Genome-Wide Analysis of the CDPK Gene Family in Populus tomentosa and Their Expressions in Response to Arsenic Stress and Arbuscular Mycorrhizal Fungi Colonization
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the CDPK Family Genes in Populus tomentosa
2.2. Sequence Alignment and Phylogenetic Analysis
2.3. Conserved Motifs, Gene Structure, and Cis-Acting Elements
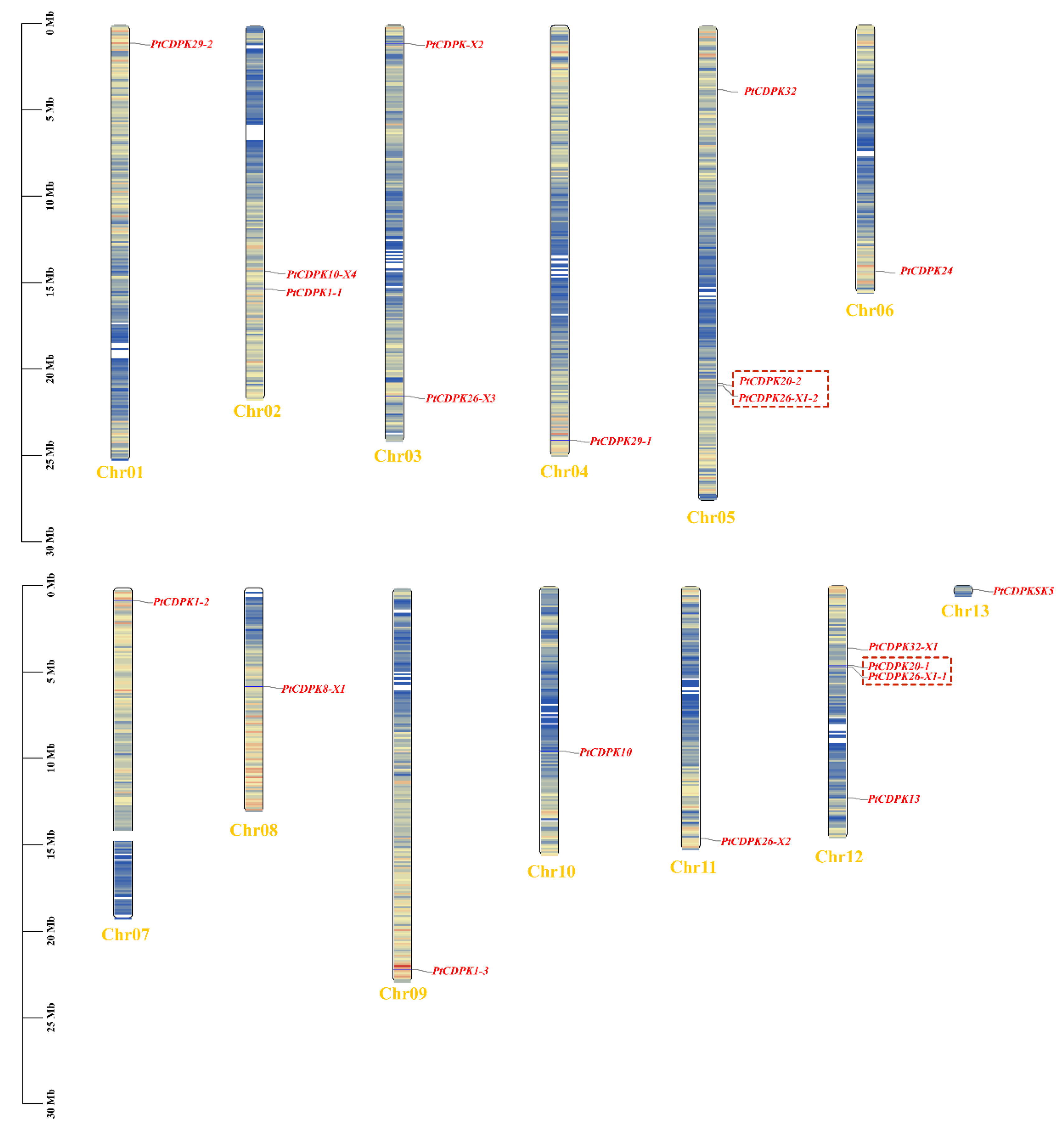
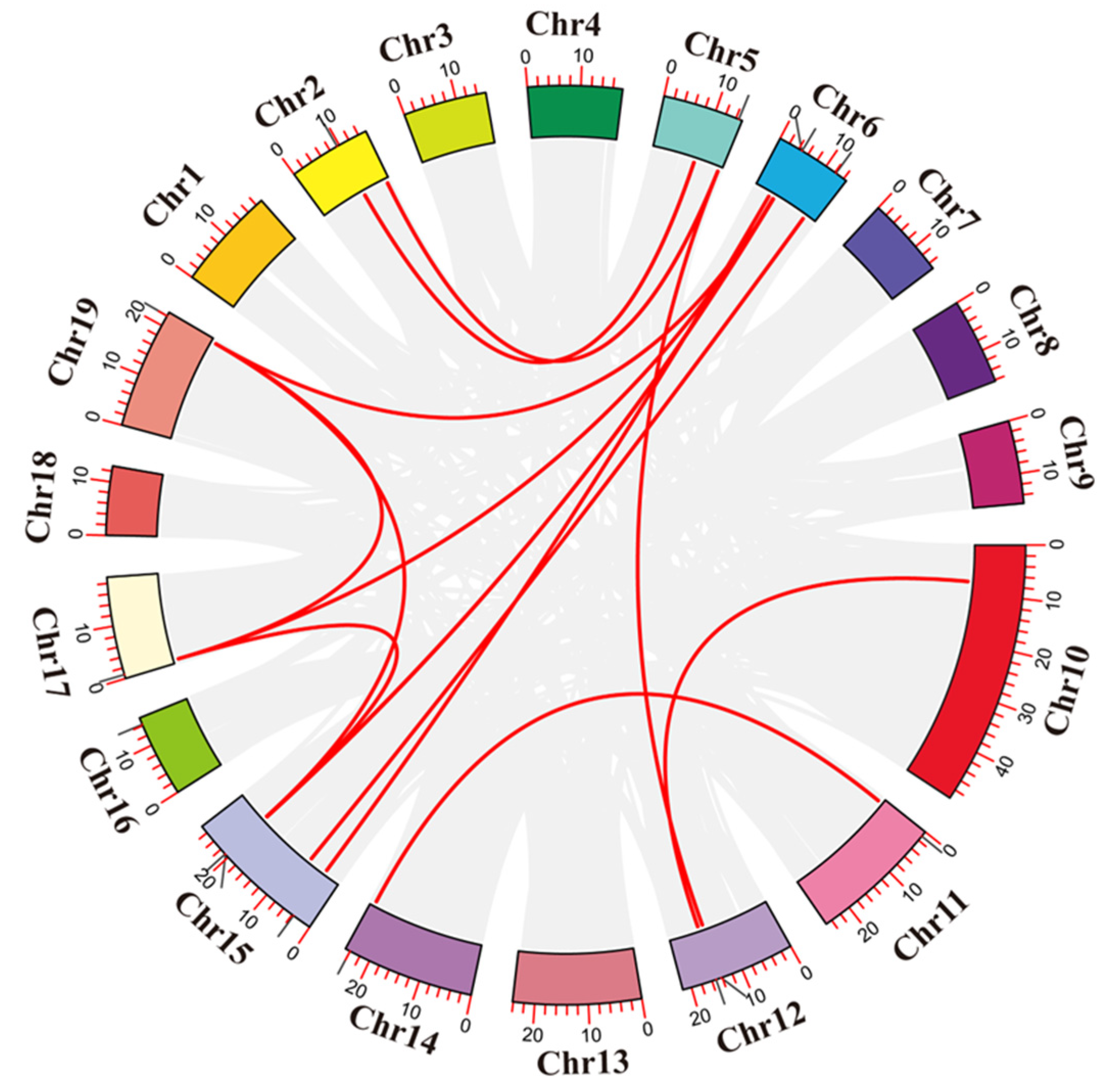
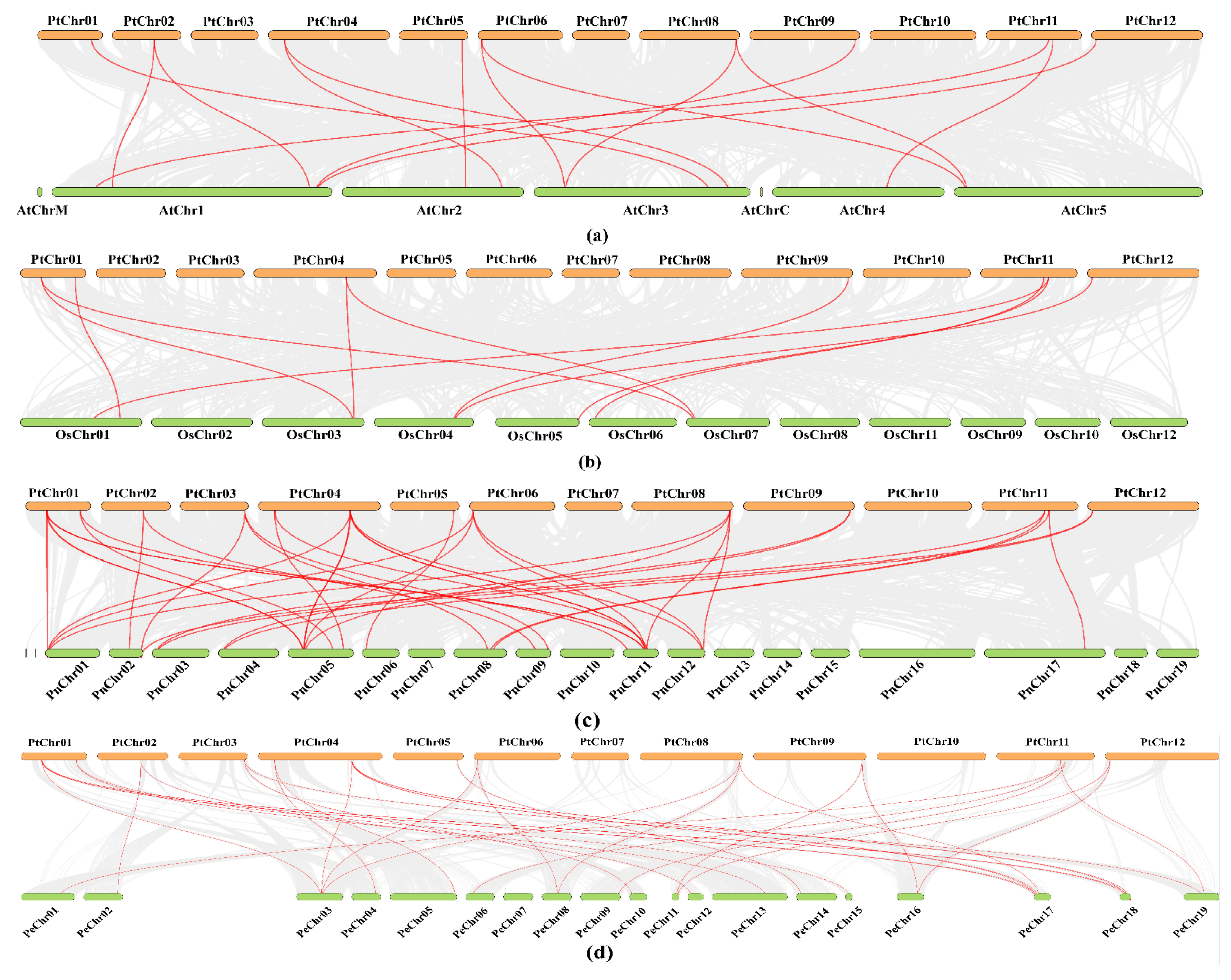
2.4. Chromosome Location, Duplication Events, and Collinearity Analysis
2.5. Plant Materials and Treatments
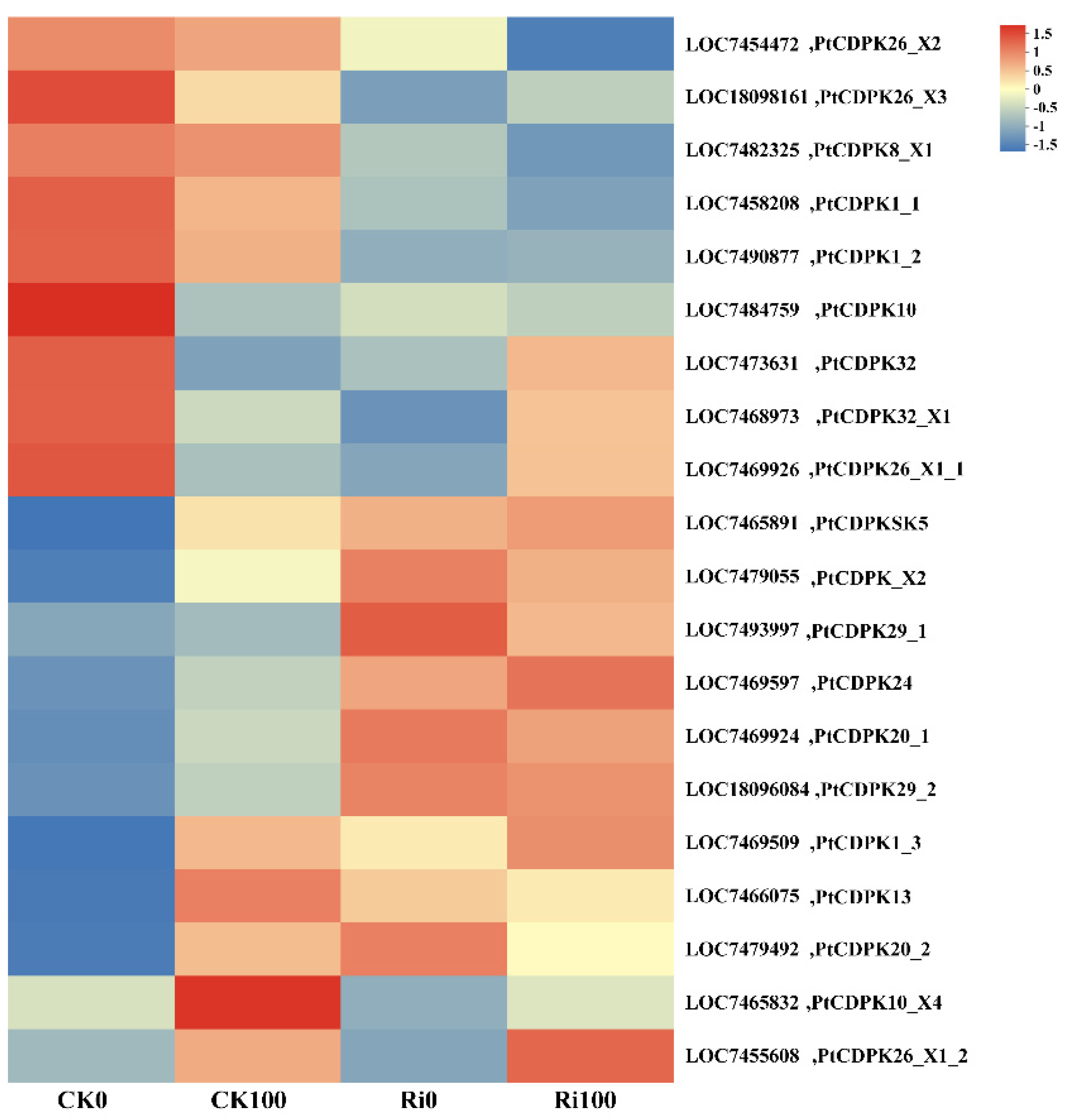
2.6. PtCDPK Expression in Response to AMF Colonization and as Stress
3. Results
3.1. Identification of CDPK Family Genes in Populus tomentosa
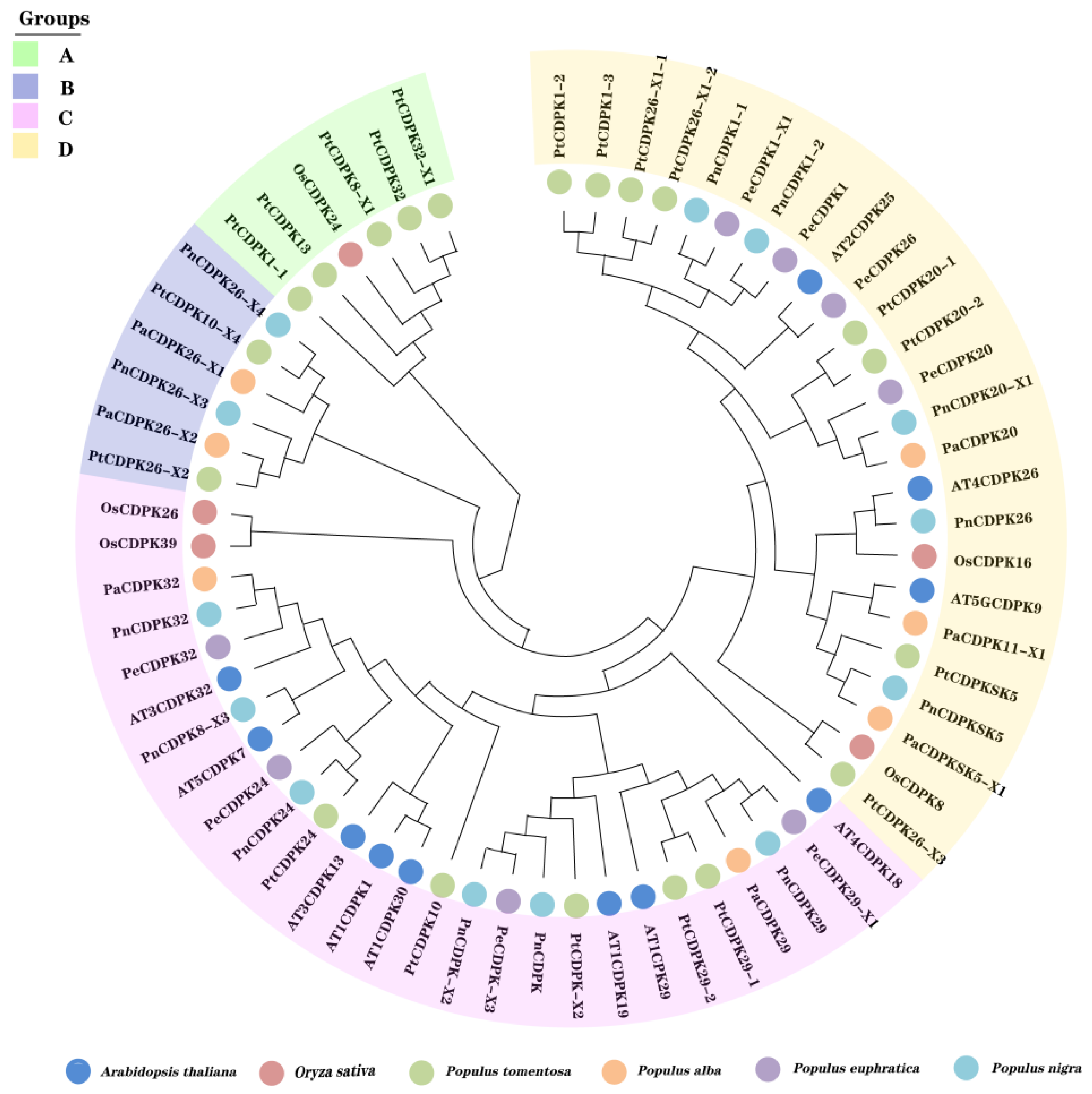
3.2. Phylogenetic Analysis of PtCDPK Genes
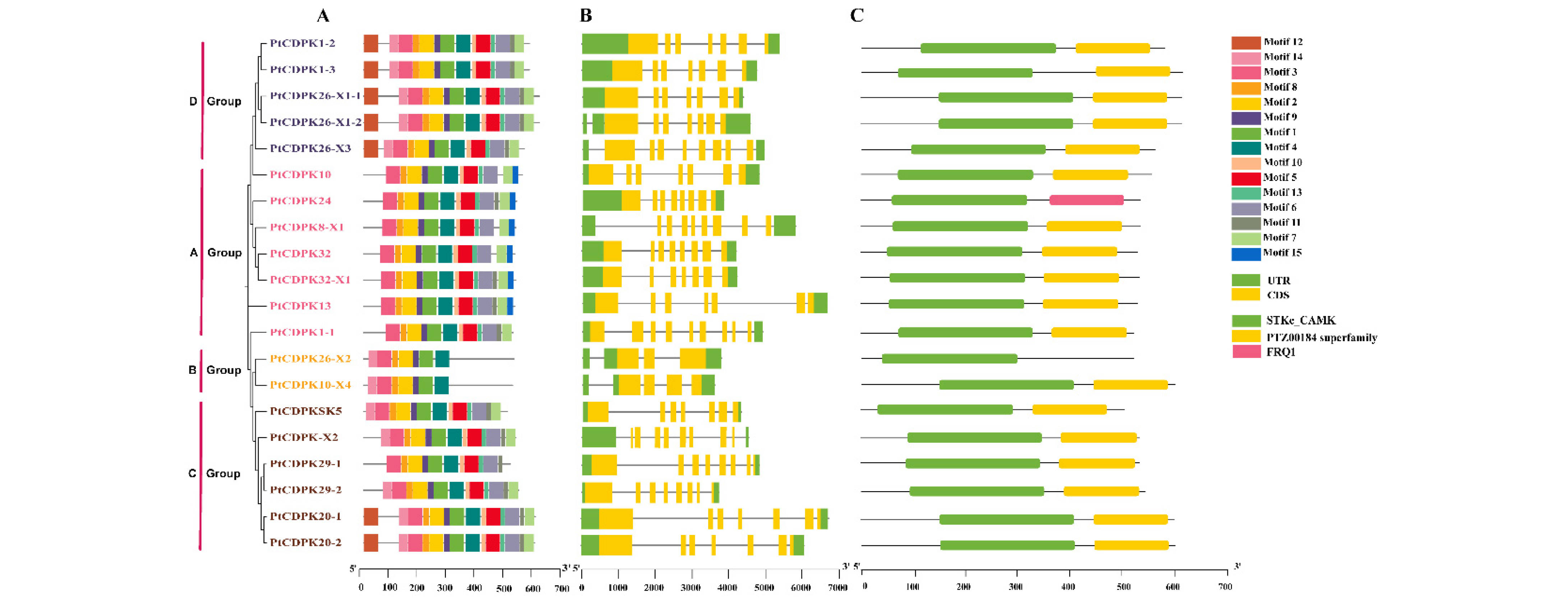
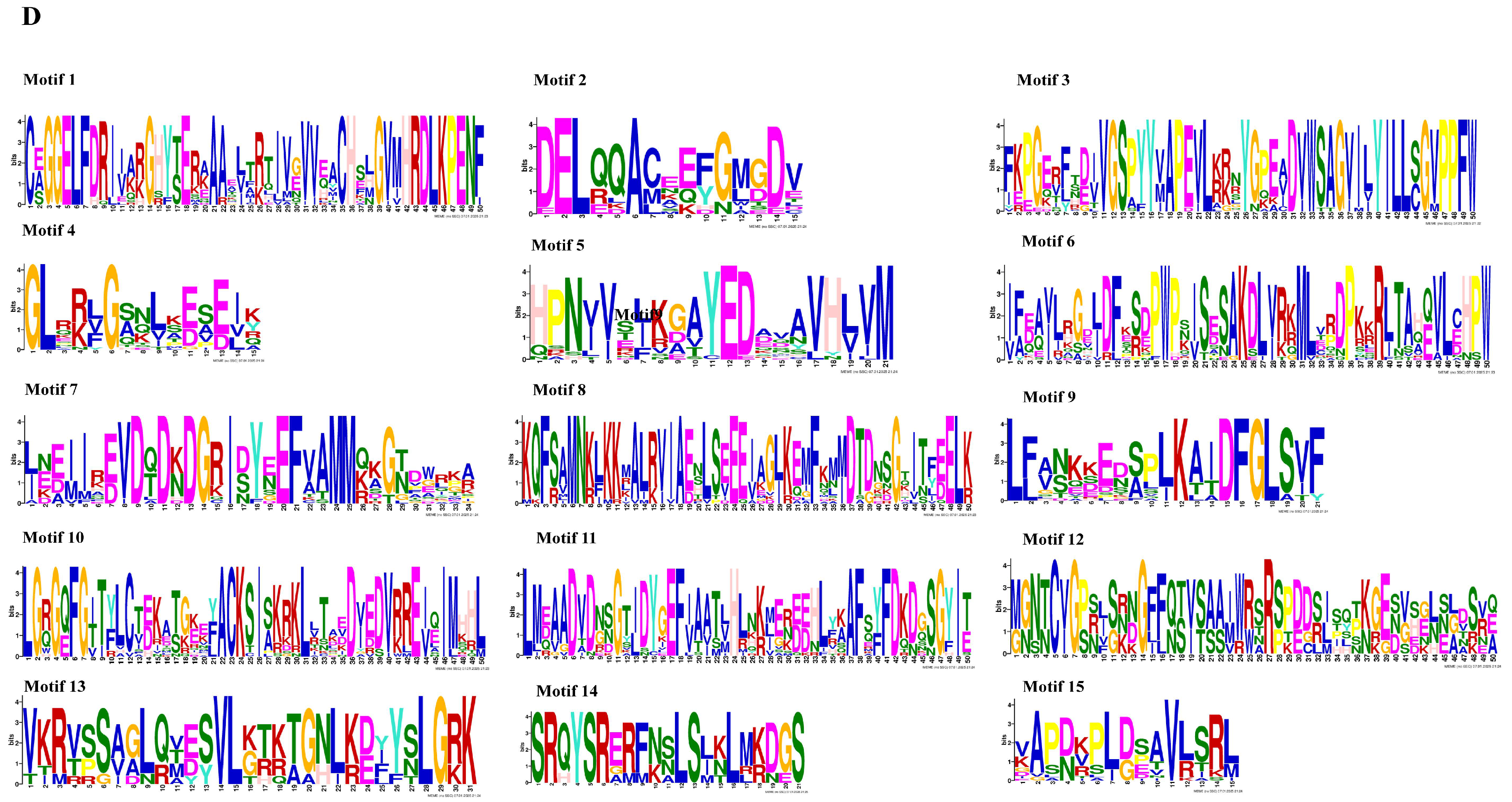
3.3. Exon–Intron Structure, Conservative Domains, and Motifs Analysis of the PtCDPK Genes
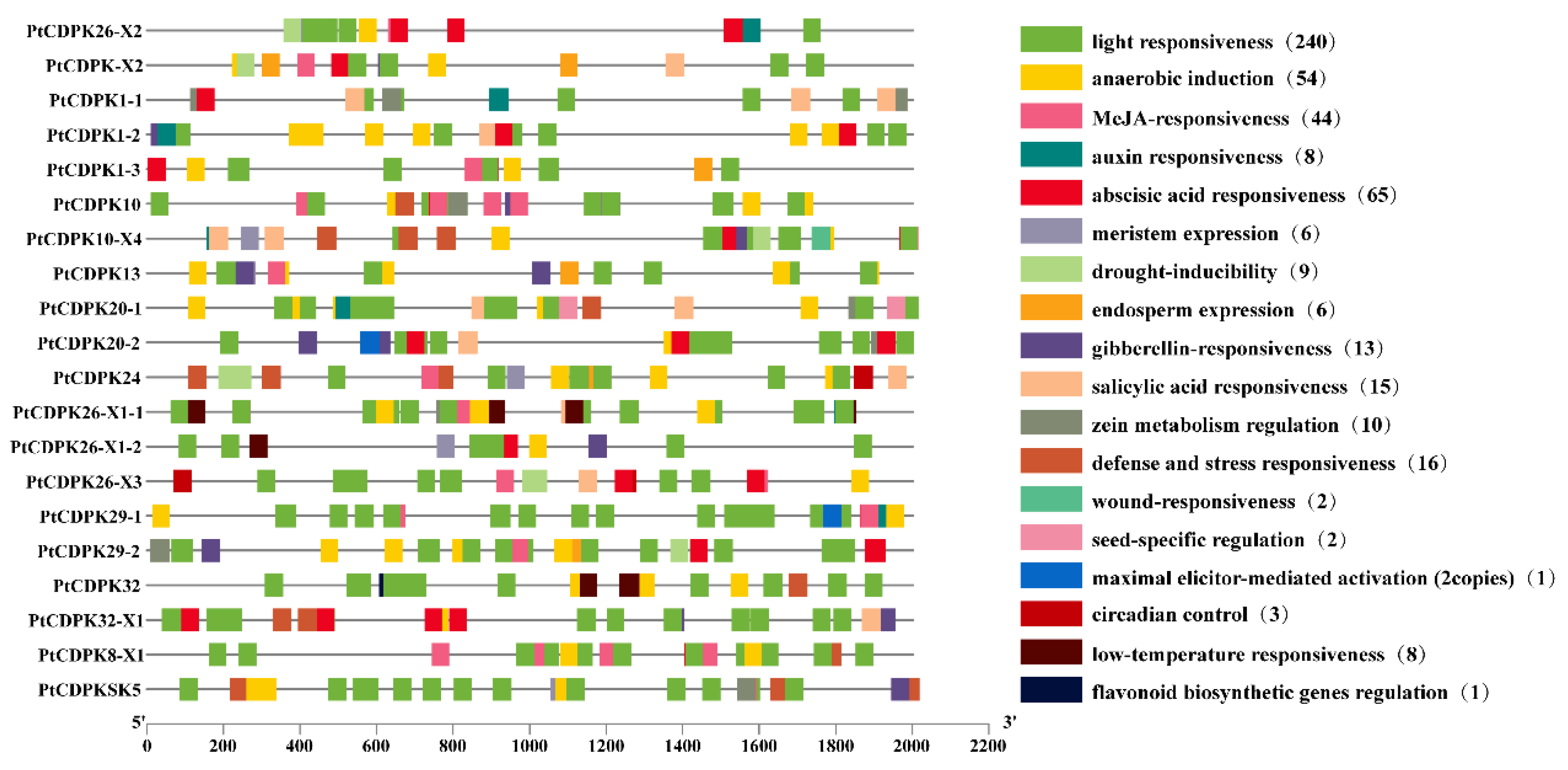
3.4. Cis-Element Analysis of the PtCDPK Genes
3.5. Chromosome Distribution and Gene Replication of the PtCDPKs
3.6. Synteny Analysis of the PtCDPK Genes
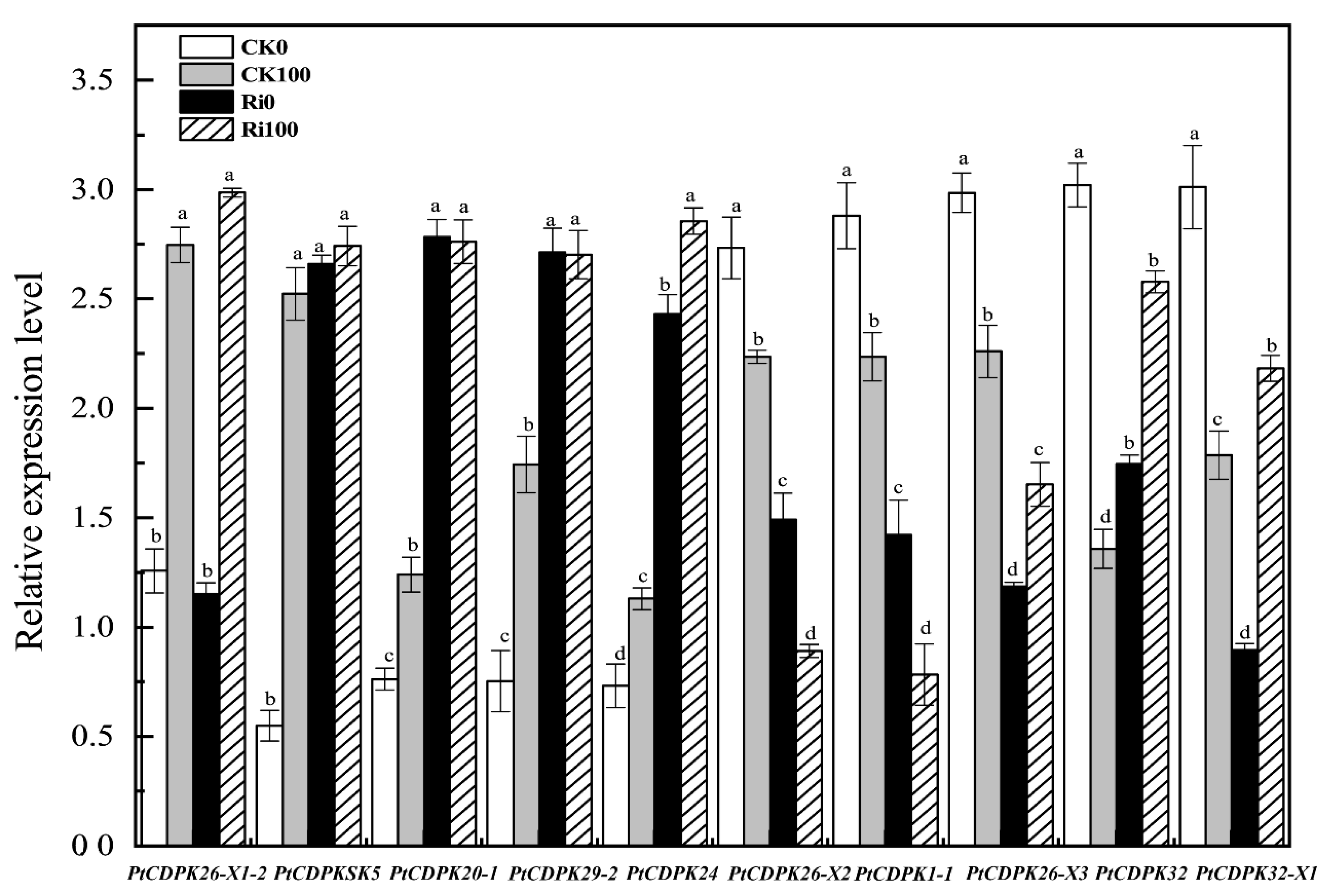
3.7. Expression Analysis of the PtCDPK Genes in Response to as Stress and AMF Colonization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDPKs | Calcium-Dependent Protein Kinases |
| AMF | Arbuscular Mycorrhizal Fungi |
| TAIR | The Arabidopsis Information Resource |
| ABA | Abscisic Acid |
| MeJA | Methyl Jasmonate |
References
