3.2. Soil and Plant Water Status
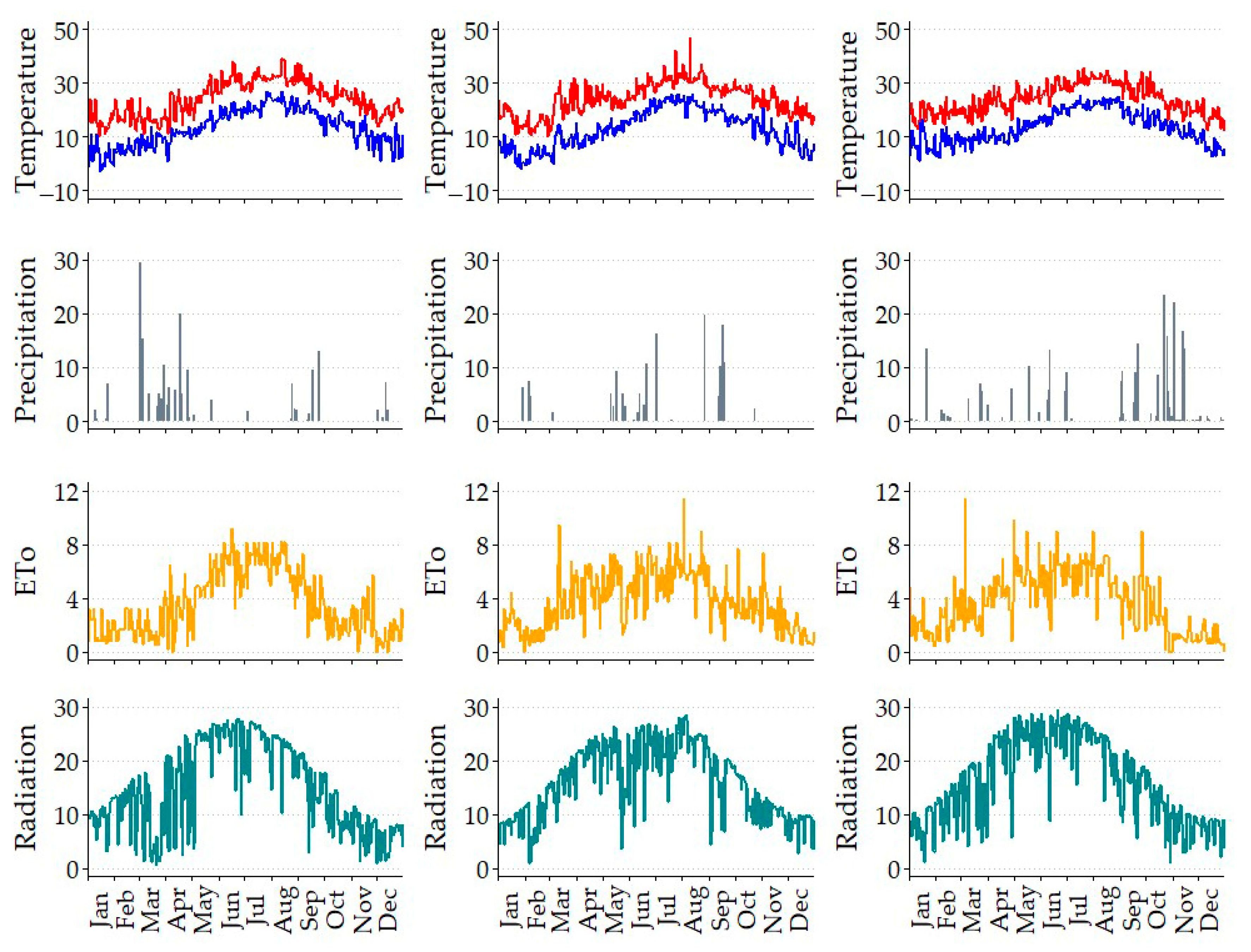
The irrigation water applied (IWA) in the Control strategy for the crop cycle in 2024 was 283 mm (
Table 2), which is 51% higher than the 188 mm used in 2023. This amount was in turn 73% greater than the 109 mm applied in 2022. The primary factors contributing to these increases were the rainfall recorded in September 2023 and the delayed start of irrigation in spring 2022 due to the rainfall during that period. The crop was three years old at the beginning of the study, and its water requirements were significantly lower than those of a mature crop. These requirements increase in parallel with plant size, which corresponds with the annual increase in crop coefficient (Kc) values. Among the established irrigation strategies, the SDI led to a 30% reduction in irrigation water compared to the Control strategy. In terms of the RDI, RDI1 and RDI2 achieved savings of 11% and 16%, respectively, when averaged over the three years.
