Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) Strains on Eggs from Different Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Location
2.2. Collection, Rearing, and Maintenance of T. absoluta in the Laboratory
2.3. Collection, Rearing, and Maintenance of T. pretiosum Strains
2.4. Selection of T. pretiosum Strains for the Control of T. absoluta
2.5. Statistical Analysis
2.6. Sampling, DNA Extraction, and Detection of Endosymbionts via Polymerase Chain Reaction (PCR) in T. absoluta Populations
2.6.1. Genomic DNA Extraction
2.6.2. PCR Reaction
3. Results and Discussion
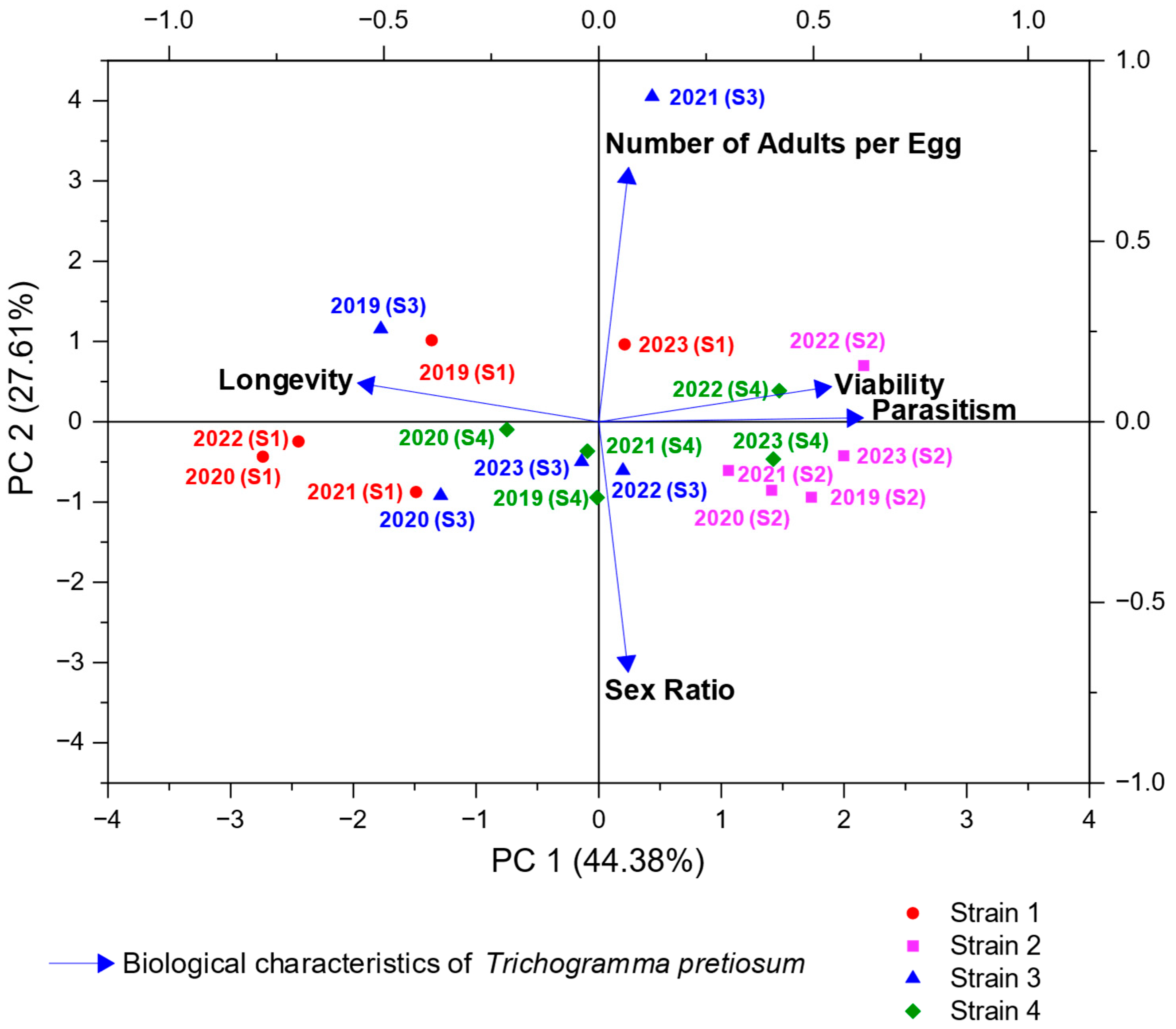
3.1. Performance and Comparative Analysis of Trichogramma pretiosum Strains Against Tuta absoluta Eggs
3.2. Detection of Endosymbionts via PCR in T. absoluta Populations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, P.H.M.; Castillo, R. Comercialização de produtos hortigranjeiros. Cad. Geogr. 2023, 33, 1–29. [Google Scholar] [CrossRef]
- Pratissoli, D.; Bueno, R.C.O.F.; Carvalho, J.R. Trichogramma da Coleta à Pesquisa Aplicada; UNICOPY: Alegre, Brazil, 2019; 211p. [Google Scholar]
- Bacci, L.; Silva, E.M.; Martins, J.C.; Silva, R.S.; Chediak, M.; Milagres, C.C.; Picanço, M.C. The seasonal dynamic of Tuta absoluta in Solanum lycopersicum cultivation: Contributions of climate, plant phenology, and insecticide spraying. Pest Manag. Sci. 2021, 77, 3187–3197. [Google Scholar] [CrossRef]
- Silva, J.E.; Silva, W.M.; Silva, T.B.M.; Campos, M.R.; Filho, A.B.E.; de Siqueira, H.Á.A. High resistance to insect growth disruptors and control failure likelihood in Brazilian populations of the tomato pinworm Tuta absoluta. Phytoparasitica 2021, 49, 689–701. [Google Scholar] [CrossRef]
- Dominguez, A.; Puigmartı, M.; Bosch, M.P.; Rosell, G.; Crehuet, R.; Ortiz, A.; Quero, C.; Guerrero, A. Synthesis, Functional Assays, Electrophysiological Activity, and Field Tests of Pheromone Antagonists of the Tomato Leafminer, Tuta absoluta. J. Agric. Food Chem. 2016, 64, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Wang, S.; Zhang, F.; Desneux, N. Biological Control with Trichogramma in China: History, Present Status and Perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.R.P.; Pinto, A.S.; Nava, D.E.; Oliveira, R.C.; Diniz, A.J.F. Controle biológico com parasitoides e predadores na agricultura brasileira. In Trichogramma spp. Um Caso de Sucesso de Controle Biológico Aplicado no Brasil; Parra, J.R.P., Coelho Junior, A., Eds.; FEALQ: Piracicaba, Brazil, 2021; pp. 203–234. [Google Scholar]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V.P. Changes in Functional and Numerical Responses of the Parasitoid Wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae) Over 45 Generations of Rearing on Ephestia kuehniella. Ann. Entomol. Soc. Am. 2022, 115, 326–335. [Google Scholar] [CrossRef]
- Hassan, S.A. Selection of suitable Trichogramma strains to control the codling moth Cydia pomonella and the two summer fruit tortrix moths Adoxophyes orana, Pnademis heparana (Lep.: Tortricidae). Entomophaga 1989, 34, 19–27. [Google Scholar] [CrossRef]
