Abstract
Alfalfa mosaic virus (AMV) is one of the most widely distributed viruses. It often exhibits combined infection with white clover mosaic virus (WCMV) and occurs with a synergistic effect at 3:1 (AMV: WCMV). This study sought to clarify whether this synergistic effect is related to the molecular mutation of the coat protein (CP) sequences of the two viruses and their interactions, as well as the effect of the WCMV CP concentration on infection with AMV. This study identified and analyzed the CP sequences of two viruses after the co-infection of AMV and WCMV in Nicotiana benthamiana and found that the CP sequences of the two viruses mutated after co-infection with AMV and WCMV compared with the sequences from separate single infections with each virus. The mutation rate of the nucleotide bases was 7.66% and 3.37% in the Co-AMV CP and Co-WCMV CP, respectively, and 9.05% and 5.77% in the amino acid, respectively. The effect of WCMV CP and AMV at different proportions antagonistically affected infection with AMV when the proportion of WCMV CP: AMV was 3:1, 2:1, and 1:1. These proportions of treatment alleviated the symptoms caused by infection with N. benthamiana and reduced the relative expression of the AMV CP by 0.56, 0.47, and 0.76-fold, respectively, compared with single infection by AMV. Thus, the CP sequences of both viruses mutated after the co-infection of AMV and WCMV, and a proportion of WCMV CP: AMV of 3:1, 2:1, and 1:1 inhibited infection by AMV.
1. Introduction
Alfalfa (Medicago sativa), a globally cultivated perennial leguminous forage grass renowned as the “King of Forages” is rich in minerals and vitamins [1]. In China, driven by the ongoing implementation of the “Revitalize the Dairy Industry with Alfalfa Initiative” and the “Grain-to-Feed” policy, the alfalfa cultivation area has continuously expanded, reaching approximately 6.5 million mu (about 433,000 hectares) [2]. However, with increasing cultivation years and frequency of mechanical harvesting, the accumulation and occurrence of viruses have risen annually, leading to significant reductions in both the yield and quality of alfalfa [3]. It is reported that over 50 different viruses infect alfalfa, with alfalfa mosaic virus (AMV) and medicago sativa alphapartitivirus 2 (MsAPV 2) being the predominant pathogens; the incidence rates were 91.7% and over 74.4%, respectively [4,5]. As the province with the largest alfalfa cultivation area in China, Gansu Province experiences widespread alfalfa viral diseases, with an average disease incidence of 32.15% and a disease index of 16.27 [6]. These diseases pose a serious threat to the alfalfa industry, causing substantial economic losses.
Synergistic co-infection by viruses is common, characterized by exacerbated disease symptoms, enhanced viral accumulation within the host, and the emergence of more severe viral diseases, including novel symptoms [7]. This co-existence stems not merely from additive effects but from complex interactions between the viruses [8,9]. AMV, the main source of viruses that infects alfalfa, is also known as Alfamovirus AMV, and it is a member of the family Bromoviridae [10]. AMV is a positive single-stranded RNA virus, infecting various plants, including Medicago sativa, Trifolium repens, Nicotiana tabacum, Vigna unguiculata, Solanum tuberosum, Capsicum annuum, and Solanum lycopersicum, and the main symptoms of infected hosts were mosaic, shrinking, and mottling [11,12]. White clover mosaic virus (WCMV/WClMV) is defined in this paper as WCMV and is also known as Potexvirus trifolii; it is a member of the family Alphaflexiviridae [5]. This primarily infects leguminous plants, such as alfalfa and clover [13], and causes leaf blight and varying degrees of mottling, bright pulse, and yellowing. Liang et al. [14] conducted a double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) on field clover. They found that the AMV and WCMV co-infection rate was as high as 83.3%, and it could enhance the disease symptoms of clover. Coat protein (CP) is the main component of the viral coat. It wraps and protects the genetic material of the virus and is involved in its replication, the translation of its RNA, its movement, the formation of symptoms, and its pathogenesis [15]. Our previous study found that inoculation with both viruses at concentrations of 75% and 25% (AMV: WCMV [3:1]), respectively, enhanced the symptoms of disease and increased the content of both viruses; there was a significant increase in the AMV CP and WCMV CP relative expression, which manifested a synergistic effect [13,16]. The increase in AMV was higher than that of WCMV; AMV was the passive synergistic virus, and WCMV was the synergistic virus. It showed that the occurrence of synergistic or antagonistic effects between the two viruses was closely related to the virus concentration [17].
