Abstract
Mungbean is grown as a summer crop in subtropical climates globally. The global demand for mungbean is increasing, and opportunities exist to expand production regions to more marginal environments, such as southern Australia, as an opportunistic summer crop to help meet the growing global demand. Mungbean has the potential to be an opportunistic summer crop when an appropriate sowing window coincides with sufficient soil water. This expansion from subtropical to temperate climates will pose challenges, including low temperatures, a longer day length and a low and variable water supply. To assess mungbean suitability to temperate, southern Australian summer rainfall patterns and soil water availability, we conducted field experiments applying a range of water treatments across four locations with contrasting rainfall patterns within the state of Victoria (in southern Australia) in 2020–2021 and 2021–2022. The water treatments were applied prior to sowing (60 mm), the vegetative stage (40 mm) and the reproductive stage (40 mm) in a factorial combination at each location. Two commercial cultivars, Celera II-AU and Jade-AU, were used. Water scarcity during flowering and the pod-filling stages were important factors constraining yield. Analysis of yield components showed that increasing water availability at critical growth stages, viz. the vegetative and reproductive stages, of mungbean was associated with increases in total biomass, HI and grain number in addition to increased water use and water use efficiency (WUE). Average WUEs ranged from 1.3 to 7.6 kg·ha−1·mm−1. The maximum potential WUE values were 6.4 and 5.1 kg·ha−1·mm−1 for Celera II-AU and Jade-AU across the sites, with the estimated soil evaporation values (x-intercept) of 83 and 74 mm, respectively. Nitrogen fixation was variable, with %Ndfa values ranging from 9.6 to 76.8%, and was significantly affected by soil water availability. This study emphasises the importance of water availability during the reproductive phase for mungbean yield. The high rainfall zones within Victoria have the potential to grow mungbean as an opportunistic summer crop.
1. Introduction
Mungbean (Vigna radiata (L.) Wilczek) is a short-duration legume grown as a summer crop in subtropical regions globally. Mungbean is a crop rich in proteins, vitamins and minerals [1] and is a very important staple food, particularly in Asia. The largest producer of mungbean is India (3 million ha under cultivation), followed by China (0.6 million ha) and Myanmar (0.7 million ha), with these three countries contributing more than 50% of annual global production [2]. Currently, global mungbean production is 5.3 million tonnes with a productivity of 0.7 t·ha−1 from 7.3 million hectares [3]. Australian production averages 90,000 tonnes annually across 120,000 hectares [4]. Recently, there has been a rise in demand for legumes worldwide, which has translated to an increase in global production. The growth in consumption of legumes has partly been driven by legumes satisfying several consumer trends—plant-based protein, non-GMO, gluten-free and clean label—with much of the growth attributed to an increasing global population [5,6]. Given the trajectory of population growth, it is projected that legume consumption will increase by 23% by 2030 compared to 2017 levels [7]. Furthermore, the global mungbean market is also expected to rise to reach USD 5.82 billion by 2033, up from USD 4.49 billion in 2024, exhibiting a growth rate of 2.79% from 2025 to 2033 [8]. Increasing the production of legumes, including mungbean, poses a significant challenge and will require expansion into new cropping regions. The importance of mungbean as a summer rotation crop in Australia is increasing due to its high export demand and market value (worth up to AUD 1200 ton−1 in 2023) [9]. The average return of mungbean is higher compared to chickpea (AUD 670 ton−1), faba bean (AUD 350 ton−1) and lentil (AUD 700 ton−1) [10]. In 2022, Australia was the third-largest exporter after China and Myanmar, accounting for 3% of the total mungbean export market share, worth AUD 144 (~USD 98) million [11].
In Australia, mungbean is typically grown in the north-eastern subtropical regions during summer, with 90% of Australian-grown mungbean exported to Asian markets, including China, India and Indonesia, as well as to American markets such as the USA and Canada [4,11,12]. While winter cereals and pulses typically dominate the rainfed farming systems of southern Australia, expanding mungbean production into this region offers the opportunity to diversify income sources, improve soil fertility via additional nitrogen fixation and utilise out-of-season rainfall with summer cropping [13]. In high rainfall areas, summer crops may enhance the productivity of subsequent winter crops by using excess soil water and mitigating waterlogging [14]. The challenge with growing summer crops in southern Australia is the winter-dominant rainfall patterns common in this region. Typical summer rainfall ranges from 50 to 200 mm, with moderate to very high variability (index of variation 0.75–2.0) [15]. In traditional growing regions, mungbean exhibits high yield variability across locations and seasons [16]. The rainfall pattern of the northern Australian mungbean growing region is summer-dominant, whereas in southern Australia, the rainfall is winter-dominant with hot and dry summers. In current production regions of north-eastern Australia, mungbean requires 350–400 mm of water to achieve maximum yield potential [17] and starting soil water (90 mm Plant Available Water—PAW) is important to overcome unpredictable in-season rainfall [18]. In mungbean, water stress during vegetative growth has been found to impose limitations on plant size, leaf area, root growth [19,20], flowering and seed set, leading to reduced yields [21,22]. Additionally, water stress during the reproductive growth stage of mungbean has been associated with decreased pod initiation and pod growth rates, shortened plant height and a reduced maturing period, ultimately affecting yield potential [23] with a yield reduction of up to 59% in subtropical conditions [22]. Wenham et al. [24] reported that drought stress during vegetative growth stages reduced yield by 10–33%, while drought stress during flowering reduced yield by 5–27% and drought stress during early podding reduced yield by 53–75% in comparison to non-stressed crops. Additionally, drought at the reproductive stage is more susceptible to yield loss compared to the vegetative stage (yield loss of up to 100% versus up to 45%) [25]. However, in another study, the vegetative stage was reported to be more susceptible to drought linked with insufficient above-ground biomass production prior to flowering [26], which emphasises the importance of biomass accumulation for yield in mungbean [27]. Additionally, the crop yield potential is influenced by phenological dynamics due to eco-physiological responses [28]. Key events, such as flowering time and grain-filling duration, are significantly impacted by agro-climatic conditions [29,30,31]. However, the effect of environmental factors on mungbean phenology at a large scale has not been assessed in southern Australian climates. Therefore, further investigation into the effects of seasonal water variability on mungbean growth and productivity across southern Australian farming systems will be necessary to expand mungbean cultivation from subtropical regions to temperate areas of southern Australia.
Under northern Australian growing conditions, inoculated mungbean has been shown to fix up to 42 kg N·ha−1, ranking lowest for nitrogen fixation potential compared to soybean (313 kg N·ha−1), lentil (111 kg N·ha−1) and field pea (227 kg N·ha−1) [32]. Soil water deficit has a significant negative impact on nitrogen fixation [22], and in soil with higher levels of nitrogen, mungbean fixes less nitrogen [33]. In soils with low nitrogen, mungbean can fix sufficient nitrogen to meet plant requirements and provide some residual N benefit for the following crop [34]. With effective nodulation, mungbean can fix up to 70 kg N·ha−1, sufficient to achieve a crop yield of 1 t·ha−1 [35]. However, while there have been limited studies regarding the adaptation of summer legumes to temperate climates such as southern Australia, there are no detailed studies on potential N fixation by mungbean in southern Australian environments.
A foundational study was therefore needed to understand how water supply and N dynamics influence the growth, development and yield potential of mungbean in southern Australian environments. This study tested the following hypotheses by growing Australian mungbean cultivars (Jade-AU and Celera II-AU) under different water availability treatments to simulate potential seasonal variability in low, medium and high rainfall zones of Victoria, Australia:
- (i)
- Increasing water supply to mungbean during the early podding period will be more beneficial to yield than increasing water supply during the vegetative growth phase.
- (ii)
- Nitrogen fixation will be strongly affected by water availability at key growth stages of mungbean grown in southern Australia.
2. Materials and Methods
2.1. Site Description
A series of field trials were conducted over two years during the summers of 2020/21 (Trial year 2020) and 2021/22 (Trial year 2021) at four locations across Victoria, Australia. Trials were located at Hamilton (37°49′38″ S, 142°04′12″ E and 207 masl (masl: metres above sea level)), Horsham (36°44′57″ S, 142°06′59″ E and 128 masl), Ouyen (35°00′32″ S, 142°15′00″ E and 52 masl) and Woomelang (35°45′49″ S, 142°30′08″ E and 85 masl) (Figure 1). The climate of three of the sites (Ouyen, Woomelang and Horsham) is semi-arid (Köppen Climate Classification System: BSk) with high evapotranspiration and low rainfall, with cool, relatively long winters and short, hot summers, while Hamilton has a cool oceanic climate (Köppen Climate Classification System: Cfb). Rainfall is winter-dominated with episodic summer rainfall typically associated with storms [36]. Sites are characterised as low rainfall (Ouyen and Woomelang), medium rainfall (Horsham) and high rainfall (Hamilton) zones with long-term total annual rainfalls of 330, 348, 378 and 619 mm, respectively. Experimental site details including soil type, cropping history, total in-crop rainfall, and sowing and harvesting dates for each site are described in Table 1. Data on rainfall and minimum and maximum air temperatures were obtained from onsite weather stations and the Australian Bureau of Meteorology [37] and are presented in Figure 1.
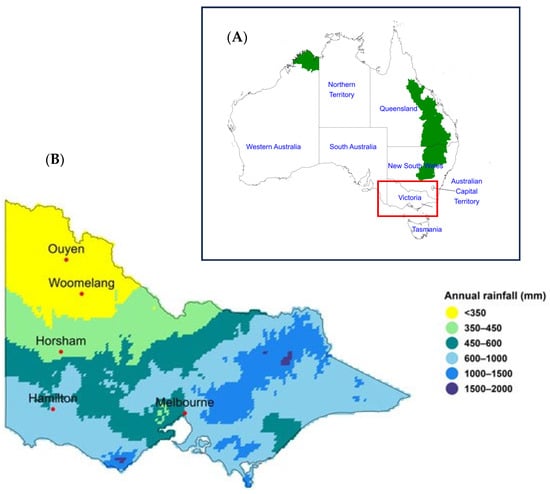
Figure 1.