- Pratissoli, D.; Parra, J.R.P. Desenvolvimento e exigências térmicas de Trichogramma pretiosum Riley, criados em duas traças do tomateiro. Pesqui. Agropecu. Bras. 2000, 35, 1281–1288. [Google Scholar] [CrossRef]
- Hassan, S.A. Strategies to select Trichogramma species for use in biological control. In Biological Control with Egg Parasitoids; Wajnberg, E., Hassan, S.A., Eds.; Oxford University: Wallingford, UK, 1994; pp. 55–71. [Google Scholar]
- Gonçalves, J.R.; Holtz, A.M.; Pratissoli, D.; Guedes, R.N.C. Avaliação da qualidade de Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) em ovos de Sitotroga cerealella (Lepidoptera: Gelechiidae). Acta Sci. Agron. 2003, 25, 485–489. [Google Scholar] [CrossRef]
- Bartlett, A.C. Genetic changes during insect-domestication. In Advances and Challenges in Insect Rearing; King, E.C., Leppla, N.C., Eds.; USDA/ARS: New Orleans, LA, USA, 1985; pp. 2–8. [Google Scholar]
- Bi, S.; Wang, X.; Tang, Y.; Lei, Y.; Guo, J.; Yang, N.; Wan, F.; Lü, Z.; Liu, W. Bacterial communities of the internal reproductive and digestive tracts of virgin and mated Tuta absoluta. Insects 2023, 14, 779. [Google Scholar] [CrossRef]
- Awori, R.M.; Hendre, P.; Amugune, N.O. The genome of a steinernematid-associated Pseudomonas piscis bacterium encodes the biosynthesis of insect toxins. Access Microbiol. 2023, 5, 000659-v3. [Google Scholar] [CrossRef] [PubMed]
- Bottega, D.B.; Boiça Junior, A.L.; Rodrigues, N.E.; Souza, B.H.S.; Forim, M.R.; Santos, T.F. Atratividade, consumo e mortalidade de Tuta absoluta (Lepidoptera: Gelechiidae) em tomateiro tratado com óleo de Melia azedarach. Rev. Cienc. Agrar. 2018, 41, 454–463. [Google Scholar] [CrossRef]
- Parra, J.R.P.; Lopes, J.R.; Zucchi, R.A.; Neto, S.S. Metodologia de criação de Anagasta kuehniella (Zeller, 1879) para produção massal de Trichogramma spp. An. Soc. Entomol. Bras. 1989, 18, 403–415. [Google Scholar] [CrossRef]
- Bueno, R.C.; Parra, J.R.P.; Bueno, A.F.; Haddad, M.L. Performance of trichogrammatids as biocontrol agents of Pseudoplusia includens Walker (Lepidoptera: Noctuidae). Neotrop. Entomol. 2009, 38, 389–394. [Google Scholar] [CrossRef]
- Stein, C.P.; Parra, J.R.P. Uso da radiação para inviabilizar ovos de Anagasta kuehniella (Zeller, 1879) visando estudos com Trichogramma spp. An. Soc. Entomol. Bras. 1987, 16, 229–231. [Google Scholar] [CrossRef]
- Bowen, W.R.; Stern, V.M. Effect of the temperature on the production of males and sexual mosaics in a uniparenteral race of Trichogramma semifumatum (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1966, 59, 823–834. [Google Scholar] [CrossRef]
- Cruz, C.D. Genes: A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- OriginPro® 2021 (Free Trial). Available online: https://www.originlab.com/ (accessed on 3 November 2023).
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 2013, 54, 506–513. [Google Scholar] [CrossRef]
- Mehrkhou, F.; Güz, N.; Korkmaz, E.M.; Çağatay, N.S. Analysis of genetic variation in an important pest, Tuta absoluta, and its microbiota with a new bacterial endosymbiont. Turk. J. Agric. For. 2021, 45, 111–123. [Google Scholar] [CrossRef]