Viruses, as one of the simplest structured forms of life, have their genetic code and their genomes mostly concentrated on strands of nucleic acid [18]. Their genetic information is exclusively located in the nucleic acid chain [19]. The mutation of bases in the nucleic acid chains can lead to changes in the genetic characteristics of the offspring viruses [20,21,22]. Currently, the common methods for analyzing base mutations in the nucleic acid chains of plant viruses include reverse transcription PCR and sequencing, Sanger sequencing, panel sequencing, third-generation sequencing technology, and bioinformatics analytical methods, among others [23,24]. During the process of viral infection, the viral CP has a critical role in the entire life process of the virus [25]. This is because it is the key part of the virus particles that is in contact with the external environment and contributes to infection, symptomatic expression, and the differentiation of pathogenicity of viruses. Thus, to some extent, it reveals the regularity of virus transmission [26,27]. In response to changing environmental conditions and the increasing prevalence of viral co-infections, the virus’s CP has undergone mutations [28]. These changes in its traits, such as replication, movement, aggregation, and mediator transmission of the virus, enables it to quickly adapt to new environments or hosts; this enhances the symptoms in the host and causes greater economic losses [29,30].
In recent years, the technology for genetically improving plants utilizing viral genes has become a new pathway to breed crops with enhanced disease resistance [31]. The primary principle is to enhance the host immunity by viral CPs, replicase, and movement-protein-mediated resistance pathways. Among these methods, the resistance mediated by CPs has become the most widely used strategy for disease resistance owing to its ability to target the core aspects of the assembly of viral coats [32,33]. It has been reported that transgenic plants resistant to tobacco mosaic virus (TMV), potato virus Y (PVY), cucumber mosaic virus (CMV), and AMV have been successfully obtained by transferring their CP genes into tobacco (Nicotiana tabacum), potato (Solanum tuberosum), cucumber (Cucumis sativa), and red clover (Trifolium pratense) [34,35,36,37], respectively.
However, whether AMV and WCMV co-infection correlates with molecular mutations in their respective CPs and how differing proportions of WCMV CP and AMV affect AMV infectivity remain systematically unstudied. To this end, we cloned the AMV CP and WCMV CP genes of AMV and WCMV that were co-infected and singly infected through sequencing the sequence mutations and interactions when the AMV CP and WCMV CP combined infection was analyzed. The effects of differing proportions of WCMV CP and AMV on AMV infection were clarified by constructing WCMV CP expression vectors. The results of the study provide a basis for in-depth research on the pathogenic mechanism of viral CP variants in co-infection. Meanwhile, it provides a theoretical basis for establishing effective prevention and control measures for viral diseases.
2. Materials and Methods
2.1. Plant and Virus Materials
Experiments were conducted between 8/2022 and 6/2024. Nicotiana benthamiana plants were used for these experiments. Seeds donated by Shaanxi Aiyouji Biotechnology Co., LTD (Shaanxi, China) were planted in seedling trays that contained sterile soil. When it grew to the two-leaf stage, it was then transplanted into 16 cm diameter plastic pots that contained sterile soil fertilized with a 1:5 (w/w) mixture of 16 N: 3.5 P: 10 K slow-release Osmocote fertilizer. N. benthamiana seedlings were grown in an artificial-intelligence-assisted climate chamber (Hangzhou Qisheng Electronic Technology Co., Ltd., Hangzhou, China) and were properly separated to avoid any contamination. The conditions of growth were fixed and included 25 °C, 50% relative humidity, a light intensity of 100 μmol·m−2·s−1, and a 16/8 h light/dark photoperiod. They were irrigated every three days.
Naturally infected alfalfa leaves collected from fields in Lanzhou, Gansu Province, served as the virus source for inoculation onto Vigna unguiculata by friction inoculation. According to the symptoms of AMV infection in V. unguiculata, it produces spots, while WCMV infection in V. unguiculata produces systematic mosaic leaves. Moreover, AMV can produce spots 48 h after inoculation, and the time of WCMV infection to produce systematic mosaic leaves is about 72 h, so the two viruses can be preliminarily separated by collecting single-spot and new leaves at different times. The two separate viruses were inoculated with cowpea three times by the same method; AMV was obtained by the single-spot separation method, and WCMV was obtained by collecting new leaves of V. unguiculata. Then, the two viruses were inoculated with N. benthamiana at the four-leaf stage, labeled, and placed in an artificial-intelligence-assisted climate chamber (Hangzhou, China) for virus propagation; the number of diseased leaves was collected after 15 days post inoculation (dpi); and AMV and WCMV were purified according to the following methods.