(A) The Australian map showing mungbean-growing local government areas in a green colour and the state of Victoria as a red inset [38]. The Australian states and territories are identified by boundaries indicated with black lines and labelled in a blue font. (B) Average annual rainfall in Victoria, Australia, 30-year climatology (1991 to 2020) [39]. Within Victoria, there are three major cropping zones, which are characterised by rainfall: low rainfall zone (Ouyen and Woomelang) < 350 mm, medium rainfall zone 350–450 mm (Horsham) and high rainfall zone > 450 mm (Hamilton).

Table 1.
Site characterisation (soil type, soil pH, growing season rainfall (GSR), and sowing and harvest dates of mungbean trials conducted at Hamilton, Horsham, Ouyen and Woomelang during the 2020 and 2021 summer seasons.
2.2. Experimental Design
The experiments were a split-plot design with four replicates [40]. The water treatment was assigned as the main plot, and variety was assigned as the subplot. The water treatments were designed to simulate various water availability scenarios that growers may encounter in temperate dryland systems during summer. To ensure establishment across all treatments, 20 mm of water was applied across all sites post-sowing. Factorial combinations of either 0 or 40 mm were then applied as a single application during vegetative growth stages (prior to floral initiation) or reproductive growth stages (start of podding, Table 2). In 2021, an additional treatment was applied at Ouyen, Woomelang and Horsham, with 60 mm of water applied as a single application prior to sowing in factorial combinations with other in-season water applications. Two commercial varieties of mungbean, Jade-AU and Celera II-AU, were utilised for the experimental program. These genotypes were selected based on their contrasting plant architecture, growth habit and grain type (market class) [38]. Jade-AU is large seeded (74 mg), while Celera II-AU is small seeded (48 mg). The seeds of these mungbean varieties were obtained from the Australian Mungbean Association, Australia.

Table 2.
Definition of the water supply treatment.
2.3. Crop Management
Trials were established at similar times during November–December across the sites (Table 1). The trials were sown using a tractor-mounted plot-scale seeder equipped with narrow-point tynes and press wheels, with seed rates to achieve a target plant density of 35 plants·m−2 as per Australian industry standard practice [17,41,42]. The plot size was 3 m in length with four rows spaced at 0.28, 0.30, 0.38 and 0.30 m at Ouyen, Woomelang, Horsham and Hamilton, respectively. The areas were 0.56, 0.60, 0.76 and 0.60 m2 at Ouyen, Woomelang, Horsham and Hamilton, respectively. The seeds were sown at a depth of 0.03 m. Prior to sowing, seeds were inoculated with NoduleN™ Mungbean Inoculant (Peat) Standard (Group I) rhizobia (New-Edge Microbials, Australia) at a rate of 5 g·kg−1 of seed.
The field was fertilised with a basal application of mono-ammonium phosphate (MAP) at a rate of 50 kg·ha−1 (5.5:11:0 kg NPK ha−1) placed below the seed at sowing. Weeds were controlled using commercial herbicides, including glyphosate, carfentrazone-ethyl and trifluralin, at label rates and timings. Similarly, insecticides, including chlorpyrifos and bifenthrin, were applied to control insect pests. Intensive manual weeding was also undertaken on two occasions each year to control weeds not removed by herbicides.
The water treatment was applied by Toro Aqua-Traxx™ drip tape (Toro, Australia) with an emitter flow rate of 1.6 L·ha−1 between each row and outlets every 20 cm. This was installed over each crop row at sowing.
2.4. Measurement of Nitrogen Fixation
The N2 fixation of mungbean was assessed in the second trial year (summer 2021) using the 15N natural abundance technique [43], which exploits naturally occurring differences in 15N composition between plant-available N sources in soil and that of the atmosphere [32]. Canola (Brassica napus L.) was selected as the non-N2-fixing reference plant. At sowing, the canola reference plants were planted at the edge of each plot. Biomass samples of the reference canola and respective mungbean plots were harvested at pod filling. The biomass samples were dried at 40 °C until constant weight and ground to <0.10 mm particle size using a CT 293 Cyclotec™ laboratory mill (FOSS, Hillerød, Denmark) and collected in 3 mL tubes. Samples were analysed for δ15N using a continuous flow system consisting of a Delta V Plus mass spectrometer connected with a Thermo Flush 1112 via Conflo IV (Thermo-Finnigan/Germany) at the West Australian Biogeochemistry Centre, University of Western Australia, Perth, Australia.
The percentage of nitrogen derived from the atmosphere (%Ndfa) was calculated as:
where and are the deviation of the reference species and the N2-fixing legume sample 15N:14N ratio from the standard (air) and β is the value of nitrogen in shoots of mungbean grown in N-free media (−2.50) [44]. The total nitrogen amount in mungbean shoots was calculated as the product of the percentage of nitrogen in mungbean shoots by shoot dry biomass. The nitrogen fixed was determined by multiplying the percentage of nitrogen derived from atmospheric nitrogen fixation by the total nitrogen content of mungbean shoots [43,44].
2.5. Data Collection
2.5.1. Soil Water
The soil water content of experimental plots was determined for all plots 3–7 days before sowing and shortly after harvest. A single soil sample from surface to 1.2 m was acquired using a 5 cm diameter corer and split into 7 intervals: 0–0.1, 0.1–0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8, 0.8–1.0 and 1.0–1.2 m. For the 0–0.1 m sample, an additional core was collected for each plot. Soil water content was measured using the oven dry method at 105 °C to constant weight. The measured gravimetric soil water content was converted to volumetric units using soil bulk densities for the same depth intervals [45].
Total water use of the crop including evaporation was calculated for each plot using the following equation:
where
Ɵs = soil water at sowing (mm).
Ɵf = soil water at harvest (mm).
R = total rainfall (mm) received from pre-sowing soil sampling (Ɵs) until harvest soil sampling (Ɵf).
I = total water applied (mm).
Water use efficiency (kg·ha−1·mm−1) was computed as the ratio of grain yield and total water use (mm), as described in the following equation:
2.5.2. Phenology
Flowering dates were recorded when the first flowering was observed in a plot. The start of flowering is defined as the reproductive growth stage. The podding date was also recorded when the first pod set was observed in a plot.
The number of days to maturity was recorded when 90% of pods were mature.
2.5.3. Crop and Yield Components
Above-ground biomass was determined at podding (podding biomass) by sampling the inner two rows over a length of 0.5 m. The areas for yield determination were 0.28, 0.30, 0.38 and 0.30 m2 at Ouyen, Woomelang, Horsham and Hamilton, respectively. The harvested plants were dried until constant weight at 40 °C. The final harvest was undertaken when the pods were mature, specifically from the inner two rows of each plot. Samples measuring 1 m long were taken at ground level from the centre two rows and dried at 40 °C. The samples were used for determining shoot biomass, plant height, grain number (grains·m−2), grain weight, harvest index and grain yield. Grain yield was adjusted to 10% moisture content and presented as kg·ha−1.
2.6. Data Analysis
Analysis of each variable was conducted separately for each site. A linear mixed model approach using the residual maximum likelihood method (REML) was used to test the effect of water treatment and variety. The experiment was conducted in a split-plot design with four replicates, where the water treatment was assigned as the main plot and variety was the subplot. Therefore, water treatment, variety and their interaction were considered fixed effects, whereas replication and whole plot were fitted as random effects. The error was divided into three levels of effect: water treatment, variety and variety × water treatment. The effect of water treatment was tested against the main plot error (replication × whole plot), and variety and variety × water treatment were tested against the residual error of the experimental units or subplots. Statistical analyses were performed using the Asreml-R package version 4.2.0.355 [46] in R version 4.4.2. The interaction effects are presented in graphs, and where there was no interaction, the main effects only are presented.
The means were compared by using Tukey’s honest significant difference (HSD) test [47] using the ‘biometryassist’ package version 1.3.0 [48] in R. The significance level was set at 5% in all comparisons. Correlation analysis was carried out to determine the relationships between yield and yield parameters using the package ‘rstatix’ version 0.7.2 [49] in R.
3. Results
3.1. Weather
The maximum temperature, minimum temperature and rainfall varied across sites and experimental years (Figure 2). For Horsham, Ouyen and Woomelang in 2020, the total rainfall prior to sowing, from August to October, was 151, 123 and 125 mm, respectively, which is above the long-term average for these sites. In 2021, the average rainfall prior to sowing, from August to October, was 178, 102, 96 and 89 mm at Hamilton, Horsham, Ouyen and Woomelang, respectively, compared with long-term averages of 201, 115, 100 and 83 mm. The total growing season rainfall for Horsham, Ouyen and Woomelang was 93, 52 and 24 mm, respectively, during the 2020 summer season. In the 2021 summer season, the totals for Hamilton, Horsham, Ouyen and Woomelang were 127, 133, 108 and 137 mm, respectively. The total rainfall received was less at Horsham, Ouyen and Woomelang in the summer of 2020 compared to the summer of 2021. The total rainfall received from sowing to 100 days (effective growing season) after sowing was 112, 62, 44, 45, 76, 18 and 30 mm for Hamilton 2021, Horsham 2020, Horsham 2021, Ouyen 2020, Ouyen 2021, Woomelang 2020 and Woomelang 2021, respectively.