- Banadkuki, S.Z.; Rahmani, S.; Bandani, A.R. Bacterial communities of two populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Asia-Pac. Entomol. 2024, 27, 102295. [Google Scholar] [CrossRef]
- Thao, M.L.; Baumann, P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl. Environ. Microbiol. 2004, 70, 3401–3406. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Perlman, S.J. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 2004, 13, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Brown, J.K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2002, 95, 711–718. [Google Scholar] [CrossRef]
- Montenegro, H.V.N.; Solferini, V.N.; Klaczko, L.B.; Hurst, G.D.D. Malekilling Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol. Biol. 2005, 14, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.M.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 2006, 72, 3646–3652. [Google Scholar] [CrossRef]
- Ribeiro, M.F. Parasitismo de Populações de Anaphes nitens (Hymenoptera: Mymaridae) em Gonipterus platensis (Coleoptera: Curculionidae) e Endossimbiontes Associados. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2019. [Google Scholar]
- Heddi, A.A.M.; Grenier, A.M.; Khatchadourian, C.; Charles, H.; Nardon, P. Four intracellular genomes direct weevil biology: Nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl. Acad. Sci. USA 1999, 96, 6814–6819. [Google Scholar] [CrossRef]
- Nováková, E.; Hypsa, V.; Moran, N.A. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol. 2009, 9, 143. [Google Scholar] [CrossRef]
- Thao, M.L.; Moran, N.A.; Abbot, P.; Brennan, E.B.; Burckhardt, D.H.; Baumann, P. Cospeciation of psyllids and their primary prokaryotic endosymbionts. Appl. Environ. Microbiol. 2000, 66, 2898–2905. [Google Scholar] [CrossRef]
- Guz, N.; Arshad, M.; Cagatay, N.; Dageri, A. High prevalence of Pantoea in Diaphorina citri (Hemiptera: Liviidae): Vector of citrus huanglongbing disease. Curr. Microbiol. 2020, 77, 1525–1531. [Google Scholar] [CrossRef]
- Sandström, J.P.; Russell, J.A.; White, J.P.; Moran, N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001, 10, 217–228. [Google Scholar] [CrossRef]
- Vossbrinck, C.R.; Baker, M.D.; Didier, E.S.; Debrunner-Vossbrinck, B.A.; Shadduck, J.A. Ribosomal DNA sequences of Encephalitozoon hellem and Encephalitozoon cuniculi: Species identification and phylogenetic construction. J. Eukaryot. Microbiol. 1993, 3, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Paes, J.P.P.; Lima, V.L.S.; Pratissoli, D.; Carvalho, J.R.; Bueno, R.C.O. Seleção de parasitóides do gênero Trichogramma (Hymenoptera: Trichogrammatidae) e parasitismo em diferentes idades de ovos de Duponchelia fovealis (Lepidoptera: Crambidae). Acta Sci. Biol. Sci. 2018, 40, 1–9. [Google Scholar] [CrossRef]
- Pratissoli, D.; Parra, J.R. Seleção de linhagens de Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) para o controle das traças Tuta absoluta (Meyrick) e Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2001, 30, 277–282. [Google Scholar] [CrossRef]
