Soil–Plant Biochemical Interactions Under Agricultural Byproduct Amendments and Potassium Humate: Enhancing Soil Function and Bioactive Compounds in Sunflower Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Characteristics and Amendments Preparation
2.2. Treatments and Growth Conditions
- Group 1: Control without any amendments (baseline).
- Group 2: 1% w/w CM alone.
- Group 3: 3% w/w RHB alone.
- Group 4: KH alone at 0.5%, 1%, and 2% w/w to assess dose-dependent effects.
- Group 5: Combination treatments pairing 1% CM or 3% RHB with each KH level.
2.3. Soil Quality Analysis
2.3.1. Soil Texture
2.3.2. pH and Electrical Conductivity
2.3.3. Organic Matter and Organic Carbon
2.3.4. Water-Stable Aggregates
2.3.5. Soil Respiration
2.3.6. Nitrate, Available Phosphorus, and Available Potassium
2.4. Plant Sampling and Measurements
2.4.1. Root-to-Shoot Ratio
2.4.2. Total Phenolic Content
2.4.3. DPPH Antioxidant Activity
2.4.4. Total Chlorophyll Content
2.5. Statistical Analyses
3. Results and Discussion
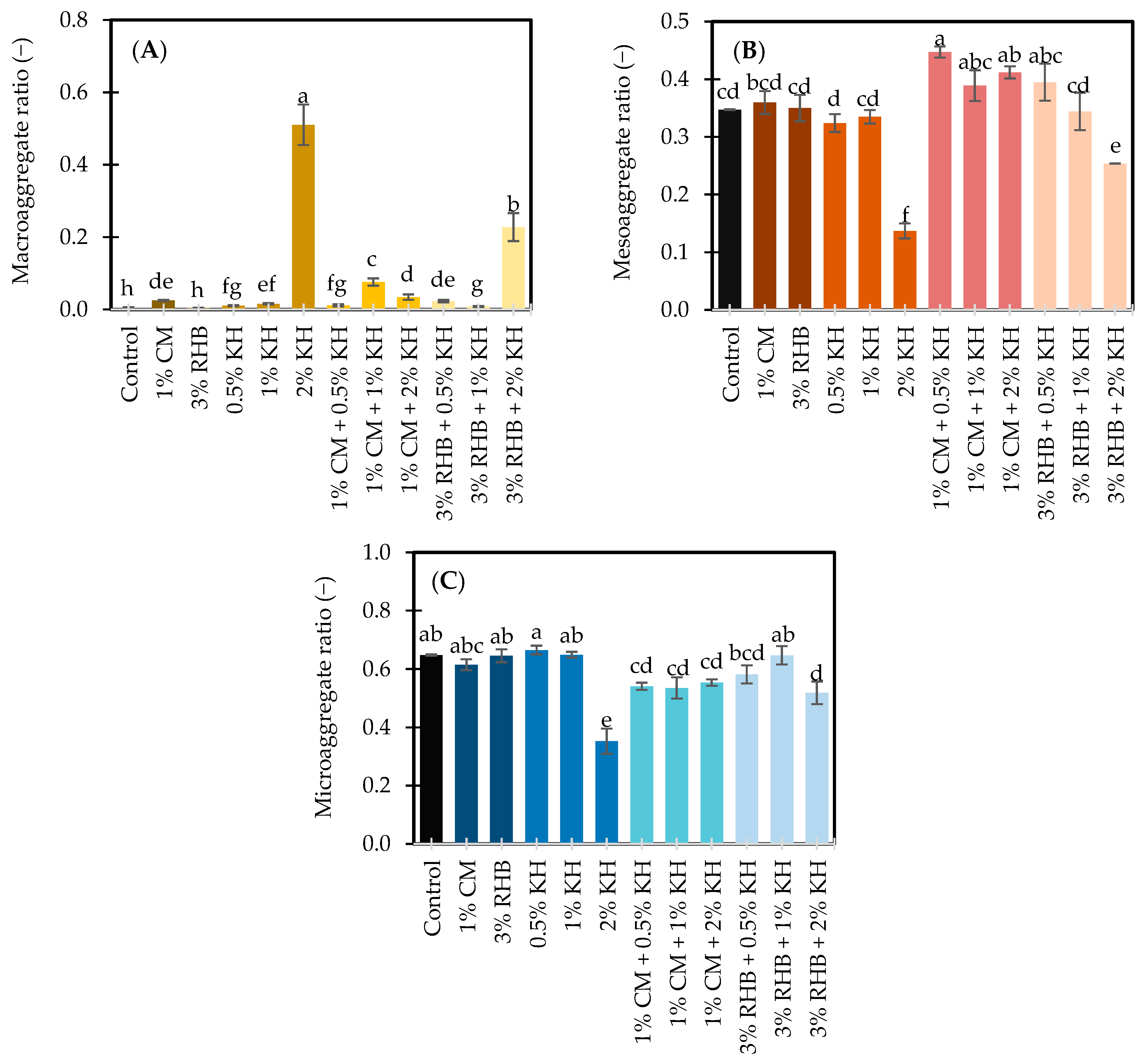