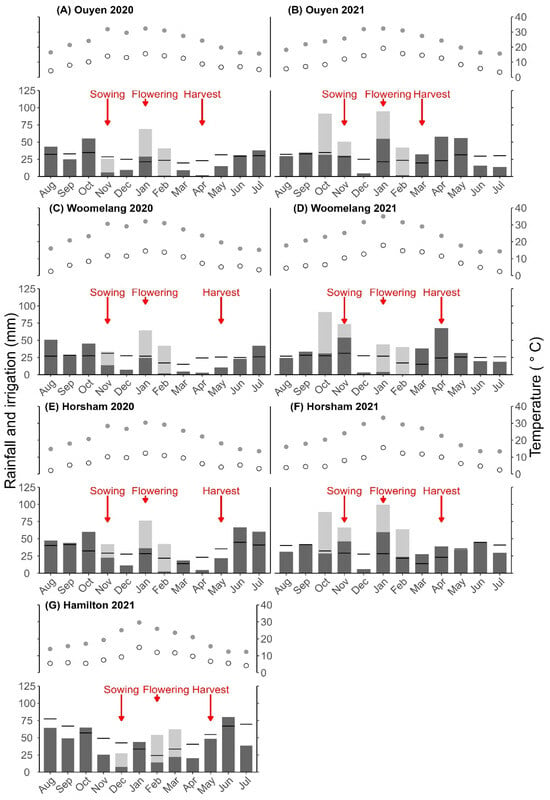
Figure 2.
Rainfall, maximum temperature and minimum temperature as monthly averages for seven trial sites: (A) Ouyen 2020, (B) Ouyen 2021, (C) Woomelang 2020, (D) Woomelang 2021, (E) Horsham, 2020, (F) Horsham 2021, (G) Hamilton 2021. Black horizontal bars represent long-term rainfall averages. The dark grey bar represents total rainfall and the light grey bar represents water applications made before sowing, at sowing and during vegetative and reproductive growth stages. Open circles represent average minimum temperature and closed grey circles represent average maximum temperature. Arrows indicate sowing, flowering and harvesting for the mungbean varieties.
The mean air temperatures in 2020 were 18.2, 21.5 and 20.1 °C at Horsham, Ouyen and Woomelang, respectively, and in 2021, 18.5, 20.0, 22.9 and 21.5 °C at Hamilton, Horsham, Ouyen and Woomelang, respectively, during the growing period. The temperature ranged from a maximum of 42.7, 45.6 and 43.9 °C to a minimum of −1.8, 2.2 and −2.8 °C at Horsham, Ouyen and Woomelang, respectively, in 2020, while in 2021 they ranged from 38.5, 40.1, 40.5 and 40.4 °C to 3.1, 1.8, 3.4 and 1.5 °C at Hamilton, Horsham, Ouyen and Woomelang, respectively. Across all sites and years, mungbean experienced between 5 and 15 days per season during which the maximum temperature exceeded 35 °C in the sensitive reproductive period (estimated as 5 days prior to flowering until 30 days after the start of flowering). Heat stress days were more prevalent at Ouyen, similar at Woomelang and Horsham and least common at Hamilton. Across all sites, temperatures gradually decreased from January to May.
3.2. Days to Flowering
There was no significant effect of water treatment and varieties on days to flowering across the sites. Average days to flowering ranged from 38 to 78 days after sowing (DAS) across the sites (Table 3). The average growing degree days (GDDs) ranged from 495 to 1195, and the average photoperiod (one week to flowering) ranged from 14.03 to 14.50 h.

Table 3.
Number of days from sowing to flowering, average growing degree days (GDDs) and average photoperiod (h) of mungbean at Hamilton, Horsham, Ouyen and Woomelang in 2020 and 2021.
3.3. Biomass and Yield Components
3.3.1. Hamilton 2021
In-season water treatment showed no significant (p > 0.05) effect on podding biomass (kg·ha−1), grain number (grains·m−2), yield (kg·ha−1) or harvest index (HI), although there was a trend towards increased biomass production where additional water was applied. However, significant differences (p < 0.05) were observed between varieties; Jade-AU tended to produce more biomass and a larger grain size but a lower grain number, HI and grain yield (Table 4). A significant interactive effect of variety and water treatment was observed for grain weight, with Celera II-AU producing a significantly higher grain weight when water was applied during the reproductive growth stages compared to application during vegetative growth stages or combined vegetative and reproductive stages (p < 0.05, Figure 3C).

Table 4.
Yield components at the Hamilton site in 2021 for podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and harvest index (HI) of mungbean varieties Celera II-AU and Jade-AU under different water treatments.
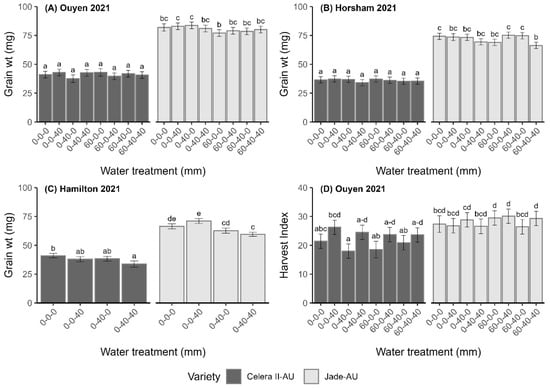
Figure 3.
Grain weight (mg) (A–C) and harvest index (HI) (D) of mungbean varieties Celera II-AU and Jade-AU under different water treatments at Ouyen 2021 (A,D), Horsham 2021 (B) and Hamilton 2021 (C). Error bars indicate the 95% confidence interval of each mean value. Data with the same letter within a treatment are not significantly different at p < 0.05.
3.3.2. Horsham 2020
Podding biomass, grain number, grain weight, yield and HI were significantly affected by water treatments (Table 5). Significant differences were observed between varieties for grain number, grain weight and HI, while grain weight was significantly affected by an interaction of variety × water treatment (Figure 3).

Table 5.
Podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and harvest index (HI) of mungbean varieties Celera II-AU and Jade-AU under different water treatments at Horsham in 2020 and 2021.
Applying 40 mm of water at both the vegetative and reproductive growth stages (0-40-40) significantly increased mungbean yield, podding biomass, grain number, grain weight and HI compared to those rainfed (0-0-0). An increased water supply at the vegetative (0-40-0) or reproductive (0-0-40) growth stages significantly increased podding biomass and grain number compared to those rainfed, while grain weight, yield and HI were significantly increased with additional water supply at reproductive growth stages but not at vegetative growth stages compared to rainfed mungbean.
Jade-AU had a significantly higher grain weight and HI compared to Celera II-AU, while grain number was significantly higher for Celera II-AU compared to Jade-AU (Table 5). Yield was higher for Jade-AU compared to Celera II-AU, but differences were not significant (p > 0.05).
3.3.3. Horsham 2021
Grain number, yield and HI were significantly affected by water treatment and variety at Horsham in 2021 (p < 0.05, Table 5). Podding biomass was significantly affected by variety while grain weight was significantly influenced by variety × water treatment interaction (Figure 3).
Applying 80 mm of water in-season significantly increased mungbean yield and grain number compared to those rainfed. Additional stored soil water (where 60 mm of water was applied at pre-sowing) had no significant effect on podding biomass, grain number, grain weight, yield or HI. Similarly, an additional water supply at the vegetative and reproductive growth stages trended towards an increased grain number, grain weight, yield and HI. However, the difference was not significant (p > 0.05). For Celera II-AU the water treatment did not have a significant effect on grain weight; for Jade-AU, the 60-0-40 treatment significantly increased grain weight by 13.6% compared to the 60-40-40 water treatment.
Comparing varieties, Jade-AU had a significantly higher podding biomass and grain weight compared to Celera II-AU. In contrast, Celera II-AU had a significantly higher grain number and HI compared to Jade-AU. The yield was higher for Jade-AU compared to Celera II-AU, but the difference was not significant.
3.3.4. Ouyen 2020
The podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and HI of mungbean were significantly affected by water treatment (Table 6). Grain number was significantly affected by variety (Table 6), while grain weight was significantly influenced by variety × water treatment interaction (Figure 3).

Table 6.
Yield components at the Ouyen site in 2020 and 2021 for podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and harvest index (HI) of mungbean varieties Celera II-AU and Jade-AU under different water treatments.
The podding biomass, grain number, yield and HI of mungbean were significantly higher where 80 mm of water was applied during the growing season (0-40-40) compared to those rainfed. Podding biomass was significantly increased with additional water applied at the vegetative growth stages compared to rainfed mungbean. Furthermore, grain number and yield were all significantly greater where water was applied during the reproductive growth stages than in rainfed conditions. However, there was no significant difference in podding biomass from water application at the vegetative and reproductive growth stages. Grain weight was significantly greater where water was applied at the reproductive growth stage (61 mg) compared to vegetative growth stages (49 mg).
Jade-AU had a significantly higher grain weight (70 mg) compared to Celera II-AU (40 mg), while Celera II-AU (529 grains·m−2) had a significantly higher grain number compared to Jade-AU (327 grains·m−2). Yield and HI were not significantly different between Jade-AU and Celera II-AU.
3.3.5. Ouyen 2021
Podding biomass (kg·ha−1) and yield (kg·ha−1) were significantly affected by water treatment and variety (Table 6). Grain number (grains·m−2) significantly differed between the water treatments. Additionally, the interaction between variety and water treatment significantly influenced grain weight (mg) and HI (Figure 3).
Podding biomass and yield were not significantly influenced by 40 mm in-season water application during the vegetative and reproductive growth stages as compared to rainfed varieties (0 mm). Additional water availability at the vegetative or reproductive growth stages had no significant effect on podding biomass, grain number or yield, even though there was a trend towards increased production with increased water availability. However, grain number was significantly higher where water was applied at vegetative and reproductive growth stages compared to those rainfed when the additional pre-sowing stored water of 60 mm was available. Biomass, grain number and yield were significantly lower with pre-sowing water and no in-season water application (60-0-0) in comparison to no pre-sowing water with water application during vegetative and reproductive stages (0-40-40).
