Abstract
This study examined the regulatory mechanism of calcium (Ca) amendment on the dynamics of soil organic carbon (SOC) fractions and extracellular enzyme activities, elucidating the role of Ca in soil carbon cycling processes. A field experiment with maize was conducted, comparing treatments of low calcium (T1), high calcium (T2), and a calcium-free control (CK). Measurements included inter-root SOC fractions—soluble organic carbon (DOC), microbial biomass carbon (MBC), and readily oxidizable organic carbon (ROC)—and the activities of the following extracellular enzymes: β-xylanase, β-glucosidase (β-glu), phenol oxidase (Phox), peroxidase (Pero), phosphatase (Phos), acetylaminoglucosidase (NAG), and urease. The main findings indicated the following: (1) Calcium addition significantly increased SOC content (115.04% and 99.22% higher in T1 and T2, respectively, than CK during the entire reproductive period) and enhanced microbial activity (elevated DOC and MBC). However, SOC decreased by 8.44% (T1) and 16.38% (T2) relative to CK in the late reproductive stage (irrigation–ripening), potentially reflecting microbial utilization (supported by the inverse correlation between SOC and MBC/DOC), and maize carbon reallocation during grain filling. (2) Calcium activated β-glu, Phox, Phos, NAG, and urease (p < 0.05), with pronounced increases in Phox (241.13 IU·L−1) and Phos (1126.65 U·L−1), indicating enhanced organic matter mineralization and phosphorus availability. (3) Calcium-driven MBC and ROC accumulation was associated with the positive regulation of Phox (path coefficient > 0.8) and the negative regulation of Phos. SOC was co-regulated by β-glu and Phos (R2 = 0.753). (4) Calcium dynamically optimized the short-term carbon distribution through enzyme activity while promoting long-term sequestration. Our study provides new evidence supporting multi-pathway interactions through which calcium mediates enzyme networks to influence the soil carbon cycle. The findings provide a theoretical foundation for calcium fertilizer management and soil carbon sequestration strategies in agriculture, advancing academic and practical goals for sustainable development and carbon neutrality.
1. Introduction
In the context of global climate change, the dynamic balance of soil organic carbon (SOC) pools has become a central issue in regulating the carbon sink functions of terrestrial ecosystems [1]. As a hotspot of plant–soil–microbe interactions, SOC transformation processes dominated by extracellular enzyme activities in the inter-root microdomains play a decisive role in soil carbon sequestration. Recent studies have shown that exogenous calcium addition significantly affects the organic carbon mineralization–sequestration balance by modulating soil physicochemical properties (e.g., pH, aggregate stability) and microbial metabolic activities [2]. Previous studies have often examined bulk soils, overlooking enzymatic spatial heterogeneity in the inter-root zone where plant–microbe interactions intensify carbon dynamics [3]. In maize cropping systems, calcium is widely used as a soil amendment, yet its regulatory mechanism on SOC fraction transformation (e.g., readily oxidizable organic carbon, soluble organic carbon) mediated by inter-root extracellular enzymes remains poorly understood. For instance, studies on field-scale SOC stabilization lacked the resolution of inter-root enzyme stoichiometry [4]. Elucidating this process could not only reveal calcium-mediated feedback mechanisms in the soil carbon cycle but also provide a theoretical basis for assessing the carbon sink effects of agricultural management practices [4]. This is of significant scientific importance for achieving the precise control of farmland ecosystems.
Previous studies have explored the effects of calcium addition on soil carbon cycling from multiple perspectives. For instance, calcium ions may enhance SOC physical protection through physicochemical pathways by promoting soil aggregate formation, increasing adsorption sites on mineral surfaces, and stimulating extracellular enzyme secretion (e.g., β-glucosidases, peroxidases) [5]. Regarding carbon fraction specificity, some studies suggest that calcium preferentially stabilizes mineral-bound carbon while potentially accelerating particulate organic carbon decomposition. These findings provide a preliminary framework linking calcium to soil carbon cycling. However, existing conclusions remain context-dependent due to the limited temporal monitoring of enzyme networks and SOC fractions. For example, short-term incubations failed to capture the feedback between microbial adaptation and carbon pool remodeling [6]. In low-pH soils, calcium addition often elevates pH, stimulating microbial activity and enhancing SOC mineralization, whereas in neutral soils, cation bridging effects may inhibit enzyme activity [7].
Despite progress, key controversies persist. Most studies focus on field-scale soils, neglecting the coupling between calcium gradient distribution and the spatial heterogeneity of enzyme activities at the inter-root scale [8]. Existing models also tend to isolate the effects of calcium on enzyme activities or SOC fractions, failing to capture the cascading effects of “calcium addition–microbial community reconfiguration–extracellular enzyme regulation–SOC fraction transformation.” [9,10]. Furthermore, short-term field incubation experiments cannot adequately reveal the long-term feedback between microbial adaptation and carbon pool remodeling post-calcium addition [11]. These limitations contribute to uncertainties in predicting calcium-mediated SOC stabilization mechanisms. Unlike isolated approaches, our integrated experimental design links calcium gradients with enzyme stoichiometry and SOC fractions across maize growth stages, addressing cascading “Ca-enzyme-SOC” interactions.
To address these gaps, multistage sampling enables the tracking of temporal enzyme–SOC coupling, while redundancy analysis (RDA) quantifies the core drivers of carbon fractions in this study to investigate two core questions through continuous calcium addition during the maize reproductive period: (1) How does calcium addition drive the dynamic partitioning of SOC fractions via the regulation of maize inter-root extracellular enzyme activities? (2) What is the coupling mechanism among calcium addition gradients, enzyme stoichiometry, and carbon fraction dynamics in this process? By uncovering the “enzyme–carbon–microbe” cascade in calcium-mediated inter-root carbon cycling, this work provides a novel theoretical foundation for understanding exogenous additives’ role in soil carbon sinks, optimizing calcium application strategies, and advancing the dual goals of yield enhancement and carbon sequestration.
2. Materials and Methods
2.1. Experimental Program
(1) Experimental site: The field scientific observation and research station of the College of Life Sciences of Yan’an University-Ansai District Experimental Demonstration Station of Ecological Agricultural Science and Technology Innovation was selected. The station, approved by the Department of Science and Technology of Shaanxi Province, is located in Ansai District, Yan’an, Shaanxi Province, with a geographic location of 36°51′30″ N, 109°19′23″ E. The average annual rainfall of 540 mm is mainly concentrated in July–September, with an average annual air temperature of 8.8 °C, annual sunshine hours of 2416 h, and an annual frost-free period of 143–174 days without irrigation, which belongs to the typical agricultural arid cropping area. In the test area, loess parent material is widely exposed at the surface, predominantly comprising homogeneous loessial soil (yellow loamy soil). The soil texture was sandy loam [12].