3.1. Soil Aggregation and Physicochemical Properties
3.1.1. Soil Aggregation in Response to Treatments
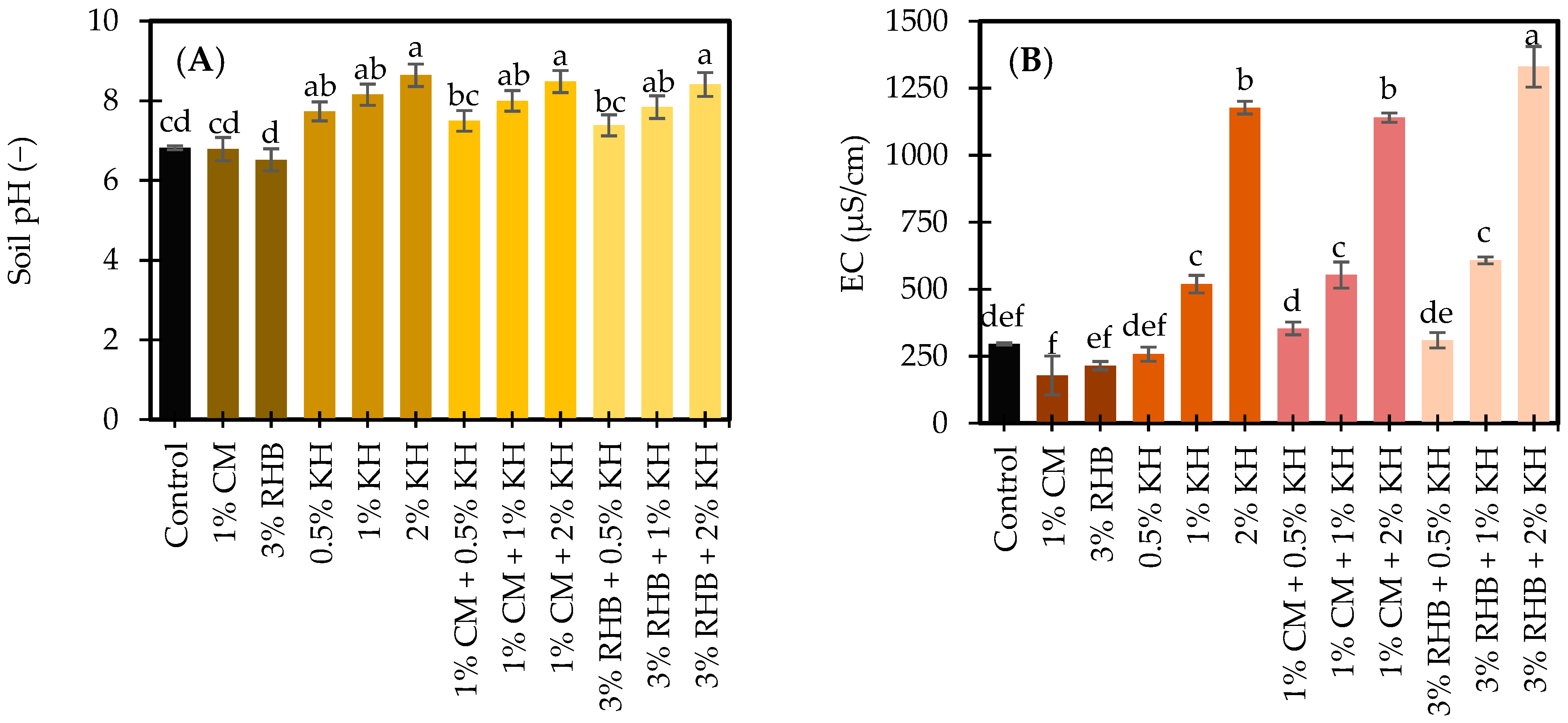
3.1.2. pH and Electrical Conductivity in Response to Treatments
3.1.3. Soil Organic Carbon in Response to Treatments
3.1.4. Soil Respiration in Response to Treatments
3.1.5. Nitrate, Available Phosphorus, and Available Potassium in Response to Treatments
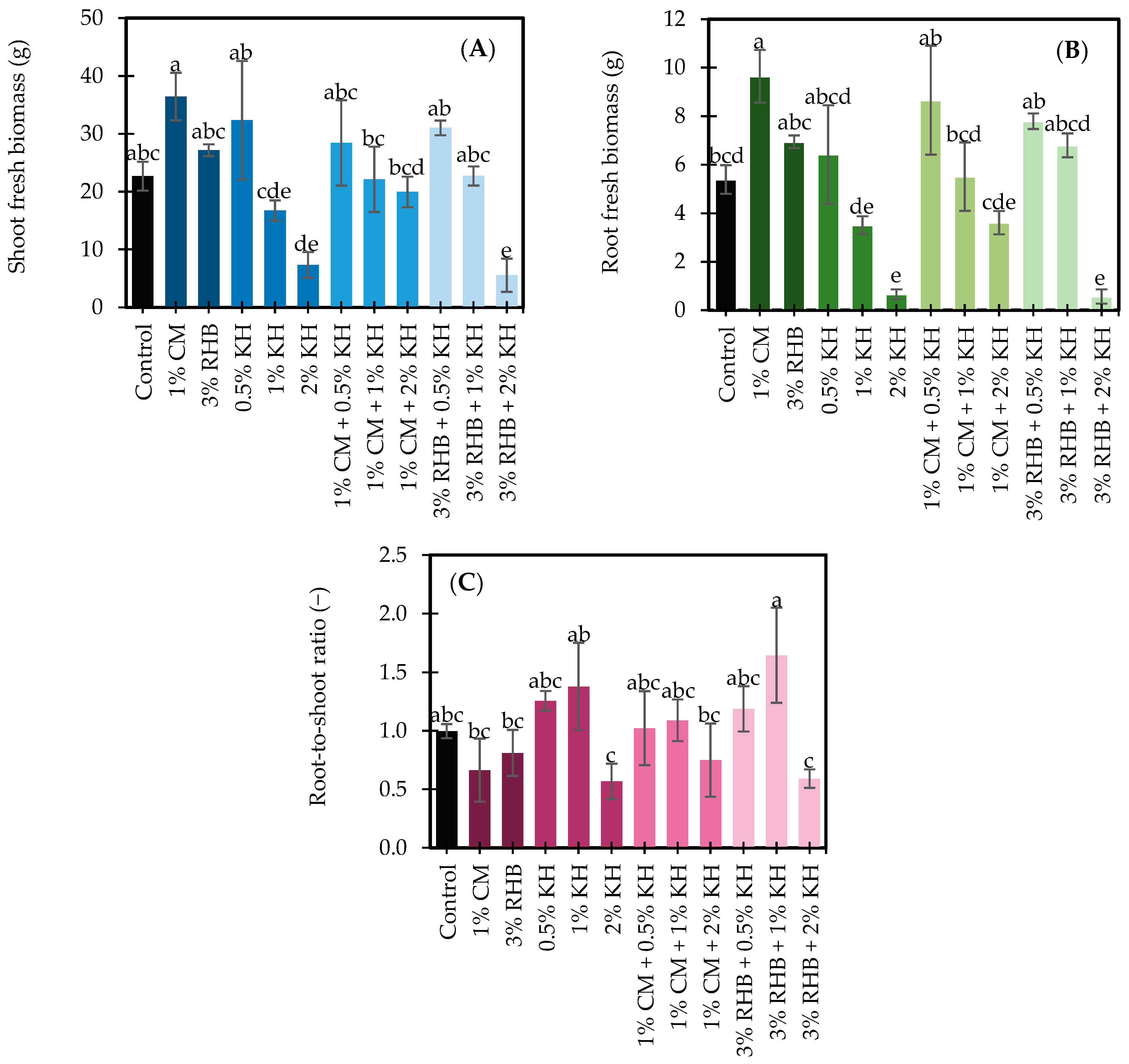
3.2. Development Traits and Biomass Distribution in Sunflower Sprouts
3.3. Secondary Metabolites in Sunflower Sprouts
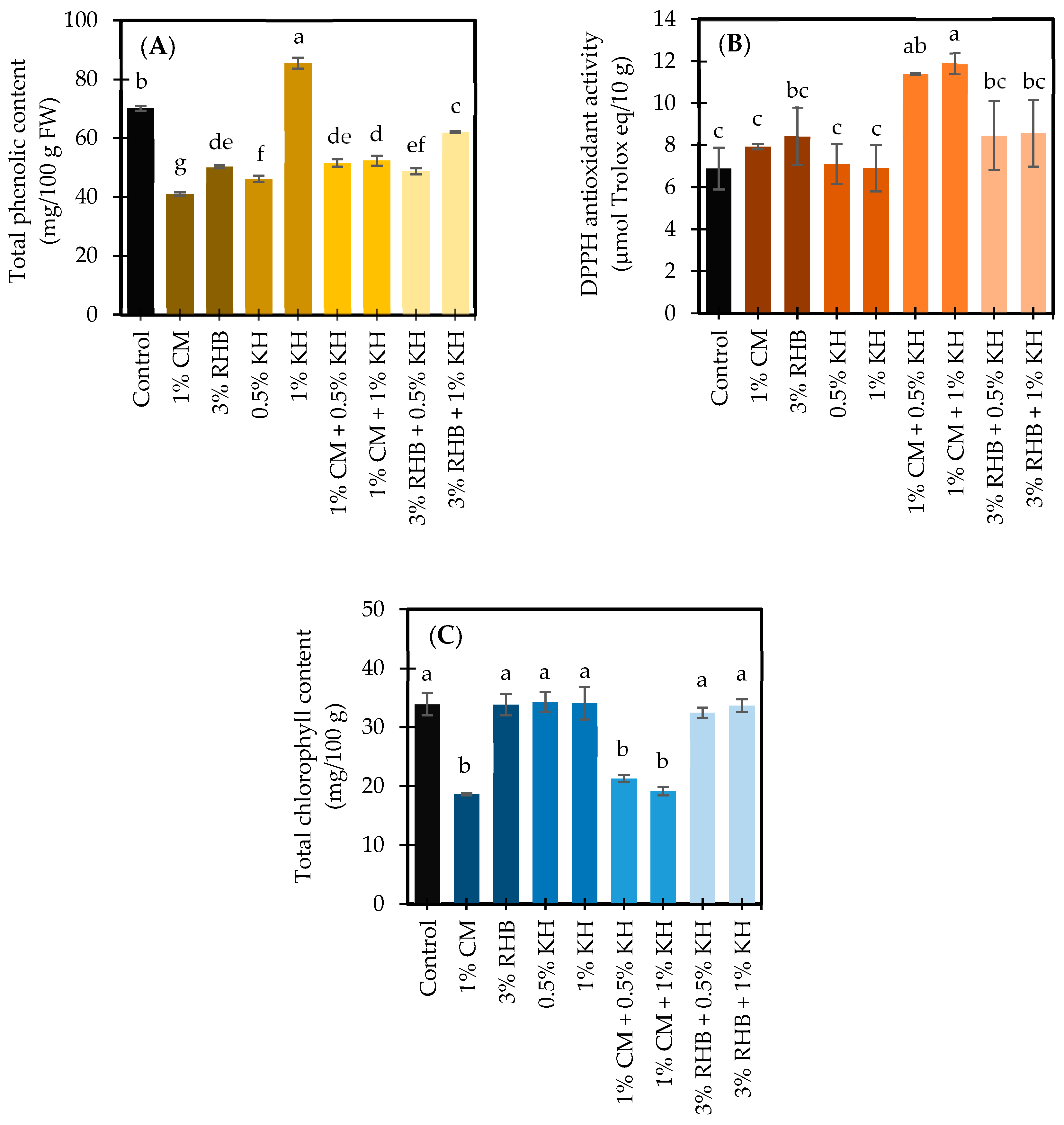
3.3.1. Total Phenolic Content in Response to Treatments
3.3.2. DPPH Antioxidant Activity in Response to Treatments
3.3.3. Chlorophyll Content in Response to Treatments
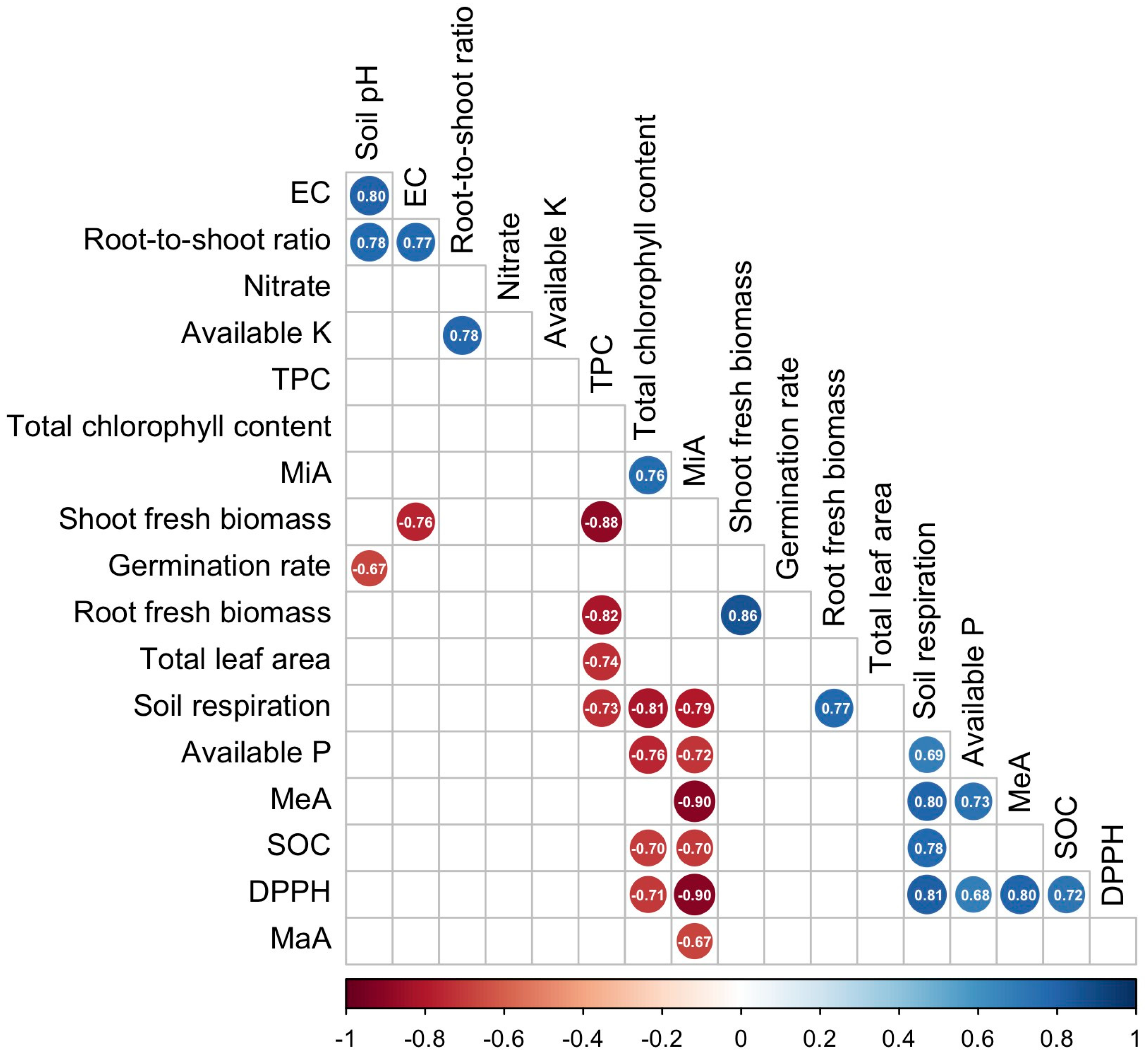
3.4. Correlation Analysis Among Soil Properties, Plant Traits, and Bioactive Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bebber, D.P.; Richards, V.R. A meta-analysis of the effect of organic and mineral fertilizers on soil microbial diversity. Appl. Soil Ecol. 2022, 175, 104450. