Dynamic Changes in Fatty Acids in Macadamia Fruit During Growth and Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Test Materials
2.1.2. Reagents and Instruments
2.2. Experimental Methods
2.2.1. Determination of Single Fruit Weight and Longitudinal and Transverse Diameter of Fruits
2.2.2. Standard Solution Preparation
2.2.3. Metabolite Extraction
2.2.4. LC-MS Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Macadamia Fruit Development
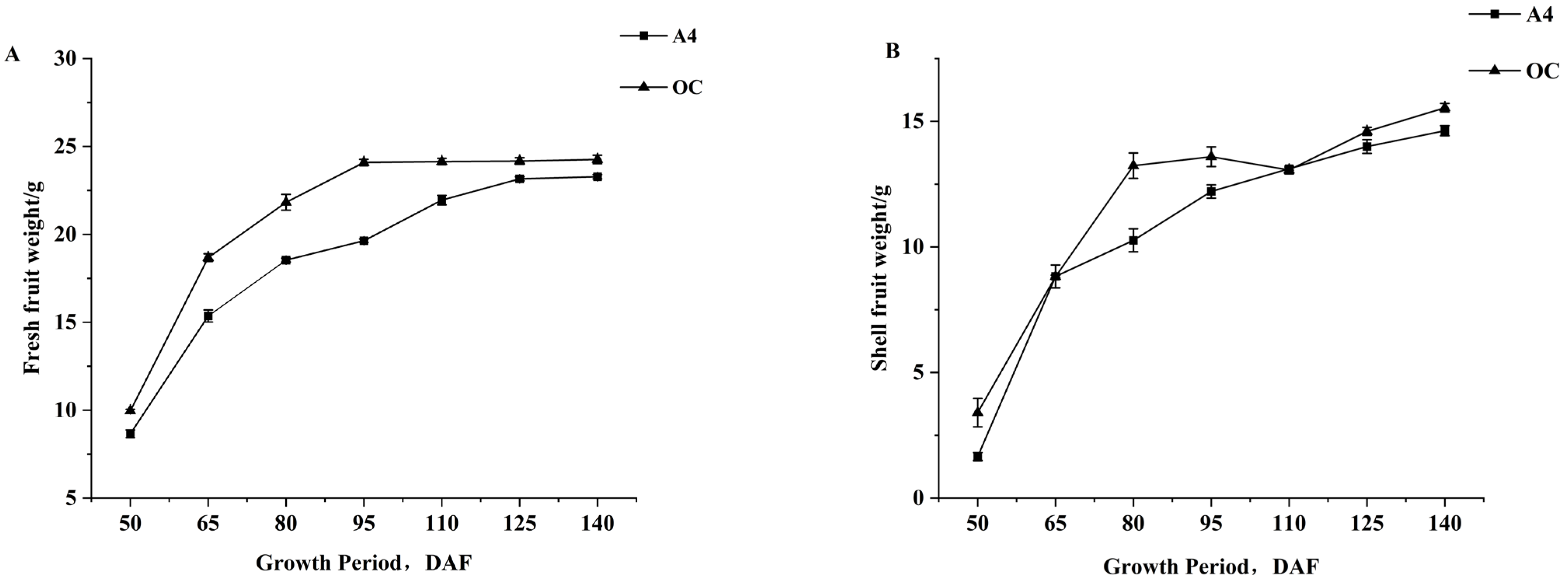
3.2. Dynamic Changes in Green Fruit Weight and Shell Fruit Weight During Growth of Macadamia
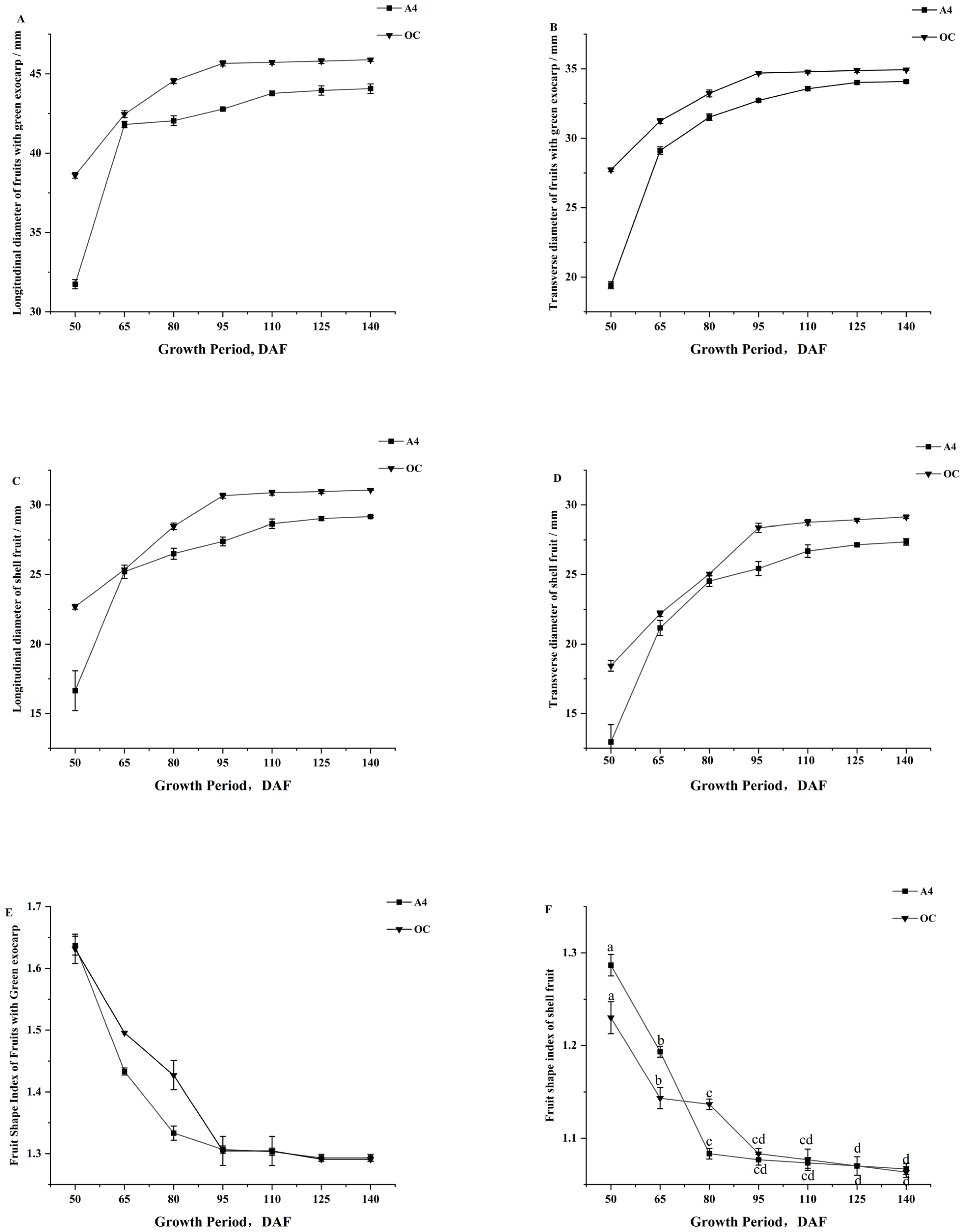
3.3. Dynamics of Longitudinal and Transverse Diameters and Fruit Shape Index of Macadamia During Growth
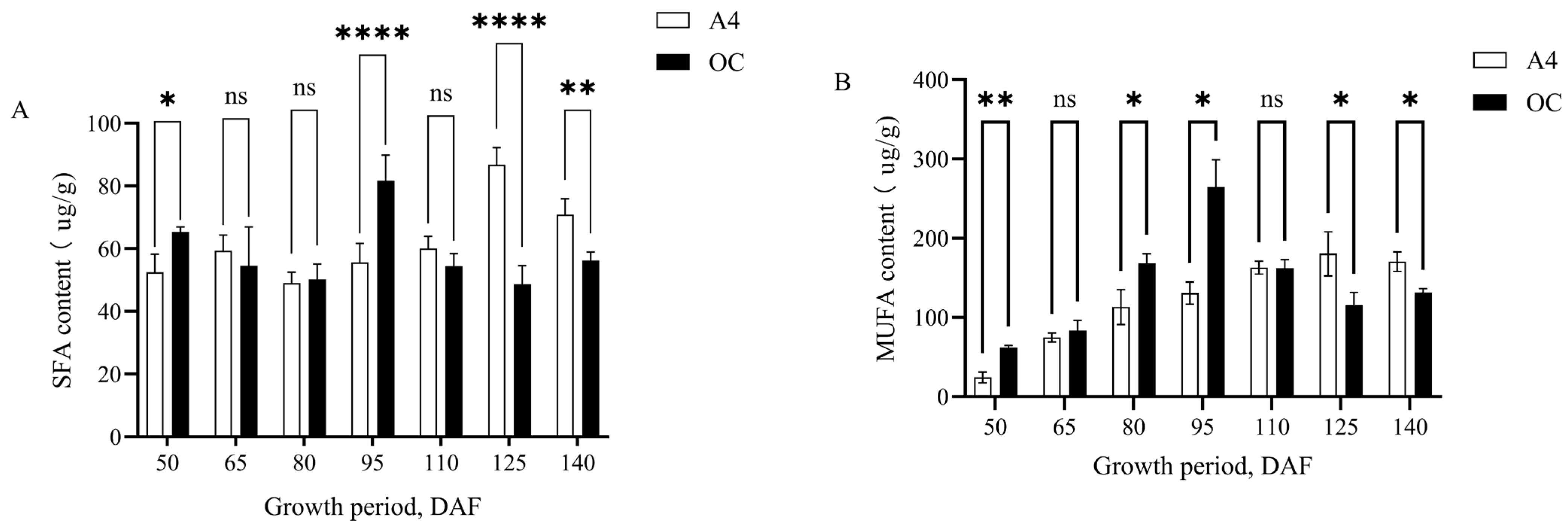
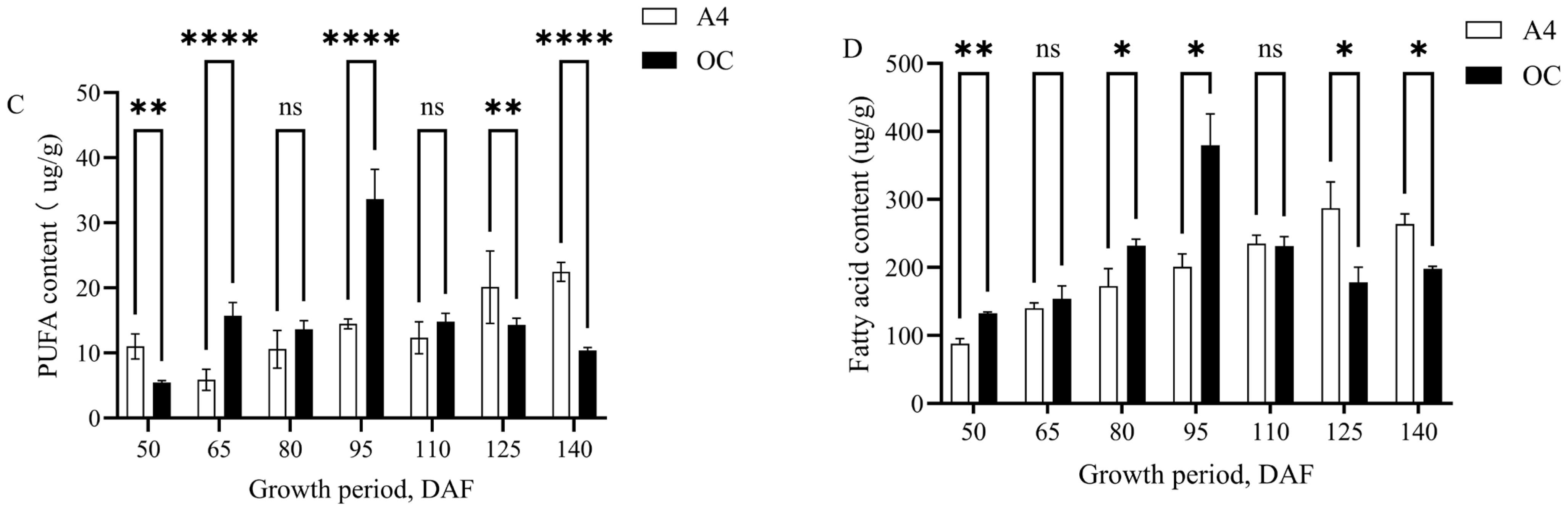
3.4. Changes in SFA, MUFA, and PUFA Contents During Growth and Development of Macadamia Kernels
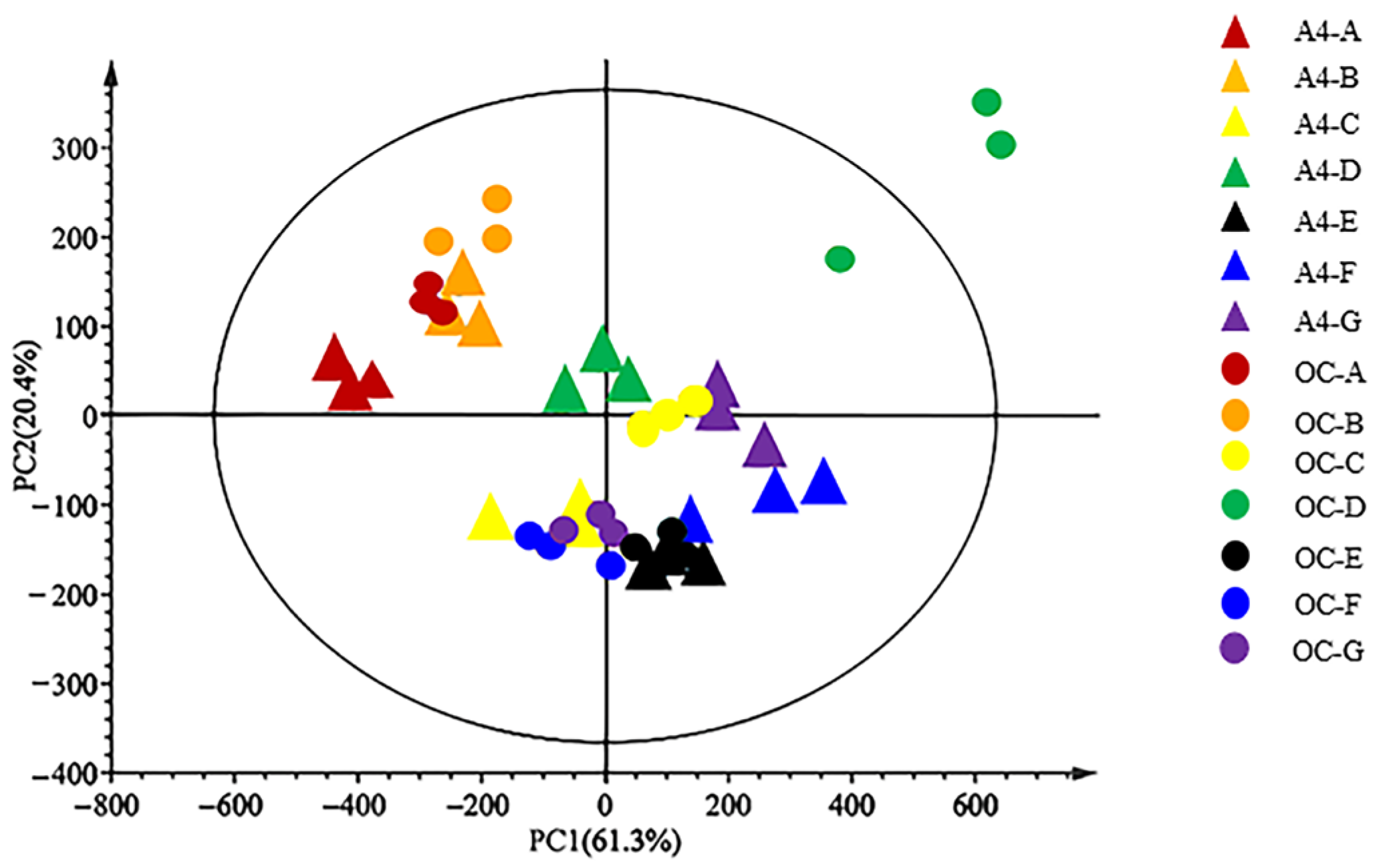
3.5. Principal Component Analysis of Fatty Acid Fractions and Contents in Macadamia Kernels at Different Developmental Stages
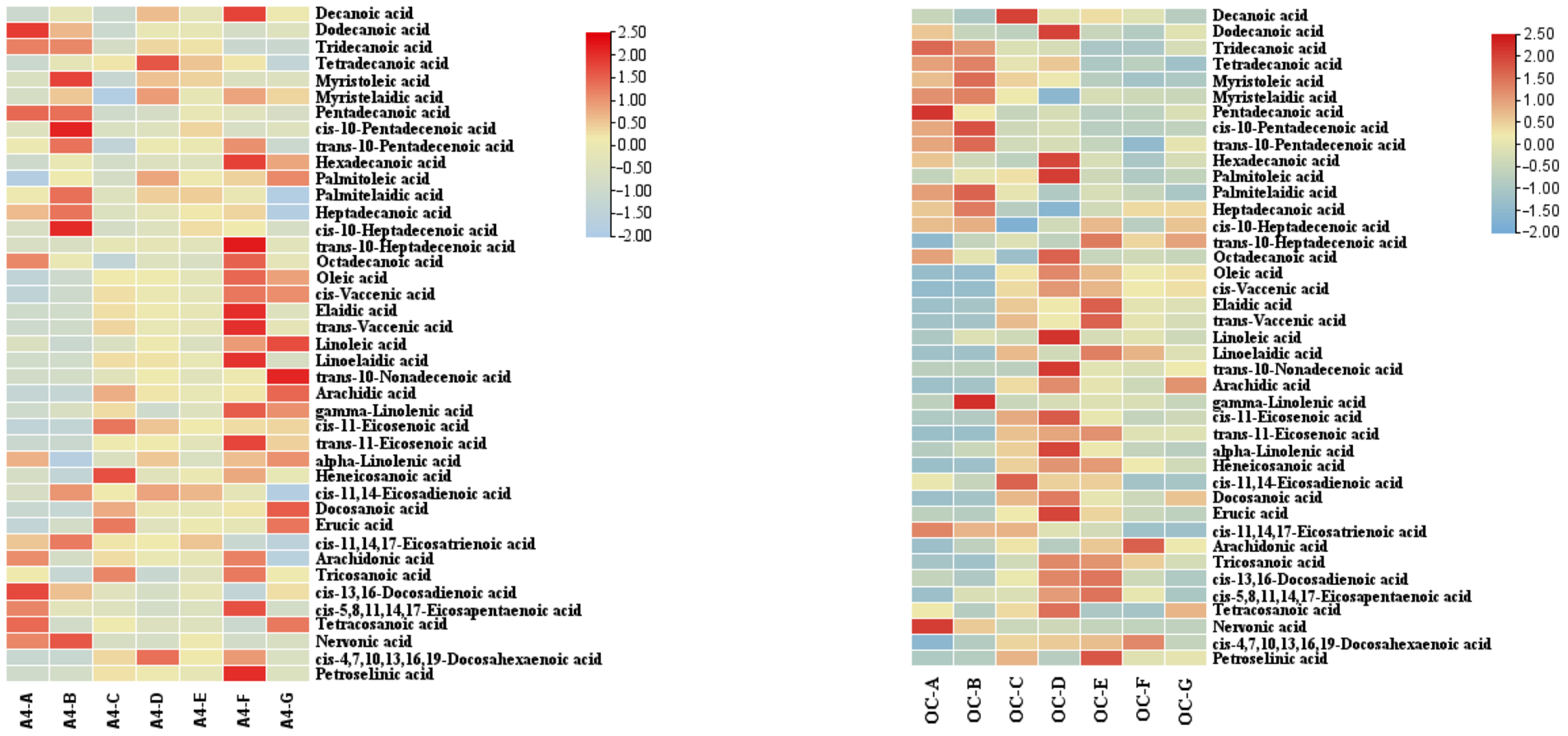
3.6. Heatmap Analysis of Fatty Acids in Macadamia Kernel at Different Developmental Stages