The grain weight for Jade-AU with the 0-40-0 water treatment was significantly higher compared to the 60-0-0 water treatment. In contrast, for Celera II-AU, irrigation had the opposite effect on grain weight for those under water treatment, with the 60-0-0 water treatment recording significantly higher grain weights compared to the 0-40-0 water treatment.
Applying additional water in-season had no significant effect on HI for Jade-AU. In contrast, for Celera II-AU, HI increased for the 0-0-40 treatment compared with 0-40-0. The remaining water treatments did not have a significant effect on the HI of Celera II-AU compared to the rainfed treatment.
Podding biomass and yield were significantly higher for Jade-AU compared to Celera II-AU.
3.3.6. Woomelang 2020
Podding biomass (kg·ha−1), grain number (grains·m−2) and yield (kg·ha−1) differed significantly between water treatments (Table 7). Grain number, grain weight, yield and HI differed significantly between varieties (Table 7).

Table 7.
Yield components at the Woomelang site in 2020 and 2021 for podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and harvest index (HI) of mungbean varieties Celera II-AU and Jade-AU under different water treatments.
In terms of water availability, treatments that received water during the vegetative and reproductive stages exhibited a significantly higher podding biomass, grain number and yield compared to rainfed treatments. While additional water during these stages was associated with increased podding biomass, grain number, grain weight, yield and harvest index, these differences were not statistically significant. Grain weight (mg) and HI showed no significant differences between the water treatments.
Celera II-AU demonstrated a significantly higher grain number, harvest index and yield compared to Jade-AU. Conversely, Jade-AU exhibited a significantly greater grain weight than Celera II-AU.
3.3.7. Woomelang 2021
Podding biomass (kg·ha−1), grain number (grains·m−2), grain weight (mg), yield (kg·ha−1) and HI (%) were significantly influenced by the water treatments (Table 7). Additionally, grain weight and yield showed significant differences between varieties tested (Table 7).
Podding biomass and grain number were significantly higher when water was applied during the vegetative and reproductive stages than when rainfed. However, grain weight and HI were significantly higher for in-season water availability than rainfed water when there was no pre-sowing water availability. The yield was significantly higher when both in-season water (80 mm) and pre-sowing water (60 mm) were available (60-40-40) compared to the rainfed treatment (0-0-0). Furthermore, the additional stored soil water from the pre-sowing application of 60 mm did not have a significant effect on podding biomass, grain number, grain weight, yield or HI. Similarly, while the extra water supply during the vegetative and reproductive stages indicated a trend towards increased grain number, grain weight, yield and harvest index, the differences were not statistically significant.
Of the varieties, Jade-AU consistently demonstrated a significantly greater podding biomass, grain weight and yield compared to Celera II-AU. There were no significant differences in grain number or harvest index between the two varieties.
3.4. Water Use and Water Use Efficiency (WUE)
Total water use differed significantly between water treatments across all trials (p < 0.01, Table 8). The effect of water treatments was significantly higher when the water supply was at both the vegetative and reproductive stage compared to rainfed treatment across the trials. However, the impact of water supply at only either the vegetative or reproductive stage was significantly higher in the drier environments of Ouyen, Woomelang and Horsham 2021. Across the trials the water use was not significantly different between the vegetative and reproductive water treatments. The impact of the pre-sowing water treatment of 60 mm was significantly greater water use compared to no pre-sowing water applications only at Ouyen 2021. At Ouyen 2020, for total water use (mm), there was a significant interactive effect of variety and water treatment (p < 0.01, Table 8). Under the rainfed condition, Jade-AU had significantly lower water use (80 mm) compared to Celera II-AU (124 mm). Conversely, Jade-AU had significantly higher water use (204 mm) with water treatment at both the vegetative and reproductive stages (0-40-40) compared to Celera II-AU (175 mm).

Table 8.
Water use (mm) of mungbean varieties Celera II-AU and Jade-AU under different water treatments at Hamilton, Horsham, Ouyen and Woomelang in 2020 and 2021.
The in-season water availability at different growth stages significantly affected WUE in drier environments (Ouyen, Woomelang and Horsham) (Table 9). In contrast, at Woomelang 2020 and in the high rainfall zone (Hamilton), the effect of water treatment on WUE was not significant. Additionally, the WUE of varieties was dependent on the water treatment in Ouyen 2021 (significant variety × water treatment interaction at p < 0.05, Table 9). The in-season water application at both the vegetative and reproductive stage without pre-sowing water application had a significantly higher WUE compared to the rainfed treatment in Horsham 2020, Horsham 2021 and Ouyen 2020, whereas at Woomelang 2021 the in-season water application at both the vegetative and reproductive stage along with pre-season water application had a significantly higher WUE compared to the rainfed treatment. Importantly, water treatment at the reproductive stage had a significantly higher WUE in Horsham 2020. For variety, Jade-AU recorded a significantly higher WUE in drier environments (Ouyen, Woomelang and Horsham), except at Ouyen 2020 and Woomelang 2020. In contrast, Celera II-AU had a higher WUE in the high rainfall zone of Hamilton compared to Jade-AU.

Table 9.
Water use efficiency (kg·ha−1·mm−1) of mungbean varieties Celera II-AU and Jade-AU under different water treatments at Hamilton, Horsham, Ouyen and Woomelang in 2020 and 2021.
The average WUE across the sites for Celera II-AU and Jade-AU was 2.3 kg·ha−1·mm−1 for both varieties (Figure 4). From the same figure, an arbitrary line which encloses the highest-yielding points of different levels of water use was used to calculate the maximum potential WUE for mungbean varieties. The maximum potential WUEs for mungbean varieties Celera II-AU and Jade-AU were 6.4 and 5.1 kg·ha−1·mm−1, respectively. The soil evaporation values of Celera II-AU and Jade were 83 and 74 mm respectively.
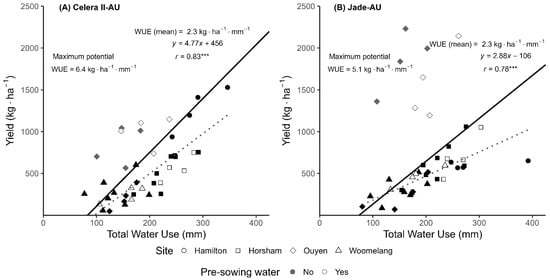
Figure 4.
Relationship between grain yield and total water use in Hamilton, Horsham, Ouyen and Woomelang for (A) Celera II-AU and (B) Jade-AU. The solid lines represent the maximum potential water use efficiency across the trials using the French and Schultz [51] frontier approach, where an arbitrary line is drawn that encloses nearly all the highest-yielding crops at varying water-use levels, thereby establishing a linear relationship between potential yield and water use. The x-intercept represents the water loss by direct evaporation and the slope represents the maximum potential water use efficiency. The dotted line shows the fitted regression line. Asterisks (***) denote a significant correlation at p < 0.001. Black points = without pre-sowing water treatment; white points = with pre-sowing water treatment. Grey-coloured diamond points represent the data from Ouyen 2021 which are excluded from calculations of the benchmark WUE and WUE (mean) across the trial.
3.5. Nitrogen Derived from the Atmosphere (%Ndfa)
The %Ndfa varied significantly across the sites ranging from 24 to 70%. The %Ndfa value was relatively higher (70%) at Woomelang 2021 compared to Ouyen 2021 (61%), Hamilton 2021 (37%) and Horsham 2021 (24%). Water treatment had a limited effect on %Ndfa apart from in Horsham and Woomelang in 2021. At Horsham 2021, a significant interactive effect of variety and water treatment on %Ndfa was observed at Horsham (p < 0.05, Table 10). Jade-AU with the 0-40-0 mm and 60-0-0 mm water treatments had a significantly higher %Ndfa compared to Celera II-AU, while there was no significant difference between the varieties for the other water treatments. The %Ndfa ranged from 9.6 to 39% at Horsham 2021. At Woomelang 2021, the water treatment had a significant effect on %Ndfa (Table 10). Water treatment at the vegetative and reproductive stage (0-40-40) had a significantly higher %Ndfa (75.8%) compared to water treatment at the pre-sowing and vegetative stage (60-40-0) with a value of 64.1%. The %Ndfa values did not differ significantly between varieties and water treatment at the other sites, Hamilton 2021 and Ouyen 2021 (Table 10).

Table 10.
%Ndfa of mungbean varieties Celera II-AU and Jade-AU under different water treatments at Hamilton, Horsham, Ouyen and Woomelang in 2021.
3.6. Total N Fixation
The total N fixed (kg·ha−1) differed significantly between water treatment and varieties in Horsham 2021, Ouyen 2021 and Woomelang 2021 (Table 11). At Horsham 2021, significantly higher N fixation was recorded with water treatment at both the vegetative and reproductive stage water treatments without pre-sowing water (0-40-40) compared to the rainfed treatment (0-0-0), whereas in Ouyen 2021, the total nitrogen fixed was significantly higher for the rainfed condition when there was no pre-sowing water available (0-0-0) compared with the rainfed treatment with pre-sowing water availability (60-0-0). In Woomelang, the total N fixation was significantly higher with 80 mm in-season water availability at the vegetative and reproductive stages, with or without pre-sowing water availability (0-40-40 and 60-40-40) compared to those rainfed (0-0-0).

Table 11.
Total amount of nitrogen (kg·ha−1) derived from fixation for mungbean varieties Celera II-AU and Jade-AU under different water treatments at Hamilton, Horsham, Ouyen and Woomelang in 2021 summer season.
