Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Cloning of TrWRKY79 Gene and Sequence Analysis
2.3. Transactivation Assay of TrWRKY79
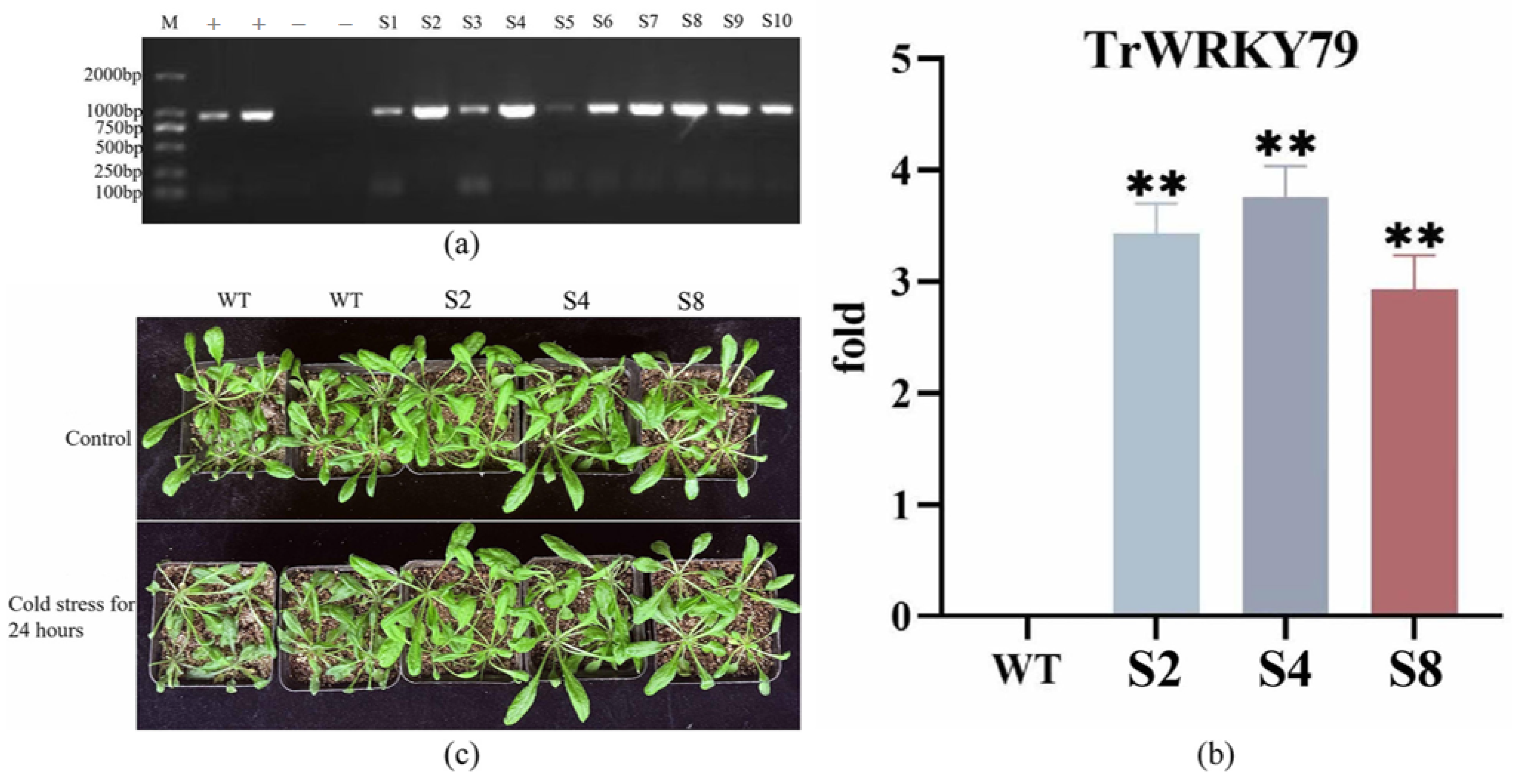
2.4. Arabidopsis Transformation and Transgenic Plant Validation
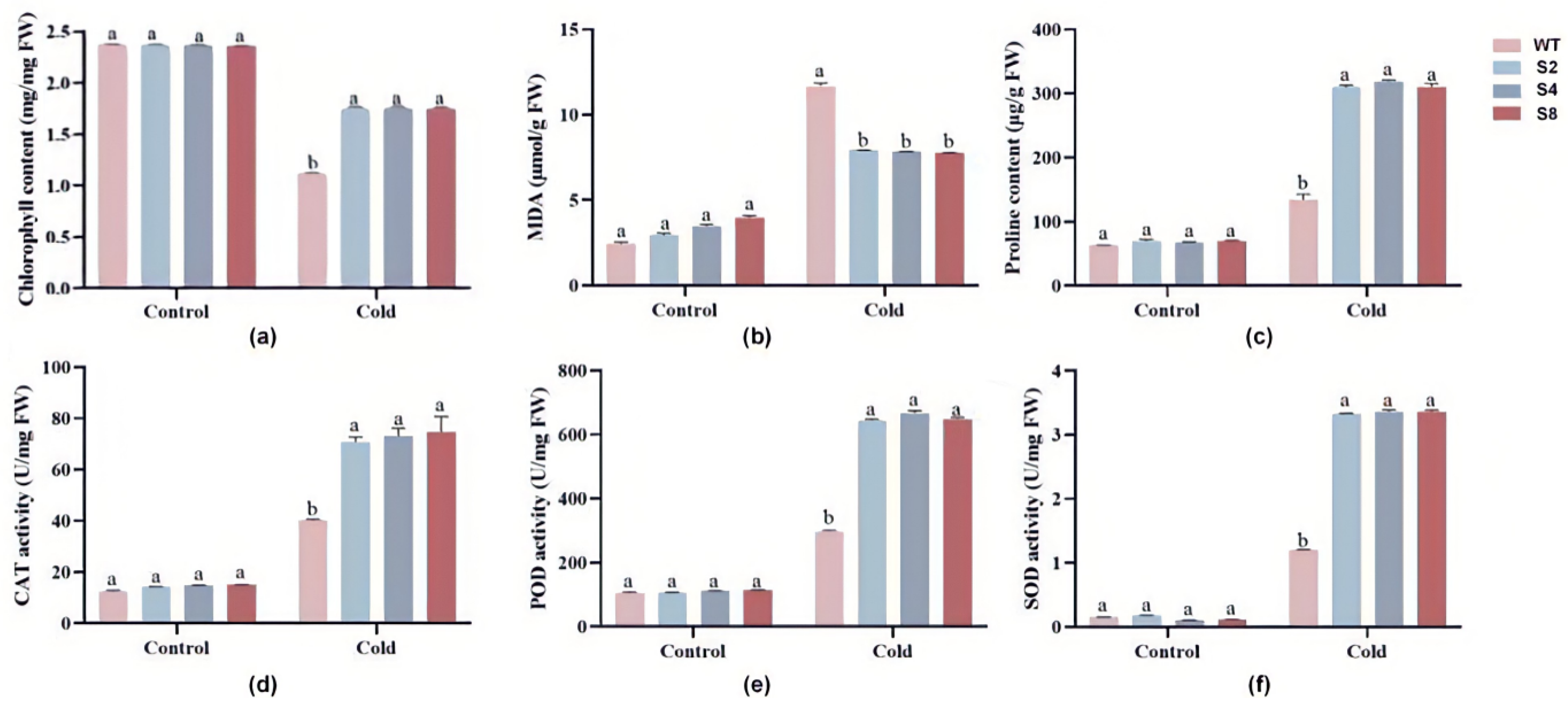
2.5. Physiological and Biochemical Characterization of TrWRKY79-Transgenic Arabidopsis
2.6. TrWRKY79 Transcription Factor Binding Site
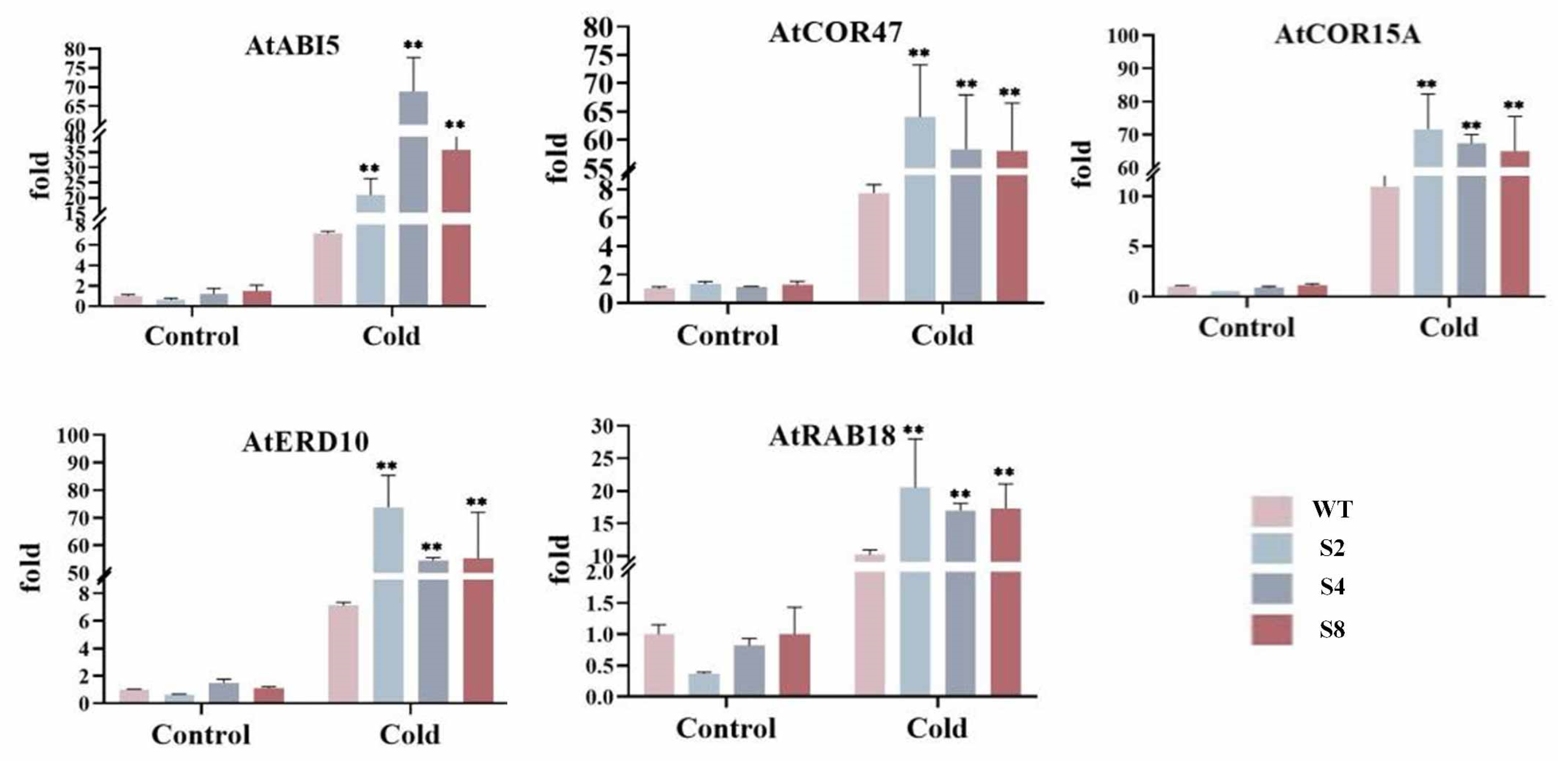
2.7. Expression Analysis of Resistance-Related Genes in Transgenic Arabidopsis thaliana
3. Results
3.1. Cloning and Sequence Analysis of TrWRKY79
3.2. Transactivation Activity Assay of TrWRKY79
3.3. Overexpression of TrWRKY79 Enhances Cold Stress Tolerance in Transgenic Arabidopsis
3.4. TrWRKY79 Enhances Physiological Tolerance to Cold Stress in Arabidopsis
3.5. Binding Site Prediction of WRKY Transcription Factors
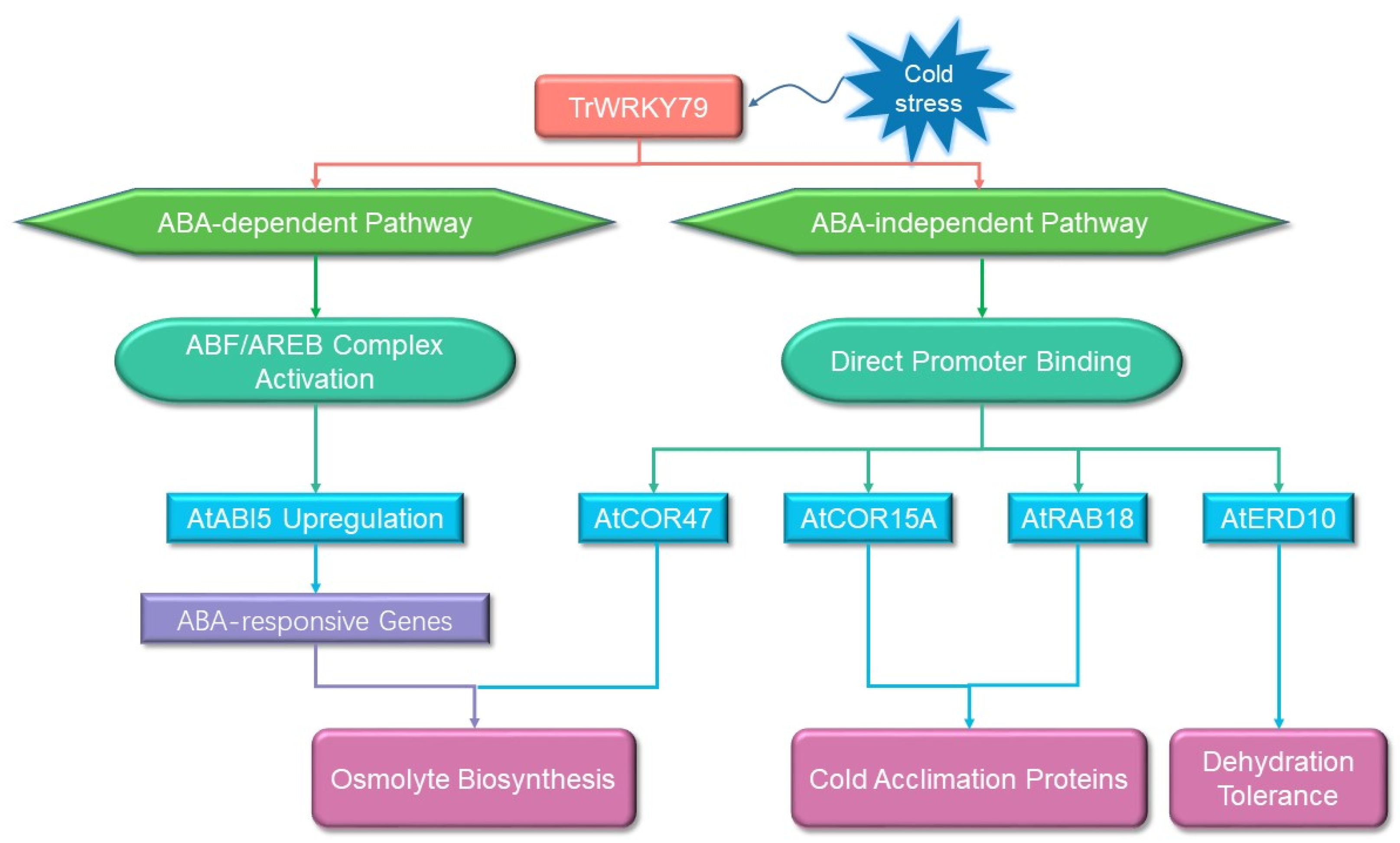
3.6. Expression Analysis of Resistance-Related Genes in Arabidopsis thaliana Transgenic for TrWRKY79 at Low Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| TrWRKY79-F | AATCAACCAACCCTATAT |
| TrWRKY79-R | ATGAATATGATTGTGTTAT |
| BD-TrWRKY79-F | CATGGAGGCCGAATTCAATCAACCAACCCTATAT |
| BD-TrWRKY79-R | GGATCCCCGGGAATTCATGAATATGATTGTGTTAT |
| BD-TrWRKY79-N-F | CATGGAGGCCGAATTCAATCAACCAACCCTATAT |
| BD-TrWRKY79-N-R | GGATCCCCGGGAATTCTCCAGATGATCCACCTCGCT |
| BD-TrWRKY79-W-F | CATGGAGGCCGAATTCAGCGAGGTGGATCATCTGGA |
| BD-TrWRKY79BW-R | GGATCCCCGGGAATTCAGCCATAACTGGATTTGGATGAGTA |
| BD-TrWRKY79-C-F | CATGGAGGCCGAATTCTACGAAGGGAAACATACTC |
| BD-TrWRKY79-C-R | GGATCCCCGGGAATTCATGAATATGATTGTGTTAT |
Appendix A.2
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| qTrWRKY79-F | TATCCTGTGGATGATGCAGT |
| qTrWRKY79-R | GATCCACCTCGCTTTTAGTC |
| qAtactin-F | AGCTAGAGACAGCCAAGAGC |
| qAtactin-R | GCTTCCATTCCGATGAGCGA |
| qAtCOR47-F | GAAACCTCAAGAGACAACGA |
| qAtCOR47-R | AGAAGAGCTGTTGGATCGG |
| qAtABI5-F | TCCAGTGGAGAAAGTAGTGG |
| qAtABI5-R | AGGTGTCTTTACCTGTTGCT |
| qAtRAB18-F | GTCAGGACAACCCGAATTT |
| qAtRAB18-R | ATACGAACTTGTTAGCGTCC |