The basic physical and chemical properties of the soil were as follows: pH 8.5 in the 0–100 cm layer, organic matter 6.36 g·kg−1, total nitrogen 0.83 g·kg−1, total phosphorus 0.57 g·kg−1, total potassium 18.20 g·kg−1, available phosphorus 15.30 mg·kg−1, fast-acting potassium 141.35 mg·kg−1, and uniform soil fertility.
(2) Experimental design: A randomized complete block design (RCBD) with calcium as the single factor was used. Blocks accounted for spatial soil heterogeneity. During the whole reproductive period of corn, according to the deficit standard of the calcium and magnesium demand of maize, the reference threshold of deficiency supplementation, and the research basis of the previous researchers, we set up two calcium (Ca) levels (low: 24.50 kg·hm−2, high: 49.00 kg·hm−2), and devised three experimental treatments: ① low calcium—T1; ② high calcium—T2; ③ no calcium—CK. Each treatment was repeated 4 times, totaling 12 experimental sample plots. We selected “Haoshan 168”, which was disease-resistant, high-yielding, highly adaptable, and had fast kernel drying, as the spring maize variety for testing [13]. Calcium was chelated with sugar alcohol (Ca, 180 g·L−1, 250 g/bottle). This kind of calcium exists in the form of an exchange state and water-soluble state, which is absorbed efficiently, green, and non-toxic.
Fertilizer application time and method: The amount of calcium fertilizers in each treatment was applied in the ratio of 1:2:3:4 in the seedling–extraction, nodulation–tasseling, tasseling–irrigating, and irrigating–ripening stages to form four fertilizer gradients in the whole maize life cycle. Calcium fertilizer was selected on windless and sunny days, and the soil around the maize root system was treated with uniform fertilization to ensure that the maize absorbed the nutrients adequately.
(3) Field management: The total number of experimental treatments was 12 sample squares, with a sample area of 6 m×6 m. All the experimental sample squares were planted with ridged furrow cultivation technology (maize grown on the surface of the furrow). The experimental design employed a maize planting density of 60,000 plants per hectare (ha−1). Ridge dimensions comprised a crown width of 10 cm, height of 15 cm, and furrow width of 30 cm, with 50 cm row spacing. Maize was spaced at 34 cm within rows. Sample plots measured 20 cm in width (spanning two adjacent rows) and were spaced 4 m apart. To maintain spatial accuracy, the nearest maize row was positioned 10 cm from the plot edge. Seeds were sown on May 2 and harvested on September 28, in 2024, with an average fertility period of about 145 days. Before the test was conducted (early to mid-April), the quick-acting nitrogen, phosphorus, and potassium content and effective calcium and magnesium content of the soil in the test area were measured. Before sowing, all sample plots were evenly spread with equal amounts of basal fertilizers according to the recommended quantities of local maize basal fertilizers, i.e., N, 130 kg·hm−2; P2O5, 120 kg·hm−2; and K2O, 38 kg·hm−2 [14]. During the whole life span of the maize, the meteorological factors, such as soil moisture, temperature, humidity, and rainfall, were observed regularly.
2.2. Specific Measurement Indexes and Methods
(1) Determination of soil organic carbon (fraction) content, soil carbon source function, and soil extracellular enzyme activity
Sample pretreatment: At the seedling, nodulation, tasseling, grouting, and maturity stages, plants with relatively good growth and uniform size were selected from each sample plot, and soil samples were collected from the inter-root zone of maize at three depths of 0–10 cm, 10–20 cm, and 20–30 cm using a sterile brush. Rhizosphere soil (0–5 cm adhering to roots) was collected using gentle brushing after root excavation, separated from bulk soil (0–30 cm). SOC fractions and enzymes were analyzed exclusively in rhizosphere samples. The whole maize plant was dug out at each fertility stage, and the soil samples in the range of 0–5 cm attached to the root surface of the plant were collected as rhizosphere soil. Four plants were dug out with their roots in each sampling area, and the rhizosphere soil samples of the four plants were mixed in equal parts and treated as a separate sample.
The soil samples from each sampling were individually placed in sterile ziplock bags, stored in an incubator with ice under low temperature conditions, and quickly transported to the laboratory. The composite sample was divided into two parts, one part was naturally air-dried and ground in the laboratory, and passed through 2 mm and 0.2 mm soil sieves for the determination of soil organic carbon (fraction) content, and the other part was used for the determination of soil extracellular enzyme activities and functional diversity of soil microbial carbon effects [15].
a. Determination of soil organic carbon (fraction) content
SOC content: Ground and sieved air-dried soil was first acidified with hydrochloric acid to remove inorganic carbon, then dried and ground, and SOC was determined using an organic carbon analyzer (Vario TOC cube, Elementar Analysensysteme GmbH, Langenselbold, Germany) [16].
DOC content: Leaching using K2SO4 solution. Weigh 10 g of fresh soil sieved through 2 mm sieve in a centrifuge tube and add 40 mL of K2SO4 solution, shake at 300 r min−1 for 1 h, centrifuge for 5 min, filter, and then dilute the leachate to determine DOC with a total organic carbon analyzer (Vario TOC cube, Elementar Analysensysteme GmbH, Langenselbold, Germany), and then convert DOC according to the measured concentration [16].
ROC content: Organic carbon that could be oxidized by potassium permanganate solution was considered as active organic carbon. The air-dried soil was ground and combined with KMNO4 solution, shaken at 60 r min−1 for 1 h, centrifuged for 5 min, and the supernatant was diluted and colorimetrically measured on a spectrophotometer at 565 nm, and the ROC of the samples was calculated based on the consumption of KMNO4 [17].
MBC content: Substrate-induced respiration method. Weigh 10 g of fresh soil through a 2 mm sieve in a wide-mouth jar, add glucose solution and talcum powder, incubate at 22 °C for 2 h, and then measure the CO2 respiration. The sample MBC was converted according to the CO2 concentration [17].
b. Determination of extracellular enzyme activities of soil microorganisms
Five hydrolase activities (URE, Bglu, NAG, Bxyl, and Phos) and two oxidase activities (Phox and Pero) were determined separately.
In the experiment, 1 g of soil sample was weighed separately, and sodium acetate buffer was added and stirred at high speed so that the soil sample and the buffer were fully mixed. Assays used 50 mM sodium acetate buffer (pH 5.0) and 200 μM MUB substrates. Incubation: 2 h at 25 °C. Data expressed per g dry soil. The five hydrolytic enzymes were determined by the microtiter plate fluorescence method, in which soil suspension was added into a 96-well fluorescence reaction plate with fluorescence substrate (4-methylumbelliferyl, MUB) and reaction substrate according to the proportion of soil suspension and fluorescence substrate (Sigma-Aldrich, St. Louis, MO, USA), and the reaction was terminated by the addition of NaOH solution after the constant temperature cultivation in a biochemical incubator at 25 °C, and then measured by multifunctional enzyme marker (Molecular Devices SpectraMax M Series; Molecular Devices LLC, San Jose, CA, USA). The activity of the two oxidative enzymes was measured by the colorimetric method: 1 g of soil sample was weighed and mixed with 125 mL of buffer and stirred, and the mixed soil suspension was centrifuged and the reaction substrate was added into a 96-well fluorescent reaction plate proportionally, and then put into a 25 °C biochemical incubator. After the constant temperature incubation was completed, NaOH solution was added to terminate the reaction, and the reaction was measured by a multifunctional enzyme labeling instrument, and the wavelength of the measurement was 450 nm [18,19].