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhu, X. Genome-wide identification and functional characterization of CDPK gene family reveal their involvement in response to drought stress in Gossypium barbadense. PeerJ 2022, 8, 12883. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M. The Arabidopsis CDPK—SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Wang, D. Mycorrhizal Symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef]
- Hamel, L.P.; Sheen, J.; Séguin, A. Ancient signals: Comparative genomics of green plant CDPKs. Trends Plant Sci. 2014, 19, 79–89. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Chen, X.H.; Huang, W.Q.; Li, Y.N.; Liu, Q.; Yan, W.; Guo, C.H.; Shu, Y.J. Genome-wide analysis of the CDPK gene family and their important roles in response to cold stress in white clover. Plant Signal. Behav. 2023, 18, 2213924. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Tang, L.; Wu, K.; Liu, J.; Yang, Y. Pb pollution altered bacterial community assembly and predicted functions in aggregate-size fractions of agricultural soil near a smelter. Rhizosphere 2024, 32, 100985. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wang, X.F.; Wu, F.Q. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y. Mycorrhizal Influence on Nutrient Uptake of Citrus Exposed to Drought Stress. Philipp. Agric. Sci. 2009, 92, 33–38. [Google Scholar]
- Coca, M.; San Segundo, B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. 2010, 63, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca2+ dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef]