| qAtCOR15A-F | GAACAAGCCTAGTGTCATCG |
| qAtCOR15A-R | ATCATCCTCTGCTGTCTTGT |
| qAtERD10-F | ACATTCGGTAGAGGATCACA |
| qAtERD10-R | TTCCTCTCCAGTGGTCTTG |
Appendix A.3
| Name | Sequence | Name | Sequence |
|---|---|---|---|
| Wbox1 | AAACTTGACTCGATT | Wbox11 | GGATTTTGACCACTG |
| Wbox2 | TTGACTTTATA | Wbox12 | TTCGATTGACCA |
| Wbox3 | TTGACTATCAC | Wbox13 | GAATCTTGACTAG |
| Wbox4 | TACATTGACCCAGAA | Wbox14 | CATTGACTCCCAC |
| Wbox5 | TTGACCTCTTC | Wbox15 | GGACCTTGACTTGAA |
| Wbox6 | TTTCATTGACTAGTT | Wbox16 | TTGACCATTTT |
| Wbox7 | GACGATTGACTGAGA | Wbox17 | CTTGTTGACTATACT |
| Wbox8 | GAAGCTTGACTCA | Wbox18 | TGCTCTTGACTTTCT |
| Wbox9 | CTGTATTGACTGGCC | Wbox19 | ATTAATTGACTAAAA |
| Wbox10 | AGTTGACTATCAC | Wbox20 | TTTTGTTGACT |
Appendix A.4
| Gene | Sequences | Reverse Complemented Sequences | ipTM |
|---|---|---|---|
| AT5G51140 | TTAGATTGACAGCGACCA | TGGTCGCTGTCAATCTAA | 0.79 |
| AT5G48953 | AGTACTTTGACCCAGATA | TATCTGGGTCAAAGTACT | 0.79 |
| AT2G07680 | TTGGATCCTTGACTTGAA | TTCAAGTCAAGGATCCAA | 0.79 |
| AT2G30050 | ATAACTTTGACCCAGATC | GATCTGGGTCAAAGTTAT | 0.79 |
| AT2G38740 | TAGGATTTTGACCACAAG | CTTGTGGTCAAAATCCTA | 0.79 |
| AT3G53810 | ATGGATTTTGACCAGTTG | CAACTGGTCAAAATCCAT | 0.79 |
| AT3G58890 | GAAGACCTTGACTTGTAC | GTACAAGTCAAGGTCTTC | 0.79 |
| AT1G58602 | TTCGTAGCTTGACTCATA | TATGAGTCAAGCTACGAA | 0.78 |
| AT1G26200 | TTGAGGAATCTTGACTAG | CTAGTCAAGATTCCTCAA | 0.78 |
| AT3G19663 | TGGGACCATGACTTGTTC | GAACAAGTCATGGTCCCA | 0.78 |
| AT3G42940 | ATAGATTTAGACCACTGT | ACAGTGGTCTAAATCTAT | 0.78 |
| AT5G67580 | GCTTCTTGACCTGCAATG | CATTGCAGGTCAAGAAGC | 0.78 |
| AT1G70944 | AGTGGATTTTGACCATGT | ACATGGTCAAAATCCACT | 0.78 |
| AT3G25855 | ATCCATGACACGATTTTG | CAAAATCGTGTCATGGAT | 0.77 |
| AT2G34630 | CCCCCCTTGACTCCATCC | GGATGGAGTCAAGGGGGG | 0.77 |
| AT4G15530 | GAGAAACTATCACCGATC | GATCGGTGATAGTTTCTC | 0.77 |
| AT5G22340 | TCAAGTTGACCAACAAAT | ATTTGTTGGTCAACTTGA | 0.77 |
| AT4G24380 | GTGGATTTGACCAGACAT | ATGTCTGGTCAAATCCAC | 0.77 |
| AT3G29020 | AAAATGACCTTGAAAATT | AATTTTCAAGGTCATTTT | 0.77 |
| AT1G70560 | AAAGGTTTTTGACCACGT | ACGTGGTCAAAAACCTTT | 0.77 |