3.7. Changes in Fatty Acid Composition in Macadamia Kernels at Different Developmental Stages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nisa, F.U.; Batool, T. The Nutritional and Therapeutic Aspects of Australian Macadamia Nuts. In Ethnobotanical Insights into Medicinal Plants; IGI Global: Hershey, PA, USA, 2024; pp. 279–307. [Google Scholar]
- Yang, F.; Tang, J.; Fu, X.M.; Tang, S.P.; Yang, H.X.; Dong, M.C.; Luo, X.P. Overview and prospect of production and marketing situation of macadamia in China. S. China Fruits 2025, 54, 1–5. [Google Scholar]
- Tu, X.-H.; Wu, B.-F.; Xie, Y.; Xu, S.-L.; Wu, Z.-Y.; Lv, X.; Wei, F.; Du, L.-Q.; Chen, H. A Comprehensive Study of Raw and Roasted Macadamia Nuts: Lipid Profile, Physicochemical, Nutritional, and Sensory Properties. Food Sci. Nutr. 2021, 9, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Kaczynski, P.; Iwaniuk, P.; Rutkowska, E.; Socha, K.; Orywal, K.; Farhan, J.A.; Perkowski, M. Nutritional compounds and risk assessment of mycotoxins in ecological and conventional nuts. Food Chem. 2024, 458, 140222. [Google Scholar] [CrossRef]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative study on the extraction of macadamia (Macadamia integrifolia) oil using different processing methods. LWT 2022, 154, 112614. [Google Scholar] [CrossRef]
