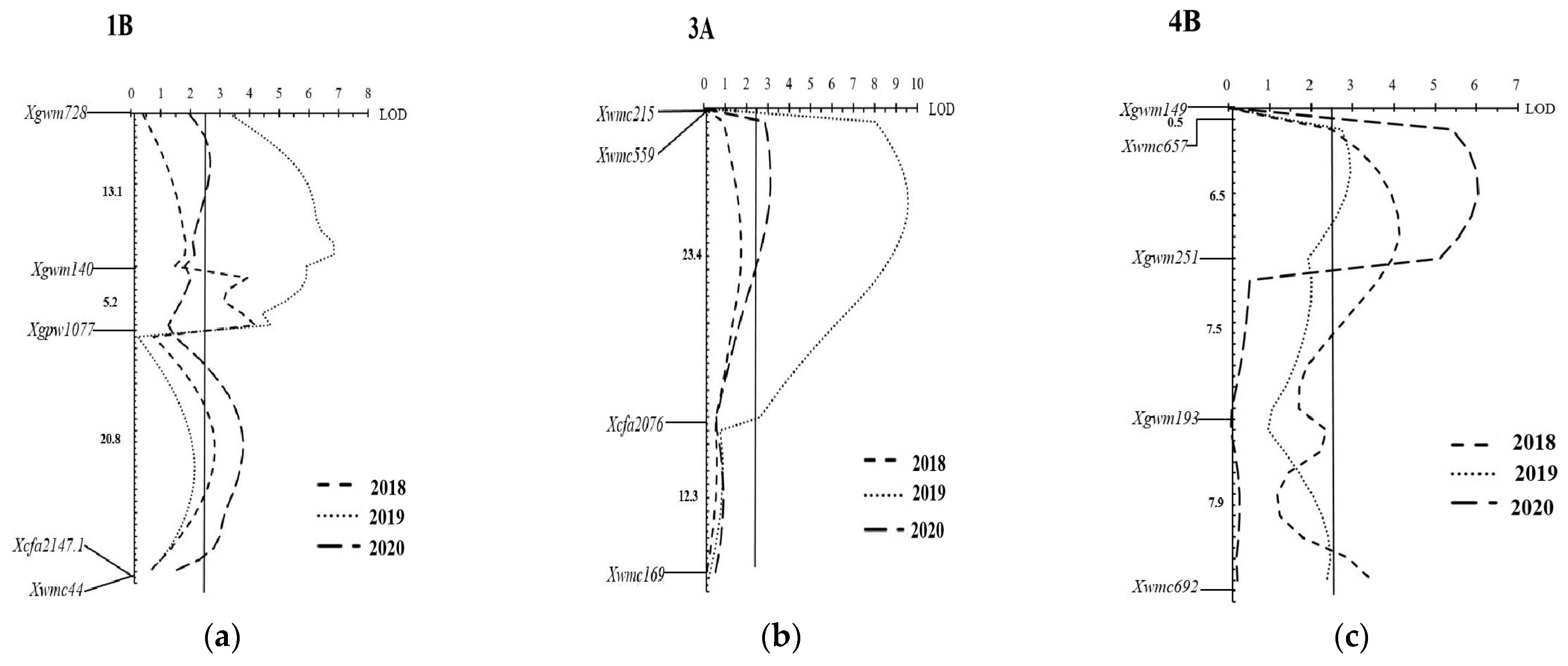
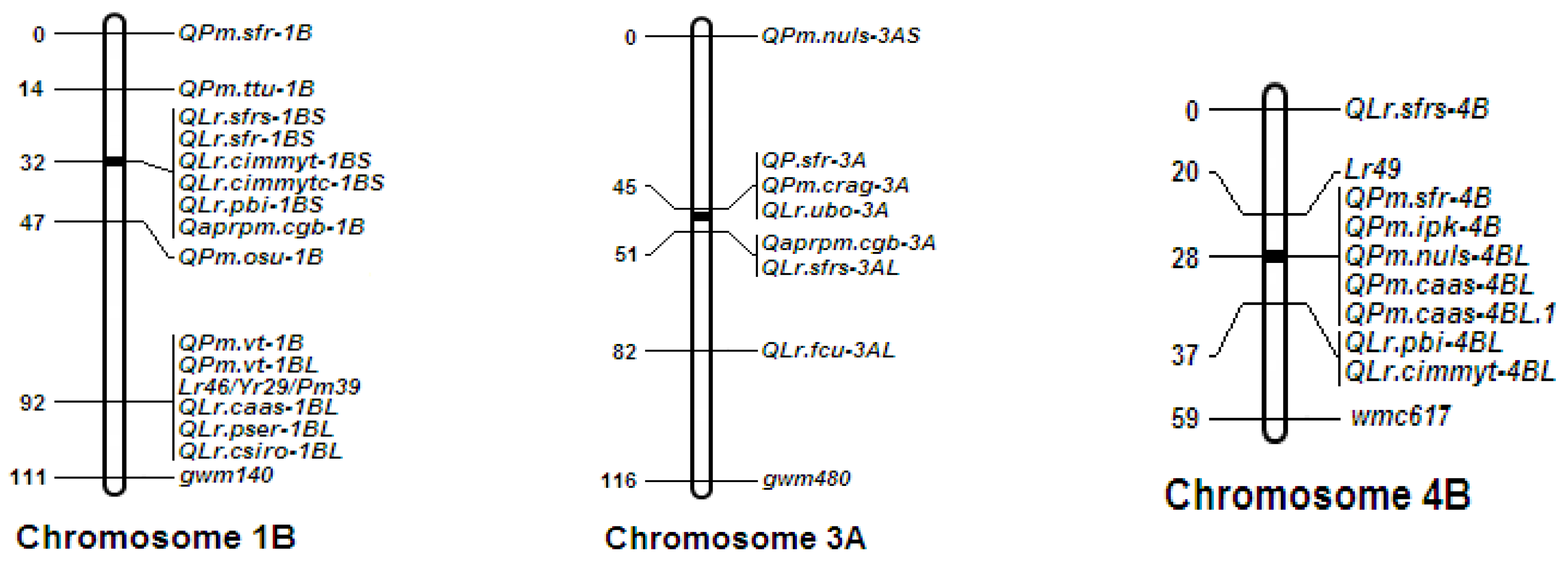
3.3. Genetic Linkage Mapping and QTL Mapping
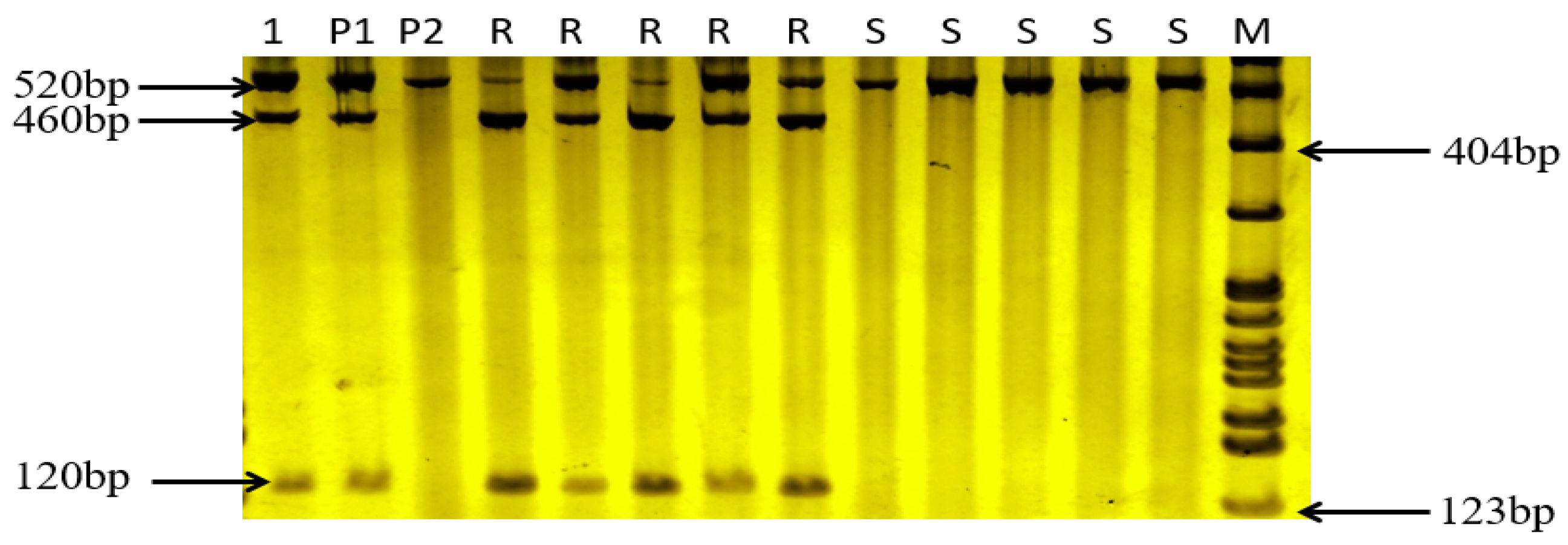
3.4. csLV46G22 Molecular Marker Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lr | Wheat leaf rust |
| Pt | Puccinia triticina Eriks |
| RILs | Recombinant inbred lines |
| QTLs | Quantitative trait loci |
| APR | Adult plant resistance |
| ASR | All-stage resistance |
| SSR | Simple sequence repeat |
| SNP | Single nucleotide polymorphism |
| GWAS | Genome-wide association studies |
| MDS | Maximum disease severity |
| ANOVA | Analysis of variance |
| LOD | Logarithm of the odds |
| PVE | Phenotypic variation explained |
| CIMMYT | International Maize and Wheat Improvement Center |
References
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Xu, X.; Duan, Z.; Su, J.; Li, X.; Wu, J.; Yao, Z. QTL mapping of adult-plant resistance to leaf rust based on SSR markers and SNP sequencing of Chinese wheat landrace Xu’ai (Triticum aestivum L.). Genet. Resour. Crop Evol. 2021, 68, 1359–1373. [Google Scholar] [CrossRef]
- Zhao, J.; Kang, Z.S. Fighting wheat rusts in China: A look back and into the future. Phytopathol. Res. 2023, 5, 6. [Google Scholar] [CrossRef]
- Khan, M.; Bukhari, A.; Dar, Z.; Rizvi, S. Status and strategies in breeding for rust resistance in wheat. Agric. Sci. 2013, 4, 292–301. [Google Scholar] [CrossRef]
- Peng, H.; Lv, G.Q.; Wang, J.R. Hénán shěng 2015 nián xiǎomài zhǔyào bìnghài fāshēng tèdiǎn jí yuányīn fēnxī [Occurrence characteristics and causes of major wheat diseases in Henan Province in 2015]. China Plant Prot. Guide 2016, 3, 91–93. [Google Scholar]
- Li, M.; Banshali, F.; Brar, G.S.; Rozek, A.; Bakkeren, G. In Vitro and in Planta Fungicide Testing for Management of Wheat Rusts. Methods Mol. Biol. 2025, 2898, 361–377. [Google Scholar] [CrossRef]
- Mourad, A.M.I.; Ahmed, A.A.M.; Baenziger, P.S.; Börner, A.; Sallam, A. Broad-spectrum resistance to fungal foliar diseases in wheat: Recent efforts and achievements. Front. Plant Sci. 2024, 13, 1516317. [Google Scholar] [CrossRef]