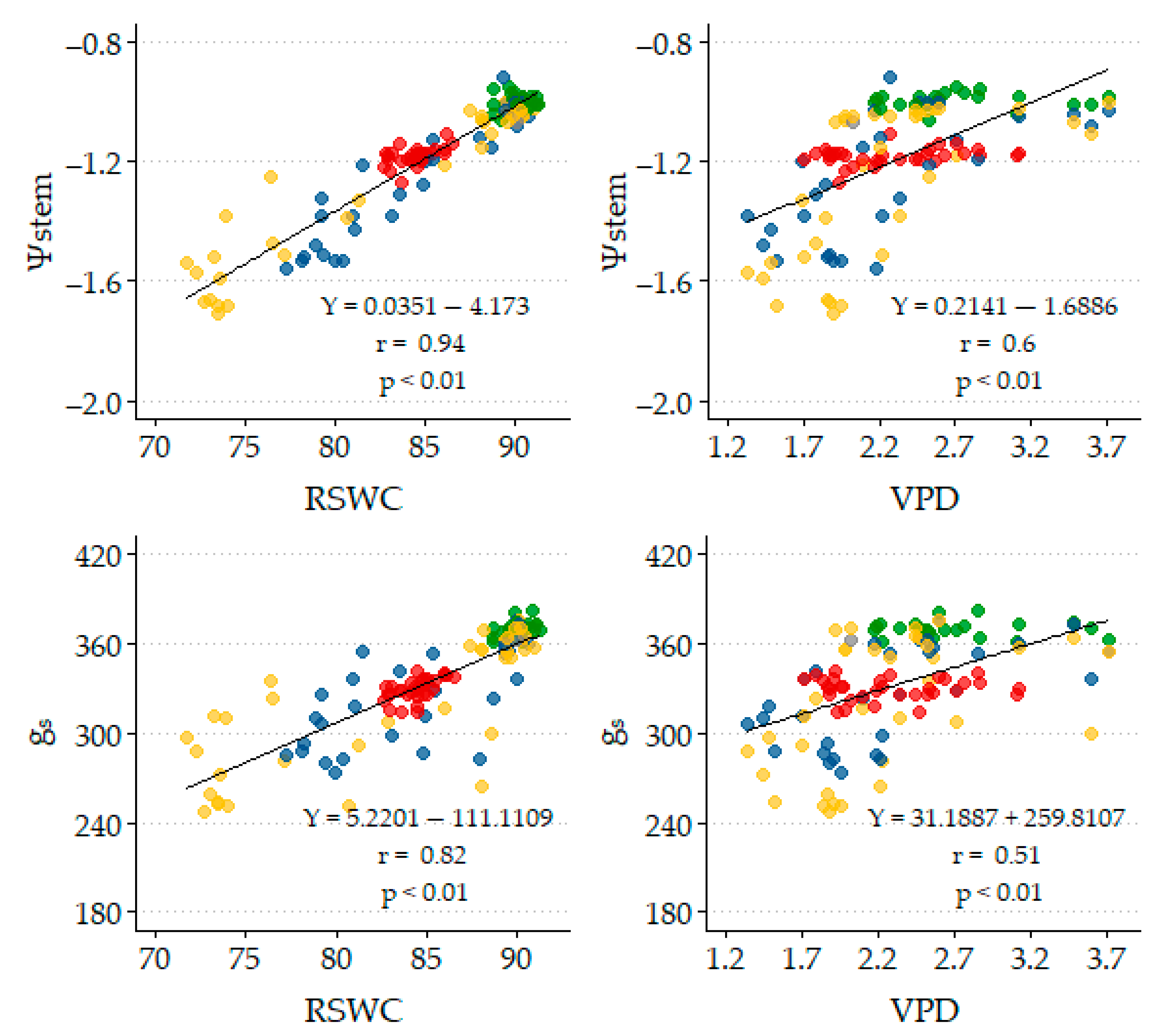
Figure 2 illustrates the seasonal variations in relative soil water content (RSWC, the VSWC expressed as a percentage of FC), air vapor pressure deficit (VPD), stem water potential (Ψstem), and stomatal conductance (gs). The objective of initiating irrigation when the VSWC reached 90% of field capacity was successfully achieved. The VPD values ranged from 1.33 to 5.26 kPa. In 2022, the maximum annual value reached 4.65 kPa on 23 June; in 2023, it increased to 5.26 kPa on 23 August; and in 2024, it was 3.29 kPa on 31 July.
The average values recorded for RSWC, Ψstem, and gs in the SDI strategy were 84.8%, −1.18 MPa, and 330 mmol m
−2 s
−1, respectively, before the evaluated irrigation events. In contrast, the values for the Control strategy were notably higher, with RSWC at 92.1%, Ψstem at −0.99 MPa, and gs at 370 mmol m
−2 s
−1. Midday Ψstem declined slightly as the season progressed, which aligns with findings from previous studies on persimmon [
15,
22,
30,
31] and other fruit crops such as pomegranate [
57]. During the water restriction phases, the values associated with RDI were lower than those of SDI, as expected due to the higher level of water restriction, particularly in the RDI2 treatment (76.6%, −1.47 MPa, 293 mmol m
−2 s
−1). These values were close to those of the Control strategy for the remainder of the crop cycle (88.7%, −1.04 MPa, 352 mmol m
−2 s
−1). In both RDI strategies, a rapid recovery of Ψstem was observed when full irrigation was resumed, consistent with observations from previous studies [
30,
31].
Midday Ψstem values recorded in previous studies on “Rojo Brillante” Control plants ranged from −0.4 to −1.1 MPa [
30,
31]. The values obtained in this study are considered extreme as they were measured just before irrigation. Consequently, the Ψstem of the Control trees indicates an adequate water status for the plants [
15]. In the case of SDI, the minimum midday Ψstem value recorded is higher (less negative) than the lowest values found in comparable studies [
15,
22,
30,
31]. This suggests that the water stress experienced by these plants was less severe, indicating a mild level of stress. On the other hand, the Ψstem values for RDI trees indicate a moderate level of stress [
15].
The gs decreased as the season progressed (
Figure 2), as reported by Griñán et al. [
33], who stated gs values oscillated between 335 and 387 mmol m
−2 s
−1 for the Control trees and between 311 and 344 mmol m
−2 s
−1 for the SDI trees. During the water restriction period, the RDI trees exhibited lower gs values compared to the SDI ones, dropping to 278 mmol m
−2 s
−1 for RDI1 and 247 mmol m
−2 s
−1 for RDI2. This decline was due to more severe water restrictions, with contributions of only 60%, 40%, and 70% of water needs for RDI1, RDI2, and SDI, respectively. In the initial and final phases of the season, the RDI trees behaved similarly to the Control plants. However, the recovery of gs values was slower than that of Ψstem. According to the literature, gs tends to respond less quickly than Ψstem to water stress [
58]. The gs values observed in this study were higher than those reported by [
32], which ranged from 250 to 300 mmol m
−2 s
−1 for Control trees and decreased to 100 mmol m
−2 s
−1 for SDI plants. Similarly, Ballester et al. [
59] recorded an average gs of 151 mmol m
−2 s
−1 for Control trees and 111 mmol m
−2 s
−1 for plants experiencing water stress. It was observed (
Figure 3) that both Ψstem and gs decrease as the RSWC decreases. Significant positive linear correlations (
p ≤ 0.01) were found between Ψstem and gs with RSWC when considering all four strategies across the three growing seasons. The correlation coefficients were 0.94 for Ψstem and 0.82 for gs.
Additionally, a significant positive linear correlation (
p ≤ 0.01; r = 0.88) was observed between gs and Ψstem (
Figure 4). This suggests that “Rojo Brillante” trees effectively manage their water status by reducing transpiration through stomatal closure, consistent with findings from [
60]. These trees maintain Ψstem within narrow limits during periods of water restriction. Thus, the “Rojo Brillante” persimmon trees exhibit a typical anisohydric behavior, as noted by previous studies [
61]. Griñán et al. [
32] reported that persimmon trees respond to mild water stress by developing a stress avoidance mechanism. When water availability is restricted, the trees reduce gs to control water loss through transpiration and prevent leaf turgor loss. Correlations have been also found between Ψstem and gs with VDP; however, these correlations have a lower value of r than those obtained for RSWC. This lower correlation may be due to two main factors: first, edaphic-based stress is more significant than atmospheric stress, as noted by [
59] and [
31], who pointed out that persimmons are not highly sensitive to VPD. Second, there is considerable tree-to-tree variability in Ψstem and gs, along with notable heterogeneity in leaf water status and stomatal conductance. This variability arises from differences in hydraulic resistance among various parts of the tree [
62].