- Bleicher, E.; Parra, J.R.P. Espécies de Trichogramma parasitóides de Alabama argillacea. II. Tabela de vida de fertilidade e parasitismo de três populações. Pesqui. Agropecu. Bras. 1990, 25, 207–214. [Google Scholar]
- Carvalho, G.A.D.; Palma, P.L.B.; Silva, E.S.; Melhado, K.Y.; Souza, S.L.; Raices, S.R. Cádmio em hortaliças: Comparando agricultura orgânica e convencional. Alim. Cienc. Tecnol. Meio Amb. 2020, 1, 35–60. [Google Scholar]
- Sá, L.A.N.; Parra, J.R.P. Biology and parasitism of Trichogramma pretiosum Riley (Hym.: Pyralidae) on Ephestia kuehniella (Zeller) and Heliothis zea (Boddie) (Lep.: Noctuidae) eggs. J. Appl. Entomol. 1994, 118, 38–43. [Google Scholar] [CrossRef]
- Molina, R.M.S.; Parra, J.R.P. Seleção de linhagens de Trichogramma (Hymenoptera: Trichogrammatidae) e determinação do número de parasitóides a ser liberado para o controle de Gymnandrosoma aurantianum Lima (Lepidoptera: Tortricidae). Rev. Bras. Entomol. 2006, 50, 534–539. [Google Scholar] [CrossRef]
- Dias, N.D.S.; Parra, J.R.P.; Lima, T.C.C. Seleção de hospedeiro alternativo para três espécies de tricogramatídeos neotropicais. Pesqui. Agropecu. Bras. 2008, 43, 1467–1473. [Google Scholar] [CrossRef]
- Poorjavad, N.; Goldansaz, S.H.; Hosseininaveh, V.; Nozari, J.; Dehghaniy, H.; Enkegaard, A. Fertility life table parameters of different strains of Trichogramma spp. collected from eggs of the carob moth Ectomyelois ceratoniae. Entomol. Sci. 2011, 14, 245–253. [Google Scholar] [CrossRef]
- Pinto, J.R.L.; Fernandes, O.A. Parasitism capacity of Telenomus remus and Trichogramma pretiosum on eggs of moth pests of peanut. Bull. Insectol. 2020, 73, 71–78. [Google Scholar]
- Pernambuco, J.C.A.; Gladenucci, J.; Favoreto, A.L.; Bueno, R.C. Tempo de permanência em campo do parasitoide Trichogramma pretiosum após a liberação. Ecossist 2014, 39, 57–61. [Google Scholar]
- Santos, N.R.; Almeida, R.P.; Padilha, I.Q.M.; Araújo, D.A.; Creão-Duarte, A.J. Molecular identification of Trichogramma species from regions in Brazil using the sequencing of the ITS2 region of ribosomal DNA. Braz. J. Biol. 2015, 75, 391–395. [Google Scholar] [CrossRef]
- Wu, L.H.; He, Y.; Hoffmann, A.A.; Thomson, L.J. Potential impact of climate change on parasitism efficiency of egg parasitoids: A meta-analysis of Trichogramma under variable climate conditions. Agric. Ecosyst. Environ. 2016, 231, 143–155. [Google Scholar] [CrossRef]
- Valente, E.C.N.; Broglio, S.M.F.; Passos, E.M.; Lima, A.S.T. Desempenho de Trichogramma galloi (Hymenoptera: Trichogrammatidae) sobre ovos de Diatraea spp. (Lepidoptera: Crambidae). Pesqui. Agropecu. Bras. 2016, 51, 293–300. [Google Scholar] [CrossRef]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V.P. Quality control of the parasitoid wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae) over 45 generations of rearing on Sitotroga cerealella. Insect Sci. 2021, 28, 180–190. [Google Scholar] [CrossRef]
- Hassan, S.A. Seleção de espécies de Trichogramma para o uso em programas de controle biológico. In Trichogramma e o Controle Biológico Aplicado; Parra, J.R.P., Zucchi, R.A., Eds.; FEALQ: Piracicaba, Brazil, 1997; pp. 183–205. [Google Scholar]