2.3. Data Standardization
In this study, to the enable cross-comparison of drivers, data were standardized using the extreme difference method. When fitting the regression function between soil organic carbon (fractions) and its core influence factors, it is necessary to standardize the data for the core influence factors. The data standardization adopts the extreme difference method. The principle and formula of the extreme difference method are as follows: normalization refers to the conversion of raw data to numbers ranging between [0, 1] or [−1, 1] by linear transformation. The formula for transformation in the range [0, 1] is as follows: x’ = (x − min)/(max − min), where min is the minimum value and max is the maximum value [10].
2.4. Statistical Analysis Methods
Two-way ANOVA was used to assess treatment differences with Tukey’s HSD test (p < 0.05) by IBM SPSS Statistics 19. Three analytical replicates per plot per growth stage were used for all SOC fraction and enzyme assays. Histograms were used to analyze the characteristics of changes in rhizosphere soil extracellular enzymes and soil organic carbon (fractions) and their differences in content from the milky to the mature stage of maize in each treatment. Correlation network heatmaps were used to analyze the correlation and trend fitting characteristics between soil organic carbon fractions, while correlation matrices used Pearson’s method. Redundancy analysis (RDA) was used to determine the core driving index (soil extracellular enzymes) of soil organic carbon (fractions) after calcium addition by Canoco 5.0. RDA employed the ‘vegan’ package using R 4.2.0. Structural equation modeling (SEM) was used to determine the core pathway of calcium addition to drive the synthesis and accumulation of soil organic carbon fractions through soil extracellular enzymes. SEM was implemented in ‘lavaan’ with maximum likelihood estimation and validated via χ2/df < 3.0, RMSEA < 0.06, CFI > 0.95. SEM can fit well when the data result meets the above standard thresholds.
3. Results and Analysis
3.1. Characteristics and Correlation of Temporal Changes in Soil Organic Carbon (Fractions)
The SOC content of treatments T1 and T2 was significantly higher than that of CK, and the difference was significant (p < 0.05). The SOC of T1 and T2 showed a decreasing trend with fertility stage (e.g., T1 decreased from 150.69 to 137.97 and T2 decreased from 146.36 to 122.39 mg·g−1) at the filling–ripening stage, while the SOC of CK increased slightly at the tasseling–ripening stage. It was found that T2 SOC decreased significantly at filling–ripening (p < 0.05), while T1 showed no statistical decline. The MBC of T1 and T2 showed a small difference from that of CK but was significantly higher at the tasseling–ripening stage of T2. DOC was generally higher in T1 and T2 than in CK, with significant differences (p < 0.05). However, the DOC of CK (1.81 mg·g−1) at the tasseling–irrigation stage was close to the DOC level of T1 and T2. The ROC of treatments T1 and T2 was lower than that of CK, but the difference was not significant, which might be related to carbon form transformation (Figure 1).
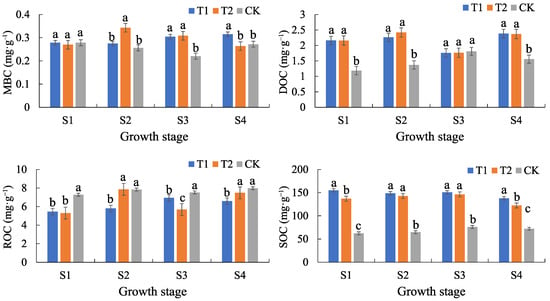
Figure 1.
Characteristics of temporal changes in soil organic carbon and its fractions. Indexes are mean ± standard deviation. Lowercase letters indicate the significance of differences in soil organic carbon (fractions) among treatments at the 0.05 level by two-way ANOVA with Tukey’s HSD test. SOC, DOC, MBC, and ROC stand for soil organic carbon, soluble organic carbon, microbial biomass carbon, and readily oxidizable organic carbon, respectively (the same below). S1–S4 denote the maize seedling emergence–nodulation, nodulation–tasseling, tasseling–grouting, and grouting–ripening stages, respectively.
The significant increase in SOC in T1 and T2 indicated that the treatments effectively enhanced the soil carbon pool, which contributed to the improvement of soil fertility and carbon sequestration capacity. The increase in DOC and MBC might reflect the enhancement of microbial activity, which promotes the decomposition of organic matter and nutrient cycling. The decrease in SOC during the irrigating to ripening period might be related to the uptake by crops or microbial consumption (Figure 1). The decrease in ROC might indicate that the addition of calcium altered the morphological distribution of carbon (e.g., from soluble to stabilized forms).
As can be seen from the bar cumulative graph, the maximum values of MBC and DOC in T1 both appeared in the stage of irrigating–ripening, which accounted for 26.85% and 27.85% of the full birth period content, respectively, and the maximum values of these two in T2 were advanced in the time series, which appeared in the stage of nodulation–sprouting. In T1, peak ROC (28.03%) and SOC (25.44%) values both occurred during the spiking–irrigation stage, representing their maximum proportions of the total growth cycle content. Notably, the ROC peak appeared earlier in the temporal sequence, coinciding with the preceding nodulation–irrigation stage. This indicates that the MBC and DOC of maize rhizosphere soil reached the maximum at the filling stage–maturity stage, and the ROC and SOC reached the maximum at the tasseling stage–filling stage after the low level of calcium addition. Compared with the low level of calcium addition, the maximum values of organic carbon content of maize rhizosphere soils with a high level of calcium addition were advanced in the time sequence of the fertility period. In the CK treatment, the maximum values of organic carbon content of maize rhizosphere soils all occurred in the grouting–maturity period (Figure 2).
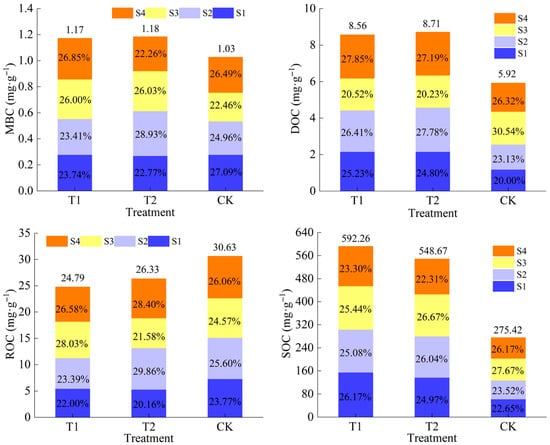
Figure 2.