| AT1G16770 | TGTTACATAGACCCAGAG | CTCTGGGTCTATGTAACA | 0.77 |
| AT5G06790 | CTTGACCTTAACTTGAAG | CTTCAAGTTAAGGTCAAG | 0.77 |
| AT5G46295 | TTACCTTGACTTGATCTT | AAGATCAAGTCAAGGTAA | 0.77 |
| AT3G50700 | GTGACCGACTTGAATACC | GGTATTCAAGTCGGTCAC | 0.76 |
| AT1G14770 | TATCATAGCTTGACTTCT | AGAAGTCAAGCTATGATA | 0.76 |
| AT4G00120 | CATGATTTTGACCACACA | TGTGTGGTCAAAATCATG | 0.76 |
| AT1G55310 | AGATTGAAGCTTGACTCA | TGAGTCAAGCTTCAATCT | 0.76 |
| AT2G03130 | TTATACATTGTCCCAGCC | GGCTGGGACAATGTATAA | 0.76 |
| AT2G21830 | GAGACCTTGTCTTGAAGC | GCTTCAAGACAAGGTCTC | 0.76 |
| AT3G20210 | TGTTACATTGACCCTGAG | CTCAGGGTCAATGTAACA | 0.76 |
| AT5G06580 | TGACTACAGTGACCCAGA | TCTGGGTCACTGTAGTCA | 0.76 |
| AT1G45403 | TGTTGCGAAGCTTGTCCA | TGGACAAGCTTCGCAACA | 0.75 |
| AT4G23100 | CGTCAAGCTTGACGAATT | AATTCGTCAAGCTTGACG | 0.75 |
| AT2G33010 | AAAAAACTATCACTCTCT | AGAGAGTGATAGTTTTTT | 0.75 |
| AT3G08820 | GACCTTGCTGTATATGTA | TACATATACAGCAAGGTC | 0.75 |
| AT2G39210 | GTTTGGACCTTGACTGAT | ATCAGTCAAGGTCCAAAC | 0.75 |
| AT3G25014 | CTCGGATTTTGTCCACTA | TAGTGGACAAAATCCGAG | 0.75 |
| AT1G43090 | AGAAGGACCTTGACTGAC | GTCAGTCAAGGTCCTTCT | 0.75 |
| AT3G16110 | AATGGTTGACTATTGAAT | ATTCAATAGTCAACCATT | 0.74 |
| AT2G30432 | TGACTTTGACCCACACAT | ATGTGTGGGTCAAAGTCA | 0.74 |
| AT5G51750 | CACTTTTGACCAATGAAA | TTTCATTGGTCAAAAGTG | 0.74 |
| AT3G18370 | CGTCACTTTGACCCAGAA | TTCTGGGTCAAAGTGACG | 0.74 |
| AT3G23123 | ACTTTTTTGACCACTGTG | CACAGTGGTCAAAAAAGT | 0.74 |
| AT1G43100 | AGAAGGACCTTGACTGAC | GTCAGTCAAGGTCCTTCT | 0.74 |
| AT4G08878 | TAATCTGTTCGATTGACC | GGTCAATCGAACAGATTA | 0.74 |
| AT2G02870 | CCTTTTGACCACTGAAAT | ATTTCAGTGGTCAAAAGG | 0.73 |
| AT2G47160 | TTGACGATTTGACTGAGA | TCTCAGTCAAATCGTCAA | 0.73 |
| AT4G34940 | ACAGATTTTGTCCACTGA | TCAGTGGACAAAATCTGT | 0.73 |
| AT5G45310 | AAAACGATTGACTGATTA | TAATCAGTCAATCGTTTT | 0.73 |
| AT5G59890 | CATGAGGACCTTGCTGCA | TGCAGCAAGGTCCTCATG | 0.73 |
| AT5G22560 | AAAATGAAGCTTGACACA | TGTGTCAAGCTTCATTTT | 0.73 |
| AT3G25011 | TCGGATTTTGTCCACTAG | CTAGTGGACAAAATCCGA | 0.72 |