Figure 2 and
Figure 3 illustrate the tendency towards anisohydric behavior in persimmons, which is further supported by the slope shown in
Figure 4, according to [
60].
3.4. Flowering
The total number of flowers (fruitlets) was affected by the growing season (
p ≤ 0.01;
Table 4). In 2024, trees produced an average of 311 fruits tree
−1, which was an increase compared to 212 fruits tree
−1 in 2023. This production level was also much higher than the 79 fruits tree
−1 recorded in 2022, which aligns with expectations due to the increase in canopy size as trees mature (
Table 3).
Flowering is triggered by both endogenous and exogenous signals [
63]. Exogenous signals include factors such as photoperiod, temperature, and stress, while endogenous signals include aspects as plant age and fruit load, as well as nutritional and hormonal status. In this experiment, the photoperiod was consistent across the three seasons observed.
Lang et al. [
64] defined dormancy as the temporary suspension of visible growth in any plant structure containing a meristem. They distinguished three phases: para-, endo- and ecodormancy. The paradormancy refers to growth suppression caused by other parts of the tree (such as apical dominance) due to the influence of “inhibitory molecules”. During the endodormancy phase, growth is not possible even under appropriate temperature conditions. This is because the buds require a period of low temperatures (chilling requirements) during the winter rest. After this endodormancy phase is completed, the buds must then be exposed to warmer temperatures during the eco-dormancy phase (heat needs) to initiate flowering.
Chill accumulations were 416, 468, and 450 chill hours (with temperatures above 7.2 °C) in 2022, 2023 and 2024, respectively. In all cases, these quantities exceeded the threshold considered necessary for astringent cultivars, considered between 200 and 400 h, and particularly for “Rojo Brillante”, which requires relatively little chilling hours, typically less than 200 h [
6,
43,
48,
65]. The slight variations in chill accumulation recorded in this study do not account for differences in flowering intensity. The delay in bud break during 2023 resulted in delayed flowering, which decreased the GDH value to 12,907, compared to 14,672 in 2022 and 14,572 in 2024.
Plant stress is the last exogenous signal noted by [
63], but no stress was recorded during bud dormancy or after this period. The only variable that differed was the irrigation treatment, which did not significantly affect (
p ≤ 0.05;
Table 4) the number of fruitlets (flowers) produced.
Concerning endogenous signals, a high number of fruits negatively affects flowering induction/differentiation [
66]. However, if natural fruit drop occurs in immature fruits 30 days after full bloom (as occurred in the present study), or if the thinning of immature fruits takes place during this period, it can prevent fruit alternation. In 2022, the total yield from the Control trees was much lower at 0.34 kg tree
−1, compared to 11.1 kg tree
−1 in 2023, and even much lower than 35.8 kg tree
−1 in 2024. Thus, the fruit load on the trees each season was independent of subsequent flowering, indicating that there was no biennial or alternate bearing, as reported [
67]. All trees involved in the study received the same fertilization treatments. As will be shown afterwards (
Table 5), the nutrient content in the leaves is within normal ranges, indicating that it is unlikely to be the cause of the varying flowering intensities observed between growing seasons. The age of the trees ranged from 3 to 5 years during the study, leading to increases in both height (37.7%) and diameter (96.9%). This growth enhanced their flowering capacity but also resulted in a decrease in their overall vigor. It is evident that the age of the plantation is the primary endogenous factor contributing to the different flowering intensities observed across the three growing seasons.
3.5. Physiological Fruit Drop
Traditionally, physiological fruit drop has been considered as a self-regulatory mechanism that adjusts the number of fruits to match the tree’s capacity for metabolite supply [
68]. This interpretation is supported by a correlation between physiological fruit drop and carbohydrate levels in leaves and fruitlets observed at the end of the physiological fruit drop period in trees where competition was altered by changing the number of fruits [
69]. Numerous studies have demonstrated that ethylene induces abscission in various tree species, including persimmon [
70]. Ethylene is recognized as the primary hormonal factor in abscission, especially under various stress conditions. There is a close relationship between ethylene levels and abscission; likewise, inhibitors of ethylene biosynthesis can prevent ethylene’s effects and reduce fruit drop in several species [
70].
The percentage of dropped fruitlets, and, consequently, the percentage of harvested fruits, was significantly affected (
p ≤ 0.01) by both the growing season and the irrigation strategy, as well as their interaction (
Table 4). Specifically, the number of dropped fruitlets decreased with the increasing age of the trees. The highest drop rates were observed in the Control trees, while the lowest drop rates were found in the RDI2 trees. In 2022, fruit dropping was particularly pronounced, primarily due to the excessive vigor of young trees (three years old) and the unfavorable weather conditions during spring. Indeed, an important abscission of fruitlets occurred in spring (
p ≤ 0.01; 98.7% on average), which resulted in a lack of fruits maturing for harvest, making it unviable to assess the impact of irrigation strategies on yield. This notable physiological drop in fruit production was widespread throughout the region that season, resulting in an average production reduction of 70% reported in the Valencian Community (Spain). In 2023, fruit abscission remained considerably high (averaging 83%), albeit lower (
p ≤ 0.01) than the previous year. This drop was also associated with the vigor of young trees (then four years old) and varying weather conditions, including rainfall, low radiation, and cooler temperatures recorded in May. In 2024, when the trees were five years old entering their adult phase [
44], the registered climate data were close to average values. As a result, the rate of fruitlet abscission was significantly lower, averaging 43% (
p ≤ 0.01). These results are similar to those reported by [
71] for their control treatment of “Rojo Brillante” and those by [
72] for “Fuyu” (non-astringent persimmon). Interaction analysis (
p ≤ 0.01) indicated that the RDI2 strategy resulted in the lowest percentage of fruit drop during the 2023 and 2024 growing seasons, leading to the highest percentage of fruit harvested. In 2022, as expected due to the large number of dropped fruitlets, there were no significant differences observed.