Accumulation characteristics of soil organic carbon and fractions.
From the correlation matrix, it would be seen that the SOC of T1 showed a significant negative correlation (p < 0.05) with MBC, and the SOC of T2 showed a highly significant negative correlation (p < 0.01) with MBC. The SOC of T1 and T2 showed a negative correlation with DOC and ROC, but did not reach a significant level. In addition, MBC showed a highly significant positive correlation with ROC in T1 (p < 0.01), and DOC showed a highly significant positive correlation with ROC in T2 (p < 0.01). In the CK treatment, SOC showed a significant negative correlation with MBC (p < 0.05), SOC showed a highly significant negative correlation with DOC (p < 0.01), and MBC and DOC also showed a highly significant negative correlation (p < 0.01) (Figure 3). From the scatter plot, it can be seen that SOC showed a linear trend relationship with MBC, DOC, and ROC. In addition, the inverse correlations between SOC and microbial biomass carbon (MBC) suggested that microbial consumption contributed to SOC depletion. While root biomass was not quantified, the timing of SOC decline coincided with peak maize grain filling, implying possible carbon reallocation to reproductive tissues.
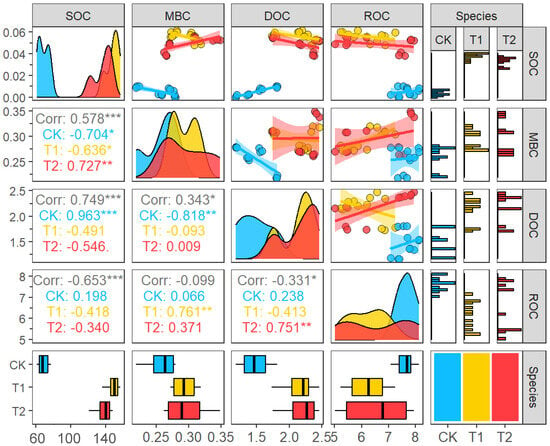
Figure 3.
Correlation of soil organic carbon and its fractions. *, **, *** denote p < 0.05, p < 0.01, p < 0.001, respectively.
3.2. Characteristics of Temporal Changes in Soil Extracellular Enzymes and Their Correlations
The β-xyl activity of T1 and T2 was generally higher than that of CK, and the β-xyl activity of T2 was significantly elevated at the emergence–extraction and tasseling–irrigation stages. The β-xyl activity of CK was the highest at the emergence–tasseling stage (122.86 IU·L−1), but fluctuated greatly. The β-glu activity of T1 and T2 was higher than that of CK, and the difference was significant (p < 0.05), and the β-glu activity of T2 increased gradually with the advancing fertility period, while the β-glu activity of CK decreased gradually with the fertility period (from 22.61 IU·L−1 to 17.88 IU·L−1). Phox activity was significantly higher (p < 0.05) in T1 and T2 than in CK, with the highest Phox activity in T2 (241.13 IU·L−1 over the whole reproductive period). Pero activity was relatively stable in T1, lower and more fluctuating in T2, and lowest in CK. Phos activity was significantly higher in T1 and T2 than in CK (p < 0.05), with the highest Phos activity in T1 (1126.65 U·L−1) and the lowest and stable Phos activity in CK. NAG activity was significantly higher in T2 than in T1 and CK (p < 0.05), and the difference between NAG activity in T1 and CK was small. Urease activity was the highest in T1 (whole-life mean value of 1389.65 IU·L−1), followed by T2 and the lowest in CK, and the urease activity of T1 was significantly (p < 0.05) different from that of T2 and CK. Thus, overall, calcium addition significantly enhanced β-glu, Phox, Pero, Phos, NAG, and urease activities (Figure 4).
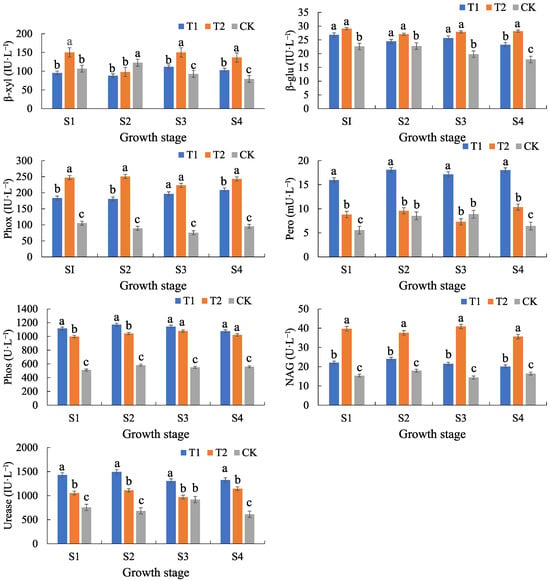
Figure 4.
Characterization of the temporal variation in soil extracellular enzymes. β-xyl, β-glu, Phox, Pero, Phos, NAG, and urease denote β-xylosidase, β-glucosidase, phenol oxidase, peroxidase, phosphatase, acetylaminoglucosidase, and urease, respectively (the same below). Indicators are mean ± standard deviation. Lowercase letters indicate the significance of differences in soil organic carbon (fractions) among treatments at the 0.05 level. S1–S4 denote the maize seedling emergence–extraction, nodulation–tasseling, tasseling–irrigation, and irrigating–ripening stages, respectively.
The elevation of β-glu and Phox activities indicated that calcium addition promoted organic carbon decomposition, which might accelerate soil organic matter mineralization. The significant elevation of Phos activity indicated that calcium addition might promote the conversion of organic phosphorus to inorganic phosphorus, and enhance the effectiveness of soil phosphorus. The elevation of urease activity indicated that calcium addition might accelerate the hydrolysis of urea, increase the supply of soil ammonium nitrogen, and promote the uptake of maize nitrogen. The elevation of NAG activity was able to decompose difficult-to-degrade organic matter, which could lead to the decomposition of organic matter. The enhancement of NAG activity can decompose difficult-to-degrade organic matter, accelerate the mineralization process of soil organic matter, and release available nutrients.
From the correlation matrix plot, it was observed that there were highly significant correlations between all soil extracellular enzymes after calcium addition. The correlation matrix showed that there were highly significant positive correlations between Phos, Pero, and urease (p < 0.001), and there were highly negative correlations among Phos, Pero with Phox, NAG, β-xyl, and β-glu (p < 0.001). Urease showed a highly significant negative correlation with Phox, NAG, β-xyl, and β-glu (p < 0.001). The correlation between Phox, NAG, β-xyl, and β-glu showed a highly significant level of positivity (p < 0.001) (Figure 5).