The variety Jade-AU had significantly higher N fixation in Horsham 2021, Ouyen 2021 and Woomelang 2021 compared to Celera II-AU in Horsham 2021, Ouyen 2021 and Woomelang 2021 (Table 11). Total N fixation was not significantly different for water treatment and variety in Hamilton 2021 where the overall site mean N fixation was 28.1 kg·ha−1 with a range of 20.0–35.2 kg·ha−1.
4. Discussion
Understanding the effect of temporal and absolute water availability on the growth and yield components of mungbean is critical to testing their suitability in new growing environments. The focus of the present work was to examine the interactive effects of variety and water supply on the growth and yield of mungbean. Mungbean yield varied between years (e.g., 227–1247 kg·ha−1 at Ouyen) and across sites (187–1247 kg·ha−1). The variability in yield across the sites is likely due to the range of weather and soil conditions across the trial environments. The addition of water during the vegetative and reproductive stages significantly influenced mungbean grain yield across all sites except at Hamilton 2021. Water use efficiency was influenced by water treatment at all sites except Hamilton 2021 and Woomelang 2020.
4.1. Relationship of Weather, Water Availability and Phenology to Mungbean Yield
Mungbean is typically adapted to subtropical climates characterised by warm summer temperatures and a shorter daylength in comparison to the semi-arid environments of south-eastern Australia. As a result, growing mungbean in this environment was expected to significantly alter the rate of phenological development. While mungbean is classified as a short-day crop [29,30,31,52,53,54], Australian commercial cultivars such as those grown in this experiment are generally considered photoperiod-insensitive [55] when grown in traditional production regions. However, in these experiments mungbean showed a level of photosensitivity, flowering at a variety of thermal time intervals across locations (Table 3). In northern Australia, mungbean flowering is typically initiated after 600 growing degree days (GDDs) [28]. However, in the present study, flowering started at a range of 829–1198 GDDs. Furthermore, sowing prior to the summer solstice (Ouyen, Woomelang and Horsham) typically resulted in a longer pre-flowering period compared with sowing after the summer solstice (Hamilton). In addition to temperature and photoperiod differences between cold semi-arid and subtropical environments, rainfall patterns and water supply are a clear point of difference between south-eastern Australia and traditional mungbean growing regions. Importantly, it was observed in the current study that water supply had no significant impact on time to flowering, which is in line with a previous study by Kumar and Sharma [56]. While this study provides an initial assessment of mungbean suitability in this environment, it highlights the need for further physiological studies to understand the phenological response to the photoperiod and temperatures typical of southern Australia.
While south-eastern Australia is a typically cooler environment than subtropical regions, the summer period is subject to extreme temperatures (>35 °C) on a semi-regular basis, with the potential to coincide with key growth stages in mungbean. Previous studies have highlighted the importance of extreme temperatures to grain set [57], as temperatures above 35 °C during the reproductive stage have a negative impact on mungbean yield [28,58]. Mungbean can undergo significant loss of flowers and pods in the field when exposed to high temperatures [59]. Rachaputi et al. [28] reported up to a 50% yield reduction in mungbean when exposed to air temperatures above 35 °C during flowering and pod set in Central Queensland, Australia. Across all sites, a minimum of 5 days per season with maximum temperatures > 35 °C from flowering to 30 days after flowering (i.e., during February) was measured during the experimental period. This high temperature may have caused significant pollen sterility, leading to a substantial reduction in grain number across the sites, resulting in yield loss in the low rainfall areas of Ouyen and Woomelang compared to the high rainfall and more temperate environment of Hamilton. Trial results suggest the decline in mungbean yield is primarily due to reduced grain set, supported by a positive relationship between grain number and yield across the sites (r = 0.96 ***). These findings align with earlier work by Kaur et al. [57], who reported that high temperatures during flowering reduce grain set by producing less viable and less vigorous pollen, ultimately leading to flower abortion. Pollen viability in high temperatures can reduce pollen fertility by 60% and flower number by 32%, resulting in the reduction in total grain number [60]. Analysis of historical climate records for the month of February indicates that a minimum of 5 days with maximum temperatures exceeding 35 °C occur in 90%, 100%, 70% and 20% of years at Ouyen, Woomelang, Horsham and Hamilton, respectively. Hence, with the current temperature patterns and existing phenology, mungbean is better suited to the climate of Hamilton. However, further research is needed to fully understand the complex interactions between temperature and reproductive processes—including pollen development, fertilisation and grain filling—to develop an agriculture system fit for mungbean cultivation during the summer season in southern Australia.
In addition to extreme temperatures, the mean daily temperature can have a significant effect on the rate of development. In the current study, growing mungbean in cooler environments led to extended flowering and grain filling, setting up higher yield potential. A negative correlation (r = −0.83 *) was observed between mean daily temperature from flowering to harvest and days to harvest from flowering, similar to previous studies [31]. As a result, mungbean grown at Hamilton exhibited an extended grain-filling period, leading to a delayed harvest compared to environments with higher temperatures (Ouyen and Woomelang). Another aspect of extended maturity is related to the non-synchronous maturity of mungbean varieties [61], which is caused by their indeterminate growth habit triggered by water availability during the reproductive period [62]. Favourable conditions result in additional flowering and pod setting, extending the duration of maturity [63]. These effects need to be considered when assessing the suitability of mungbean to southern Australia since uneven maturity could have a variety of negative effects ranging from yield and grain quality to the logistics of growing mungbean in a broader farming system.
4.2. The Relationship Between Water Availability and Yield-Determining Attributes
Changing water availability at different growth stages had a significant influence on yield and components. Each additional water application after sowing significantly increased yield across all sites except in Hamilton 2021, where supplementary water treatment had no yield advantage over those rainfed alone. This might be due to higher in-season rainfall before sowing (178 mm), and during flowering and pod setting at Hamilton 2021 (Figure 2). The soil water available at sowing in Hamilton 2021 was 71 mm with an additional 112 mm of rainfall during the first 100 days after sowing, whereas at other sites, the soil water at sowing was less than 65 mm and rainfall during the first 100 days after sowing was less than 76 mm. The long-term summer rainfall at Ouyen, Woomelang, Horsham and Hamilton was 69, 72, 78 and 100 mm, respectively, with Hamilton receiving the highest average annual rainfall [39]. Analysis by Christy et al. [64] assessed the probability of receiving sufficient rainfall to support summer cropping across Victorian grain-growing regions. Defined as a minimum of 30 mm of rainfall to enable sowing between October and December, followed by 150 mm of follow-up rainfall within a 90-day growing season, the probability of sufficient rainfall was 28, 19, 33 and 55% at Ouyen, Woomelang, Horsham and Hamilton, respectively [64]. Therefore, before deciding on the cultivation of mungbean, the status of stored water needs to be considered.
Water availability during the reproductive stage of mungbean had a significant effect on grain number, as previously observed by Wenham et al. [24]. Water stress can reduce pod set by 10–35%, resulting in yield losses of up to 75% in plants stressed during podding [24]. Grain number is primarily determined by pod set and pollen fertility [60]. In our study, the yield and grain number at Hamilton were not significantly affected by the water treatment, indicating that stored soil water and in-season rainfall likely met the crops’ requirements. In contrast, at the lower rainfall sites (Ouyen and Woomelang), water stress had a significant negative impact on both mungbean grain yield and grain number, likely due to lower soil water availability and less in-season rainfall compared to Hamilton. The grain numbers recorded for water treatments during the reproductive stage at Ouyen and Woomelang were lower compared to Hamilton. The lower grain yield in low rainfall sites, Ouyen and Woomelang, could be a result of a reduction in grain number caused by water stress, as evidenced by the significant correlation between grain yield and grain number (r = 0.96 ***) across the sites and at each site. These findings align with previous research, which has shown that mungbean yields decrease by 53–75% under water stress during reproduction, with a strong correlation between grain yield and grain number [24,65]. Analysis of yield components showed that the yield response to additional water was significantly correlated with harvest index (r = 0.45 ***). The positive response of an increased HI to water treatment was consistent with other studies [21]. The effect was significantly greater when water was applied at both the vegetative and reproductive stages compared to rainfed treatments across the sites, except at the high rainfall zone (Hamilton 2021). Increased water availability across the growing season allows mungbean to maximise leaf area index and consequently achieve higher photosynthetic efficiency [25]. Water availability during the vegetative stage is essential to support leaf emergence and expansion [66,67]. Similarly, a higher HI with each additional water treatment resulted in a higher yield through a greater grain number. Furthermore, the yield was greater when water was available during the reproductive stage compared to the vegetative stage, even though the difference was not significant across the sites. The significant effect of each additional water treatment at critical stages on grain number is consistent with the findings of earlier studies [26,65,68,69]. Importantly, the higher grain number likely resulted from the higher availability of sinks and increased biomass during the grain-filling phase in the irrigated treatments [69]. The sensitivity of grain number and seed size to mungbean yield under water stress has been well documented [21,22,24,65]. Water stress during microsporogenesis will affect pollen viability and plant fertility, resulting in a lower grain set [70,71]. A greater grain number was due to the lesser stress resulting from the availability of soil water with each additional water treatment [26]. Therefore, sufficient water availability during the reproductive stage for flowering and pod development is critical for mungbean yield [72,73], emphasising the need to select sites with a higher probability of receiving follow-up rainfall during the growing period, particularly at the reproductive growth stage (e.g., Hamilton).
Yields with water supply at the vegetative and reproductive stages in the current study of southern Australia for mungbean are similar to those observed by Rachaputi et al. [28] under rainfed conditions in Queensland, where yields up to 2000 kg·ha−1 were achieved with supplementary irrigation for the mungbean variety Jade-AU. With water supply during the vegetative and reproductive stages, mungbean can be successfully grown in southern Australia, highlighting its potential as a rotation crop in dryland systems during years with sufficient spring soil water, or within irrigation regions as a pulse phase. Most of the irrigated land in southern Australia is in northern Victoria and southern New South Wales, forming part of the Murray–Darling Basin and the Goulburn River irrigation systems [74]. These systems support more than 400,000 ha of irrigated crops such as corn, oilseed and barley in northern Victoria [75]. Across these irrigated regions, there is already some production of other summer crops such as soybean, navy bean and corn [76]. Given its high market value and relatively low irrigation requirement (3.5–4.5 ML·ha−1), mungbean has the potential to expand into these regions as a reliable summer crop [17]. However, further research on mungbean agronomy is needed to support expansion in these systems.