| AT5G19640 | AATTGGACCTTGACTCAA | TTGAGTCAAGGTCCAATT | 0.72 |
| AT5G17167 | GGCACTTGACTCGGTTGC | GCAACCGAGTCAAGTGCC | 0.72 |
| AT5G11350 | CAATTCGATTGACCAATG | CATTGGTCAATCGAATTG | 0.71 |
| AT3G56270 | CTCCATTGACCCAGAGAA | TTCTCTGGGTCAATGGAG | 0.71 |
| AT4G26270 | CTTGACCTTGACTTGATA | TATCAAGTCAAGGTCAAG | 0.71 |
| AT2G14080 | TTACATTGTCCCAGAGCA | TGCTCTGGGACAATGTAA | 0.70 |
| AT3G51530 | CCACGGTTGACTGAGAAG | CTTCTCAGTCAACCGTGG | 0.70 |
| AT4G23130 | AGTTGACCTTGACTTGCA | TGCAAGTCAAGGTCAACT | 0.70 |
| AT4G34930 | CAGATTTTGTCCACTGAT | ATCAGTGGACAAAATCTG | 0.70 |
| AT3G57760 | TTCGATTGACCAAAATCA | TGATTTTGGTCAATCGAA | 0.70 |
| AT4G28150 | AGTCATTGACCCAGTTTC | GAAACTGGGTCAATGACT | 0.70 |
Appendix B


References
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (bHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.; Zhang, N.; Li, S.; Si, H. Role of bZIP Transcription Factors in Plant Salt Stress. Int. J. Mol. Sci. 2023, 24, 7893. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Diao, P.; Chen, C.; Zhang, Y.; Meng, Q.; Lv, W.; Ma, N. The role of NAC transcription factor in plant cold response. Plant Signal Behav. 2020, 15, 1785668. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, B.; Wang, N.; Zheng, Z.; Yang, L.; Zhong, S.; Fang, Q.; Xiao, Z.; Zhao, H. A WRKY Transcription Factor PmWRKY57 from Prunus mume Improves Cold Tolerance in Arabidopsis thaliana. Mol. Biotechnol. 2023, 65, 1359–1368. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Wang, X. bZIP transcription factor OsbZIP52/RISBZ5: A potential negative regulator of cold and drought stress response in rice. Planta 2012, 235, 1157–1169. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Yang, X.; Li, Q.; Ling, J.; Wang, H.; Gu, X.; Huang, S.; Jiang, W. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016, 108, 478–487. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5’ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, M.; Zhang, X.; Hao, B.; Kaushik, S.K.; Pan, Y. WRKY gene family evolution in Arabidopsis thaliana. Genetica 2011, 139, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Abdullah-Zawawi, M.R.; Ahmad-Nizammuddin, N.F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef]