- Wei, S.; Hu, W.; Deng, X.; Zhang, Y.; Liu, X.; Zhao, X.; Luo, Q.; Jin, Z.; Li, Y.; Zhou, S. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014, 14, 133. [Google Scholar]
- Szczegielniak, J.; Borkiewicz, L.; Szurmak, B.; Lewandowska-Gnatowska, E.; Statkiewicz, M.; Klimecka, M.; Muszynska, G. Maize calcium-dependent protein kinase (ZmCPK11): Local and systemic response to wounding, regulation by touch and components of jasmonate signaling. Physiol. Plant. 2012, 146, 1–14. [Google Scholar]
- Jiang, S.; Zhang, D.; Wang, L.; Pan, J.; Liu, Y.; Kong, X.; Zhou, Y.; Li, D. A maize calcium-dependent protein kinase gene, ZmCPK4, positively regulated abscisic acid signaling and enhanced drought stress tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2013, 71, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Kinoshita, N.; Ishiyama, K.; Hata, S.; Kyozuka, J.; Hayakawa, T.; Nakamura, T.; Shimamoto, K.; Yamaya, T.; Izui, K. A Ca2+-dependent protein kinase that endows rice plants with cold- and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol. 2001, 42, 1228–1233. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S. Expression of calcium-dependent protein kinase (CDPK) genes under abiotic stress conditions in wild-growing grapevine Vitis amurensis. J. Plant Physiol. 2013, 170, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Jue, D.; Zhang, F.; Zhang, D.; Liu, C.; Wu, Q.; Luo, C. Genome-wide identification and expression analysis of the citrus calcium-dependent protein kinase(CDPK) genes in response to arbuscular mycorrhizal fungi colonization and drought. Biotechnol. Biotechnol. Equip. 2020, 34, 1304–1314. [Google Scholar] [CrossRef]