For the purification of AMV, we referred to the method of Jin et al. [38]. Diseased leaves were collected and washed; 50 g of tissue was weighed; and potassium phosphate buffer (pH 7.5, 0.5 M) containing 0.01 M EDTA, 1% Triton X-100, and 1% mercaptoethanol was added at 1:2 (w/v). After grinding and homogenizing in a frozen mortar, 5% chloroform-n-butanol (equal volume mixing) was added, and after 40 min of standstill, low-temperature high-speed refrigerated centrifuge (5430R, Eppendorf, Germany) was carried out, the supernatant was taken, and 6% PEG6000 and NaCl were added and stirred for 4 h. After centrifugation, the precipitation was dissolved in 0.01 M EDTA, 1% Triton X-100 potassium phosphate buffer (pH 7.4, 0.02 M), and after multiple centrifugations, the precipitation was suspended in phosphate buffer (pH 7.0, 0.02 M), divided into 2 mL sterile centrifuge tubes, labeled, and stored in a −80 °C freezer (Gansu Agricultural University, Lanzhou, China) for later use.
The purification of WCMV referred to the method of Wetter et al. [39]. Diseased leaves were collected and washed; 50 g of tissue was weighed; and phosphate buffer (pH 7.8, 0.05 M) containing 0.2 EDTA and 0.2% Na2SO3 was added at 1:4 (w/v). After grinding and homogenizing in a frozen mortar, the supernatant was taken after low-temperature high-speed refrigerated centrifuge (5430R, Eppendorf, Germany), the supernatant was adjusted to pH 5.0 by acetic acid, the supernatant was taken by centrifugation, 4% PEG6000 and 0.3 M NaCl were added and allowed to stand for 2 h, and the precipitation was suspended in boron acid borax buffer (pH 8.6, 0.13 M). After several centrifugations, the precipitation was suspended in phosphate buffer (pH 7.0, 0.02 M), aliquoted into 2 mL sterile centrifuge tubes, labeled, and stored in a −80 °C freezer (Gansu Agricultural University, Lanzhou, China) for later use.
The concentrations of the purified AMV and WCMV were 300 pg·mL−1. The accession numbers for AMV CP and WCMV CP are PV867012 and PV867011, respectively.
2.2. Virus Inoculation
AMV and WCMV were mixed at a ratio of 3:1 (AMV: WCMV) (v/v). The viral mixture was then inoculated on the N. benthamiana leaves by friction. The leaves were thoroughly rinsed with sterile water until clean, and then a small amount of carborundum was delicately sprinkled onto the leaves. Each leaf was then inoculated with 100 µL of virus solution using friction. Briefly, the virus solution was applied on the leaf surface and then gently rubbed using fingertips. Three leaves were inoculated per plant. After 15 days post inoculation (dpi), the diseased leaves were collected, ground into a grinding solution, and inoculated onto N. benthamiana again. This process was repeated for 10 generations.
The leaves of N. benthamiana seedlings that were co-inoculated with AMV and WCMV were used as the treatments. AMV (60 pg·mL−1) and WCMV (100 pg·mL−1) were inoculated singly as positive controls, and 0.02 moL·L−1 of phosphate-buffered solution (PBS, pH 7.0) was used as the negative control (CK) [17]. Each treatment inoculated 10 seedlings, repeated three times. The seedlings were labeled and placed in the climate chamber to observe the symptoms of disease daily. Samples were collected at 15 dpi, the total RNA of the plant was extracted, 10 parts of RNA from each treatment were mixed together. Finally, there were 3 parts of RNA for each treatment, the CP of the two viruses was used as primers for PCR amplification, the product was detected by 1% gel electrophoresis, and the size of the 3 repeated CP bases was found to be basically the same through verification. Then, we collected these three strips into one sterile centrifuge tube and sent them to Beijing Qingke Science and Technology Biological Co., Ltd. (Beijing, China) for sequencing and compared the sequences of AMV and WCMV co-infection and single infection and counted the mutation rate and amino acid mutation rate of the CP base of the two viruses in the co-infection.