4.3. Relationship Between Yield and Water Use
Total water use varied between the sites, ranging from 134 mm at Woomelang in 2020 to 304 mm at Hamilton in 2021. Crops with water applied at both the vegetative and reproductive growth stages extracted significantly more water compared to those rainfed across all sites (Table 8). Additional water availability at both growth stages likely led to higher water extraction due to increased water demand from the increased canopy [42,66,67] and reduced loss from soil evaporation, which ultimately improved WUE [42]. Additional water supply at vegetative or reproductive stages did not result in significant differences in total water use at any sites. However, the total water use was greater when applied at both the vegetative and reproductive stages compared to rainfed conditions across the sites. Mungbean was able to extract water from up to 1.2 m deep at some sites where additional water was applied pre-sowing, which was consistent with observations from tropical environments [42]. Additionally, a significant positive correlation was observed between total water use and both podding biomass (r = 0.38 **) and yield (r = 0.34 ***) across all the treatments and sites. Previous studies have also reported positive relationships between total water use and biomass and grain yield in various grain crops [77,78].
The maximum potential WUEs observed in the current study (6.4 and 5.1 kg·ha−1·mm−1 for Celera II-AU and Jade-AU, respectively) were lower than previous estimates (7.1 kg·ha−1·mm−1) by Collins et al. [79] under subtropical growing conditions in Queensland and New South Wales, Australia. However, the average WUE observed by Collins et al. [79] was similar to the present study (4.4 kg·ha−1·mm−1). For both varieties, the yield was driven by the higher water use across the locations. Additionally, the greater maximum potential WUE of Celera II-AU compared to Jade-AU could be driven by its response to higher total water use in Hamilton, producing a significantly higher yield. The lower WUE values measured in this study could be attributed to the increased transpiration efficiency in subtropical environments associated with rapid canopy cover, leading to reduced soil exposure and improved effective water use [26]. Another factor could be the timing of water availability, as Bourgault et al. [80] reported that insufficient water availability during flowering and podding had a negative effect on yield, resulting in a lower yield. Therefore, it is important to consider the size of rainfall events that drive soil evaporation in southern Australia, where rainfall during summer is likely to be highly episodic compared to the subtropical growing conditions of Queensland and New South Wales. However, in the current study conducted at Ouyen in 2021, the yield was disproportionately elevated in relation to the corresponding water use. This phenomenon may have arisen from the effects of in-season lateral flow within the soil associated with a dune/swale topography [81], resulting in an underestimation of water use calculations. Consequently, it could have inflated the Water Use Efficiency (WUE) values in Ouyen 2021.
4.4. Effect of Water Availability at Different Growth Stages on %Ndfa and Total N Fixation
The %Ndfa values observed in this study (9.6–76.8%, Table 10) are consistent with the range (15–90%) observed by Peoples et al. [82]. It has also been reported that the %Ndfa in mungbean can be up to 100% [83], indicating a wide variation in mungbean atmospheric N fixation. The significantly higher %Ndfa and N accumulation with the additional water availability at vegetative and reproductive stages compared to the rainfed treatment at Horsham 2021 and Woomelang 2021 was consistent with the results of Thomas et al. [22]. Water availability plays a significant role in nitrogen fixation and N accumulation, as demonstrated at Horsham in 2021, where biomass production was reduced by 55% under rainfed conditions compared to water application at vegetative and reproductive growth stages, resulting in a 65% reduction in N fixation. Water availability is a major contributor to yield and supporting rhizobia and nodule function. Therefore, water availability during the vegetative and reproductive growth stages of mungbean could have created conducive conditions for rhizobia to fix nitrogen, while a lack of water during these stages might have caused nodule senescence under rainfed treatment [84]. In contrast, at Hamilton in 2021 and Ouyen in 2021, nitrogen fixation was not affected by water treatment. This could be due to water availability throughout the season from stored soil water, resulting in reduced effects of water treatment or increased soil N levels [85,86], as measured at Hamilton in 2021. In the current study, total N fixation was not strongly linked to %Ndfa across the sites, while the variable N fixation observed across the sites appears to be associated with differences in biomass production, as indicated by a significant positive correlation between biomass and N fixation (r = 0.78 *). This relationship has also been reported in earlier studies in soybean [86] and mungbean [22]. The increased %Ndfa values on Calcarosols (Ouyen and Woomelang) compared to the Vertosols and Chromosols of Horsham and Hamilton could be due to soil texture [87]. Therefore, mungbean cultivation in southern Australia will require inoculation of seeds with the appropriate rhizobia strain [88,89] and selecting sites with a higher likelihood of receiving follow-up rainfall during the growing period, particularly at the reproductive growth stage. However, further investigation of soil N fixation with below-ground N in roots could reveal new insights into the nitrogen fixation of mungbean under water-limited soils in southern Australia.
5. Conclusions
The growth of mungbean differed markedly across sites, water treatments and varieties. Yields of more than 1000 kg·ha−1 were observed at Hamilton and Ouyen when the crop received sufficient water during critical growth stages, viz. the vegetative and reproductive stages of mungbean. Crops that received 52 mm of rainfall during the growing period (i.e., Ouyen) yielded 300 kg·ha−1, while in a year with 108 mm of rainfall, grain yield exceeded 1000 kg·ha−1. Rainfall during February and March would be critical to avoid the effects of drought, which could lead to a significant reduction in grain number and HI, resulting in lower yields. Phenology was largely influenced by photoperiod and had no significant effect on yield. Photoperiod-insensitive varieties could improve the adaptation of mungbean to the environments of southern Australia, by avoiding cooler temperatures from the onset of autumn, thereby constraining the reproductive period and bringing forward maturity. Total water use was influenced by the environment, particularly seasonal rainfall and stored soil water. Assessment of nitrogen fixation across the sites indicated that nitrogen accumulation differed across water treatments. Due to the critical nature of stored soil water and in-season water availability to mungbean growth, the high rainfall zone within Victoria (Hamilton) appears the best option for considering mungbean as an opportunistic summer crop. For southern Australia, this study demonstrates that mungbean sowing must be tailored to regional conditions, particularly by aligning sowing with rainfall events and avoiding the risk of high temperatures (>35 °C) during the reproductive phase. Regions receiving more than 450 mm of annual rainfall, such as Hamilton and Rutherglen, are better suited to opportunistic mungbean cultivation within an October–December sowing, provided there is adequate soil water at sowing (more than 100 mm) and sufficient rainfall (more than 200 mm) to support crop growth.
Author Contributions
Conceptualization, J.G.N. and A.J.D.; methodology, J.G.N., A.J.D., A.J.W. and S.S.; software, S.S.; validation, S.S. and J.G.N.; formal analysis, S.S.; investigation, S.S.; resources, J.G.N.; data curation, S.S. and A.J.W.; writing—original draft preparation, S.S.; writing—review and editing, S.S., J.G.N., A.J.D., A.J.W., A.P. and S.N.; visualization, S.S.; supervision, J.G.N., A.J.D., A.P. and S.N.; project administration, J.G.N.; funding acquisition, J.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through the Victorian Grains Innovation Partnership, as a part of the “VGIP1A—Alternative Legume Crops—Southern Region” project, in a collaboration between Agriculture Victoria and the Grains Research and Development Corporation (GRDC). The scholarship to S.S. for this research was provided by The University of Melbourne, Agriculture Victoria and GRDC.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks to the Centre for Agricultural Innovation (CAI), a joint initiative between The University of Melbourne and Agriculture Victoria, for supporting the Ph.D. programme. The authors are grateful to the Crop Agronomy team at the Grains Innovation Park for providing technical and operational support during the experiment across the sites. Thanks are due to the Agriculture Victoria team at Hamilton (Penny Riffkin, Frank Henry and Jamie Smith) for hosting and managing trials. Likewise, the authors would like to acknowledge the West Australian Biogeochemistry Centre, The University of Western Australia, for their analytical support for nitrogen assessment.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zhu, Y.-S.; Sun, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62, 1290. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Schafleitner, R.; Kenyon, L.; Srinivasan, R.; Easdown, W.; Ebert, A.; Hanson, P. Genetic improvement of mungbean. SABRAO J. Breed. Genet. 2012, 44, 177–190. [Google Scholar]
- Nair, R.; Schreinemachers, P. Global status and economic importance of mungbean. In The Mungbean Genome; Nair, R.M., Schafleitner, R., Lee, S.-H., Kole, C., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–8. [Google Scholar]
- Australian Mungbean Association. Industry Size and Value. Available online: http://www.mungbean.org.au/about-us.html (accessed on 25 March 2021).
- Nair, R.M.; Alam, A.M.; Douglas, C.; Gowda, A.; Pratap, A.; Win, M.M.; Karimi, R.; Emmanuel, M.K.; Binagwa, P.; Boddepalli, V.; et al. Establishing the International Mungbean Improvement Network; Australian Centre for International Agricultural Research: Canberra, Australia, 2022; p. 118.
- Tarahi, M. The potential application of mung bean (Vigna radiata L.) protein in plant- based food analogs: A review. Legume Sci. 2024, 6, e70011. [Google Scholar] [CrossRef]
- Diego, R. Multidisciplinary Research: A Key to Unlocking Effective Innovation for Pulse Crops. Available online: https://iyp2016.org/news/320-multidisciplinary-research-a-key-to-unlocking-effective-innovation-for-pulse-crops (accessed on 11 April 2024).