- Ciolkowski, I.; Wanke, D.; Birkenbihl, R.P.; Somssich, I.E. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol. Biol. 2008, 68, 81–92. [Google Scholar] [CrossRef]
- Lei, R.; Li, X.; Ma, Z.; Lv, Y.; Hu, Y.; Yu, D. Arabidopsis WRKY2 and WRKY34 transcription factors interact with VQ20 protein to modulate pollen development and function. Plant J. 2017, 91, 962–976. [Google Scholar] [CrossRef]
- Tang, H.; Bi, H.; Liu, B.; Lou, S.; Song, Y.; Tong, S.; Chen, N.; Jiang, Y.; Liu, J.; Liu, H. WRKY33 interacts with WRKY12 protein to up-regulate RAP2.2 during submergence induced hypoxia response in Arabidopsis thaliana. New Phytol. 2021, 229, 106–125. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63-OsWRKY76-OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef]
- Niu, C.F.; Wei, W.; Zhou, Q.Y.; Tian, A.G.; Hao, Y.J.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, Z.B.; Zhang, J.S.; et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012, 35, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, J.S.; Ness, R.W.; Cohan, B.; Fitzpatrick, C.R.; Innes, S.G.; Koch, S.; Miles, L.S.; Munim, S.; Peres-Neto, P.R.; Prashad, C.; et al. Global urban environmental change drives adaptation in white clover. Science 2022, 375, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Zhang, T.; Bai, Y.; Chen, C.; Guo, D.; Guo, C.; Shu, Y. Genome-wide analysis of the WRKY genes and their important roles during cold stress in white clover. PeerJ 2023, 11, e15610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A Novel Sweetpotato WRKY Transcription Factor, IbWRKY2, Positively Regulates Drought and Salt Tolerance in Transgenic Arabidopsis. Biomolecules 2020, 10, 506. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise estimation of chlorophyll a, b and carotenoid content by deconvolution of the absorption spectrum and new simultaneous equations for Chl determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Liu, F.; Zhu, J. Responses of Antioxidant Enzymes to Chronic Free-Air Ozone Stress in Rice (Oryza sativa L.) Cultivars with Different Ozone-Sensitivities. Bull. Environ. Contam. Toxicol. 