Phenolic Profiles of Different Apricot Varieties Grown in Spain: Discrimination Among Cultivars During the Harvest Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Extraction and UHPLC Analysis
2.3. Statistical Analysis
3. Results and Discussion
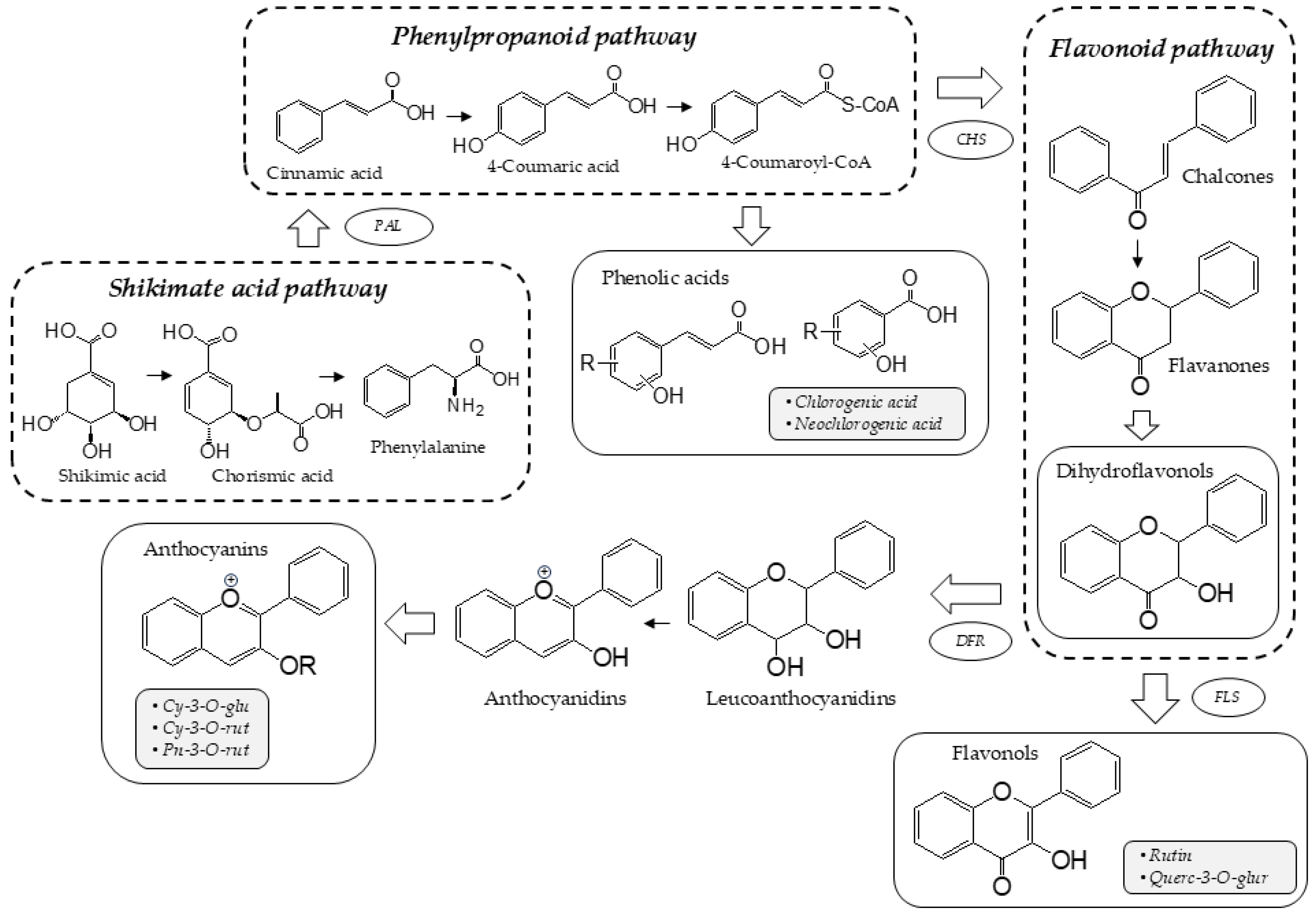
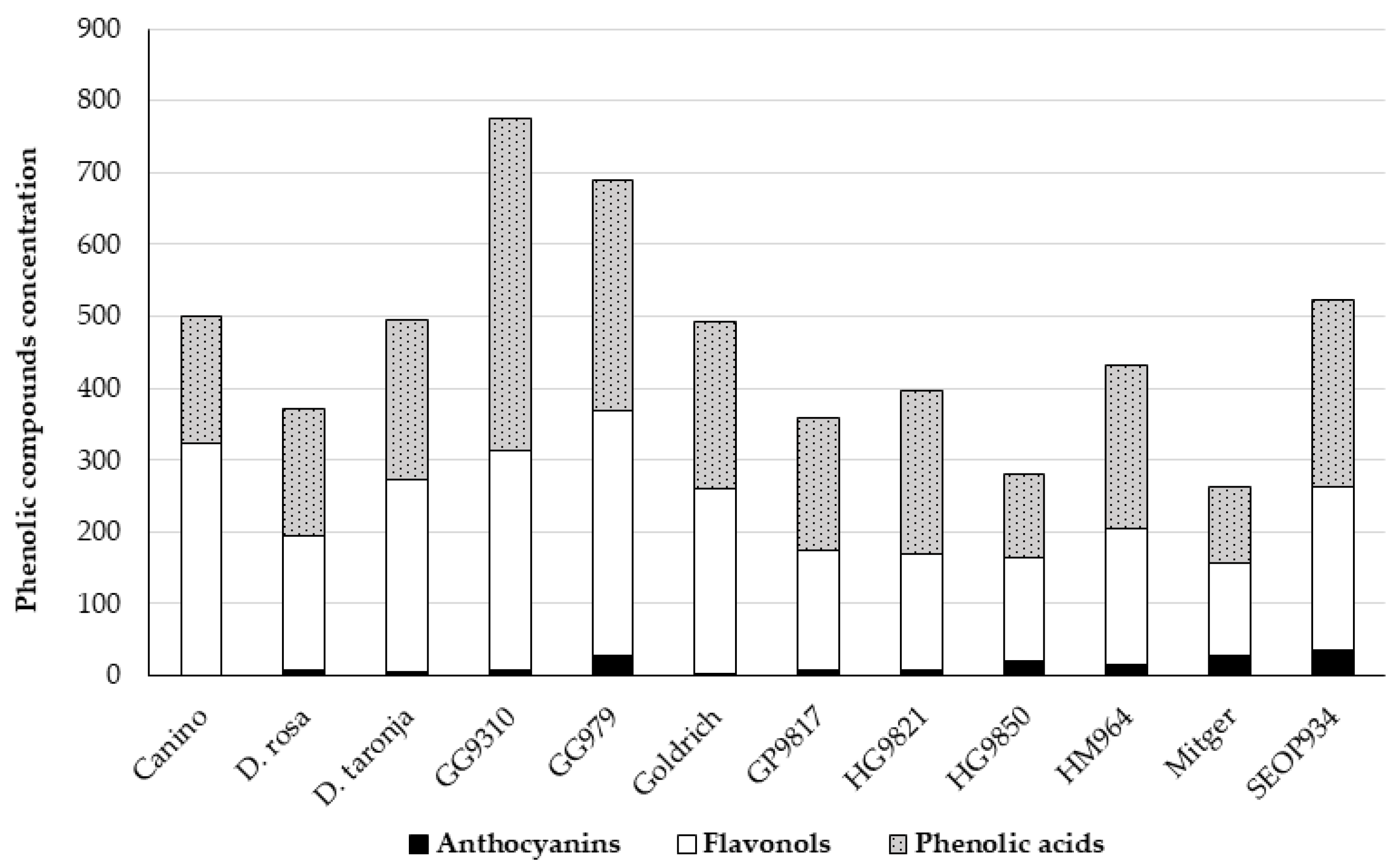
3.1. The Profile of Phenolic Compounds
3.1.1. Anthocyanin Content
3.1.2. Flavonol and Phenolic Acid Content
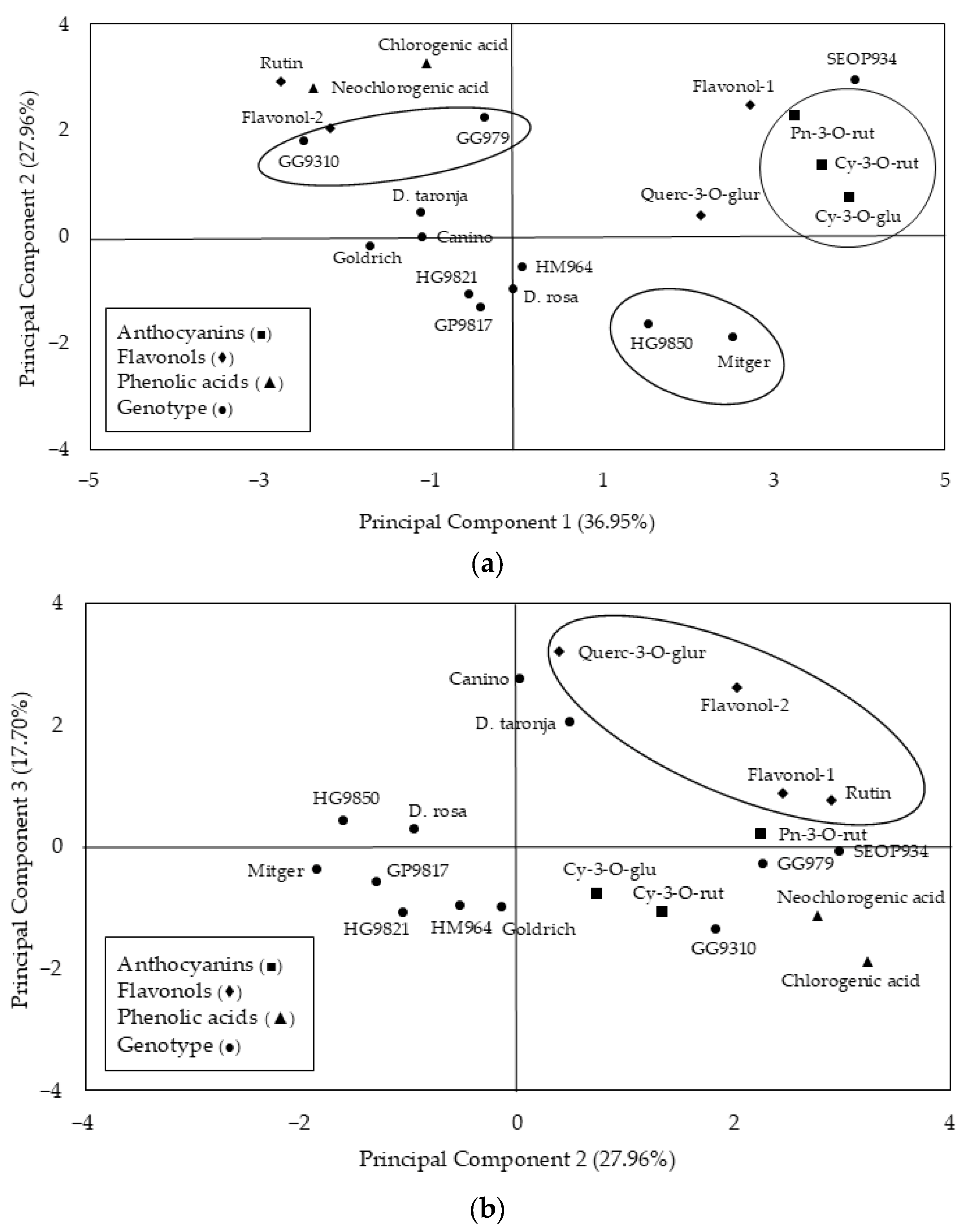
3.2. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry Weight |
| PCA | Principal Component Analysis |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
References
- Ali, S.; Masud, T.; Abbasi, S.K.; Mahmood, T.; Hussain, A. Apricot: Nutritional potentials and health benefits-a review. Ann. Food Sci. Technol. 2015, 16, 175–189. [Google Scholar]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Göttingerová, M.; Kumsta, M.; Rampácková, E.; Kiss, T.; Necas, T. Analysis of Phenolic Compounds and Some Important Analytical Properties in Selected Apricot Genotypes. Hortscience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Schmitzer, V.; Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Krskab, B.; Stampar, F. Comparative study of primary and secondary metabolites in apricot (Prunus armeniaca L.) cultivars. J. Sci. Food. Agric. 2011, 91, 860–866. [Google Scholar] [CrossRef]
- Vicente, A.R.; Manganaris, G.A.; Cisneros-Zevallos, L.; Crisosto, C.H. Prunus. In Health-Promoting Properties of Fruit and Vegetables; Terry, L.A., Ed.; CABI: Wallingford, UK, 2011; pp. 238–259. [Google Scholar] [CrossRef]