- Yadav, J.K.; Sinha, S.; Shukla, H.; Singh, A.; Sahu, T.K.; Jha, S.K.; Kumari, J.; Verma, M.; Kumar, S.; Singh, R.; et al. Genetic dissection of leaf rust resistance in a diversity panel of tetraploid wheat (Triticum turgidum). BMC Plant Biol. 2025, 25, 406. [Google Scholar] [CrossRef]
- Spychała, J.; Noweiska, A.; Tomkowiak, A.; Bobrowska, R.; Szewczyk, K.; Kwiatek, M.T. Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2025, 26, 665. [Google Scholar] [CrossRef]
- Lin, F.; Chen, X.M. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor. Appl. Genet. 2007, 114, 1277–1287. [Google Scholar] [CrossRef]
- Zhang, P.; Lan, C.; Asad, M.A.; Gebrewahid, T.W.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf rust in the Chinese landraces Pingyuan 50/Mingxian 169 using the wheat 55K SNP array. Mol. Breed. 2019, 39, 98. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Bajgain, P.; Rouse, M.N.; Li, J.; Zhang, P. Mapping and characterization of the recessive leaf rust resistance gene Lr83 on wheat chromosome arm 1DS. Theor. Appl. Genet. 2023, 136, 115. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.M.I.; Draz, I.S.; Omar, G.E.; Börner, A.; Esmail, S.M. Genome-Wide Screening of Broad-Spectrum Resistance to Leaf Rust (Puccinia triticina Eriks) in Spring Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 921230. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Mujeeb-Kazi, A.; Huerta-Espino, J. Lr46: A gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology 1998, 88, 890–894. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Lillemo, M.; Huerta-Espino, J.; Bhavani, S.; Singh, S.; Lan, C.; Calvo-Salazar, V.; Lagudah, E.S. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 2014, 127, 781–789. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Rosewarne, G.M.; Periyannan, S.K.; Viccars, L.; Calvo-Salazar, V.; Lan, C.; Lagudah, E.S. Lr68: A new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 2012, 124, 1475–1486. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Chao, S.; Brown-Guedira, G.; Bansal, U.; Bariana, H. Adult plant leaf rust resistance derived from the soft red winter wheat cultivar ‘Caldwell’ maps to chromosome 3BS. Crop Sci. 2018, 58, 152–158. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Su, Z.; Bernardo, A.; Bai, G.; Chao, S. Mapping and characterization of the new adult plant leaf rust resistance gene Lr77 derived from Santa Fe winter wheat. Theor. Appl. Genet. 2018, 131, 1553–1560. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Bernardo, A.; Bai, G.; Hayden, M.J.; Chao, S. Adult plant leaf rust resistance derived from Toropi wheat is conditioned by Lr78 and three minor QTL. Phytopathology 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Singla, J.; Lüthi, L.; Wicker, T.; Bansal, U.; Krattinger, S.G.; Keller, B. Characterization of Lr75: A partial, broad–spectrum leaf rust resistance gene in wheat. Theor. Appl. Genet. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Rouse, M.N. Adult plant leaf rust resistance QTL derived from wheat line CI13227 maps to chromosomes 2AL, 4BS, and 7AL. Plant Genome 2022, 15, e20215. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B.A. putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Spychała, J.; Tomkowiak, A.; Noweiska, A.; Bobrowska, R.; Bocianowski, J.; Sobiech, A.; Kwiatek, M.T. Diversity of Expression Patterns of Lr34,Lr67, and Candidate Genes towards Lr46 with Analysis of Associated miRNAs in Common Wheat Hybrids in Response to Puccinia triticina Fungus. Curr. Issues Mol. Biol. 2024, 46, 5511–5529. [Google Scholar] [CrossRef]
- Pinto da Silva, G.B.; Zanella, C.M.; Martinelli, J.A.; Chaves, M.S.; Hiebert, C.W.; McCallum, B.D.; Boyd, L.A. Quantitative trait loci conferring leaf rust resistance in hexaploid wheat. Phytopathology 2018, 108, 1344–1354. [Google Scholar] [CrossRef]
- Singh, R.; Huerta-Espino, J.; Rajaram, S. Achieving near-immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathol. Entomol. Hung. 2000, 35, 133–139. [Google Scholar]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Sharp, P.J.; Kreis, M.; Shewry, P.R.; Gale, M.D. Location of β-amylase sequences in wheat and its relatives. Theor. Appl. Genet. 1988, 75, 286–290. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, G.; Zhou, Y.; Qi, A.; Gao, F.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf rust in the wheat cross Zhou 8425B/Chinese spring using high–density SNP Markers. Front. Plant Sci. 2017, 8, 793. [Google Scholar] [CrossRef]