- Gnoni, G.V.; Natali, F.; Geelen, M.J.H.; Siculella, L. Oleic acid as an inhibitor of fatty acid and cholesterol synthesis. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: New York, NY, USA, 2010; pp. 1365–1373. [Google Scholar]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Lim, H.; Lim, S. The potential cardiometabolic effects of long-chain ω-3 polyunsaturated fatty acids: Recent updates and controversies. Adv. Nutr. 2023, 14, 612–628. [Google Scholar] [CrossRef]
- Bozbas, E.; Zhou, R.; Soyama, S.; Allen-Redpath, K.; Mitchell, J.L.; Fisk, H.L.; Calder, P.C.; Jones, C.; Gibbins, J.M.; Fischer, R. Dietary n-3 polyunsaturated fatty acids alter the number, fatty acid profile and coagulatory activity of circulating and platelet-derived extracellular vesicles: A randomized, controlled crossover trial. Am. J. Clin. Nutr. 2024, 119, 1175–1186. [Google Scholar] [CrossRef]
- Leaf, A. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. J. Cardiovasc. Med. 2007, 8 (Suppl. S1), S27–S29. [Google Scholar] [CrossRef]
- Poggioli, R.; Hirani, K.; Jogani, V.G.; Ricordi, C. Modulation of inflammation and immunity by omega-3 fatty acids: A possible role for prevention and to halt disease progression in autoimmune, viral, and age-related disorders. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7380–7400. [Google Scholar]