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Shyam, S.; Ahmed, S.; Joshi, S.J.; Sarma, H. Biochar as a soil amendment: Implications for soil health, carbon sequestration, and climate resilience. Discov. Soil 2025, 2, 18. [Google Scholar] [CrossRef]
- Giagnoni, L.; Renella, G. Effects of biochar on the C use efficiency of soil microbial communities: Components and mechanisms. Environments 2022, 9, 138. [Google Scholar] [CrossRef]
- Pandian, K.; Vijayakumar, S.; Mustaffa, M.R.A.F.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A sustainable soil conditioner for improving soil health, crop production and environment under changing climate: A review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Mendes, L.W.; Alleoni, L.R.F. Potassium organomineral fertilizer alters the microbiome of a sandy loam tropical soil. Appl. Soil Ecol. 2025, 207, 105960. [Google Scholar] [CrossRef]
- Liu, C.; Shang, H.; Han, L.; Sun, X. Effect of alkali residue and humic acid on aggregate structure of saline-alkali soil. Soil Sci. Soc. Am. J. 2024, 88, 291–303. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, X.; Zhong, D.; Lian, H. Potassium humate supplementation improves photosynthesis and agronomic and yield traits of foxtail millet. Sci. Rep. 2024, 14, 9508. [Google Scholar] [CrossRef]
- Zaghloul, E.A.; Awad, E.S.A.; Mohamed, I.R.; El-Hameed, A.M.A.; Feng, D.; Desoky, E.S.M.; Algopishi, U.B.; Al Masoudi, L.M.; Elrys, A.S.; Mathew, B.T.; et al. Co-application of organic amendments and natural biostimulants on plants enhances wheat production and defense system under salt-alkali stress. Sci. Rep. 2024, 14, 29742. [Google Scholar] [CrossRef]
- Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kintl, A.; Radziemska, M.; Baltazar, T.; Jaskulska, I.; Malicek, O.; Latal, O.; Brtnicky, M. Carbon-enriched organic amendments differently affect the soil chemical, biological properties and plant biomass in a cultivation time-dependent manner. Chem. Biol. Technol. Agric. 2022, 9, 52. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Rupngam, T.; Udomkun, P.; Boonupara, T.; Kaewlom, P. Enhancing soil health, growth, and bioactive compound accumulation in sunflower sprouts using agricultural byproduct-based soil amendments. Agronomy 2025, 15, 1213. [Google Scholar] [CrossRef]
- Fichtner, T.; Goersmeyer, N.; Stefan, C. Influence of soil pore system properties on the degradation rates of organic substances during soil aquifer treatment (SAT). Appl. Sci. 2019, 9, 496. [Google Scholar] [CrossRef]
- Kroetsch, D.; Wang, C. Particle size distribution. Soil Sampl. Methods Anal. 2008, 2, 713–725. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis. FAO Fertilizer and Plant Nutrition Bulletin, 19. 2008. Available online: https://www.fao.org/4/i0131e/i0131e.pdf (accessed on 11 April 2025).
- Smallholder Agriculture. Soil Health Evaluation Manual (Version 6.4). 2020. Available online: https://smallholder-sha.org/wp-content/uploads/2020/08/soiltoolkitmanual_sv6.4_edits_8_2020_pom_rewrite_changeaccepted.pdf (accessed on 27 December 2024).