- Venkatesan, T.; Jalali, S.K. Trichogrammatids: Adaptation to stresses. In Biological Control of Insect Pests Using Egg Parasitoids; Sithanantham, S., Ballal, C.R., Jalali, S.K., Bakthavatsalam, N., Eds.; SPRINGER: New Delhi, India, 2013; pp. 105–125. [Google Scholar]
- Twort, V.G.; Blande, D.; Duplouy, A. One’s trash is someone else’s treasure: Sequence read archives from Lepidoptera genomes provide material for genome reconstruction of their endosymbionts. BMC Microbiol. 2022, 22, 209. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.; Chen, Y.; Lu, Z.; Gui, F. Tracking adaptive pathways of invasive insects: Novel insight from genomics. Int. J. Mol. Sci. 2023, 24, 8004. [Google Scholar] [CrossRef]
- Nedoluzhko, A.V.; Kadnikov, V.V.; Beletsky, A.V.; Sharko, F.S.; Tsygankova, S.V.; Mardanov, A.V.; Ravin, N.V.; Skryabin, K.G. Microorganisms associated with microscopic insects Megaphragma amalphitanum and Scydosella musawasensis. Microbiology 2017, 86, 533–535. [Google Scholar] [CrossRef]
- Wang, H.; Xian, X.; Gu, Y.; Castañé, C.; Arnó, J.; Wu, S.; Wan, F.; Liu, W.; Zhang, G.; Zhang, Y. Similar bacterial communities among different populations of a newly emerging invasive species, Tuta absoluta (Meyrick). Insects 2022, 13, 252. [Google Scholar] [CrossRef]
- Kikuchi, Y. Endosymbiotic bacteria in insects: Their diversity and culturability. Microbes Environ. 2009, 24, 195–204. [Google Scholar] [CrossRef]
- Whittle, M.; Barreaux, A.M.; Bonsall, M.B.; Ponton, F.; English, S. Insect-host control of obligate, intracellular symbiont density. Proc. R. Soc. B 2021, 288, 20211993. [Google Scholar] [CrossRef] [PubMed]
- Siozios, S.; Jimenez, P.N.; Azagi, T.; Sprong, H.; Frost, C.; Parratt, S.R.; Taylor, G.; Brettell, L.; Liew, K.C.; Croft, L.; et al. Genome dynamics across the evolutionary transition to endosymbiosis. bioRxiv 2023, 5, 1–16. [Google Scholar] [CrossRef]
- Foray, V.; Grigorescu, A.S.; Sabri, A.; Haubruge, E.; Lognay, G.; Francis, F.; Fauconnier, M.L.; Hance, T.; Thonart, P. Whole-genome sequence of Serratia symbiotica strain cwbi-2.3T, a free-living symbiont of the black bean aphid Aphis fabae. Genome Announc. 2014, 2, e00767-14. [Google Scholar] [CrossRef] [PubMed]
- Perreau, J.; Patel, D.K.; Anderson, H.; Maeda, G.P.; Elston, K.M.; Barrick, J.; Moran, N.A. Vertical transmission at the pathogen–symbiont interface: Serratia symbiotica and aphids. mBio 2021, 12, e00359-21. [Google Scholar] [CrossRef]
- Nadal-Jimenez, P.; Parrat, S.R.; Siozios, S.; Hurst, G.D. Isolation, culture and characterization of Arsenophonus symbionts from two insect species reveal loss of infectious transmission and extended host range. Front. Microbiol. 2023, 14, 1089143. [Google Scholar] [CrossRef]
- Thierry, M.; Becker, N.; Reynaud, B.; Lett, J.M.; Delatte, H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011, 20, 2172–2187. [Google Scholar] [CrossRef]
- Wu, L.H.; Hoffmann, A.A.; Thomson, L.J. Taiwanese Trichogramma of Asian corn borer: Morphology, ITS-2 rDNA characterization, and natural Wolbachia infection. Insect Sci. J. 2016, 16, 22. [Google Scholar] [CrossRef]