2.3. Total RNA Isolation
The N. benthamiana leaves with AMV and WCMV combined infection (Co-AMV CP and Co-WCMV CP), AMV single infection (Si-AMV CP), and WCMV single infection (Si-WCMV CP) were collected in 1.5 mL enzyme-free centrifuge tubes, frozen in liquid nitrogen, and stored at −80 °C until use for total RNA isolation. The total RNA was extracted from the sample using the TRIzol reagent (Tiangen, Beijing, China) according to the manufacturer’s instructions. The quality and quantity of the extracted total RNA sample were assessed using a NanoPhotometer-N50 spectrophotometer (GE Healthcare, Wiesbaden, Germany). Only RNA samples with an OD260/280 absorption ratio between 1.80 and 2.20 and RIN values >8.0 were used for subsequent tests. There were three replicates per sample.
2.4. Cloning of the AMV CP and WCMV CP Sequences During Co-Infection and Single Infection
The pairs of gene-specific primers were designed by Primer3 v.4.1 (https://primer3.ut.ee, accessed on 6 May 2023) to clone the target genes Co-AMV CP, Si-AMV CP, and Co-WCMV CP, Si-WCMV CP (Table 1). Next, the extracted RNA was reverse-transcribed to cDNA using a One-Step cDNA kit (Tiangen) in 20 μL that contained 4 μL 5× FastKing-RT SuperMin, 1 μL total RNA, and 15 μL RNase-free water at 42 °C for 15 min and 95 °C for 3 min. The CP genes were subsequently amplified using RT-PCR with viral cDNA as the template (Tiangen) [40]. The PCR products were detected on 1% agarose gels and were recovered using a TaKaRa MiniBEST Agarose Gel DNA Extraction Kit Ver. 4.0 (TaKaRa, Dalian, China), ligated into the pMD-T vector (Tiangen), and then transformed into DH5α Escherichia coli cultures (Tiangen). Positive clones were sequenced at Sangon Biotech Co., Ltd. (Shanghai, China). The cloned gene sequences were compared with the reference sequences to test and verify the correctness of the cloning results.

Table 1.
Primer sequences used in this study.
2.5. Bioinformatic Analysis in Co-Infection and Single Infection of the AMV CP and WCMV CP Sequences
All the cDNA sequences of the AMV CP and WCMV CP were further edited with DNAMAN 9.0 and verified using the online software NCBI BLAST (https://www.ncbi.nlm.nih.gov/, accessed on 6 June 2023) after referral to sequences and other strains isolated from different regions. Additionally, other regions of the AMV CP and WCMV CP amino acid sequences were obtained from the NCBI database and aligned using GENEDOC v.2.7 to examine the relationships among the sequences. A phylogenetic tree was constructed using the neighbor-joining algorithm and MEGA 7.0. A bootstrap analysis with 1000 replicates was used to evaluate the reliability of the internal branches.
Moreover, the online software ExPASy-ProtParam tool (http://web.expasy.org/protparam, accessed on 6 June 2023) was used to calculate the physicochemical properties related to the amino acid sequences. The N-glycosylation and phosphorylation sites of the proteins were predicted using the NetNGlyc 1.0 Server (https://services.healthtech.dtu.dk/services/NetNGlyc-1.0/, accessed on 6 June 2023) and the NetPhos 3.1 Server (https://services.healthtech.dtu.dk/services/NetPhos-3.1/, accessed on 6 June 2023), respectively. In addition, the transmembrane structure of the protein was predicted using the TMHMM 2.0 Server (https://services.healthtech.dtu.dk/services/TMHMM-2.0/, accessed on 6 June 2023), and its hydrophilicity was analyzed using the ExPASy-Protscale tool (https://www.expasy.org/, accessed on 6 June 2023). The secondary structure was predicted using the online software SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_ seccons.html, accessed on 6 June 2023).