- Gong, M.G.; Bai, N.; Su, J.J.; Wang, Y.; Wei, Y.N.; Zhang, Q.M. Transcriptome analysis of Gossypium reveals the molecular mechanisms of Ca2+ signaling pathway on arsenic tolerance induced by arbuscular mycorrhizal fungi. Front. Microbiol. 2024, 15, 1362296. [Google Scholar] [CrossRef]
- Huang, T.L.; Nguyen, Q.T.T.; Fu, S.F.; Lin, C.Y.; Chen, Y.C.; Huang, H.-J. Transcriptomic changes and signaling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Chen, Y.Q.; Li, Z.B.; Tan, X.T.; Xin, G.R.; He, C.T. Defense guard: Strategies of plants in the fight against cadmium stress. Adv. Biotechnol. 2024, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: New York, NY, USA, 2008; pp. 13–41. [Google Scholar]
- Cao, M.; Xiang, Y.; Huang, L.; Li, M.; Jin, C.; He, C.; Xin, G. Winter forage crops influence soil properties through establishing different arbuscular mycorrhizal fungi communities in paddy field. Adv. Biotechnol. 2024, 2, 30. [Google Scholar] [CrossRef]
- Cao, M.Y.; Ye, S.P.; Jin, C.; Cheng, J.K.; Xiang, Y.; Song, Y.; Xin, G.R.; He, C.T. Arbuscular mycorrhizal fungi communities established by different winter green manures in paddy field promotes the post-cropping rice production. J. Integr. Agric. 2024, 24, 1588–1605. [Google Scholar]
- Zhang, Q.; Yang, W.; Wang, M.; Chen, J.; Zhang, Z.; Wei, Y.; Chang, Q.; Gong, M. Transcriptome analysis reveals the molecular mechanisms for mycorrhiza-enhanced drought tolerance in maize by regulating the Ca2+ signaling pathway. J. Fungi 2025, 11, 375. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, Z.; Dai, X.; Li, Y.; Wang, L. The response of the species diversity pattern of Populus to climate change in China. Phys. Chem. Earth Parts A/B/C 2020, 116, 102858. [Google Scholar] [CrossRef]