This result aligns with findings from other researchers: Intrigliolo et al. [
73] reported that applying a 50% water restriction strategy during spring led to a lower fruit drop, necessitating more thinning compared to Control trees and those subjected to the same water restriction during the summer or autumn months. Similarly, [
31] found that implementing a 50% water restriction in June and July reduced fruit drop, suggesting that moderate stress can decrease fruit drop as water stress increases. They also identified a correlation between the tree’s water status (measured by the average Ψstem during the water restriction period) and fruit drop in two of the three years studied. Furthermore, Buesa et al. [
31] observed that the same water restriction applied in spring and, particularly in summer, resulted in reduced fruit drop and an increased number of harvested fruits compared to the Control treatment. However, George et al. [
74] highlight that, in studies conducted in Japan, severe water stress, specifically at leaf water potentials below −1.8 MPa, has been shown to increase fruitlet drop. It is believed that fruitlet abscission occurs due to heightened ethylene production, which is triggered by the blockage of photosynthate transport [
70]. They reported that the transport of photosynthates, along with water to the fruit, helps inhibit fruit drop by preventing the induction of ethylene synthesis in young persimmon fruits. The apparent contradiction between these findings may arise from the fact that [
70,
74] focus on severe water stress, whereas the present study, as well as studies by [
30,
31,
73], addresses moderate or mild water stress conditions.
The physiological fruit drop observed in this study aligns with the early drop described by [
75]. During this early fruitlet drop, an abscission layer develops between the peduncle and the parent branch [
74,
75]. At this stage, the persimmon pedicel detaches along with the young fruit [
76]. The young fruits fall from the connection points between the stalk and the persimmon calyx because the vascular bundles are present only in the stalk, not in the calyx [
76]. The late fruit drop typically occurs from mid-August to September, during which an abscission layer forms between the peduncle and the flesh [
75]. However, this late drop has not been recorded in our experiments.
There are two types of physiological fruit drop that have been linked to various causes [
75]. For seedless cultivars like “Rojo Brillante”, key factors include insufficient accumulation of assimilates due to low sunlight and excessive rainfall, reduced root activity caused by excessive soil moisture, and inadequate nutrition for all the fruits when there is a high fruit set. In March 2022, the maximum temperature was significantly below average, coinciding with a period of heavy rainfall, with up to 342 mm recorded in March. This substantial rainfall contributed to reduced solar radiation, which was about 50% of the average solar radiation value. Consequently, this decline in solar radiation may have led to decreased photosynthesis, resulting in a lower supply of carbohydrates for the developing fruits, which could promote their abscission. Although to a lesser extent, the reduced solar radiation and higher rainfall compared to average levels continued into April 2022. In contrast, in March and April 2023, maximum temperatures were higher than average. During these months, rainfall was negligible (
Figure 1), leading to sunny days that doubled the solar radiation recorded in March 2022. However, in May 2023, rainfall occurred, which lowered temperatures and radiation. In 2024, weather conditions returned closer to average, resulting in normal levels of fruitlet drop.
The RSWC in the Control strategy ranged from 90% to 100% of filed capacity (