2.6. Determination of the Inhibitory Effect of the WCMV CP on Infection with AMV
A prepared pCAM-WCMV CP-EGFP Agrobacterium infiltration solution was mixed with ratios of AMV at 1:3, 2:3, 2:1, 3:1, and 1:1 (WCMV CP: AMV). Healthy 5-leaf-stage N. benthamiana plants were selected and cultivated in the dark for 3 h. Subsequently, the back of the leaf blades of N. benthamiana were stab-wounded with a disposable sterile needle. The syringe tube without the needle was pointed at the wound of the leaf blade, and the piston was slowly pushed so that the agrobacterium infiltration solution slowly penetrated into the N. benthamiana leaf blades from the wound. Three leaves of each plant were infiltrated, and each leaf was infiltrated with 100 μL; pCAM-WCMV CP-EGFP, in the same way, inoculated with AMV and PBS, was used as the AMV, WCMV single infection control and negative control. There were at least three biological replicates of each treatment, and for each replicate, at least 10 seedlings. The plants were placed in the climate chamber, and their symptoms were observed daily. Samples were collected at 5 dpi, 10 dpi, and 15 dpi for RNA extraction.
The RNA was extracted as described in Section 2.3, and the extracted RNA was reverse-transcribed into cDNA using a PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (Tiangen). The AMV CP genes were subsequently amplified using quantitative reverse-transcription PCR (RT-qPCR) (Table 1) with the viral cDNA as a template (Tiangen). The CP relative expression was quantified in a 20 μL volume that contained 10 μL 2× Super Real PerMix Plus, 1 μL upstream primer, 1 μL downstream primer, 1 μL template cDNA, 0.5 μL 50× ROX Reference Dye, and 6.5 μL RNase-free water. The reaction conditions were as follows: pre-denaturation at 95 °C for 15 min, denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 32 s for 40 cycles. Finally, a melting curve analysis was performed at 60 to 95 °C per 0.6 °C increment. Each treatment was repeated three times. The results of the RT-qPCR were analyzed using the 2-ΔΔct method [41].
The synergism promotion rate/antagonism inhibition rate (%) was as follows:
AMV CP relative expression in the control − AMV CP relative expression in the treatment/AMV CP relative expression in the control × 100.
The criteria of synergism and antagonism were as follows: (-) was a synergistic effect, and (+) was an antagonistic effect.
2.7. Statistical Analysis
Each treatment was repeated three times, and the data were analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) was employed to analyze the data, and significant differences between the treatment groups were determined using a Duncan’s multiple range test at a significance level of p < 0.05. Microsoft Excel 2023 (Microsoft, Redmond, WA, USA) was used to plot the graphs and tables.
3. Results
3.1. Symptoms of N. benthamiana After Co-Infection with AMV and WCMV
The N. benthamiana leaves that were co-infected with AMV and WCMV had more pronounced symptoms than those that were singly infected with each virus. The manifest symptoms of co-infection included severe mosaic, chlorosis, and shrinkage chlorosis. Single infection with AMV resulted in mottling, mild mosaics, and crumpling. N. benthamiana that was singly infected was characterized by mild yellow macular mosaic symptoms, crumpling, and deformity on the leaves. The CK remained healthy and symptomless (Figure 1).
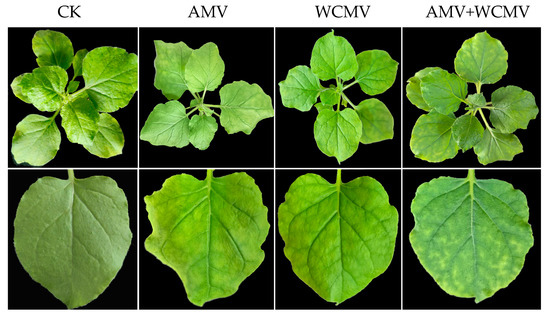
Figure 1.
Symptoms in N. benthamiana after AMV and WCMV co-infection and single infection. (CK) healthy N. benthamiana. (AMV) AMV infection. (WCMV) WCMV infection. (AMV+WCMV) AMV and WCMV co-infection.
The results of gel electrophoresis showed that the AMV CP was 666 bp and that of WCMV CP was 624 bp in AMV and WCMV co-infection and single infection, which were consistent with the fragment size expected (Figure 2).
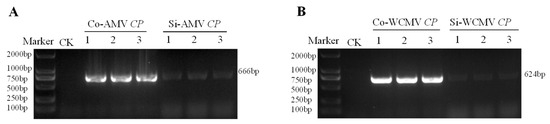
Figure 2.