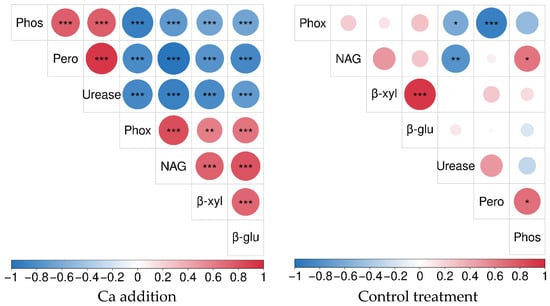
Figure 5.
Correlation of soil extracellular enzymes. *, **, *** denote p < 0.05, p < 0.01, p < 0.001, respectively.
In the control treatment, a highly significant negative correlation was observed between Phox and Pero (p < 0.001) and also between NAG and urease. β-xyl and β-glu showed a highly significant positive correlation (p < 0.001). The correlations between other soil extracellular enzymes were not significant (Figure 5).
3.3. Mechanism of Calcium Addition to Regulate Soil Organic Carbon Accumulation
From the redundancy analysis, Ca and Phox showed a significant positive regulation of DOC, and Phos showed a significant negative regulation of DOC after calcium addition; Ca, Phox, and Phos were also the main indexes affecting the SOC content, and their regulation of SOC was opposite to that of DOC. Ca, Phox, and β-xyl were the main indexes affecting the ROC content, while Ca and Phox positively and significantly regulated ROC content and β-xyl negatively and significantly regulated ROC content. β-xyl was the main indicator affecting MBC content and showed significant negative regulation (Figure 6).
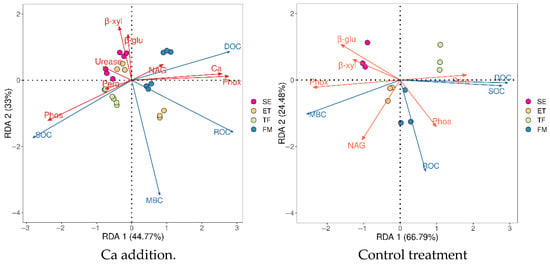
Figure 6.
Redundancy analysis of soil extracellular enzymes regulating soil organic carbon and its fractions after calcium addition. SE, ET, TF, and FM denote the corn seedling–extraction stage, nodulation–tasseling stage, tasseling–irrigation stage, and irrigating–ripening stage, respectively.
In the control treatment, Pero, β-glu, Phox, and NAG were the main indicators affecting DOC and SOC, among which, Pero showed highly significant positive regulation, and the regulation was opposite to the other indicators. Phos, NAG, and β-glu emerged as primary factors regulating ROC content. Phos and NAG significantly promoted ROC accumulation, contrasting with Phox which exhibited inhibitory effects. For MBC, key regulatory enzymes were NAG and Pero, where Phox and NAG stimulated MBC formation, whereas Pero strongly suppressed it (Figure 6).
After constructing the structural equation modeling, it was found that MBC and ROC were mainly regulated by Ca affecting Phox and Phos enzyme activities, in which Ca first positively regulated Phox enzyme activity, and at the same time, Ca negatively regulated Phos enzyme activity, and subsequently, both Phox and Phos positively regulated the accumulation of MBC and ROC contents. In addition, the path coefficients of Phox regulating MBC and ROC were both higher than 0.800, which were significantly higher than those of Phos regulating MBC and ROC, indicating that Ca was associated with MBC and ROC accumulation via Phox regulation, and Phox regulated MBC and ROC much more than Phos (Figure 7).
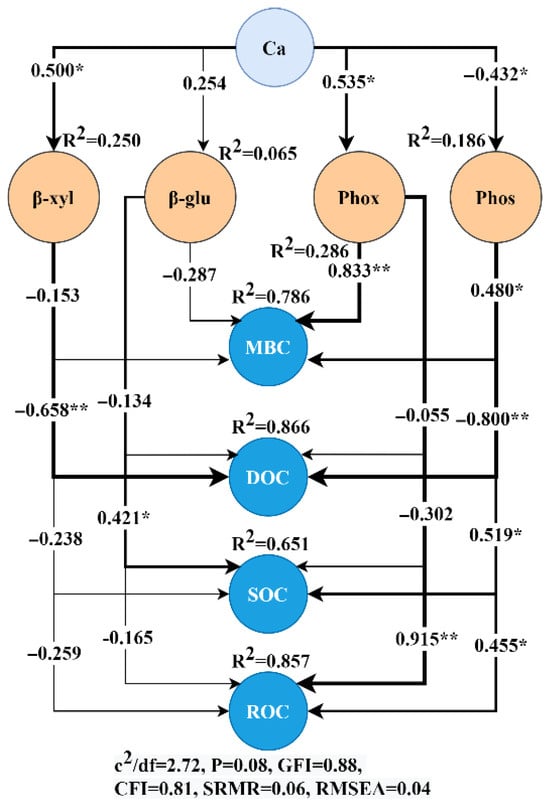
Figure 7.
Structural equation modeling of soil extracellular enzymes regulating soil organic carbon and its fractions after calcium addition. * represents p < 0.05, ** represents p< 0.01; χ2 represents the chi-square; df represents the degree of freedom; GFI represents the goodness of fit index; CFI represents the comparative fit index; and RMSEA represents the root mean square error of approximation.
DOC was mainly regulated by Ca affecting β-xyl and Phos enzyme activities, in which Ca first positively regulated β-xyl enzyme activity and, at the same time, Ca negatively regulated Phos enzyme activity, and subsequently, both β-xyl and Phos negatively regulated DOC content. SOC was mainly regulated by Ca affecting β-glu and Phos enzyme activities, in which Ca first positively regulated β-glu enzyme activity; meanwhile, Ca negatively regulated Phos enzyme activity, and subsequently, both β-glu and Phos positively regulated SOC content (Figure 7).
The trend relationship between soil organic carbon fractions and their core driving indicators was established using scatter fitting plots. As can be seen from the figure, MBC showed a nonlinear relationship with Phox and Phos, and MBC showed a trend of increasing and then decreasing with the increase in Phox and Phos enzyme activities. DOC exhibited linear negative relationships with both β-xyl and Phos, decreasing progressively as their enzymatic activities increased. ROC demonstrated a positive linear correlation with Phox, but a unimodal response to Phos, peaking at intermediate activity levels before declining. SOC showed a nonlinear association with β-glu coupled with a positive linear relationship with Phos (Figure 8).