Figure 2). Consequently, there was never any excess soil moisture. Regarding the nutritional status of the trees, all received the same fertilization treatment. The nutrient content of the leaves (
Table 5) fell within normal ranges [
37,
45]. Significant differences (
p ≤ 0.01;
Table 5) were observed between the growing seasons, with higher nutrient levels recorded in 2024 compared to 2023, except for phosphorus (P). These differences between the two growing seasons align with findings reported by [
45]. Additionally, Ref. [
77] correlated an unusual and significant fruit drop of the “Loutian” persimmon tree in Luotian County, China, with low levels of available P in the soil, specifically noting values of 5.80 mg kg
−1. However, at the experimental station where the study was conducted, the level of available P in the soil was high, exceeding 170 mg kg
−1. The P content in the leaves ranged from 0.14% to 0.22%, which is slightly above the range considered normal (0.08% to 0.14%) by experts for interpreting foliar analysis, as well as the maximum level observed in high-yield orchards [
37,
45]. Therefore, it does not appear to be the cause of the fruit drop between the growing seasons.
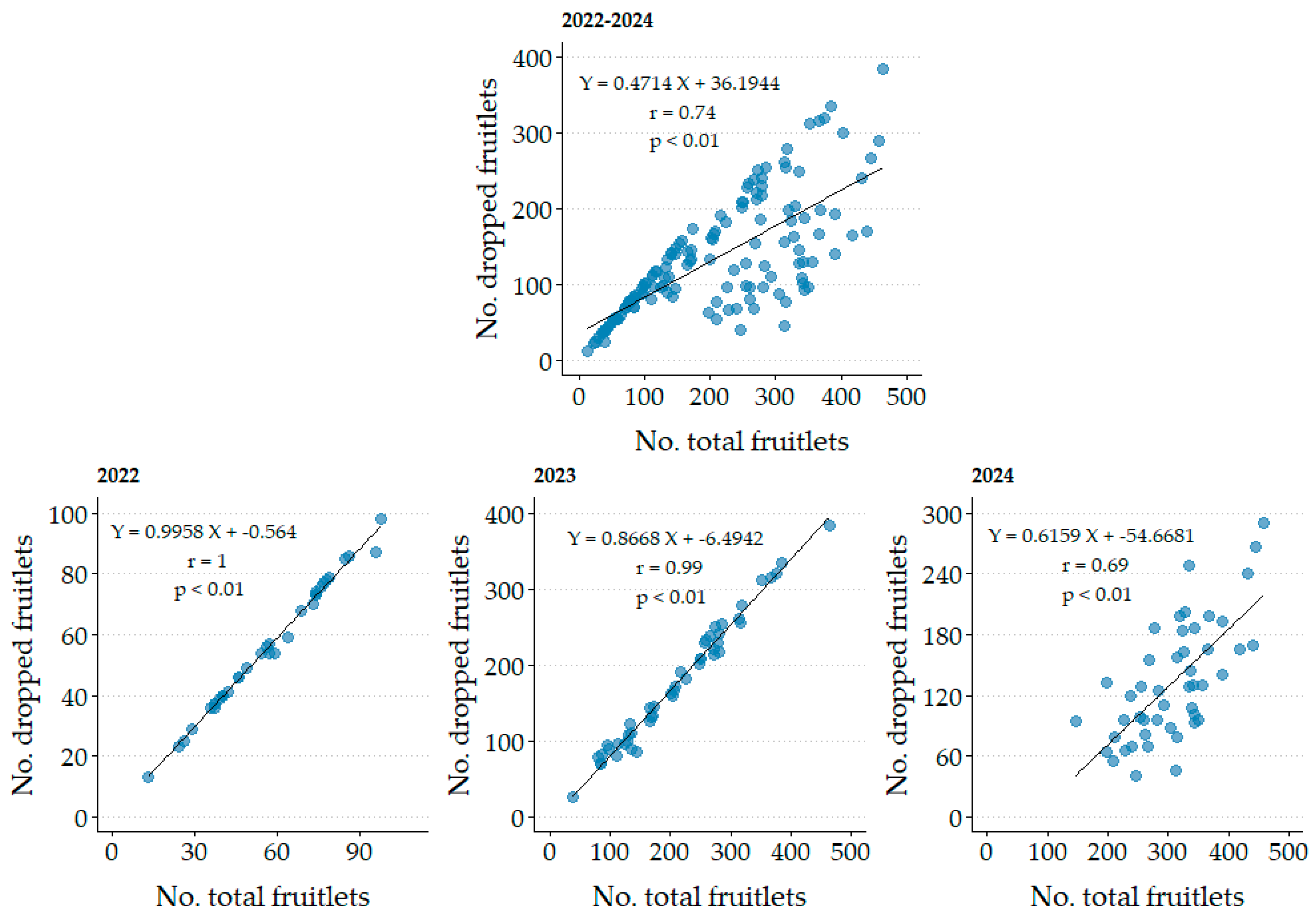
A positive linear correlation (
p ≤ 0.01; r = 0.74;
Figure 5) was found between the number of dropped fruitlets and the total number of fruitlets over the three years of the study. However, due to variations in the age and size of the trees, as well as differences in weather conditions during each growing season, separate analyses were conducted for each growing season. Coefficients for 2022 (r = 1.00) and 2023 (r = 0.99) were highlighted, given that in those years fruit drop was notably high, with rates of 98.7% and 83.0%, respectively. A high rate of fruit set indicates significant competition for photoassimilates among the fruits; when fruit set is abundant and nutritional resources are insufficient for all fruits, it leads to substantial fruitlet drop [
75]. This physiological fruit drop is understood as a self-regulatory mechanism that adjusts the number of fruits to remain within the tree’s capacity to supply necessary metabolites [
68].
Fruitlet drop may not have a single cause. Reig et al. [
71] found a peak in ethylene production at anthesis, and they propose that this increase in ethylene at that stage likely is a cause to physiological fruitlet abscission as there is no shortage of hormones or carbohydrates present at that time. The elevated ethylene production is linked to increased abscission in both vegetative and reproductive organs [
71]. However, Gómez-Cadenas et al. [
78] suggest that the abscission following anthesis, in the transition from ovary to fruit, is primarily hormonal in nature and is largely driven by the action of gibberellic acid (GA), which starts the process of fruit growth. The subsequent development of fruit requires nutrient availability, particularly the supply of carbon, as has been indicated for certain crops [
78].
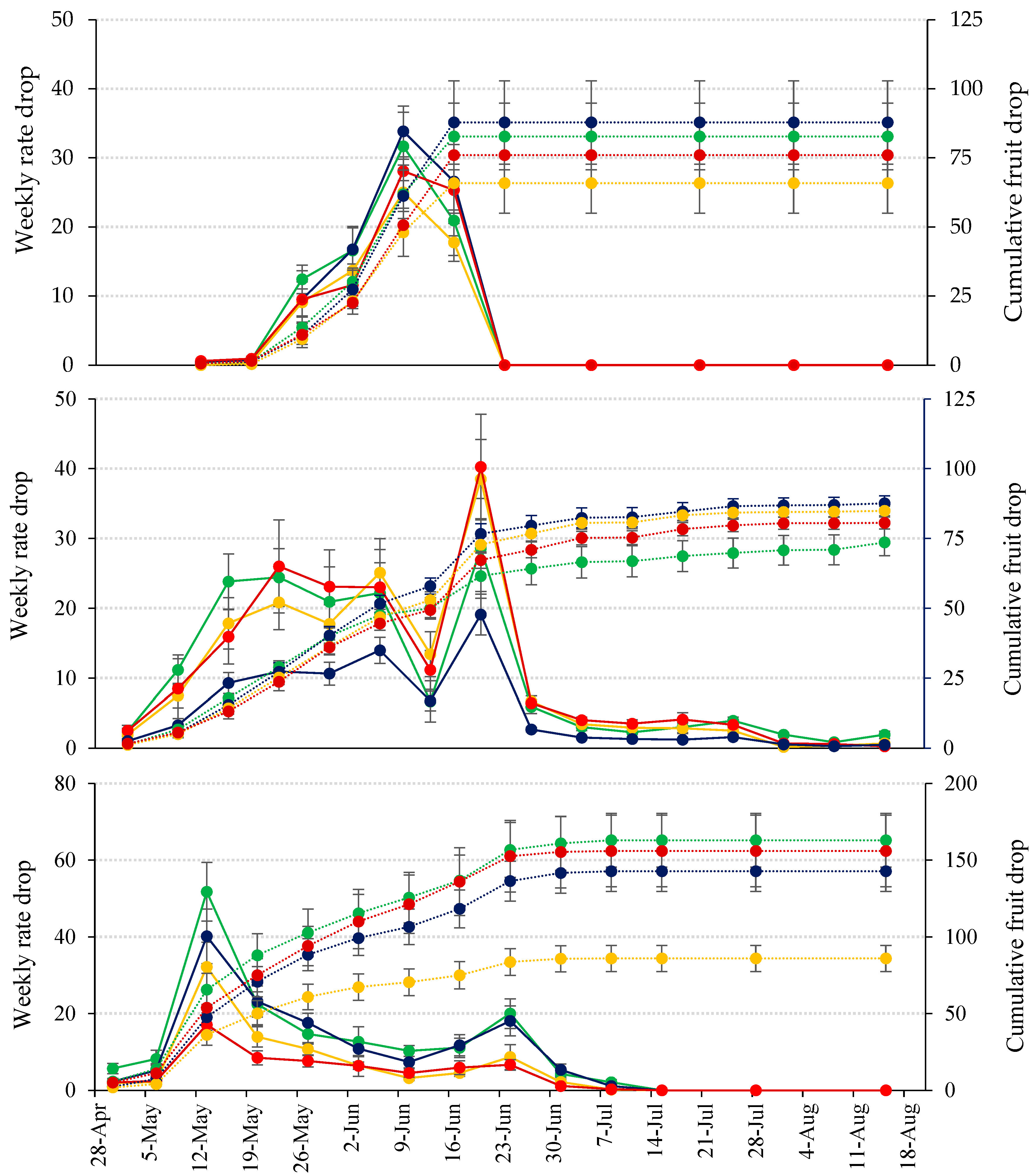
Figure 6 illustrates the seasonal variations in the weekly rates of fruitlet drop. The physiological fruitlet drop observed in this study displayed two waves, which is typical for seedless fruits [
30,
74,
75]. This phenomenon generally occurs from anthesis to early July [
74,
75], coinciding with maximum shoot growth and starch depletion [
79]. In 2022, there was only one wave of fruit drop, with nearly all fruit (98.7%) falling during this single wave. The onset of fruitlet drop began 8 to 18 days after anthesis, occurring mainly over a 35-day period in 2022, when only one wave was present. In contrast, during 2023 and 2024, the fruitlet drop extended over 60 days, reflecting the presence of two waves. The delay in fruit abscission relative to anthesis is attributed to the time required for the synthesis and secretion of hydrolytic enzymes induced by ethylene [
71].