- Baccichet, I.; Chiozzotto, R.; Spinardi, A.; Gardana, C.; Bassi, D.; Cirilli, M. Evaluation of a large apricot germplasm collection for fruit skin and flesh acidity and organic acids composition. Sci. Hortic. 2022, 294, 110780. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Nutraceutical profiles of apricots (Prunus armeniaca L.) as a source of fruit quality traits for breeding. Span. J. Agric. Res. 2021, 19, e0703. [Google Scholar] [CrossRef]
- Xi, W.P.; Zheng, H.; Zhang, Q.; Wenhui, L.I. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef]
- Ortuño-Hernandez, G.; Silva, M.; Toledo, R.; Ramos, H.; Reis-Mendes, A.; Ruiz, D.; Martinez-Gomez, P.; Ferreira, I.M.P.L.V.O.; Salazar, J.A. Nutraceutical Profile Characterization in Apricot (Prunus armeniaca L.) Fruits. Plants 2025, 14, 1000. [Google Scholar] [CrossRef]
- Veberic, R.; Stampar, F. Selected polyphenols in fruits of different cultivars of genus Prunus. Phyton Ann. Rei Botan. 2005, 45, 375–383. [Google Scholar]
- Paul, A.; Acharya, K.; Chakraborty, N. Involvement of Phenylpropanoid pathway and Shikimic acid pathway in environmental stress response. In Biology and Biotechnology of Environmental Stress Tolerance in Plants; Roychoudhury, A., Ed.; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press (Taylor & Francis): Boca Raton, FL, USA, 2023; Volume 1, pp. 27–66. [Google Scholar]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kanwal, F.; Ullah, S.; Fahad, M.; Tariq, L.; Altaf, M.T.; Riaz, A.; Zhang, G. Plant secondary metabolites—Central regulators against abiotic and biotic stresses. Metabolites 2025, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Renard, C.M.G.C.; Reich, M.; Ginies, C.; Audergon, J.M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT-Food Sci. Technol. 