- Yamagata, K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017, 16, 118. [Google Scholar] [CrossRef]
- Bouali, I.; Trabelsi, H.; Abdallah, I.B.; Albouchi, A.; Martine, L.; Grégoire, S.; Bouzaien, G.; Gandour, M.; Boukhchina, S.; Berdeaux, O. Changes in fatty acid, tocopherol and xanthophyll contents during the development of Tunisian-grown pecan nuts. J. Am. Oil Chem. Soc. 2013, 90, 1869–1876. [Google Scholar] [CrossRef]
- Rezanejad, F.; Shekari, M. Studies of growth, oil, and fatty acids in seeds of two cultivars of Pistacia vera L. in relation with developmental stages. Trees 2019, 33, 577–586. [Google Scholar] [CrossRef]
- Koaze, H.; Karanja, P.N.; Kojima, M.; Baba, N.; Ishibashi, K. Lipid accumulation of macadamia nuts during kernel development. Food Preserv. Sci. 2002, 28, 67–73. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Hong, H.T.; Pun, S.; Liu, D.; Torrisi, C.; Mai, T.; Alam, M.B.; Russell, D.; Topp, B. Macadamia nuts—Good Fats, Bad Fats, and Biofortification. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2020; Volume 1292, pp. 377–382. [Google Scholar]
- O’Hare, T.J.; Trieu, H.H.; Topp, B.; Russell, D.; Pun, S.; Torrisi, C.; Liu, D. Assessing fatty acid profiles of macadamia nuts. HortScience 2019, 54, 633–637. [Google Scholar] [CrossRef]