- Page, A.L.; Robert, H.; Miller, D.; Keeney, R. (Eds.) Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Agronomy Monograph No. 9, Part 2; American Society of Agronomy: Madison, WI, USA, 1982; Front Matter—1982—Agronomy Monographs—Wiley Online Library. [Google Scholar]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ozcan, M.M.; AL-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. LWT—Food Sci. Technol. 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Barua, U.; Das, R.P.; Gogoi, B. Chlorophyll estimation in some minor fruits of Assam. Ecol. Environ. Conserv. 2016, 22, 1787–1789. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D.; Zhou, B.; Cai, S.; Li, Y.; Kuang, E.; Sun, L.; Li, L.J. Impacts of long-term fertilization on the molecular structure of humic acid and organic carbon content in soil aggregates in black soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef] [PubMed]
- Quaggiotti, S.; Ruperti, B.; Pizzeghello, D.; Francioso, O.; Tugnoli, V.; Nardi, S. Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize (Zea mays L.). J. Exp. Bot. 2004, 55, 803–813. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus chemistry: Genesis, composition, reactions. J. Chem. Educ. 1995, 72, A93. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Van Dang, L.; Ngoc, N.P.; Hung, N.N. Soil quality and pomelo productivity as affected by chicken manure and cow dung. Sci. World J. 2021, 2021, 6289695. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Ghorbani, M.; Rezaei-Rashti, M.; Abrishamkesh, S.; Amirahmadi, E.; Chengrong, C.H.E.N.; Gorji, M. Application of rice husk biochar for achieving sustainable agriculture and environment. Rice Sci. 2021, 28, 325–343. [Google Scholar] [CrossRef]
- Da Silva Mendes, J.; Fernandes, J.D.; Chaves, L.H.G.; Guerra, H.O.C.; Tito, G.A.; de Brito Chaves, I. Chemical and physical changes of soil amended with biochar. Water Air Soil Pollut. 2021, 232, 338. [Google Scholar] [CrossRef]
- Chen, X.; Lewis, S.; Heal, K.V.; Lin, Q.; Sohi, S.P. Biochar engineering and ageing influence the spatiotemporal dynamics of soil pH in the charosphere. Geoderma 2021, 386, 114919. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar] [CrossRef]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Moustafa, Y.; Hammam, A.; Haddad, S. Potassium humate application and cutting immature flowers affect soil properties, microbial activity and jerusalem artichoke yield components. J. Soil Sci. Agric. Eng. 2018, 9, 33–41. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lin, Y.-Q.; Huang, H.-J. Valorization of Rice Husk for the Production of Porous Biochar Materials. Fermentation 2021, 7, 70. [Google Scholar] [CrossRef]
- Fatima, S.; Riaz, M.; Al-Wabel, M.I.; Arif, M.S.; Yasmeen, T.; Hussain, Q.; Roohi, M.; Fahad, S.; Ali, K.; Arif, M. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J. Soils Sediments 2021, 21, 148–162. [Google Scholar] [CrossRef]
- Liu, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M.; Wu, J. Mineral-ions modified biochars enhance the stability of soil aggregate and soil carbon sequestration in a coastal wetland soil. Catena 2020, 193, 104618. [Google Scholar] [CrossRef]
- Han, Z.; Xu, P.; Li, Z.; Guo, S.; Li, S.; Liu, S.; Wu, S.; Wang, J.; Zou, J. Divergent effects of biochar amendment and replacing mineral fertilizer with manure on soil respiration in a subtropical tea plantation. Biochar 2023, 5, 73. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Mahal, N.K.; Butail, N.P.; Dhaliwal, J.K.; Gautam, A.; Bawa, A.; Kovács, P.; Kumar, S. Soil labile carbon and nitrogen fractions after eleven years of manure and mineral fertilizer applications. Arch. Agron. Soil Sci. 2023, 69, 875–890. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chakrabarti, K.; Chakraborty, A.; Ghosh, S. Effect of long-term fertilizers and manure application on microbial biomass and microbial activity of a tropical agricultural soil. Biol. Fertil. Soils 2011, 47, 227–233. [Google Scholar] [CrossRef]
- López-López, G.; Lobo, M.C.; Negre, A.; Colombàs, M.; Rovira, J.M.; Martorell, A.; Reolid, C.; Sastre-Conde, I. Impact of fertilisation practices on soil respiration, as measured by the metabolic index of short-term nitrogen input behaviour. J. Environ. Manag. 2012, 113, 517–526. [Google Scholar] [CrossRef]
- Afzal, S.; Muhammad, D.; Ullah, R.; Adnan, M.; Saeed, B.; Alzayed, R.M.; Alhajouj, S.A.; Alaida, M.F.; Ahmad, M.; Altalhi, A.; et al. Interactive effect of humic acid and farmyard manure on soil health and microbial activity in calcareous soil. Pak. J. Bot. 2025, 57, 3. [Google Scholar] [CrossRef]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef]