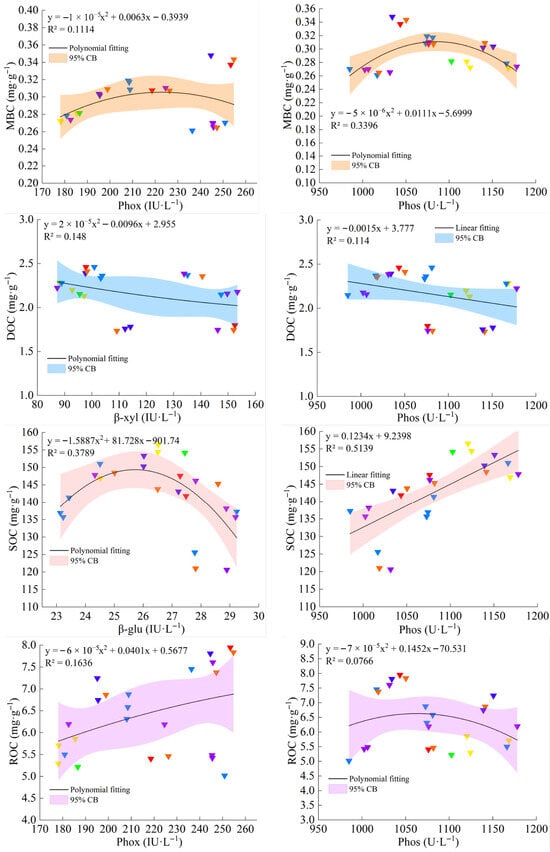
Figure 8.
Trend relationships between soil organic carbon fractions and their core driver indexes after Ca addition. All the relationships on the left and right panels are the results of combining T1 and T2 data. Different symbolic colors represent the dynamic characteristics of the gradual change process of the relationship between the two variables.
Ultimately, a regression function between soil organic carbon fractions and their core influence factors was fitted using partial least squares to quantify the driving relationship between them and determine the contribution of the core factors to the carbon fractions. The specific functional relationships were as follows:
MBC = 0.295 − 0.003 × Ca + 0.033 × Phox + 0.029 × Phos R2 = 0.246
DOC = 2.16 + 0.047 × Ca−0.243 × Phos − 0.277 × β-xyl R2 = 0.748
SOC = 142.617 − 4.809 × Ca + 6.726 × Phos + 2.63 × β-glu R2 = 0.753
ROC = 6.39 + 0.343 × Ca + 0.948 × Phos + 1.065 × Phox R2 = 0.462
4. Discussion
4.1. Effects of Calcium Addition on Soil Organic Carbon and Its Fractions
This study elucidates the mechanism by which calcium addition enhances soil organic carbon (SOC) stability through calcium–enzyme interactions and physical aggregate protection, while clarifying its associated microbial metabolic effects [20]. Calcium ions facilitated the conversion of active carbon to a stabilized state via organic–mineral complex formation. However, concurrent increases in microbial activity—evidenced by elevated soluble organic carbon and microbial biomass carbon—resulted in net SOC loss during the late plant reproductive stage [21]. The SOC decrease may indicate microbial or plant uptake, though root biomass and respiration data would strengthen this interpretation. This reveals a dynamic equilibrium in agroecosystem carbon fixation: a spatiotemporal trade-off between mineral-driven chemical stabilization and biological reactivation processes. Crucially, we demonstrate for the first time that plant–microbe carbon competition can reactivate stable carbon pools at farm-scale resolution, offering a novel framework for predicting SOC stability under global change scenarios [22].
These findings significantly advance the understanding of mineral–biotic interactions in carbon cycling, with important theoretical and practical implications. While confirming calcium-mediated carbon stabilization mechanisms in agricultural soils, our results highlight critical SOC fertility dynamics—notably a 16.38% SOC decline at maize maturity following high calcium inputs [23,24]. This underscores the pivotal role of regional nutrient management in modulating carbon sequestration outcomes. The work provides direct evidence for optimizing calcium fertilization strategies and refining carbon cycle models, particularly through the “dynamic stabilization” concept. This paradigm points to innovative pathways for reconciling carbon sequestration targets with agricultural productivity goals.
The SOC decrease during irrigation–ripening aligns with two non-exclusive mechanisms: (1) microbial utilization evidenced by elevated MBC/DOC and their inverse correlation with SOC, (2) maize carbon reallocation to grain development. While root biomass and respiration were not monitored, the synchronous SOC decline with reproductive maturity supports potential plant-mediated partitioning [24].
Current limitations include short experimental durations restricting the assessment of long-term calcium–carbon sequestration effects. Future research should prioritize multifactorial experiments to unravel calcium–carbon–climate change interactions and conduct cross-validation studies across diverse cropping systems. Such efforts will enable the development of precision calcium-regulated carbon sequestration technologies, ultimately supporting science-based strategies for enhancing SOC stocks in regional agricultural systems.
4.2. Effect of Calcium Addition on Soil Extracellular Enzyme Activities
The significant enhancement of soil enzyme activities by calcium addition may be linked to its key role in regulating soil chemical properties and microbial processes. Calcium influences enzyme activities through multiple mechanisms; acting as a cofactor, it directly activates enzymes such as phosphatases, while indirectly increasing phenoloxidase and peroxidase activities by altering the physicoprotective properties of organic matter and releasing substrates via cation bridging effects [25]. Concurrently, calcium may exacerbate phosphorus fixation and nitrogen limitation, driving the synergistic expression of phosphatases and ureases to alleviate nutrient constraints. The significant positive correlations among enzymes (e.g., phenol oxidase, phosphatase, and urease) reflect soil microorganisms’ adaptive strategies of coupled carbon–nitrogen–phosphorus metabolism [26]. In contrast, the negative correlation between phenol oxidase and cellulases (β-xylosidase and β-glucosidase) highlights a metabolic trade-off in nutrient partitioning, potentially leading to a “carbon partitioning effect” characterized by enhanced recalcitrant carbon decomposition and the suppressed metabolism of easily degradable carbon. This dynamic could influence the stability of the soil carbon pool [27].
This study supports the established paradigm that calcium induces phosphatase activity through phosphorus limitation and corroborates competition between the lignin and cellulose decomposition pathways under the “enzyme-partitioning hypothesis.” [28]. However, the observed activation of β-glucosidase by calcium conflicts with prior findings, which may reflect soil type variability or calcium dosage effects. Similarly, inconsistent urease responses underscore the critical role of substrate availability in regulating enzyme activity. These results emphasize calcium’s non-equilibrium regulatory effects on soil function, challenging the traditional view that cation impacts are mediated solely via pH [29]. Furthermore, incorporating enzyme interactions into soil elemental cycling models could enhance predictions, offering insights for optimizing calcium amendment strategies and soil carbon sequestration.
Current conclusions are constrained by indirect mechanistic evidence (e.g., the absence of microbial community or functional gene validation) and the insufficient consideration of abiotic interactions (e.g., Mg2+ competition and organic matter composition) [30]. Future work should (1) resolve functional microorganism contributions using metagenomic and transcriptomic approaches, (2) conduct long-term field experiments to evaluate calcium leaching dynamics, (3) design multi-ion interaction studies to isolate calcium-specific effects. Such advancements will refine our understanding of ionic regulation in soil biogeochemistry and improve the predictive capacity of ecological models.