2009, 42, 373–377. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Polyphenol content in apricot fruits. Sci. Hortic. 2021, 277, 109828. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Gil-Muñoz, F.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Insights of phenolic pathway in fruits. Transcriptional and metabolic profiling in apricot (Prunus armeniaca). Int. J. Mol. Sci. 2021, 22, 3411. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Vianello, F.; Correa, C.R.; Campos, R.A.D.S.; Borguini, M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Jia, K.; Liao, K.; Liu, L.; Fan, G.; Zhang, S.; Wang, Y. Metabolomic and transcriptomice analyses of flavonoid biosynthesis in apricot fruits. Front. Plant Sci. 2023, 14, 1210309. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Dondini, L.; Martínez-Gómez, P.; Ruiz, D. Identification of QTLs linked to fruit quality traits in apricot (Prunus armeniaca L.) and biological validation through gene expression analysis using qPCR. Mol. Breed. 2019, 39, 28. [Google Scholar] [CrossRef]
- Luo, P.; Ning, G.; Wang, Z.; Shen, Y.; Jin, H.; Li, P.; Huang, S.; Zhao, J.; Bao, M. Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. Red color flower formation in plants. Front. Plant Sci. 2016, 6, 1257. [Google Scholar] [CrossRef] [PubMed]
- Badenes, M.L.; Martínez-Calvo, J.; Gómez, H.; Zuriaga, E. ‘Dama Taronja’ and ‘Dama Rosa’ apricot cultivars that are resistant to sharka (Plum pox virus). HortScience 2018, 53, 1228–1229. [Google Scholar] [CrossRef]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of