- Kaijser, A.; Dutta, P.; Savage, G. Oxidative stability and lipid composition of macadamia nuts grown in New Zealand. Food Chem. 2000, 71, 67–70. [Google Scholar] [CrossRef]
- Kong, G.H.; Tao, L.; He, X.Y.; Ni, S.B.; Chen, L.L.; Xiao, X.M. Study on arrangement of pollination varieties for dominant Macadamia variety Own Chioce. S. China Fruits 2024, 53, 74–79. [Google Scholar]
- Xu, H.; Li, R.; Zhao, J.; Xu, Y.; Chen, Z. Fruit development and abscission of macadamia nuts. Chin. J. Trop. Crops 1995, 02, 78–83. [Google Scholar]
- Jing, M.; Gong, L.; Yue, H.; Li, Z.; Wu, C.; Chen, Y.; Ning, Y.; Guo, G.; He, X. Fruit development and quality change of ‘O.C’ Cultivar in Macadamia. West. For. Sci. 2024, 5, 76–84. [Google Scholar]
- Stephenson, R.A.; Gallagher, E.C. Effects of temperature during latter stages of nut development on growth and quality of macadamia nuts. Sci. Hortic. 1986, 30, 219–225. [Google Scholar] [CrossRef]
- Sakai, W.S.; Nagao, M.A. Fruit growth and abscission in Macadamia imtegrifolia. Physiol. Plant. 1985, 64, 455–460. [Google Scholar] [CrossRef]
- Han, S.; Luo, L.; Lu, J.; He, E.; Lu, Z. Fruit Development Dynamics and Model Establishment of Four Macadamia spp. Varieties in Guizhou. Seed 2023, 42, 79–85+97. [Google Scholar]