- Mu, Z.Y.; Xu, M.Y.; Manda, T.; Yang, L.M.; Hwarari, D.; Zhu, F.Y. Genomic survey and evolution analysis of calcium-dependent protein kinases in plants and their stress-responsive patterns in Populus. BMC Genom. 2024, 25, 1108. [Google Scholar] [CrossRef]
- Hu, Z.; Lv, X.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Genome-wide identification and expression analysis of calcium-dependent protein kinase in tomato. Front. Plant Sci. 2016, 7, 469. [Google Scholar] [CrossRef]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Scott, J.M. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2020, 44, 1044–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Livak Kenneth, J.; Schmittgen Thomas, D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Du, H.; Chen, J.; Zhan, H.; Li, S.; Wang, Y.; Wang, W.; Hu, X. The Roles of CDPKs as a Convergence Point of Different Signaling Pathways in Maize Adaptation to Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 2325. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Corke, F.; Smith, C.; Bevan, M.W. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008, 22, 1331–1336. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.; Chai, G.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Mol. Biol. Rep. 2013, 40, 2645–2662. [Google Scholar] [CrossRef]
- Hématy, K.; Höfte, H. Novel receptor kinases involved in growth regulation. Curr. Opin. Plant Biol. 2008, 11, 321–328. [Google Scholar] [CrossRef]