Reverse transcription PCR amplification of AMV CP and WCMV CP genes. (CK) negative control. (A), (Co-AMV CP) amplification of AMV CP transcripts during AMV and WCMV co-infection. (Si-AMV CP) amplification of AMV CP transcripts during AMV single infection. (B), (Co-WCMV CP) amplification of WCMV CP transcripts during AMV and WCMV co-infection. (Si-WCMV CP) amplification of WCMV CP transcripts during WCMV single infection.
3.2. Mutation Analysis of the AMV CP and WCMV CP During Co-Infection
The AMV CP and WCMV CP encode 221 and 207 amino acids, respectively, and both are composed of 20 different amino acids (Figure 3). DNAMAN was used to analyze and compare the sequence mutation of the AMV CP and WCMV CP during co-infection and single infection. There were multiple nucleotide base mutations in sites of the Co-AMV CP. Among them, the bases at 121, 123, 138, 186, 212, 243, 249, 257, 264, 279, 309, 312, 317, 408, 452, 504, 515, 575, 577, and 609 were missense mutations, which changed the amino acids encoded. The remaining sites of mutation were synonymous mutations, and the amino acids were not changed. Compared with the Si-AMV CP, 7.66% of the nucleotide bases of Co-AMV CP were mutated. There was a frequency of mutations in which nucleotide base C mutated to T at rates of 22.00%. A mutated to C at lower frequencies, at a rate of 2.00%. The rest of the bases had mutation rates in between these values. The frequency of mutations in the amino acids was 9.05%. The amino acids mutated from Val mutated to Ala, Glu mutated to Asp, Tyr mutated to Ser, and His mutated to Tyr at higher frequencies of 10.53%, while the rest of the mutated amino acids had a mutation rate of 5.00% (S1, S3).
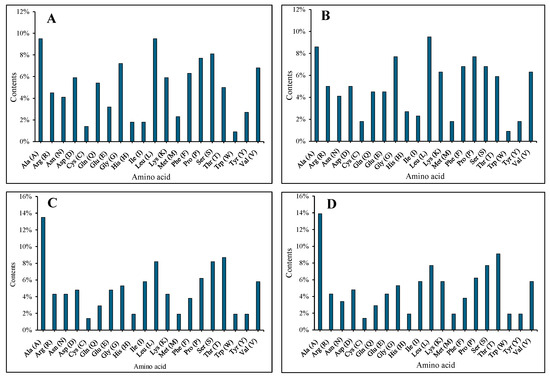
Figure 3.
Proportion of amino acids of the AMV CP and WCMV CP proteins. ((A) Co-AMV CP, (B) Si-AMV CP, (C) Co-WCMV CP, (D) Si-WCMV CP).
Moreover, mutations in the nucleotide bases were also found in multiple sites of the WCMV CP gene during co-infection. Among them, the bases at 54, 158, 219, 241, 246, 252, 275, 278, 306, 458, 510, and 531 were missense mutations, which changed the amino acids encoded. The remaining mutation sites were synonymous mutations, and the amino acids were not changed. Compared with the Si-WCMV CP, the Co-WCMV CP had nucleotides mutated at 3.37%. Nucleotide base A mutated to T at a higher frequency of 33.33%, while A mutated to G, G mutated to A, and C mutated to T at a lower frequency of 4.76%. The rest of the bases had mutation rates in between these values. The amino acid mutated at 5.77%. Ala mutated to Gly at higher frequencies with rates of 16.67%, while the rest of the amino acids mutated at 8.33% (S2, S3). The nucleotides and amino acids of the Co-AMV CP mutated at higher rates than those of the Co-WCMV CP.
The phylogenetic analyses of the AMV CP and WCMV CP in co-infection and single infection showed that the AMV CP sequence can be divided into group III, while the Co-AMV CP and Si-AMV CP are members of group I. The Co-AMV CP was highly homologous with the French (AF0157161.1) and UK (AJ130709.1) isolates. The WCMV CP sequence can be categorized into group II. The Co-WCMV CP and Si-WCMV CP were members of group II, while the Co-WCMV CP was highly homologous with the New Zealand isolate (X16636.1) (Figure 4).