Figure 7 illustrates the seasonal variations in fruit growth during the 2023 and 2024 growing seasons. The data show that “Rojo Brillante” persimmon fruits exhibit a sigmoid growth curve in both seasons, as indicated by [
31]. Therefore, fruit diameter growth does not stop at any point during growth, which does occur in fruits with a double sigmoid growth curve. Water restriction during the growth stop period in fruits with double sigmoid growth is crucial to ensure optimal growth and prevent unit weight loss, but in fruits with a sigmoid growth curve, the determination of the ideal timing for the application of RDI is more complicated [
31].
The fruits harvested in 2024 were smaller than those from 2023. This difference is attributed to the significantly higher number of fruits collected in 2024 compared to 2023. No significant differences in growth were observed among the different irrigation strategies. However, during the fruit maturation phase, noted in the final measurements, the Control group exhibited a higher value compared to the deficit irrigation (DI) strategies, as shown in
Table 6 (
p ≤ 0.01).
In the 2024 growing season, shoot growth was measured on all three branch types (spurs, mixed-fruit-bearing, and woody branches;
Figure 8). Based on visual observation rather than an accurate count, it can be stated that the majority of the harvested fruit came from the spurs, while a smaller amount came from branches with mixed fruit. Both mixed-fruit-bearing and woody branches exhibited strong growth from 3 May to 13 May. This growth likely reduced the carbohydrate supply to the fruitlets, potentially causing stress that could lead to increased ethylene biosynthesis in the fruit and subsequent fruit drop. Sun et al. [
70] noted that persimmon fruit does not produce ethylene while still attached to healthy trees. However, when the transport of photosynthates from the parent tree is blocked, ethylene synthesis begins in the fruit. This process can lead to fruit drop, which may explain the highest weekly rate of fruitlet drop observed between 3 May and 13 May.
3.6. Yield and Fruit Characteristics
Both total and marketable yields were affected by the growing season and irrigation strategy (
p ≤ 0.01;
Table 6). Specifically, the marketable yield in 2024 reached 36.5 kg tree
−1, which is three times greater than the 12.4 kg tree
−1 recorded in 2023. The average yield in 2024 aligns with the average reported for that season by commercial plantations [
80]. However, it is lower than the yields reported in other seasons by [
30,
31] for the same “Rojo Brillante” variety, which were from plantations that were eight years old at the start of their studies. Given that the plantation in question was only five years old in 2024, the yield can be considered normal [
44].
In terms of irrigation strategies, the RDI2 strategy produced significantly higher total and commercial yields compared to the Control strategy, with
p-values lower than 0.01. Specifically, the RDI2 strategy yielded 12.9% and 62.6% more in kg and in the total number of fruits, respectively. This improvement is largely attributed to a lower physiological fruit drop observed in the RDI2 strategy. While the fruit drop was also reduced in the RDI1 strategy, it was not as pronounced as in RDI2 (
Table 4).
Due to the varying number of total fruits harvested per tree in different growing seasons (on average 34.8 in 2023 and 174.1 in 2024), the average unit weight of the marketable fruits obtained in 2024 was significantly lower (
p ≤ 0.01) at 228.6 g compared to the 2023 harvest, which averaged 379.8 g. Additionally, the unit weight of fruits from the Control strategy was higher (
p ≤ 0.01; 337.6 g) than that of the deficit irrigation (DI) strategies, which averaged 293.0 g. This difference is related to the number of marketable fruits harvested, with an average of 75.1 in the Control strategy compared to 108.5 in the DI strategies, since the greater the number of fruits per tree, the greater competition between them for carbohydrates [
81].
In terms of size, as expected (given that there is a significant positive linear relationship between average diameter (D) and the UW; D = 0.1105x + 45.244, r: 0.99,
p ≤ 0.01), the fruits harvested in 2023 were larger (
p ≤ 0.01; D = 87.5 mm) than those harvested in 2024 (D = 70.2 mm). Furthermore, the Control fruits exhibited a greater equatorial diameter (
p ≤ 0.01; average D = 80.8 mm) compared to fruits from the deficit irrigation strategies (average D = 78.2 mm). A quadratic relationship was established between the diameter (Y; mm) and the number of fruits harvested per tree (X), as shown in
Figure 9. This finding is consistent with results obtained by [
81] in peach trees. According to Agustí et al. [
81], this inverse relationship is likely due to competition among fruits for carbohydrates, which reduces their availability when the number of fruits is excessively high.
The growing season and irrigation strategy did not significantly affect the shape of the fruit (
Table 6). All the fruits maintained a similar shape, with the shape index (calculated as D/L, L denotes the length from the calyx to the base, both in mm) ranging from 0.96 to 1.03.
In 2024, the average WP (
Table 6) was higher (
p ≤ 0.01) than in 2023, with values of 6.27 kg m
−3 in 2024 compared to 1.96 kg m
−3 in 2023. Additionally, the WP achieved with DI strategies was also higher (
p ≤ 0.01; averaging 4.37 kg m
−3) than that of the Control strategy, which had an average of 3.34 kg m
−3. The differences between the growing seasons can be attributed to the substantially higher marketable yield in 2024 compared to 2023, with yields of 12.4 kg tree
−1 in 2023 and 36.5 kg tree
−1 in 2024. Regarding irrigation strategies, the Control strategy resulted in both the lowest marketable yield (9.5 kg tree
−1 in 2023 and 33.1 kg tree
−1 in 2024) and the highest IWA, which was 188 mm in 2023 and 283 mm in 2024. These findings align with the results reported by [
31], who observed higher WP with deficit irrigation strategies than with the Control, noting no significant differences among the DI treatments. However, Intrigliolo et al. [
73] found that water productivity only increased when water restrictions were applied during the latter part of the growing season.