- Singh, D.; Simmonds, J.; Park, R.F.; Bariana, H.S.; Snape, J.W. Inheritance and QTL mapping of leaf rust resistance in the European winter wheat cultivar ‘Beaver’. Euphytica 2009, 169, 253–261. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Zhang, L.; Wang, J. Estimation of recombination frequency in bi-parental genetic populations. Genet. Res. 2012, 94, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Lillemo, M.; Joshi, A.K.; Prasad, R.; Chand, R.; Singh, R.P. QTL for spot blotch resistance in bread wheat line Saar co-locate to the biotrophic disease resistance loci Lr34 and Lr46. Theor. Appl. Genet. 2013, 126, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; William, H.M.; Bouchet, S.; Cloutier, S.; McFadden, H.; Lagudah, E.S. Leaf tip necrosis, molecular markers and β1-proteasome subunits associated with the slow rusting resistance genes Lr46/Yr29. Theor. Appl. Genet. 2006, 112, 500–508. [Google Scholar] [CrossRef]
- Singh, S.; Bowden, R.L. Molecular mapping of adult-plant race-specific leaf rust resistance gene Lr12 in bread wheat. Mol. Breed. 2011, 28, 137–142. [Google Scholar] [CrossRef]
- Bansal, U.K.; Hayden, M.J.; Venkata, B.P.; Khanna, R.; Saini, R.G.; Bariana, H.S. Genetic mapping of adult plant leaf rust resistance genes Lr48 and Lr49 in common wheat. Theor. Appl. Genet. 2008, 117, 307–312. [Google Scholar] [CrossRef]
- William, H.M.; Singh, R.P.; Huerta-Espino, J.; Palacios, G.; Suenaga, K. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar] [CrossRef]
- Yu, H.; Zhiying, D.; Xiang, C.; Tian, J. Analysis of diversity and linkage disequilibrium mapping of agronomic traits on B-genome of wheat. J. Genom. 2014, 2, 20–30. [Google Scholar] [CrossRef][Green Version]
| Environment a | Parents | |||
|---|---|---|---|---|
| Xinong1163-4 | Thatcher | Mean ± SD | ||
| 2018 BD | 1 | 80 | 24.23 ± 15.58 | |
| 2019 BD | 1 | 80 | 32.38 ± 23.78 | |
| 2020 BD | 1 | 80 | 35.14 ± 24.05 | |
| Environment a | 195 RILs | |||
| Range | Variance coefficient (%) | Skewness | Kurtosis | |
| 2018 BD | 1–80 | 64.31 | 0.77 | −0.04 |
| 2019 BD | 1–90 | 73.43 | 0.80 | −0.29 |