- Russell, J.E.; Nunney, L.; Saum, M.; Stouthamer, R. Host and symbiont genetic contributions to fitness in a Trichogramma–Wolbachia symbiosis. PeerJ 2018, 6, e4655. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; Boyd, B.M.; Dale, C. The life of an insect endosymbiont from the cradle to the grave. Curr. Biol. 2019, 29, R485–R495. [Google Scholar] [CrossRef]
- Gupta, A.; Nair, S. Dynamics of insect–microbiome interaction influence host and microbial symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef]
- Sabater-Muñoz, B.; Toft, C. Evolution from free-living bacteria to endosymbionts of insects: Genomic changes and the importance of the chaperonin GroEL. In Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects; SPRINGER: Cham, Switzerland, 2020; pp. 77–103. [Google Scholar] [CrossRef]
- Skaljac, M.; Zanic, K.; Kostanjsek, R. The presence of Wolbachia in Tuta absoluta (Lepidoptera: Gelechiidae) populations from coastal Croatia and Montenegro. Afr. Entomol. 2012, 20, 191–194. Available online: https://hdl.handle.net/10520/EJC119288 (accessed on 25 November 2023). [CrossRef]
- Duman, M. DNA barcoding of sunn pest adult parasitoids using cytochrome c oxidase subunit I (COI). Biochem. Syst. Ecol. 2015, 59, 70–77. [Google Scholar] [CrossRef]
- Shashank, P.R.; Twinkle, S.; Chandrashekar, K.; Meshram, N.M.; Suroshe, S.S.; Bajracharya, A.S. Genetic homogeneity in South American tomato pinworm, Tuta absoluta: A new invasive pest to oriental region. 3 Biotech 2018, 8, 350. [Google Scholar] [CrossRef]
- Hagenblad, J.; Hülskötter, J.; Acharya, K.P.; Brunet, J.; Chabrerie, O.; Cousins, S.A.O.; Dar, P.A.; Diekmann, M.; De Frenne, P.; Hermy, M.; et al. Low genetic diversity despite multiple introductions of the invasive plant species Impatiens glandulifera in Europe. BMC Genet. 2015, 16, 103. [Google Scholar] [CrossRef]



| Strain | Host Insect (Eggs) | Host Plants | Collection Origin |
|---|---|---|---|
| S1 | Ephestia kuehniella | Brassicas | Pardinho, São Paulo, Brazil |
| S2 | Ephestia kuehniella | Tomato | Mogi Mirim, São Paulo, Brazil |
| S3 | Spodoptera frugiperda | Maize | Botucatu, São Paulo, Brazil |
| S4 | Ephestia kuehniella | Atemoya | Pardinho, São Paulo, Brazil |
| Endosymbiont/ Microsporidium | Target Gene | Primer Sequence 5′ > 3′ | Size (bp) * | Reference |
|---|---|---|---|---|
| Arsenophonus | 16S rRNA | F-CGTTTGATGAATTCATAGTCAAA R-GTCCTCCAGTTAGTGTTACCCAAC | 600 | [26] |
| Cardinium | 16S rRNA | F-TACTGTAAGAATAAGCACCGGC R-GTGGATCACTTAACGCTTTCG | 900 | [27] |
| Hamiltonella | 16S rRNA | F-TGAGTAAAGTCTGGAATCTGG R-AGTTCAAGACCGCAACCTC | 700 | [28] |
| Spiroplasma | 16S rRNA | F-GCTTAACTCCAGTTCGCC R-CCTGTCTCAATGTTAACCTC | 800 | [29] |
| Rickettsia | 16S rRNA | F-GCTCAGAACGAACGCTATC R-GAAGGAAAGCATCTCTGC | 900 | [30] |
| Serratia | 16S rRNA | F-CGCAGGCGGTTTGTTAAGTC R-CTTCAAGGGCACAACCTCCA | 268 | [31] |
| Wolbachia | 16S rRNA | F-CGGGGGAAAAATTTATTGCT R-AGCTGTAATACAGAAAGTAAA | 700 | [32] |
| Sodalis | 16S rRNA | F-ACCGCATAACGTCGCAAGACCR-TAACCCAACATTTCTCAACACGAG | 1000 | [33] |
| Carsonela | 16S rRNA | F-CACGTGCTACAATGAGTAAAACAA R-GGTTCCCCTACAGCTACCTTG | 279 | [34] |