2019, 103, 428–434. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cannon, C.H.; Cobb, G.P.; Anderson, T.A. Conservation and divergence of plant microRNA genes. Plant J. 2006, 46, 243–259. [Google Scholar] [CrossRef]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Lu, C.; Ma, W.; Guan, J.; Gong, C.; Yang, J.; Zhang, H.; Zhang, K.; Wu, S.; et al. Protenix-Advancing Structure Prediction Through a Comprehensive AlphaFold3 Reproduction. Bytedance 2025. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002, 129, 706–716. [Google Scholar] [CrossRef]
- Garcýa-Laynes, S.; Herrera-Valencia, V.A.; Tamayo-Torres, L.G.; Limones-Briones, V.; Barredo-Pool, F.A.; Baas-Espinola, F.M.; Alpuche-Solís, A.G.; Puch-Hau, C.; Peraza-Echeverria, S. The Banana MaWRKY18, MaWRKY45, MaWRKY60 and MaWRKY70 Genes Encode Functional Transcription Factors and Display Differential Expression in Response to Defense Phytohormones. Genes 2022, 13, 1891. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kwon, S.I.; Jang, J.Y.; Fang, I.L.; Lee, H.; Choi, C.; Park, S.; Ahn, I.; Bae, S.C.; Hwang, D.J. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2016, 35, 1975–1985. [Google Scholar] [CrossRef]
- Bataller, S.; Davis, J.A.; Gu, L.; Baca, S.; Chen, G.; Majid, A.; Villacastin, A.J.; Barth, D.; Han, M.V.; Rushton, P.J.; et al. Disruption of the OsWRKY71 transcription factor gene results in early rice seed germination under normal and cold stress conditions. BMC Plant Biol. 2024, 24, 1090. [Google Scholar] [CrossRef]
- Robatzek, S.; Somssich, I.E. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001, 28, 123–133. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Hsin, K.T.; Hsieh, M.C.; Lee, Y.H.; Lin, K.C.; Cheng, Y.S. Insight into the Phylogeny and Binding Ability of WRKY Transcription Factors. Int. J. Mol. Sci. 2022, 23, 2895. [Google Scholar] [CrossRef] [PubMed]