4.3. Mechanism of Calcium Addition on Soil Organic Carbon Fraction Regulation
Calcium forms an indirect control network for microbial carbon and easily oxidizable organic carbon through the bidirectional regulation of phenol oxidase and phosphatase activities. The high path coefficient (>0.8) indicates that the positive regulation of phenol oxidase by calcium is the dominant pathway [31]. This likely occurs because phenol oxidase, as an oxidizing enzyme, directly participates in the decomposition of recalcitrant carbon (e.g., lignin), thereby promoting the conversion of macromolecular carbon to stable forms such as readily oxidizable organic carbon [32]. In contrast, the negative regulation of phosphatase may inhibit the mineralization of readily degradable organophosphorus compounds, reducing the competition for carbon and phosphorus and indirectly enhancing carbon sequestration. This mechanism reveals that calcium acts as a “two-way gate” in carbon partitioning, optimizing carbon pool stability by activating recalcitrant carbon decomposition pathways while suppressing the metabolism of readily degradable carbon [33]. The ecological significance of this study lies in calcium’s potential role as a key node in the biogeochemical cycle (C-P-Ca) through its regulation of enzyme activity balance, providing a theoretical foundation for soil carbon sequestration technologies.
This study aligns with the classical “enzyme stoichiometry hypothesis,” which posits that β-glucosidase and phosphatase synergistically drive soil organic carbon accumulation, consistent with microbial resource allocation strategies involving carbon–phosphorus enzymes [34]. However, the novel contribution here is the identification of a unique calcium-mediated regulatory pathway for soluble organic carbon stability: negative regulation via β-glucosidase and phosphatase. This contrasts with conventional views that attribute soluble organic carbon stability primarily to direct complexation by pH or metal ions. The observed discrepancies may arise from the specificity of the study system (e.g., calcium-rich soils), suggesting that calcium’s enzyme-mediated regulation extends beyond its direct chemical role in certain environments [35].
Notably, the predominance of phenol oxidase in this study challenges the conventional emphasis on β-glucosidase as the central carbon cycling enzyme. This implies that recalcitrant carbon-decomposing enzymes may play specialized roles in calcium-enriched ecosystems, diverging from the recent findings on metal–enzyme interactions [36].
However, this study does not account for calcium’s competitive interactions with other cations under field conditions. Further research should integrate isotope tracer techniques and spatially resolved enzyme profiling to elucidate the molecular mechanisms underlying calcium’s regulation of soil enzyme activities.
5. Conclusions
This study revealed the key mechanism of calcium regulation in the soil carbon cycle by analyzing the effects of calcium addition on soil organic carbon, its fractions and extracellular enzyme activities. The main conclusions are as follows:
- (1).
- Calcium addition significantly increased the content of SOC and its fractions, effectively enhancing the soil carbon sequestration capacity. The increase in DOC and MBC reflected heightened microbial activity, which promoted the decomposition of organic matter and facilitated nutrient cycling. However, the decrease in SOC during the late reproductive stage (irrigation stage to maturity stage) might be attributed to crop uptake or microbial depletion, while the reduction in ROC suggests that calcium addition drives the conversion of carbon forms toward a steady state.
- (2).
- Calcium addition significantly enhanced the activities of β-glu, Phox, Pero, Phos, NAG, and urease. Among these, the increased activities of Phox and Phos promoted lignin degradation and organophosphorus mineralization, respectively, while elevated urease activity improved nitrogen availability. The correlation analysis revealed significant positive synergy between Phox, Phos, and urease, but negative correlations with β-xyl and β-glu, highlighting the complex interplay within the enzyme network under calcium addition.
- (3).
- The key pathways of calcium-regulated carbon fractions were identified as follows: Calcium indirectly affected the accumulation of MBC and ROC (path coefficient > 0.8) through the positive regulation of Phox and the negative regulation of Phos, underscoring Phox’s dominant role in carbon partitioning. SOC accumulation was primarily driven by combined β-glu and Phos activities. Additionally, DOC content was negatively regulated by β-xyl and Phos activities, indicating that calcium dynamically modulates dissolved carbon stability via enzymatic control.
Calcium addition optimized the distribution of soil carbon forms by enhancing specific enzyme activities, thereby improving short-term nutrient availability and fostering long-term carbon sequestration. These results provide a theoretical foundation for the rational application of calcium fertilizers in farmland management, supporting the synergistic goals of enhancing soil fertility and achieving carbon neutrality.
Author Contributions
Writing—original draft, Funding acquisition, and Supervision, Z.H.; Data collection, Writing—Review and Editing, and Conceptualization, X.S.; Methodology, Validation, Investigation, and Formal analysis, X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32201902) and the Science Research Launch Project of Ph.D. (205040305), of which the funder was Zhaoquan He.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| SOC | Soil organic carbon |
| DOC | Soluble organic carbon |
| MBC | Microbial biomass carbon |
| ROC | Readily oxidizable organic carbon |
| β-xyl | β-xylosidase |
| β-glu | β-glucosidase |
| Phox | Phenol oxidase |
| Pero | Peroxidase |
| Phos | Phosphatase |
| NAG | Acetylaminoglucosidase |
References
- Witzgall, K.; Vidal, A.; Schubert, D.; Höschen, C.; Schweizer, S.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Hall, S.; Ye, C.; Weintraub, S.; Hockaday, W. Molecular trade-offs in soil organic carbon composition at continental scale. Nat. Geosci. 2020, 13, 687–692. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.; Torn, M.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The Influence of organic amendment source on carbon and nitrogen mineralization in different soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Liu, E.; Liu, Z.; Sun, Z.; Li, J.; Gong, H.; Peng, J.; Zhu, O. Soil inorganic carbon stocks increase non-synergistically with soil organic carbon after ecological restoration practices in drylands. J. Environ. Manag. 2023, 348, 119070. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Goldsmith, A.; Herold, I.; Lecha, S.; Toor, G. Assessing soil organic carbon in soils to enhance and track future carbon stocks. Agronomy 2020, 10, 1139. [Google Scholar] [CrossRef]