- Chen, S.; Krinsky, B.H.; Long, M. New genes as drivers of phenotypic evolution. Nat. Rev. Genet. 2013, 14, 645–660. [Google Scholar] [CrossRef]
- Mittal, S.; Mallikarjuna, M.G.; Rao, A.R.; Jain, P.A.; Dash, P.K.; Thirunavukkarasu, N. Comparative analysis of CDPK family in maize, Arabidopsis, rice, and sorghum revealed potential targets for drought tolerance improvement. Front. Chem. 2017, 5, 115. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, H.L.; Mei, C.; Wang, X.J.; Yan, L.; Liu, R.; Zhang, X.F.; Wang, X.F.; Zhang, D.P. The Arabidopsis Ca2+-dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth. New Phytol. 2011, 192, 61–73. [Google Scholar] [CrossRef]
- Redman, J.C.; Haas, B.J.; Tanimoto, G.; Town, C.D. Development and evaluation of an Arabidopsis whole genome Affymetrix probe array. Plant J. 2004, 38, 545–561. [Google Scholar] [CrossRef]
- Ivashuta, S.; Liu, J.; Liu, J.; Lohar, D.P.; Haridas, S.; Bucciarelli, B.; VandenBosch, K.A.; Vance, C.P.; Harrison, M.J.; Gantt, J.S. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 2005, 17, 2911–2921. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-wide identifcation of the rice calcium-dependent protein kinase and its closely related kinase gene families: Comprehensive analysis of the CDPKsgene family in rice. Plant Cell Physiolopy 2005, 46, 356–366. [Google Scholar] [CrossRef]
- Kamiyoshihara, Y.; Iwata, M.; Fukaya, T.; Tatsuki, M.; Mori, H. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant Cell Environ. 2010, 64, 140–150. [Google Scholar] [CrossRef]