Phenolic Compounds and Antioxidant Capacity in Fruits of Apricot Genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calvo, J.; Font, A.; Llácer, G.; Badenes, M.L. Apricot and peach breeding programs from the IVIA. In XII EUCARPIA Symposium on Fruit Breeding and Genetics, ISHS Acta Horticulturae 814; ISHS: Leuven, Belgium, 2009; Volume 814, pp. 185–188. [Google Scholar] [CrossRef]
- Orazem, P.; Stampar, F.; Hudina, M. Fruit Quality of Redhaven and Royal Glory Peach Cultivars on Seven Different Rootstocks. J. Agric. Food. Chem. 2011, 59, 9394–9401. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomás-Barberán, F.A. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Extraction Solvents Affect Anthocyanin Yield, Color, and Profile of Strawberries. Plants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of extraction methods for characterization of phenolic compounds in apricot fruit (Prunus armeniaca). Food Funct. 2019, 10, 6492–6502. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Xia, L.; Hui, J.; Li, J.; Feng, Y.; Chen, Y. Preliminarily exploring of the association between sugars and anthocyanin accumulation in apricot fruit during ripening. Sci. Hortic. 2019, 248, 112–117. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
| Genotype | Pedigree | Origin | Peel Color 1 |
|---|---|---|---|
| Canino | Unknown | Spain | yellow green to orange |
| Dama rosa | Goldrich × Ginesta | IVIA | yellow green to orange |
| Dama taronja GG9310 | Goldrich × Katy | IVIA | yellow green to orange |
| Goldrich × Ginesta | IVIA | yellow green to orange | |
| GG979 | Goldrich × Ginesta | IVIA | yellowish to orange |