- Huang, C.; Li, Y.; Wang, K.; Zhang, J.; Xu, Y.; Si, X.; Pei, D. Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development. Food Chem. 2022, 370, 130975. [Google Scholar] [CrossRef]
- Parcerisa, J.; Codony, R.; Boatella, J.; Rafecas, M. Triacylglycerol and phospholipid composition of hazelnut (Corylus avellana L.) lipid fraction during fruit development. J. Agric. Food Chem. 1999, 47, 1410–1415. [Google Scholar] [CrossRef]
- Han, S.; Luo, L.; Lu, J.; Zhang, Z.; Zhu, W.; Wang, G.; Liu, F. Yield Performance of four macadamia nut varieties of different ages in Guizhou. S. China Fruit Tree 2022, 51, 109–113. [Google Scholar]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M.G. Changes in fatty acid and tocopherol content during almond (Prunus dulcis, cv. Nonpareil) kernel development. Sci. Hortic. 2017, 225, 150–155. [Google Scholar] [CrossRef]
- Zeng, W.; Lei, B.; Chen, Y.; Chen, J.; Li, P.; Jiang, L.; Li, C.; Liu, Q.; Yang, Y. Regulatory mechanism of carbohydrate metabolism pathways on oil biosynthesis of oil plant Symplocos paniculata. Front. Plant Sci. 2025, 16, 1452533. [Google Scholar] [CrossRef]