- Charpentier, M.; Sun, J.; Vaz, M.T.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Very, A.A.; Sanders, D.; Morris, R.; et al. Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef]
- Shu, B.; Cai, D.; Zhang, F.; Zhang, D.J.; Liu, C.Y.; Wu, Q.S.; Luo, C. Identifying citrus CBL and CIPK gene families and their expressions in response to drought and arbuscular mycorrhizal fungi colonization. Biol. Plant. 2020, 64, 773–787. [Google Scholar] [CrossRef]
| Gene | Original ID | Length (aa) | MW (kDa) | pI | N-Yristoylation | S-Almitoylation | EF-Hand | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| PtCDPK1-1 | LOC7458208 | 521 | 58.84 | 6.14 | Yes | Yes | 5 | CS |
| PtCDPK1-2 | LOC7490877 | 579 | 64.67 | 5.59 | No | Yes | 5 | CS |
| PtCDPK1-3 | LOC7469509 | 579 | 65.12 | 5.36 | No | Yes | 5 | CS |
| PtCDPK-X2 | LOC7479055 | 532 | 60.09 | 6.03 | Yes | Yes | 5 | C |
| PtCDPK8-X1 | LOC7482325 | 533 | 59.99 | 6.56 | No | Yes | 5 | C |
| PtCDPK10 | LOC7484759 | 555 | 63.16 | 5.96 | Yes | Yes | 5 | ER |
| PtCDPK10-X4 | LOC7465832 | 520 | 57.34 | 5.99 | Yes | Yes | 5 | C |
| PtCDPK13 | LOC7466075 | 528 | 59.71 | 5.87 | No | Yes | 5 | C |
| PtCDPK20-1 | LOC7469924 | 599 | 66.35 | 5.49 | No | Yes | 5 | Cs |
| PtCDPK20-2 | LOC7479492 | 598 | 66.49 | 5.23 | No | Yes | 5 | Cs |
| PtCDPK24 | LOC7469597 | 534 | 60.71 | 5.78 | Yes | Yes | 5 | C |
| PtCDPK26-X1-1 | LOC7469926 | 613 | 68.51 | 5.97 | No | Yes | 5 | Cs |
| PtCDPK26-X1-2 | LOC7455608 | 613 | 69.03 | 5.84 | No | Yes | 5 | C |
| PtCDPK26-X2 | LOC7454472 | 526 | 57.83 | 5.72 | No | Yes | 5 | N |
| PtCDPK26-X3 | LOC18098161 | 560 | 62.53 | 5.38 | No | Yes | 5 | C |
| PtCDPK29-1 | LOC7493997 | 513 | 57.63 | 5.86 | Yes | Yes | 5 | Cs |