- Research and Markets. Mung Beans Market. Available online: https://www.researchandmarkets.com/report/mung-bean?srsltid=AfmBOopkR7J8AKnj2bPOPr_d8o66eVjRjbes1vvvCTgxICjv21Ke8yXW (accessed on 27 June 2025).
- Wells, L. Rain, Firming Prices Buoy Outlook for CQ Mungbeans. Available online: https://www.graincentral.com/cropping/pulses/rain-firming-prices-buoy-outlook-for-cq-mungbeans/ (accessed on 30 June 2023).
- Wells, L. Pulse update: Prices steady, demand strong. Grain Cent. 2021. [Google Scholar]
- McCarrol, A.P. Exporting Food Quality Mungbeans to Taiwan: The Importance of Supply Chain Entities; Seoul National University: Seoul, Republic of Korea, 2024. [Google Scholar]
- AEGIC. Australian Pulses. Available online: https://www.aegic.org.au/wp-content/uploads/2024/11/AEGIC-Australian-pulses-brochure-2024-LR.pdf (accessed on 4 June 2025).
- Delahunty, A.; Wallace, A.; Norton, S.; Henry, F.; Riffkin, P.; Christy, B.; Brand, J.; Clancy, A.; Silwal, S.; Partington, D.; et al. New legume species as opportunistic summer crops for southern Australia–Part 2 Exploring global germplasm for increasing crop adaptation. In Proceedings of the 20th Australian Agronomy Conference, Toowoomba, QLD, Australia, 18–22 September 2022. [Google Scholar]
- Harris, R.H.; Armstrong, R.D.; Wallace, A.J.; Belyaeva, O.N. Delaying nitrogen fertiliser application improves wheat 15N recovery from high rainfall cropping soils in south eastern Australia. Nutr. Cycl. Agroecosyst. 2016, 106, 113–128. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Rainfall Variability Maps. Available online: http://www.bom.gov.au/climate/maps/averages/rainfall-variability/?period=dec (accessed on 18 April 2025).
- Shanice, V.H.; Dudley, C.; Kang, Y.; Smith, D.; Nair, R.M.; Douglas, C.A.; Potgieter, A.; Robinson, H.; Hickey, L.T.; Smith, M.R. Building a better Mungbean: Breeding for reproductive resilience in a changing climate. Food Energy Secur. 2023, 12, e467. [Google Scholar] [CrossRef]
- Grains Research and Development Corporation. Mungbeans. Grownotes 2017, 282. [Google Scholar]
- Gentry, J. Mungbean Management Guide; The state of Queensland, Department of Employment, Economic Development and Innovation: Brisbane, Australia, 2010.
- Sadasivan, R.; Natrajaratnam, N.; Dabu, R.; Muralidharan, V.; Rangasmay, S. Response of mungbean cultivars to soil moisture stress at different growth phases. In Proceedings of the Mungbean, second international symposium, Bangkok, Thailand, 16–20 November 1987; pp. 260–262. [Google Scholar]
- Nielsen, D.C.; Nelson, N.O. Black bean sensitivity to water stress at various growth stages. Crop Sci. 1998, 38, 422–427. [Google Scholar] [CrossRef]
- Pannu, R.K.; Singh, D.P. Effect of irrigation on water use, water-use efficiency, growth and yield of mungbean. Field Crops Res. 1993, 31, 87–100. [Google Scholar] [CrossRef]
- Thomas; Robertson, M.J.; Fukai, S.; Peoples, M.B. The effect of timing and severity of water deficit on growth, development, yield accumulation and nitrogen fixation of mungbean. Field Crops Res. 2004, 86, 67–80. [Google Scholar] [CrossRef]
- El-Nakhlawy, F.S.; Ismail, S.M.; Basahi, J.M. Optimizing mungbean productivity and irrigation water use efficiency through the use of low water-consumption during plant growth stages. Legume Res. 2018, 41, 108–113. [Google Scholar] [CrossRef]
- Wenham, K.; Williams, A.; Rachaputi, R.; Rossignol, T.; Collins, M. Critical Period of Moisture Vulnerability in Mungbeans. GRDC Grains Research Update. 2020. Available online: https://grdc.com.au/__data/assets/pdf_file/0030/430887/GRDC-Update-Paper-Wenham-Kylie-July-2020.pdf (accessed on 9 June 2025).
- De Costa, W.A.J.M.; Shanmugathasan, K.N.; Joseph, K.D.S.M. Physiology of yield determination of mung bean (Vigna radiata (L.) Wilczek) under various irrigation regimes in the dry and intermediate zones of Sri Lanka. Field Crops Res. 1999, 61, 1–12. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Geetika, G.; Collins, M.; Singh, V.; Hammer, G.; Mellor, V.; Smith, M.; Rachaputi, R.C.N. Canopy and reproductive development in mungbean (Vigna radiata). Crop Pasture Sci. 2022, 73, 1142–1155. [Google Scholar] [CrossRef]
- Rachaputi, R.C.N.; Sands, D.; McKenzie, K.; Agius, P.; Lehane, J.; Seyoum, S. Eco-physiological drivers influencing mungbean [Vigna radiata (L.) Wilczek] productivity in subtropical Australia. Field Crops Res. 2019, 238, 74–81. [Google Scholar] [CrossRef]
- Lamichaney, A.; Parihar, A.K.; Hazra, K.K.; Dixit, G.P.; Katiyar, P.K.; Singh, D.; Singh, A.K.; Kumar, N.; Singh, N.P. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L.). Front. Plant Sci. 2021, 12, 635868. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Sarker, B.C.; Alam, M.A.; Javed, T.; Alam, M.J.; Zaman, M.S.U.; Azam, M.G.; Shabbir, R.; Raza, A.; Habib-ur-Rahman, M.; et al. Yield stability and genotype environment interaction of water deficit stress tolerant mung bean (Vigna radiata l. Wilczak) genotypes of bangladesh. Agronomy 2021, 11, 2136. [Google Scholar] [CrossRef]
- Parihar, A.K.; Gupta, S.; Hazra, K.K.; Lamichaney, A.; Sen Gupta, D.; Singh, D.; Kumar, R.; Singh, A.K.; Vaishnavi, R.; Jaberson, M.S.; et al. Multi-location evaluation of mungbean (Vigna radiata L.) in Indian climates: Ecophenological dynamics, yield relation, and characterization of locations. Front. Plant Sci. 2022, 13, 984912. [Google Scholar] [CrossRef] [PubMed]
- Unkovich, M.J.; Baldock, J.; Peoples, M.B. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant Soil 2010, 329, 75–89. [Google Scholar] [CrossRef]
- Gentry, J.; Sands, D.J.; Silburn, C.; Dunn, M.; Seymour, N.P.; Bell, K.L. Mungbeans: Response to applied nitrogen. In Proceedings of the 20th Agronomy Australia Conference, Toowoomba, Queensland, 18–22 September 2022. [Google Scholar]
- Martin, R.; Montgomery, S.; Phan, S.; Im, S. Mungbean Production Guide for Cambodian Conditions; ACIAR Monograph No. 162; ACIAR: Bruce, ACT, Australia, 2015; pp. 1–52. [Google Scholar]
- McIntosh, P. Protecting Mungbean Inoculant. Available online: http://pulseaus.com.au/blog/post/protecting-mungbean-inoculant (accessed on 28 March 2021).
- Hauser, S.; Grams, C.M.; Reeder, M.J.; McGregor, S.; Fink, A.H.; Quinting, J.F. A weather system perspective on winter–spring rainfall variability in southeastern Australia during El Niño. Q. J. R. Meteorol. Soc. 2020, 146, 2614–2633. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Climate Data Online. Available online: https://reg.bom.gov.au/climate/data/index.shtml (accessed on 13 November 2023).
- Australian Mungbean Association. Australian Mungbean Association Strategic Plan 2020–2025; Australian Mungbean Association: Dalby, QLD, Australia, 2021; pp. 1–26. [Google Scholar]
- Bureau of Meteorology. Average Annual, Seasonal and Monthly Rainfall. Available online: http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall/index.jsp?period=an&area=vc#maps (accessed on 27 March 2021).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- NSW DPI. Summer Cropping Options for Northern and Central NSW. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/1415388/summer-crop-options-2022.pdf (accessed on 19 March 2025).
- Rachaputi, R.C.N.; Sands, D.; McKenzie, K.; Lehane, J.; Agius, P.; Seyoum, S.; Peak, A. Water extraction patterns of mungbean (Vigna radiata) in diverse subtropical environments. Agric. Water Manag. 2019, 219, 109–116. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2008.
- Diatta, A.A.; Thomason, W.E.; Abaye, O.; Thompson, T.L.; Battaglia, M.L.; Vaughan, L.J.; Lo, M.; Filho, J.F.D.C.L. Assessment of nitrogen fixation by mungbean genotypes in different soil textures using 15N natural abundance method. J. Soil Sci. Plant Nutr. 2020, 20, 2230–2240. [Google Scholar] [CrossRef]
- Gan, Y.; Hamel, C.; O’Donovan, J.T.; Cutforth, H.; Zentner, R.P.; Campbell, C.A.; Niu, Y.; Poppy, L. Diversifying crop rotations with pulses enhances system productivity. Sci. Rep. 2015, 5, 14625. [Google Scholar] [CrossRef] [PubMed]
- The VSNi Team. Asreml: Fits Linear Mixed Models Using REML, R package version 4.2.0.355; VSN International: Hemel Hempstead, UK, 2023.
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Rogers, S.; Conway, A. Biometryassist: Functions to Assist Design and Analysis of Agronomic Experiments, R package version 1.3.0; The University of Adelaide: Adelaide, Australia, 2025.