- Lucchetti, S.; Ambra, R.; Pastore, G. Effects of peeling and/or toasting on the presence of tocopherols and phenolic compounds in four Italian hazelnut cultivars. Eur. Food Res. Technol. 2018, 244, 1057–1064. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Mapel-Velazco, L.; Martín-del-Campo, S.T.; Chávez-Servia, J.L.; Martínez, A.J.; Verdalet-Guzmán, I. Fatty acids profile of oil from nine varieties of macadamia nut. Int. J. Food Prop. 2017, 20, 1262–1269. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, C.; Niu, Y. Analysis of fatty acid synthesis in macadamia nuts based on transcriptome sequencing. Trop. Agric. Sci. Technol. 2022, 45, 12–18+23. [Google Scholar]
- Wenya, G. Molecular Regulation of Fatty Acid Synthesis in Siberian Almond Seed Kernels. Master’s Thesis, Shenyang Agricultural University, Liaoning, China, 2023. [Google Scholar]
- Jin, F.; Zhou, Y.; Zhang, P.; Huang, R.; Fan, W.; Li, B.; Li, G.; Song, X.; Pei, D. Identification of key lipogenesis stages and proteins Involved in walnut kernel development. J. Agric. Food Chem. 2023, 71, 4306–4318. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, R.; Lai, H.; He, Y.; Chen, Y.; Xun, C.; Zhang, Y.; He, Z. Comparative transcriptomic and lipidomic analysis of fatty acid accumulation in three Camellia oleifera varieties during seed maturing. J. Agric. Food Chem. 2024, 72, 18257–18270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Du, S.; Gu, Q.; Fu, J.; Wang, Z. Screening of key genes in fatty acid synthesis in seed kernel of Elaeagnus mollis diels. Acta Bot. Boreali-Occident. Sin. 2024, 44, 938–949. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Zhang, B.; Duan, M.; Zhang, H.; Yan, S.; Yang, F.; Shi, W.; Fu, X.; Yang, H.; Li, J.; et al. Dynamic Changes in Fatty Acids in Macadamia Fruit During Growth and Development. Agronomy 2025, 15, 1682. https://doi.org/10.3390/agronomy15071682
Cao M, Zhang B, Duan M, Zhang H, Yan S, Yang F, Shi W, Fu X, Yang H, Li J, et al. Dynamic Changes in Fatty Acids in Macadamia Fruit During Growth and Development. Agronomy. 2025; 15(7):1682. https://doi.org/10.3390/agronomy15071682
Chicago/Turabian StyleCao, Mingqun, Birong Zhang, Minxian Duan, Hanyao Zhang, Suyun Yan, Fan Yang, Wenbin Shi, Xiaomeng Fu, Hongxia Yang, Jinxue Li, and et al. 2025. "Dynamic Changes in Fatty Acids in Macadamia Fruit During Growth and Development" Agronomy 15, no. 7: 1682. https://doi.org/10.3390/agronomy15071682
APA StyleCao, M., Zhang, B., Duan, M., Zhang, H., Yan, S., Yang, F., Shi, W., Fu, X., Yang, H., Li, J., & Zhou, X. (2025). Dynamic Changes in Fatty Acids in Macadamia Fruit During Growth and Development. Agronomy, 15(7), 1682. https://doi.org/10.3390/agronomy15071682