| 2020 BD | 1–90 | 68.44 | 0.54 | −0.92 |
| Environment | R |
|---|---|
| 2017–2018 BD | 0.495 ** |
| 2018–2019 BD | 0.510 ** |
| 2019–2020 BD | 0.629 ** |
| Environment b | Chromosome | QTL a | Marker Interval c | LOD d | PVE (%) e | Additive Effect f | Dominate Effect | DR |
|---|---|---|---|---|---|---|---|---|
| 2018BD | 1B | QLr.hbau-1BL.1 | Xgwm140-Xgpw1077 | 4.20 | 10.66 | −2.06 | 46.57 | OD |
| 2018BD | 1B | QLr.hbau-1BL.2 | Xgpw1077-Xcfa2147.1 | 2.82 | 3.37 | −3.63 | −5.92 | OD |
| 2018BD | 4B | QLr.hbau-4BL | Xwmc657-Xgwm251 | 4.13 | 8.13 | −6.23 | 5.06 | D |
| 2018BD | 4B | QLr.hbau-4BL.1 | Xgwm193-Xwmc692 | 3.46 | 12.18 | −0.55 | 38.54 | OD |
| 2019BD | 1B | QLr.hbau-1BL.3 | Xgwm728-Xgwm140 | 6.85 | 21.88 | −10.61 | 25.35 | OD |
| 2019BD | 3A | QLr.hbau-3A | Xwmc215-Xcfa2076 | 9.57 | 24.95 | −17.15 | −3.83 | PD |
| 2019BD | 4B | QLr.hbau-4BL | Xwmc657-Xgwm251 | 2.95 | 5.31 | −5.60 | −7.68 | OD |
| 2020BD | 1B | QLr.hbau-1BL.3 | Xgwm728-Xgwm140 | 2.68 | 6.16 | −5.79 | −9.43 | OD |
| 2020BD | 1B | QLr.hbau-1BL.2 | Xgpw1077-Xcfa2147.1 | 3.79 | 5.44 | −7.68 | −4.41 | PD |
| 2020BD | 3A | QLr.hbau-3A | Xwmc215-Xcfa2076 | 3.13 | 6.63 | −7.94 | −6.62 | D |
| 2020BD | 4B | QLr.hbau-4BL | Xwmc657-Xgwm251 | 6.03 | 12.23 | −10.36 | −5.94 | PD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Kang, Z.; Li, X.; Li, M.; Xue, L.; Li, X. QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4×Thatcher RIL Population. Agronomy 2025, 15, 1717. https://doi.org/10.3390/agronomy15071717
Zhang J, Kang Z, Li X, Li M, Xue L, Li X. QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4×Thatcher RIL Population. Agronomy. 2025; 15(7):1717. https://doi.org/10.3390/agronomy15071717
Chicago/Turabian StyleZhang, Jiaqi, Zhanhai Kang, Xue Li, Man Li, Linmiao Xue, and Xing Li. 2025. "QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4×Thatcher RIL Population" Agronomy 15, no. 7: 1717. https://doi.org/10.3390/agronomy15071717
APA StyleZhang, J., Kang, Z., Li, X., Li, M., Xue, L., & Li, X. (2025). QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4×Thatcher RIL Population. Agronomy, 15(7), 1717. https://doi.org/10.3390/agronomy15071717