| Pantoea | 16S rRNA | F-ACGGAGGGTGCAAGCGTTAAT R-AGGTAAGGTTCTTCGCGTTGCA | 630 | [35] |
| Regiella | 16S rRNA | F-ATCGGGGAGTAGCTTGTCAT R-TACGGYTACCTTGTTACGACTT | 1000 | [36] |
| Nosema | 16S rRNA | F-CACCAGGTTGATTCTGCC R-TTATAGTCCTGCTAATGGTTC | 222 | [37] |
| Endosymbionts | PCR Cycle Conditions |
|---|---|
| Arsenophonus and Hamiltonella | Initial denaturation at 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 5 min. |
| Spiroplasma and Regiella | Initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, 52 °C for 1 min, 72 °C for 2 min, and a final extension at 72 °C for 5 min. |
| Rickettsia and Cardinium | Initial denaturation at 95 °C for 2 min, followed by 30 cycles of 92 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. |
| Serratia | Initial denaturation at 95 °C for 10 min, followed by 35 cycles of 95 °C for 1 min, 62 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 1 min. |
| Wolbachia | Initial denaturation at 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. |
| Sodalis | Initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, 62 °C for 1 min, 72 °C for 2 min, and a final extension at 72 °C for 5 min. |
| Carsonela | Initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min. |
| Pantoea | Initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, 72 °C for 90 s, and a final extension at 72 °C for 10 min. |
| Nosema | Initial denaturation at 95 °C for 4 min, followed by 45 cycles of 95 °C for 1 min, 48 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 4 min. |
| Sources of Variation | Variables | ||||
|---|---|---|---|---|---|
| P | V | L | NAE | SR | |
| T. absoluta populations (A) | 8.72 * | 0.92 ns | 8.66 * | 3.09 * | 3.72 * |
| T. pretiosum strains (B) | 30.30 * | 6.44 * | 37.30 * | 2.56 ns | 5.94 * |
| A × B | 2.32 * | 1.47 ns | 2.01 ns | 4.30 * | 8.81 * |
| Overall mean | 47.30 | 97.50 | 12.40 | 1.01 | 0.68 |
| CV (%) | 27.70 | 6.11 | 6.97 | 0.35 | 0.49 |
| Biological Characteristics | T. absoluta Populations Collected in Different Years | Trichogramma pretiosum Strains | |||
|---|---|---|---|---|---|
| S1 1,2 | S2 1,2 | S3 1,2 | S4 1,2 | ||
| Parasitism (%) | 2019 | 32.2 ± 9.07 abB | 67.7 ± 4.59 abA | 30.0 ± 9.03 aB | 42.9 ± 6.84 bcB |
| 2020 | 16.6 ± 2.98 bB | 66.3 ± 7.32 abA | 37.7 ± 8.23 aB | 22.0 ± 4.44 cB | |
| 2021 | 30.6 ± 8.23 bB | 58.3 ± 6.71 bA | 47.8 ± 8.92 aAB | 35.0 ± 6.23 bcAB | |
| 2022 | 26.0 ± 5.57 bB | 77.2 ± 5.35 abA | 44.3 ± 9.42 aB | 69.2 ± 4.08 aA | |
| 2023 | 58.3 ± 6.92 aB | 85.0 ± 3.51 aA | 41.2 ± 5.51 aB | 56.6 ± 7.45 abB | |
| Viability (%) | 2019 | 96.8 ± 0.63 aB | 98.7 ± 0.62 aA | 96.8 ± 1.20 aA | 96.5 ± 2.58 aA |
| 2020 | 95.1 ± 2.12 aB | 97.8 ± 1.14 aA | 97.1 ± 1.66 aA | 97.1 ± 1.31 aA | |
| 2021 | 95.6 ± 2.44 aB | 100 ± 0.00 aA | 98.9 ± 0.41 aA | 97.6 ± 1.27 aA | |
| 2022 | 91.6 ± 1.98 aB | 99.2 ± 0.45 aA | 99.6 ± 0.26 aA | 97.9 ± 0.75 aA | |
| 2023 | 97.5 ± 1.18 aB | 98.8 ± 0.69 aA | 96.8 ± 1.39 aA | 100 ± 0.00 aA | |
| Longevity (days) | 2019 | 15.1 ± 0.90 abA | 10.7 ± 0.89 aB | 16.4 ± 0.52 aA | 10.8 ± 0.79 aB |
| 2020 | 17.7 ± 0.72 aA | 10.9 ± 1.15 aB | 16.7 ± 0.88 aA | 11.5 ± 0.98 aB | |