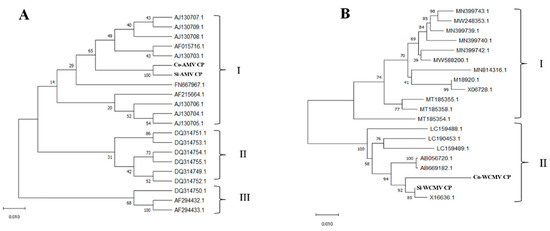
Figure 4.
Phylogenetic relationships of the AMV CP and WCMV CP genes. (A) Co-AMV CP and Si-AMV CP; (B) Co-WCMV CP and Si-WCMV CP during the co-infection and single infection, compared with strains from different regions. The Co-AMV CP, Si-AMV CP, Co-WCMV CP, and Si-WCMV CP are labeled in bold.
3.3. Nucleotide and Amino Acid Analyses of the AMV CP and WCMV CP DuringCo-Infection and Single Infection
The sequence analysis revealed that Co-AMV CP and Co-WCMV CP have predicted protein molecular weights of 24.189 and 22.424 kDa, respectively, with theoretical isoelectric point values of 8.73 and 6.07, respectively. These were lower than the molecular weight and isoelectric point of Si-AMV CP (24.311 kDa and 8.88) and Si-WCMV CP (22.424 kDa and 8.43). Moreover, the fat coefficients of 73.03 and 84.57 were higher than those of Si-AMV CP (72.85) and Si-WCMV CP (83.17); the instability coefficients were 40.04 and 43.61, and the extinction coefficients were 18,575 and 28,085 (S4).
In addition, the hydrophilicity values of Co-AMV CP and Co-WCMV CP were predicted to be −0.283, and −0.068, which were higher than those of Si-AMV CP (−0.319) and Si-WCMV CP (−0.083), respectively. They were all identified as hydrophilic proteins. Based on the prediction of protein stability, Si-AMV CP was determined to be a stable protein, while Co-AMV CP, Co-WCMV CP, and Si-WCMV CP were determined to be unstable proteins (S5). The genes Co-AMV CP and Co-WCMV CP were confirmed to lack transmembrane helical regions based on the predicted transmembrane domains, which were all nuclear and cytoplasmic; this was consistent with the findings on Si-AMV CP and Si-WCMV CP (S5).
The online prediction protein secondary structure software indicated that in both the combined and single infections, the Co-AMV CP had 36 α-helices, 41 extended chains, and 144 random coils. In contrast, the Si-AMV CP had 42 α-helices, 41 extended chains, and 138 random coils. The α-helices decreased by 6, and the random coils increased by 6. The Co-WCMV CP gene had 88 α-helices, 16 extended chains, and 104 random coils, compared to the Si-WCMV CP, which had 91 α-helices, 15 extended chains, and 102 random coils. Thus, the α-helices were reduced by 3, and the extended chains and random coils increased by 1 and 2, respectively (Figure 5).
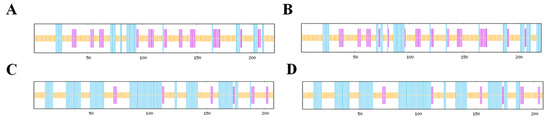
Figure 5.
Prediction of the secondary structures of AMV CP and WCMV CP genes during the co-infection and single infection. (A) Co-AMV CP, (B) Si-AMV CP, (C) Co-WCMV CP, and (D) Si-WCMV CP.
The docking score of the Co-AMV CP with Co-WCMV CP was −67.44, the contact area was 1158.7 Å2, and the binding free energy was −12.7 kcal/mol. This indicates that there were large binding interface interactions. Residues Arg95 and Ala135 of the Co-AMV CP form hydrogen bonds with residues Gln208 and Gln168 of Co-WCMV CP. This interaction is often the key to stable binding. In addition, residues Arg95 and Glu145 of Co-AMV CP formed salt bridges with residues Gln208 and His176 of Co-WCMV CP, respectively, which further enhanced the stability of the interaction (Figure 6). These findings provide important insights into the mechanism and function of the interactions of AMV and WCMV.
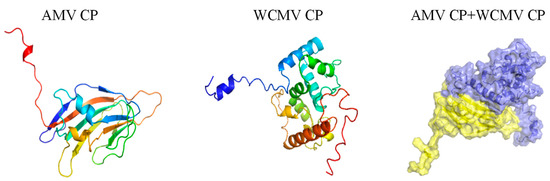
Figure 6.