- Hammargren, J.; Rosenquist, S.; Jansson, C.; Knorpp, C. A novel connection between nucleotide and carbohydrate metabolism in mitochondria: Sugar regulation of the Arabidopsis nucleoside diphosphate kinase 3a gene. Plant Cell Rep. 2008, 27, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Pan, Y.; Li, J.; Chen, X.; Pan, Y.; Wang, Y.; Tian, S.; Zhang, X. Heterologous expression of Arabidopsis C-repeat binding factor 3 (AtCBF3) and cold-regulated 15A (AtCOR15A) enhanced chilling tolerance in transgenic eggplant (Solanum melongena L.). Plant Cell Rep. 2014, 33, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Bai, H.; Yan, Y.; Liu, Y.; Wang, X.; Zhang, Z. Exploring ABI5 regulation: Post-translational control and cofactor interactions in ABA signaling. Plant J. 2025, 121, e17232. [Google Scholar] [CrossRef]

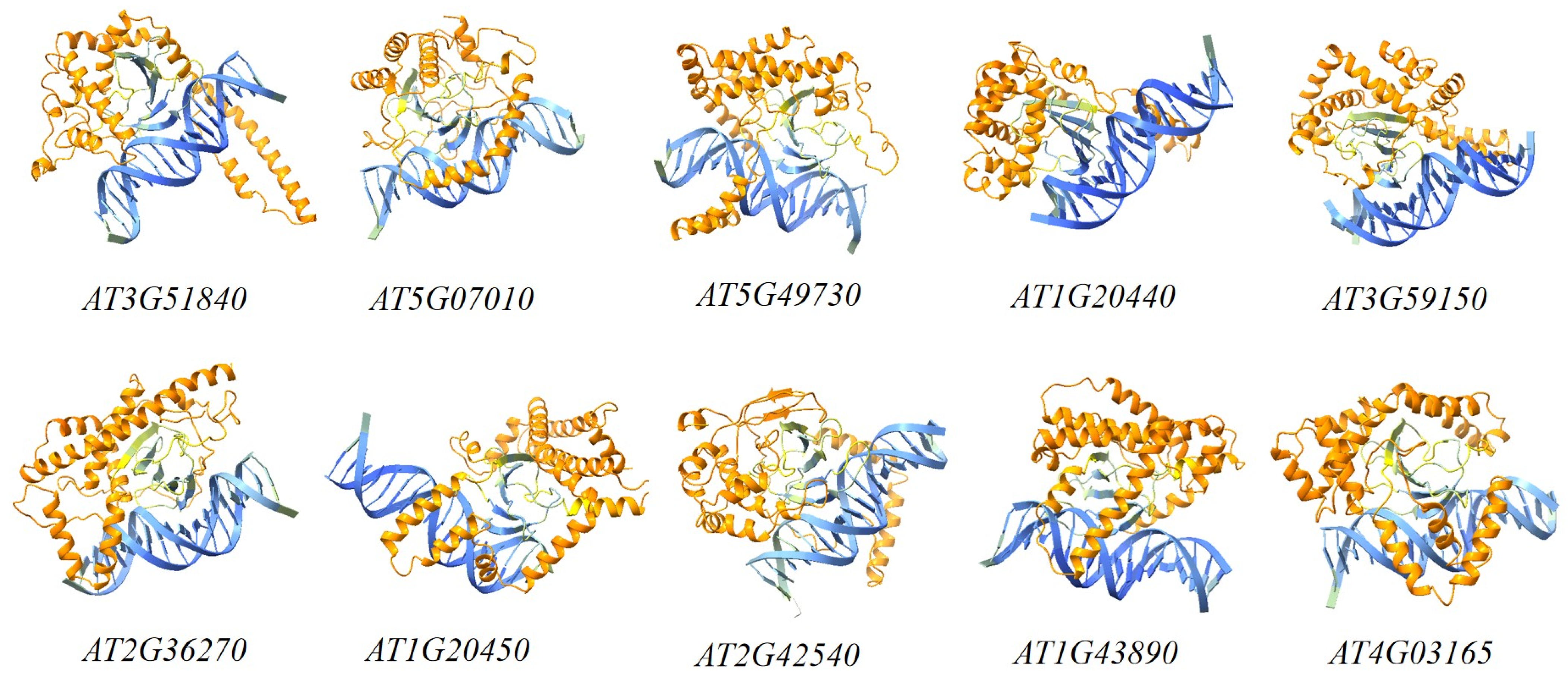
| Gene | Sequences | Reverse Complemented Sequences | ipTM |
|---|---|---|---|
| AT1G35340 | GAAATCGAGTCAAGAAAT | ATTTCTTGACTCGATTTC | 0.86 |
| AT2G26180 | ACTCTTGACTCGATTTTT | AAAAATCGAGTCAAGAGT | 0.85 |
| AT1G67770 | AAAGAACCTTGACTTGTT | AACAAGTCAAGGTTCTTT | 0.85 |
| AT5G17970 | GTAAAAGCTTGACTCAGC | GCTGAGTCAAGCTTTTAC | 0.85 |
| AT3G51840 | TTCTTTGCTTGACCGCTT | AAGCGGTCAAGCAAAGAA | 0.84 |
| AT2G40120 | GTTTCTTGACTCGATTCT | AGAATCGAGTCAAGAAAC | 0.84 |
| AT5G07010 | ACTTAAGCTTGATGAATA | TATTCATCAAGCTTAAGT | 0.83 |
| AT1G48330 | AAAACTTGACCCGATTAA | TTAATCGGGTCAAGTTTT | 0.83 |
| AT5G49730 | TTTTACTTGACTCTTCTA | TAGAAGAGTCAAGTAAAA | 0.82 |
| AT1G20440 | AGAGTGACCACCCCAATG | CATTGGGGTGGTCACTCT | 0.82 |
| AT2G39350 | GATAACTTGACTCGAACC | GGTTCGAGTCAAGTTATC | 0.82 |
| AT3G19090 | GATAACTTGACTCGTTTG | CAAACGAGTCAAGTTATC | 0.82 |
| AT1G09530 | ATTAAACTTGTCTCGATA | TATCGAGACAAGTTTAAT | 0.82 |
| AT3G59150 | TTTGTTTGACTGAAATTT | AAATTTCAGTCAAACAAA | 0.81 |
| AT1G69020 | GACACCTTGACTTGATGT | ACATCAAGTCAAGGTGTC | 0.81 |
| AT2G04630 | CTGAACTTGACTCGAAAT | ATTTCGAGTCAAGTTCAG | 0.81 |
| AT2G30910 | ATGAAGCTTGACTCAATC | GATTGAGTCAAGCTTCAT | 0.81 |
| AT3G45525 | TTACATTTTGACCACTGT | ACAGTGGTCAAAATGTAA | 0.81 |