Various indices have been utilized to assess the skin color of persimmons, based on the CIE L*C*h* color space parameters L*, a*, and b* (or from Hunter L, a, and b coordinates). These indices include the a/b ratio [
50,
82]; Chroma [
18,
83,
84,
85]; hue angle [
18,
83,
84,
85,
86,
87]; color index (CI), which is likely the most commonly used index for both “Rojo Brillante” [
10,
18,
88,
89,
90,
91,
92] and other cultivars [
86]. Additionally, color charts such as the Japanese standard color chart [
93] and the chart used by the Council Regulation “Kaki Ribera del Xúquer” are also employed in evaluations. Asakuma and Shiraishi [
87] discovered a strong positive correlation between hue angle and the color chart. For this reason, the hue angle and CI values are presented in
Table 7.
Significant differences (
p ≤ 0.01) were observed between the color indices of the fruits harvested in the two growing seasons. Fruits from 2024 exhibited a lower hue angle (68.8, indicating they were redder) and a higher CI value (6.6, indicating they were more orange) compared to those harvested in 2023, which had hue angles of 74.7 and CI values of 4.4, respectively. Regarding irrigation strategies, the SDI, and to a lesser extent the RDI2, resulted in the lowest (
p ≤ 0.01) average hue angle (70.5, indicating a redder color) and the highest average CI value (5.9, indicating a more orange color). The analysis of the interaction between both factors (
p ≤ 0.01) shows that the impact of the irrigation strategy was more pronounced in 2024 than in 2023. Specifically, fruits associated with the SDI strategy and RDI2, which imposed more intense water restrictions than RDI1, exhibited the lowest hue angle values (ranging from 66.6 to 67.1, indicating they were more reddish) and the highest CI values (ranging from 7.4 to 7.2, indicating they were more orange). These CI values are consistent with those reported by [
10,
94] for “Rojo Brillante”. These results suggest that the fruits harvested in 2024, particularly those from the SDI and RDI2 strategies, were more mature than those harvested in 2023.
The ripening of persimmon fruit involves a change in color from yellow to deep orange or red, passing through light orange. This color change results from the degradation of chlorophyll and the biosynthesis of carotenoids [
95]. Lutein is the primary carotenoid present in the skin during the green stage, while β-cryptoxanthin and zeaxanthin accumulate during the coloring stage [
83,
96]. Plaza et al. [
83] found that the main carotenoids present in the fruit of the “Rojo Brillante” persimmon were β-cryptoxanthin and β-carotene, along with lutein and lycopene, and that the lycopene content increased with ripening, particularly when the fruit showed a reddish-orange color.
The levels of secondary metabolites, including carotenoids, can be influenced by different factors (temperature, water availability, ultraviolet radiation, nutrient levels, atmospheric pollution, mechanical stress, pathogen attack, cultivar, ripening stage, and, when applicable, de-astringency treatment) that may vary seasonally [
83,
97]. Variations in these factors can lead to differences in skin color, which may have occurred in this study. It should be noted that, following the Council Regulation “Kaki de la Ribera del Xúquer” recommendations, the fruits should have been harvested when the color chart value was 4. The yield obtained in this study was intended for commercial use, and, according to the marketing company, the harvest was initially scheduled for Friday, October 11th. However, due to company issues, it was postponed until Monday, October 14th, resulting in a higher level of ripeness.
The irrigation strategy and its interaction with the growing season influenced the flesh dry matter content (DM) of the fruits (p ≤ 0.01). Fruits from the most severe DI strategies, namely, RDI2 and SDI, exhibited higher DM values of 19.6% and 19.2%, respectively, compared to those from the Control and moderate deficit irrigation strategy (RDI1), which had DM values of 17.2% and 18.1%, respectively. Interaction analysis indicated that these differences were significant (p ≤ 0.01) in 2023.
Estimating the DM of fruits is essential for optimizing harvest times, particularly for climacteric fruits that continue to ripen after harvest. These fruits must be harvested with a minimum DM content to ensure adequate starch accumulation and the desired post-harvest sugar content. If harvested too early, the fruits may not achieve good quality later on. Conversely, if the fruits ripen excessively, they may become overly soft, complicating transportation and storage.
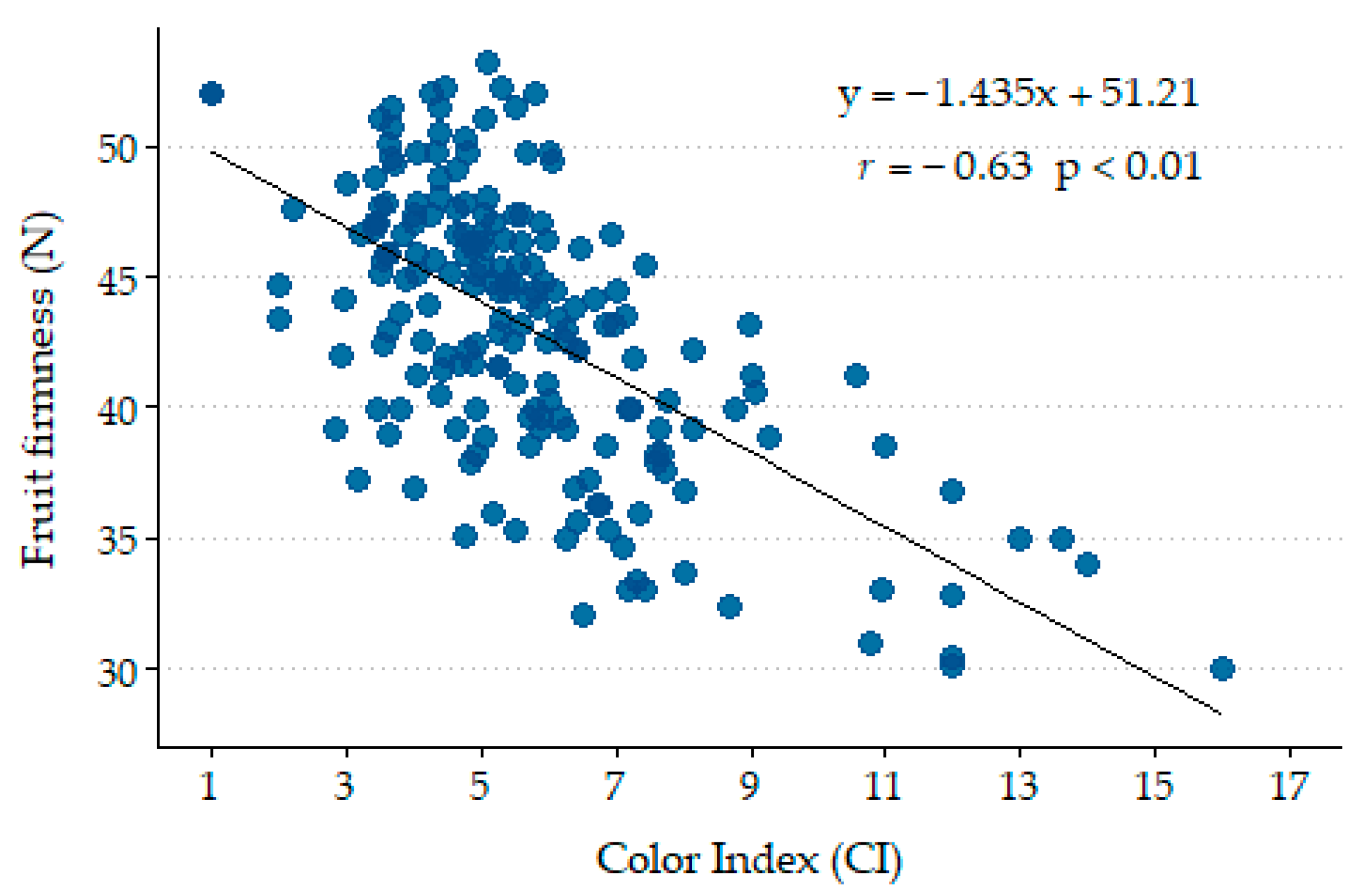
The firmness values of the fruits ranged from 38.2 N for SDI fruits harvested in 2024 to 46.0 N for Control fruits harvested in 2023. These values align with those reported by [
98] and are considered optimal for marketing. Changes in firmness, and consequently texture, during fruit ripening are mainly the result of the degradation of the primary cell wall [