| Goldrich | Goldrich Sunglo × Perfection | USA | orange |
| GP9817 | Goldrich × Palau | IVIA | yellow green |
| HG9821 | Harcot × Ginesta | IVIA | yellow green to orange |
| HG9850 | Harcot × Ginesta | IVIA | yellow green to orange |
| HM964 | Harcot × Mitger | IVIA | yellow green to orange |
| Mitger | Unknown | Spain | yellowish |
| SEOP934 | Seo × Palau | IVIA | yellowish to orange |
| Genotype | Anthocyanins | Flavonols | Phenolic Acids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cy-3-O-glu 1 | Cy-3-O-rut 1 | Pn-3-O-glu 2 | Rutin 1 | Querc-3-O-g 1 | Flavonol-1 3 | Flavonol-2 3 | Neochlorog 1 | Chlorog 1 | |
| Canino | nd | 0.46 ± 0.6 | 0.10 ± 0.0 | 267.48 ± 7.9 | 26.79 ± 1.3 | 7.05 ± 0.4 | 22.33 ± 1.3 | 104.36 ± 4.1 | 70.69 ± 4.5 |
| Dama rosa | 1.95 ± 1.1 | 6.60 ± 5.9 | nd | 146.96 ± 10.6 | 20.44 ± 1.6 | 5.21 ± 0.6 | 12.10 ± 0.9 | 88.44 ± 6.6 | 89.51 ± 3.8 |
| Dama taronja | 0.69 ± 1.0 | 2.46 ± 2.0 | 0.88 ± 1.0 | 210.75 ± 12.9 | 22.50 ± 0.7 | 6.03 ± 0.1 | 29.00 ± 2.1 | 139.23 ± 6.5 | 84.10 ± 1.7 |
| GG9310 | 1.22 ± 2.5 | 5.50 ± 4.9 | 0.46 ± 0.6 | 275.75 ± 37.5 | 15.27 ± 7.8 | 0.89 ± 0.5 | 15.02 ± 2.3 | 328.24 ± 25.2 | 132.10 ± 11.3 |
| GG979 | 5.68 ± 2.0 | 21.99 ± 11.9 | nd | 294.93 ± 22.4 | 21.10 ± 2.6 | 2.23 ± 0.6 | 22.39 ± 3.6 | 168.37 ± 8.9 | 152.41 ± 3.5 |
| Goldrich | 0.29 ± 0.0 | 2.16 ± 1.7 | 0.39 ± 0.0 | 230.14 ± 3.7 | 12.27 ± 0.6 | 3.02 ± 0.7 | 12.79 ± 0. 9 | 119.12 ± 1.8 | 113.32 ± 4.7 |
| GP9817 | 1.36 ± 1.5 | 5.70 ± 5.7 | nd | 138.70 ± 10.3 | 17.18 ± 1.6 | 1.79 ± 0.3 | 8.89 ± 0.9 | 86.81 ± 7.1 | 97.18 ± 8.2 |
| HG9821 | 1.03 ± 1.6 | 5.70 ± 2.6 | nd | 137.18 ± 6.5 | 16.75 ± 2.4 | 1.99 ± 0.8 | 6.17 ± 0.9 | 118.25 ± 6.8 | 109.34 ± 13.3 |