| AT5G13320 | AAAACATTGACCCAGACT | AGTCTGGGTCAATGTTTT | 0.81 |
| AT5G23903 | GATACCTTGACTTGACGG | CCGTCAAGTCAAGGTATC | 0.81 |
| AT3G05220 | AAATTATTGACCATATTA | TAATATGGTCAATAATTT | 0.81 |
| AT5G47910 | AAGGATTTTGACCAGACC | GGTCTGGTCAAAATCCTT | 0.81 |
| AT2G36270 | CAAACTTTGACTATTTCT | AGAAATAGTCAAAGTTTG | 0.80 |
| AT1G20450 | AGTCCGATTGGCCCACAT | ATGTGGGCCAATCGGACT | 0.80 |
| AT2G42540 | ATGTACTTGACGAGATCG | CGATCTCGTCAAGTACAT | 0.80 |
| AT1G43890 | CTTTTGACTAATTAGTTT | AAACTAATTAGTCAAAAG | 0.80 |
| AT4G03165 | AAAGAATCTGGACTGAAA | TTTCAGTCCAGATTCTTT | 0.80 |
| AT1G75700 | GATTGTTGACTATTTAAA | TTTAAATAGTCAACAATC | 0.80 |
| AT4G13730 | GTTTGCTTGTCTCAACTT | AAGTTGAGACAAGCAAAC | 0.80 |
| AT3G58130 | TTTAACTTAACTCGTGAA | TTCACGAGTTAAGTTAAA | 0.80 |
| AT2G14920 | TTTACATTGACCCCAGCT | AGCTGGGGTCAATGTAAA | 0.80 |
| AT1G20180 | TGATACATTGACCCATTC | GAATGGGTCAATGTATCA | 0.80 |
| AT2G25905 | ATGGACCTTAACTAGAAG | CTTCTAGTTAAGGTCCAT | 0.80 |
| AT2G44140 | CGTAACTTGACTCGATAT | ATATCGAGTCAAGTTACG | 0.80 |
| AT3G52900 | ACGGATTTTGACCTCTCA | TGAGAGGTCAAAATCCGT | 0.80 |
| AT3G61710 | CTACGGAAACTTGACTCG | CGAGTCAAGTTTCCGTAG | 0.80 |
| AT4G33390 | TGGAAGCTTGGCTCATAT | ATATGAGCCAAGCTTCCA | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Guo, M.; Hong, W.; Li, M.; Zhu, X.; Guo, C.; Shu, Y. Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis. Agronomy 2025, 15, 1700. https://doi.org/10.3390/agronomy15071700
Li S, Guo M, Hong W, Li M, Zhu X, Guo C, Shu Y. Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis. Agronomy. 2025; 15(7):1700. https://doi.org/10.3390/agronomy15071700
Chicago/Turabian StyleLi, Shuaixian, Meiyan Guo, Wei Hong, Manman Li, Xiaoyue Zhu, Changhong Guo, and Yongjun Shu. 2025. "Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis" Agronomy 15, no. 7: 1700. https://doi.org/10.3390/agronomy15071700
APA StyleLi, S., Guo, M., Hong, W., Li, M., Zhu, X., Guo, C., & Shu, Y. (2025). Overexpression of a White Clover WRKY Transcription Factor Improves Cold Tolerance in Arabidopsis. Agronomy, 15(7), 1700. https://doi.org/10.3390/agronomy15071700