| PtCDPK29-2 | LOC18096084 | 542 | 60.81 | 5.51 | Yes | Yes | 5 | Cs |
| PtCDPK32 | LOC7473631 | 528 | 59.85 | 6.12 | No | Yes | 5 | N |
| PtCDPK32-X1 | LOC7468973 | 532 | 60.46 | 6.32 | No | Yes | 5 | C |
| PtCDPKSK5 | LOC7465891 | 503 | 56.61 | 5.54 | No | Yes | 5 | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.; Su, J.; Wang, S.; Wang, Y.; Wang, W.; Chen, X.; Zhang, Q. Genome-Wide Analysis of the CDPK Gene Family in Populus tomentosa and Their Expressions in Response to Arsenic Stress and Arbuscular Mycorrhizal Fungi Colonization. Agronomy 2025, 15, 1655. https://doi.org/10.3390/agronomy15071655
Gong M, Su J, Wang S, Wang Y, Wang W, Chen X, Zhang Q. Genome-Wide Analysis of the CDPK Gene Family in Populus tomentosa and Their Expressions in Response to Arsenic Stress and Arbuscular Mycorrhizal Fungi Colonization. Agronomy. 2025; 15(7):1655. https://doi.org/10.3390/agronomy15071655
Chicago/Turabian StyleGong, Minggui, Jiajie Su, Shuaihui Wang, Youjia Wang, Weipeng Wang, Xuedong Chen, and Qiaoming Zhang. 2025. "Genome-Wide Analysis of the CDPK Gene Family in Populus tomentosa and Their Expressions in Response to Arsenic Stress and Arbuscular Mycorrhizal Fungi Colonization" Agronomy 15, no. 7: 1655. https://doi.org/10.3390/agronomy15071655
APA StyleGong, M., Su, J., Wang, S., Wang, Y., Wang, W., Chen, X., & Zhang, Q. (2025). Genome-Wide Analysis of the CDPK Gene Family in Populus tomentosa and Their Expressions in Response to Arsenic Stress and Arbuscular Mycorrhizal Fungi Colonization. Agronomy, 15(7), 1655. https://doi.org/10.3390/agronomy15071655