- Tan, W.; Jia, Y.; Huang, C.; Zhang, H.; Li, D.; Zhao, X.; Wang, G.; Jiang, J.; Xi, B. Increased suppression of methane production by humic substances in response to warming in anoxic environments. J. Environ. Manag. 2018, 206, 602–606. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Holatko, J.; Hammerschmiedt, T.; Datta, R.; Baltazar, T.; Kintl, A.; Latal, O.; Pecina, V.; Sarec, P.; Novak, P.; Balakova, L.; et al. Humic acid mitigates the negative effects of high rates of biochar application on microbial activity. Sustainability 2020, 12, 9524. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.P. Efficacy of potassium humate and chemical fertilizers on yield and nutrient availability patterns in soil at different growth stages of rice. Commun. Soil Sci. Plant Anal. 2017, 48, 245–261. [Google Scholar] [CrossRef]
- Chen, Z.X.; Elrys, A.S.; Zhang, H.M.; Tu, X.S.; Wang, J.; Cheng, Y.; Zhang, J.B.; Cai, Z.C. How does organic amendment affect soil microbial nitrate immobilization rate? Soil Biol. Biochem. 2022, 173, 108784. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- El-Naqma, K. The role of humate substances in controlling synergism and antagonism of nutrients uptake by potato plants. Environ. Biodivers. Soil Secur. 2020, 4, 149–165. [Google Scholar] [CrossRef]
- Garg, S.; Bahl, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Abdelkader, A.E. Effect of different levels of farmyard manure, mineral fertilization and potassium humate on growth and productivity of garlic. Sciences 2019, 9, 287–296. [Google Scholar]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Gatabazi, A. Nitrogen, Phosphorus and Potassium Availability as Influenced by Humate and Fulvate Soil Amendment. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2014. [Google Scholar]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef] [PubMed]
- Reitemeier, R.F. Soil potassium. Adv. Agron. 1951, 3, 113–164. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Minkina, T.; Mandzhieva, S. Assessment of ecological condition of haplic chernozem calcic contaminated with petroleum hydrocarbons during application of bioremediation agents of various natures. Land 2021, 10, 169. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, X.; Wang, S.; Pu, X. Benefits of organic manure combined with biochar amendments to cotton root growth and yield under continuous cropping systems in Xinjiang, China. Sci. Rep. 2020, 10, 4718. [Google Scholar] [CrossRef]
- Ibrahim, M.; Yamin, M.; Sarwar, G.; Anayat, A.; Habib, F.; Ullah, S. Tillage and farm manure affect root growth and nutrient uptake of wheat and rice under semi-arid conditions. Appl. Geochem. 2011, 26, S194–S197. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Zou, Z.; Fan, L.; Li, X.; Dong, C.; Zhang, L.; Zhang, L.; Fu, J.; Han, W.; Yan, P. Response of plant root growth to biochar amendment: A meta-analysis. Agronomy 2021, 11, 2442. [Google Scholar] [CrossRef]
- El-Masry, T.; El-Sawah, N.; Osman, A.; Abd El-Ghany, S.; Abed El-Hamed, G. Influence of potassium humate and calcium phosphate on production of pepper seedlings. Fayoum J. Agric. Res. Dev. 2021, 35, 363–379. [Google Scholar] [CrossRef]
- Nemeata Alla, H.; Sasy, A.; Helmy, S.A. Effect of potassium humate and nitrogen fertilization on yield and quality of sugar beet in sandy soil. J. Plant Prod. 2018, 9, 333–338. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Malik, Z.; Malik, N.; Noor, I.; Kamran, M.; Parveen, A.; Ali, M.; Sabir, F.; Elansary, H.O.; El-Abedin, T.K.Z.; Mahmoud, E.A.; et al. Combined effect of rice-straw biochar and humic acid on growth, antioxidative capacity, and ion uptake in maize (Zea mays L.) grown under saline soil conditions. J. Plant Growth Regul. 2023, 42, 3211–3228. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- de Moura, O.V.T.; Berbara, R.L.L.; de Oliveira Torchia, D.F.; Da Silva, H.F.O.; de Castro, T.A.V.T.; Tavares, O.C.H.; Rodrigues, N.F.; Zonta, E.; Santos, L.A.; García, A.C. Humic foliar application as sustainable technology for improving the growth, yield, and abiotic stress protection of agricultural crops. A review. J. Saudi Soc. Agric. Sci. 2023, 22, 493–513. [Google Scholar] [CrossRef]
- Farhadian, M.; Fallah, S.; Kaul, H.P.; Salehi, A. Effects of cow manure and humic acid on Echinacea purpurea (L.) performance and essential oils accumulation under drought conditions. Ind. Crops Prod. 2024, 222, 119826. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendás, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Thepsilvisut, O.; Chutimanukul, P.; Sae-Tan, S.; Ehara, H. Effect of chicken manure and chemical fertilizer on the yield and qualities of white mugwort at dissimilar harvesting times. PLoS ONE 2022, 17, e0266190. [Google Scholar] [CrossRef]