| HG9850 | 4.00 ± 0.9 | 14.30 ± 4.1 | 0.95 ± 0.6 | 113.44 ± 3.8 | 23.22 ± 1.3 | 1.31 ± 0.1 | 8.01 ± 0.3 | 51.83 ± 1.7 | 63.61 ± 3.9 |
| HM964 | 2.86 ± 0.1 | 12.63 ± 5.9 | nd | 159.76 ± 4.1 | 18.08 ± 0.9 | 2.83 ± 0.2 | 7.83 ± 0.1 | 116.14 ± 3.9 | 110.35 ± 6.3 |
| Mitger | 8.56 ± 1.9 | 18.75 ± 4.9 | 0.69 ± 0.1 | 101.97 ± 2.9 | 21.15 ± 0.6 | 1.38 ± 0.1 | 4.42 ± 0.3 | 58.06 ± 0.9 | 47.56 ± 5.3 |
| SEOP934 | 7.53 ± 2.9 | 23.31 ± 5.9 | 4.81 ± 4.9 | 167.61 ± 14.6 | 23.31 ± 0.9 | 26.47 ± 0.8 | 9.95 ± 1.9 | 128.53 ± 3.8 | 130.81 ± 4.8 |
| p-value | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, J.; Gómez-Martínez, H.; Bermejo, A. Phenolic Profiles of Different Apricot Varieties Grown in Spain: Discrimination Among Cultivars During the Harvest Season. Agronomy 2025, 15, 1652. https://doi.org/10.3390/agronomy15071652
Morales J, Gómez-Martínez H, Bermejo A. Phenolic Profiles of Different Apricot Varieties Grown in Spain: Discrimination Among Cultivars During the Harvest Season. Agronomy. 2025; 15(7):1652. https://doi.org/10.3390/agronomy15071652
Chicago/Turabian StyleMorales, Julia, Helena Gómez-Martínez, and Almudena Bermejo. 2025. "Phenolic Profiles of Different Apricot Varieties Grown in Spain: Discrimination Among Cultivars During the Harvest Season" Agronomy 15, no. 7: 1652. https://doi.org/10.3390/agronomy15071652
APA StyleMorales, J., Gómez-Martínez, H., & Bermejo, A. (2025). Phenolic Profiles of Different Apricot Varieties Grown in Spain: Discrimination Among Cultivars During the Harvest Season. Agronomy, 15(7), 1652. https://doi.org/10.3390/agronomy15071652