98]. While there is no universally accepted minimum degree of firmness, values below 10 N after storage and marketing are generally considered commercially inadequate [
98]. The lowest firmness values correspond to the ripest fruits, which have the highest CI. A negative linear correlation was found between firmness and the CI (
p ≤ 0.01; r = −0.63;
Figure 10), which is very close to the correlations reported by [
98] for the same cultivar. Although the correlation coefficient is not particularly high, as indicated by [
90], the relationship between firmness and color varies depending on the harvest date and treatment. The equation provided was derived from fruits harvested on different dates in 2023 and 2024. This correlation supports the validity of the CI as a criterion for determining harvest timing.
Growing season, irrigation strategy, and their interaction affected the soluble solids content (SSC) of persimmon fruit flesh (
p ≤ 0.01;
Table 7). Fruits harvested in 2023 had a higher average SSC, 18.1° Brix, compared to those harvested in 2024, which averaged 17.2° Brix. These values are notably higher than those reported by [
18] for the same cultivar, which ranged between 14.7 and 15.9° Brix across two maturity stages. Among the irrigation strategies, RDI2 produced a higher average SSC, of 17.8° Brix (
p ≤ 0.01), compared to RDI1 and Control, which averaged 17.6° and 17.5° Brix, respectively. Analyzing the interaction further (
p ≤ 0.01), there were no significant differences between the irrigation strategies in 2023; however, in 2024, the RDI2 strategy resulted in a higher SSC value, of 17.6° Brix, than the SDI strategy, which averaged 17.3° Brix, and both of these values exceeded those of the RDI1 and Control strategies, averaging 17.0° and 16.7° Brix, respectively.
The differences observed in SSC, both in comparison to the study conducted by [
99] with the same cultivar (ranging between 14.7 and 15.9 °Brix in two maturity stages) and across different growing seasons (with an average difference of 0.94° Brix), are important as an increase of just 1° Brix is considered a notable enhancement in the perception of the fruit’s flavor [
100]. The predominant sugars in the flesh of the “Rojo Brillante” persimmon are sucrose (a non-reducing sugar), glucose, and fructose (reducing sugars), in that order [
101]. This pattern is typical for PVA cultivars. Notably, sucrose hydrolyzes into fructose and glucose as the fruit ripens [
50].
Titratable acidity was influenced only by the growing season, not by the irrigation strategy. This may be due to the significant variability indicated by the high residual sum of squares (70.0%). The main organic acids present in persimmon fruits are malic acid, followed by citric and fumaric acids [
75,
102].
Acidity measurements provide a reliable estimate of acidity intensity, but SSC is not a good predictor of the sweetness perceived by consumers, particularly in astringent persimmon cultivars, because in addition to sugars and organic acids, SSC includes soluble tannins [
101]. The presence of both acidity and tannin content in astringent fruits, where soluble tannin levels remain high, complicates the relationship between SSC and sweetness perception. The maturity index (MI) interprets SSC in conjunction with acidity as a de-astringency treatment can eliminate astringency before consumption. The MI ratio balances SSC and acidity values, showing similar values in different strategies. The MI was only influenced by the growing season (
p ≤ 0.01), being the highest MI that obtained in 2024 (78.6) compared to 2023 fruits (71.8). Notably, the same harvesting criteria were applied in both seasons. In 2024, the harvest date was advanced by 16 days compared to 2023, although it was still delayed by three days compared to the initially proposed date.
During the ripening process of fleshy fruits, a series of physical, biochemical, and physiological changes occur. These changes include alterations in color, flavor, texture, and the production of aroma, organic acids, and polyphenols [
103]. Our study found that the application of DI resulted in an increase in color and a decrease in fruit firmness, suggesting that its effects on fruit flavor and aroma should not be overlooked. While fruit flavor is associated with non-volatile compounds, aroma is linked to volatile compounds [
103], particularly flavonoids [
104,
105], where researchers have examined the chemical characteristics of various persimmon cultivars, including Fuyu, Hachiya, Chocolate, and Sharon. Their analysis revealed unique combinations of compounds, such as alcohols, aldehydes, esters, ketones, and terpenes for each cultivar. Consequently, it would be interesting to analyze, using liquid chromatography–mass spectrometric analysis, the potential effects of DI strategies on these components in the “Rojo Brillante” cultivar.