- Rowley, M.; Grand, S.; Verrecchia, É. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2017, 137, 27–49. [Google Scholar] [CrossRef]
- Duan, S.; Wu, Y.; Zhang, C.; Wang, L.; Song, S.; Zhang, C.; Xu, W.; Bondada, B.; Wang, S. Differential regulation of enzyme activities and physio-anatomical aspects of calcium nutrition in grapevine. Sci. Hortic. 2020, 272, 109423. [Google Scholar] [CrossRef]
- Ma, S.; Chen, G.; Tian, D.; Du, E.; Xiao, W.; Jiang, L.; Zhou, Z.; Zhu, J.; He, H.; Zhu, B.; et al. Effects of seven-year nitrogen and phosphorus additions on soil microbial community structures and residues in a tropical forest in Hainan Island, China. Geoderma 2020, 361, 114034. [Google Scholar] [CrossRef]
- Huang, B.; Xing, Y.; Luo, W.; Yan, G.; Liu, G.; Wang, X.; Wang, Q. Effects of long-term nitrogen addition and throughfall reduction on extracellular enzyme activity and ecoenzymatic stoichiometry in a temperate forest. J. Soil Sci. Plant Nutr. 2024, 24, 1534–1546. [Google Scholar] [CrossRef]
- Li, D.; Ping, Q.; Mo, R.; Guo, W.; Zhang, S.; Wang, L.; Li, Y. Revealing synergistic mechanisms of biochar-assisted microbial electrolysis cells in enhancing the anaerobic digestion performance of waste activated sludge: Extracellular polymeric substances characterization, enzyme activity assay, and multi-omics analysis. Water Res. 2024, 267, 122501. [Google Scholar] [PubMed]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- He, H.; Hu, Q.; Li, R.; Pan, X.; Huang, B.; He, Q. Regional gap in maize production, climate and resource utilization in China. Field Crop. Res. 2020, 254, 107830. [Google Scholar] [CrossRef]
- Ren, X.; Sun, D.; Wang, Q. Modeling the effects of plant density on maize productivity and water balance in the Loess Plateau of China. Agric. Water Manag. 2016, 171, 40–48. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Change Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef]
- Azam, A.; Akhtar, M.; Rukh, S.; Mehmood, A.; Imran, M.; Khan, A.; Qayyum, A.; Ahmad, W.; Gurmani, A. Changes in soil organic carbon fractions across a Loess Toposequence. J. Soil Sci. Plant Nutr. 2020, 20, 1193–1202. [Google Scholar] [CrossRef]
- Begill, N.; Don, A.; Poeplau, C. No detectable upper limit of mineral-associated organic carbon in temperate agricultural soils. Glob. Change Biol. 2023, 29, 4662–4669. [Google Scholar] [CrossRef]
- Calabrese, S.; Mohanty, B.; Malik, A. Soil microorganisms regulate extracellular enzyme production to maximize their growth rate. Biogeochemistry 2021, 158, 303–312. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; García-Palacios, P.; Cao, J.; Dacal, M.; Zhou, X.; Li, J.; Xia, J.; Niu, S.; Yang, H.; et al. Differential responses of carbon-degrading enzyme activities to warming: Implications for soil respiration. Glob. Change Biol. 2018, 24, 4816–4826. [Google Scholar] [CrossRef]
- Huang, X.; Jia, Z.; Guo, J.; Li, T.; Sun, D.; Meng, H.; Yu, G.; He, X.; Ran, W.; Zhang, S.; et al. Ten-year long-term organic fertilization enhances carbon sequestration and calcium-mediated stabilization of aggregate-associated organic carbon in a reclaimed Cambisol. Geoderma 2019, 355, 113880. [Google Scholar] [CrossRef]
- Rowley, M.; Grand, S.; Spangenberg, J.; Verrecchia, É. Evidence linking calcium to increased organo-mineral association in soils. Biogeochemistry 2021, 153, 223–241. [Google Scholar] [CrossRef]
- Rowley, M.; Nico, P.; Bone, S.; Marcus, M.; Pegoraro, E.; Castanha, C.; Kang, K.; Bhattacharyya, A.; Torn, M.; Peña, J. Association Between soil organic carbon and calcium in acidic grassland soils from Point Reyes National Seashore, CA. Biogeochemistry 2023, 165, 91–111. [Google Scholar] [CrossRef]
- Feng, W.; Jiang, J.; Lin, L.; Wang, Y. Soil calcium prompts organic carbon accumulation after decadal saline-water irrigation in the Taklamakan desert. J. Environ. Manag. 2023, 344, 118421. [Google Scholar] [CrossRef] [PubMed]
- Sowers, T.; Adhikari, D.; Wang, J.; Yang, Y.; Sparks, D. Spatial associations and chemical composition of organic carbon sequestered in Fe, Ca, and organic carbon ternary systems. Environ. Sci. Technol. 2018, 52, 6936–6944. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, P.; Piccolo, A. Reduced catalytic activity of an exogenous extracellular β-D-glucosidase due to adsorption on a model humic-clay complex and different soils under wetting and drying cycles. Biol. Fert. Soils 2019, 55, 617–627. [Google Scholar] [CrossRef]
- Xie, X.; Wu, T.; Zhu, M.; Jiang, G.; Xu, Y.; Wang, X.; Pu, L. Comparison of random forest and multiple linear regression models for estimation of soil extracellular enzyme activities in agricultural reclaimed coastal saline land. Ecol. Indic. 2021, 120, 106925. [Google Scholar] [CrossRef]
- Raiesi, F.; Salek-Gilani, S. The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 2018, 126, 140–147. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Kou, Y.; Liu, D.; Liu, Q.; Zhang, Z.; Jiang, Z.; Yin, H. Differential effects of N addition on the stoichiometry of microbes and extracellular enzymes in the rhizosphere and bulk soils of an alpine shrubland. Plant Soil 2020, 449, 285–301. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Du, X.; Wu, J.; Zhang, Z.; Jin, H.; Liang, H.; Gao, D. Performance enhancement of white rot fungi extracellular enzymes via new hydrogel microenvironments for remediation of benzo[a]pyrene contaminated soil. J. Hazard. Mater. 2023, 454, 131505. [Google Scholar] [CrossRef]
- Gomez, E.; Delgado, J.; Gonzalez, J. Persistence of microbial extracellular enzymes in soils under different temperatures and water availabilities. Ecol. Evol. 2020, 10, 10167–10176. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Bing, H.; Zhou, J.; Wang, J. Vegetation type rather than climate modulates the variation in soil enzyme activities and stoichiometry in subalpine forests in the eastern Tibetan Plateau. Geoderma 2020, 374, 114424. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, Z.; Jiang, D.; Jiang, N.; Jiang, H.; Chen, L. Oxidases and hydrolases mediate soil organic matter accumulation in chernozem of northeastern China. Geoderma 2021, 403, 115206. [Google Scholar] [CrossRef]
- Sánchez-Julia, M.; Turner, B. Abiotic contribution to phenol oxidase activity across a manganese gradient in tropical forest soils. Biogeochemistry 2020, 153, 33–45. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob. Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, J.; Zeng, H.; Wang, W. Vertical pattern and its driving factors in soil extracellular enzyme activity and stoichiometry along mountain grassland belts. Biogeochemistry 2018, 141, 23–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).