| 2021 | 14.5 ± 0.79 abA | 9.65 ± 0.86 aC | 13.1 ± 1.00 bAB | 11.2 ± 0.89 aBC | |
| 2022 | 13.2 ± 0.92 bA | 9.75 ± 0.99 aB | 14.0 ± 0.96 abA | 9.15 ± 0.52 aB | |
| 2023 | 12.5 ± 0.78 bA | 10.6 ± 0.56 aA | 11.3 ± 0.67 bA | 10.6 ± 0.67 aA | |
| Number of adults per egg (NAE) | 2019 | 1.01 ± 0.00 aA | 1.00 ± 0.00 aA | 1.01 ± 0.00 bA | 1.00 ± 0.00 aA |
| 2020 | 1.02 ± 0.02 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 bA | 1.00 ± 0.00 aA | |
| 2021 | 1.00 ± 0.00 aB | 1.00 ± 0.00 aB | 1.14 ± 0.11 aA | 1.00 ± 0.01 aB | |
| 2022 | 1.02 ± 0.01 aA | 1.10 ± 0.03 aA | 1.00 ± 0.00 bA | 1.00 ± 0.00 aA | |
| 2023 | 1.00 ± 0.00 aA | 1.00 ± 0.01 aA | 1.00 ± 0.00 bA | 1.00 ± 0.00 aA | |
| Sex Ratio | 2019 | 0.54 ± 0.04 bB | 0.76 ± 0.02 aA | 0.52 ± 0.06 bB | 0.76 ± 0.03 aA |
| 2020 | 0.76 ± 0.04 aAB | 0.73 ± 0.03 aAB | 0.79 ± 0.02 aA | 0.65 ± 0.06 abB | |
| 2021 | 0.77 ± 0.03 aA | 0.78 ± 0.02 aA | 0.53 ± 0.05 bB | 0.69 ± 0.02 abA | |
| 2022 | 0.70 ± 0.02 aAB | 0.72 ± 0.02 aAB | 0.75 ± 0.04 aA | 0.59 ± 0.02 bB | |
| 2023 | 0.52 ± 0.03 bB | 0.71 ± 0.02 aA | 0.71 ± 0.03 aA | 0.72 ± 0.03 abA | |
| Endosymbionts | Tuta absoluta Populations (Collection Years) | ||||
|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | |
| Rickettsia | - | - | - | - | - |
| Hamiltonella | - | - | - | - | - |
| Wolbachia | - | - | - | - | - |
| Arsenophonus | + | + | - | - | - |
| Cardinium | - | - | - | - | - |
| Sodalis | - | - | - | - | - |
| Nosema | - | - | - | - | - |
| Carsonella | - | - | - | - | - |
| Spiroplasma | - | - | - | - | - |
| Serratia | - | + | + | - | - |
| Pantoea | - | - | - | - | - |
| Regiella | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalbianco, A.B.; Daniel, D.F.; Pratissoli, D.; Alvarez, D.d.L.; Silva, N.N.P.d.; Santos, D.M.; Seabra Júnior, S.; Oliveira, R.C.d. Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) Strains on Eggs from Different Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Agronomy 2025, 15, 1692. https://doi.org/10.3390/agronomy15071692
Dalbianco AB, Daniel DF, Pratissoli D, Alvarez DdL, Silva NNPd, Santos DM, Seabra Júnior S, Oliveira RCd. Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) Strains on Eggs from Different Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Agronomy. 2025; 15(7):1692. https://doi.org/10.3390/agronomy15071692
Chicago/Turabian StyleDalbianco, Alessandro Bandeira, Diego Fernando Daniel, Dirceu Pratissoli, Daniel de Lima Alvarez, Nadja Nara Pereira da Silva, Daniel Mariano Santos, Santino Seabra Júnior, and Regiane Cristina de Oliveira. 2025. "Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) Strains on Eggs from Different Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)" Agronomy 15, no. 7: 1692. https://doi.org/10.3390/agronomy15071692
APA StyleDalbianco, A. B., Daniel, D. F., Pratissoli, D., Alvarez, D. d. L., Silva, N. N. P. d., Santos, D. M., Seabra Júnior, S., & Oliveira, R. C. d. (2025). Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) Strains on Eggs from Different Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Agronomy, 15(7), 1692. https://doi.org/10.3390/agronomy15071692