- Tu, Y.; Shen, J.; Peng, Z.; Xu, Y.; Li, Z.; Liang, J.; Wei, Q.; Zhao, H.; Huang, J. Biochar-dual oxidant composite particles alleviate the oxidative stress of phenolic acid on tomato seed germination. Antioxidants 2023, 12, 910. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, F.; Wang, G.; Zhang, G.; Wang, Y.; Chen, X.; Mao, Z. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Al-Otaibi, H.H.; Parmar, A.; Ramadan, K.M.A.; Lobato, A.K.S.; El-Mogy, M.M. Application of potassium humate and salicylic acid to mitigate salinity stress of common bean. Life 2023, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasheed, K.G.; Mazrou, Y.; Omara, A.E.D.; Osman, H.S.; Nehela, Y.; Hafez, E.M.; Rady, A.M.S.; El-Moneim, D.A.; Alowaiesh, B.F.; Gowayed, S.M. Soil amendment using biochar and application of K-humate enhance the growth, productivity, and nutritional value of onion (Allium cepa L.) under deficit irrigation conditions. Plants 2021, 10, 2598. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Rahimzadeh, S. The biochar-based nanocomposites influence the quantity, quality and antioxidant activity of essential oil in dill seeds under salt stress. Sci. Rep. 2022, 12, 21903. [Google Scholar] [CrossRef] [PubMed]
- Chrząszcz, M.; Krzemińska, B.; Celiński, R.; Szewczyk, K. Phenolic composition and antioxidant activity of plants belonging to the Cephalaria (Caprifoliaceae) genus. Plants 2021, 10, 952. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Cong, M.; Hu, Y.; Sun, X.; Yan, H.; Yu, G.; Tang, G.; Chen, S.; Xu, W.; Jia, H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Linna, C.; Ma, S.; Ma, Q.; Guo, J.; Wang, F.; Wang, L. Effects of biochar with inorganic and organic fertilizers on agronomic traits and nutrient absorption of soybean and fertility and microbes in purple soil. Front. Plant Sci. 2022, 13, 871021. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Rana, N.; Sharma, U.; Gudeta, K.; Alharbi, K.; Ameen, F.; Bhat, S.A. Effect of organic manures on growth, yield, leaf nutrient uptake and soil properties of kiwifruit (Actinidia deliciosa Chev. ) cv. Allison. Plants 2022, 11, 3354. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2021, 45, 695–704. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant growth-defense trade-offs: Molecular processes leading to physiological changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Yan, S.; Yi-Quan, W.; Rui-qian, T. Effects of soil compaction stress on photosynthesis, chlorophyll fluorescence parameters of cucumber (Cucumis sativus L.) leaves. J. Plant Nutr. Fertil. 2009, 15, 638–642. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]


| Property | Soil | CM | RHB | KH |
|---|---|---|---|---|
| Soil texture | Sandy loam | - | - | - |
| pH value (–) | 6.6 (0.2) 1 | 8.7 (0.1) | 6.7 (0.0) | 10.8 (0.1) |
| Electrical conductivity (EC, mS/cm) | 0.6 (0.0) | 1.6 (0.1) | 0.1(0.0) | 10.8 (0.2) |
| Organic matter (OM, %) | 4.3 (0.1) | 65.3 (2.1) | 13.2 (1.2) | 36.7 (0.8) |
| Soil organic carbon (SOC, %) | 2.5 (0.2) | 37.2 (0.8) | 8.1 (0.5) | 21.3 (0.5) |
| Moisture (%) | 0.4 (0.0) | 5.2 (0.0) | 5.0 (0.3) | 8.7 (0.0) |
| Water holding capacity (WHC, %) | 16.1 (1.8) | 481.2 (19.5) | 225.2 (12.1) | - |
| Nitrate (NO3−, mg/kg) | 120.2 (6.1) | 117.1 (16.5) | 40.8 (5.6) | 3473.6 (89.7) |
| Available phosphorus (P, mg/kg) | 15.9 (1.0) | 2481.7 (202.0) | 75.3 (7.3) | 75.3 (19.2) |
| Available potassium (K, mg/kg) | 460.5 (12.3) | 10215.3 (482.5) | 2525.4 (142.5) | 7276.9 (771.4) |
| Humic acid (%) 2 | - | - | - | 67.5 (2.5) |
| Water solubility (%) 2 | - | - | - | 97.5 (2.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupngam, T.; Udomkun, P.; Boonupara, T.; Kaewlom, P. Soil–Plant Biochemical Interactions Under Agricultural Byproduct Amendments and Potassium Humate: Enhancing Soil Function and Bioactive Compounds in Sunflower Sprouts. Agronomy 2025, 15, 1651. https://doi.org/10.3390/agronomy15071651
Rupngam T, Udomkun P, Boonupara T, Kaewlom P. Soil–Plant Biochemical Interactions Under Agricultural Byproduct Amendments and Potassium Humate: Enhancing Soil Function and Bioactive Compounds in Sunflower Sprouts. Agronomy. 2025; 15(7):1651. https://doi.org/10.3390/agronomy15071651
Chicago/Turabian StyleRupngam, Thidarat, Patchimaporn Udomkun, Thirasant Boonupara, and Puangrat Kaewlom. 2025. "Soil–Plant Biochemical Interactions Under Agricultural Byproduct Amendments and Potassium Humate: Enhancing Soil Function and Bioactive Compounds in Sunflower Sprouts" Agronomy 15, no. 7: 1651. https://doi.org/10.3390/agronomy15071651
APA StyleRupngam, T., Udomkun, P., Boonupara, T., & Kaewlom, P. (2025). Soil–Plant Biochemical Interactions Under Agricultural Byproduct Amendments and Potassium Humate: Enhancing Soil Function and Bioactive Compounds in Sunflower Sprouts. Agronomy, 15(7), 1651. https://doi.org/10.3390/agronomy15071651