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R package version 0.7.2. 2023. Available online: https://rpkgs.datanovia.com/rstatix/authors.html#citation (accessed on 10 June 2025).
- Chauhan, Y.; Williams, R. Physiological and agronomic strategies to increase mungbean yield in climatically variable environments of northern Australia. Agronomy 2018, 8, 83. [Google Scholar] [CrossRef]
- French, R.; Schultz, J. Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate. Aust. J. Agric. Res. 1984, 35, 743–764. [Google Scholar] [CrossRef]
- Bashandi, M.M.H.; Poehlman, J.M. Photoperiod response in mungbeans (Vigna radiata (L.) Wilczek). Euphytica 1974, 23, 691–697. [Google Scholar] [CrossRef]
- Imrie, B.C.; Lawn, R.J. Time to flowering of mung bean (Vigna radiata) genotypes and their hybrids in response to photoperiod and temperature. Exp. Agric. 1990, 26, 307–318. [Google Scholar] [CrossRef]
- Lawn, R. Agronomic studies on Vigna spp. in south-eastern Queensland. I. Phenological response of cultivars to sowing date. Aust. J. Agric. Res. 1979, 30, 855–870. [Google Scholar] [CrossRef]
- Ellis, R.H.; Lawn, R.J.; Summerfield, R.J.; Qi, A.; Roberts, E.H.; Chay, P.M.; Brouwer, J.B.; Rose, J.L.; Yeates, S.J.; Sandover, S. Towards the reliable prediction of time to flowering in six annual crops. IV. cultivated and wild mung bean. Exp. Agric. 1994, 30, 31–43. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.D. Physiological responses and dry matter partitioning of summer mungbean (Vigna radiata L.) genotypes subjected to drought conditions. J. Agron. Crop Sci. 2009, 195, 270–277. [Google Scholar] [CrossRef]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Jha, U.C.; Shafi, S.; Tallury, S.; Nayyar, H.; Ciampitti, I.A.; Siddique, K.H.M.; Prasad, P.V.V. Differential physiological and yield responses of selected mung bean (Vigna radiata (L.) R. Wilczek) genotypes to various high-temperature stress regimes. Sci. Rep. 2025, 15, 1034. [Google Scholar] [CrossRef] [PubMed]
- Geetika, G.; Hammer, G.; Smith, M.; Singh, V.; Collins, M.; Mellor, V.; Wenham, K.; Rachaputi, R.C. Quantifying physiological determinants of potential yield in mungbean (Vigna radiata (L.) Wilczek). Field Crops Res. 2022, 287, 108648. [Google Scholar] [CrossRef]
- Patriyawaty, N.R.; Rachaputi, R.C.N.; George, D. Physiological mechanisms underpinning tolerance to high temperature stress during reproductive phase in mungbean (Vigna radiata (L.) Wilczek). Environ. Exp. Bot. 2018, 150, 188–197. [Google Scholar] [CrossRef]
- Iqbal, J.; Ahsan, M.; Saleem, M.; Ali, A. Appraisal of gene action for indeterminate growth in mungbean [Vigna radiata (L.) Wilczek]. Front. Plant Sci. 2015, 6, 665. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kwon, H.; Cho, K.-H.; Yoon, M.Y.; Kim, M.Y.; Lee, S.-H. Identification of epigenetic variation associated with synchronous pod maturity in mungbean (Vigna radiata L.). Sci. Rep. 2020, 10, 17414. [Google Scholar] [CrossRef] [PubMed]
- Marwiyah, S.; Suwarno, W.B.; Wirnas, D.; Trikoesoemaningtyas; Sutjahjo, S.H. Genotype by environment interaction on phenology and synchronous maturity of mungbean. Agron. J. 2021, 113, 2321–2334. [Google Scholar] [CrossRef]
- Christy, B.; Delahunty, A.; Norton, S.; Wallace, A.; Riffkin, P.; O’Leary, G.J.; Nuttall, J. New legume species as opportunistic summer crops for southern Australia–Part 1: Environmental suitability. In Proceedings of the 20th Australian Agronomy Conference, Toowoomba, QLD, Australia, 18–22 September 2022. [Google Scholar]
- Gölgül, İ.; Kırnak, H.; Ali İrik, H. Yield components and crop water stress index (CWSI) of mung bean grown under deficit irrigations. Gesunde Pflanz. 2023, 75, 271–281. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Squire, G.R. The Physiology of Tropical Crop Production; C. A. B. International: Wallingford, UK, 1990; p. 236. [Google Scholar]
- Singh, D.P.; Singh, P.; Sharma, H.C.; Turner, N.C. Influence of water deficits on the water relations, canopy gas exchange, and yield of chickpea (Cicer arietinum). Field Crops Res. 1987, 16, 231–241. [Google Scholar] [CrossRef]
- Jamieson, P.D.; Martin, R.J.; Francis, G.S.; Wilson, D.R. Drought effects on biomass production and radiation-use efficiency in barley. Field Crops Res. 1995, 43, 77–86. [Google Scholar] [CrossRef]
- Goel, K.; Kundu, P.; Sharma, P.; Zinta, G. Thermosensitivity of pollen: A molecular perspective. Plant Cell Rep. 2023, 42, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Iovane, M.; Aronne, G. High temperatures during microsporogenesis fatally shorten pollen lifespan. Plant Reprod 2022, 35, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Zeleke, K.; Luckett, D.J. Irrigated agricultural production dynamics in response to rainfall variability and water policy reforms in the southern Murray-Darling Basin of Australia. Agric. Water Manag. 2025, 315, 109539. [Google Scholar] [CrossRef]
- Goulburn-Murray Water. Goulburn-Murray Water at a Glance. Available online: https://www.g-mwater.com.au/about/waterclass/resources/videos/the-gmid.html (accessed on 29 June 2025).
- Delahunty, A.; Henry, F.; Wallace, A.; Christy, B.; Brand, J.; Norton, S.; Perry, E.; Riffkin, P.; Walker, C.; O’Leary, G.; et al. Alternative Legume Crop Options for Southern Australia—A Review; Agriculture Victoria, Department of Jobs, Precincts and Regions (DJPR): Melbourne, VIC, USA, 2020; pp. 1–152.
- Matthews, R.B.; Harris, D.; Williams, J.H.; Rao, R.C.N. The physiological basis for yield differences between four genotypes of groundnut (Arachis hypogaea) in response to drought. II. Solar radiation interception and leaf movement. Exp. Agric. 1988, 24, 203–213. [Google Scholar] [CrossRef]
- Silwal, S.; Bhattarai, S.P.; Midmore, D.J. Aerobic rice with or without strategic irrigation in the subtropics. Agronomy 2020, 10, 1831. [Google Scholar] [CrossRef]
- Collins, M.; Anderson, B.; Bell, L. Yield Gaps in Mungbean Crops Across the Northern Grains Region. GRDC Grains Research Update 2020, pp. 100–106. Available online: https://grdc.com.au/__data/assets/pdf_file/0023/433823/GRDC-Grains-Research-Update-Toowoomba-2020.pdf (accessed on 31 July 2022).
- Bourgault, M.; Madramootoo, C.A.; Webber, H.A.; Stulina, G.; Horst, M.G.; Smith, D.L. Effects of deficit irrigation and salinity stress on common bean (Phaseolus vulgaris L.) and mungbean (Vigna radiata (L.) wilczek) grown in a controlled environment. J. Agron. Crop Sci. 2010, 196, 262–272. [Google Scholar] [CrossRef]
- Lu, S.; Liu, M.; Yi, J.; Li, S.; Xu, Y.; Zhang, H.; Ding, F. Lateral partition patterns and controlling factors of soil infiltration at a steep, near-stream, and humid hillslope scale. Catena 2024, 239, 107917. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Shah, Z.; Shah, S.H.; Peoples, M.B.; Schwenke, G.D.; Herridge, D.F. Crop residue and fertiliser N effects on nitrogen fixation and yields of legume–cereal rotations and soil organic fertility. Field Crops Res. 2003, 83, 1–11. [Google Scholar] [CrossRef]
- Djekoun, A.; Planchon, C. Water status effect on dinitrogen fixation and photosynthesis in soybean. Agron. J. 1991, 83, 316–322. [Google Scholar] [CrossRef]
- Raji, S.G.; Tzanakakis, V.; Dörsch, P. Bradyrhizobial inoculation and P application effects on haricot and mung beans in the Ethiopian Rift Valley. Plant Soil 2019, 442, 271–284. [Google Scholar] [CrossRef]
- Herridge, D.F.; Robertson, M.J.; Cocks, B.; Peoples, M.B.; Holland, J.F.; Heuke, L. Low nodulation and nitrogen fixation of mungbean reduce biomass and grain yields. Aust. J. Exp. Agric. 2005, 45, 269–277. [Google Scholar] [CrossRef]
- Diatta, A.A.; Thomason, W.E.; Abaye, O.; Vaughan, L.J.; Thompson, T.L.; Lo, M.; Chim, B.K.; Bateman, S. Inoculation and soil texture effects on yield and yield components of mungbean. J. Agric. Sci. 2018, 10, 6. [Google Scholar] [CrossRef]
- Mott, J.; Abaye, O.; Reiter, M.; Maguire, R. Evaluating effects of Bradyrhizobium and arbuscular mycorrhizal fungi inoculation on yield components of mung bean (Vigna radiata (L.) wilczek) and nitrogen fixation. Agronomy 2022, 12, 2358. [Google Scholar] [CrossRef]
- Christopher, M.; Macdonald, B.; Yeates, S.; Ziegler, D.; Seymour, N. Wild bradyrhizobia that occur in the Burdekin region of Queensland are as effective as commercial inoculum for mungbean (Vigna radiata (L.)) and black gram (Vigna mungo (L.)) in fixing nitrogen and dry matter production. Appl. Soil Ecol. 2018, 124, 88–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).