AMV CP and WCMV CP interactions during the AMV and WCMV co-infection. AMV CP tertiary structure. WCMV CP tertiary structure. AMV CP + WCMV CP interactions, AMV CP in yellow, and WCMV CP in purple.
3.4. Diseased Symptoms and the WCMV CP Fluorescence Intensity of N. benthamiana After Infection with Different Proportions of WCMV CP and AMV
The results showed that at proportions of 1:3 and 2:3, compared with AMV single infection, N. benthamiana leaves exhibited more obvious symptoms; the symptoms included severe yellowing, and the leaves became thinner (Figure 7). At proportions of 3:1, 2:1, and 1:1, there were yellowing bulges in the infiltration site, and other places were greener. Moreover, the WCMV CP was expressed at 5 dpi, and the fluorescence intensity was the strongest when WCMV CP was singly infiltrated, followed by the fluorescence intensity when 3:1 and 2:1 were infected; the fluorescence intensity was the weakest when proportions of 1:1, 1:3, and 2:3 were infected (Figure 8).

Figure 7.
Symptoms in N. benthamiana plants infected at different proportions of WCMV CP and AMV. (A) healthy N. benthamiana, (B) AMV single infection, (C) WCMV CP single infiltration, (D) 2:3 (WCMV CP: AMV), (E) 1:3 (WCMV CP: AMV), (F) 2:1 (WCMV CP: AMV), (G) 3:1 (WCMV CP: AMV), and (H) 1:1 (WCMV CP: AMV).
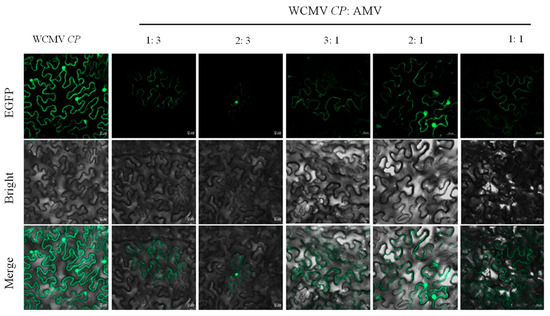
Figure 8.
Fluorescence intensity in N. benthamiana leaves infected with different proportions of WCMV CP and AMV. (WCMV CP) WCMV CP single infiltration, (1:3) (WCMV CP: AMV), (2:3) (WCMV CP: AMV), (3:1) (WCMV CP: AMV), (2:1) (WCMV CP: AMV), (1:1) (WCMV CP: AMV). EGFP stands for green fluorescence channel; bright stands for bright field; and merge stands for merged field. Scale bars: 20 μm.
3.5. Changes in the AMV CP Relative Expression After Infection with Different Proportions of WCMV CP and AMV
The AMV CP relative expression was determined in N. benthamiana. The result showed that when the AMV CP relative expression was normalized to 1.0 for different days post inoculation in the AMV single infection, the AMV CP relative expression increased significantly by a WCMV CP: AMV of 1:3 and 2:3, and the AMV CP was expressed at relatively higher levels at 15 dpi. They were 4.13- and 2.66-fold greater than that of AMV single infection, respectively. The AMV CP relative expression decreased significantly in WCMV CP: AMV at proportions of 3:1, 2:1, and 1:1; at 15 dpi, they were 0.56-, 0.47-, and 0.76-fold lower than that of AMV single infection, respectively (Figure 9A). When 5 dpi AMV CP relative expression was normalized to 1.00 for each treatment, the AMV CP relative expression showed an overall increasing trend with the increase of days post inoculation (Figure 9B). Generally, when there was a high concentration of WCMV CP in the WCMV CP: AMV, the AMV CP relative expression decreased.
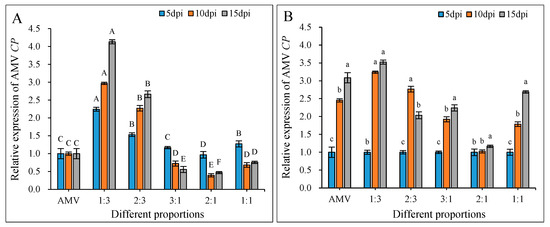
Figure 9.
Changes in the relative expression of AMV CP. (A) The AMV CP relative expression at different days post inoculation under AMV single infection treatment was normalized to 1.0. The AMV CP relative expression was calculated with co-infections at WCMV CP: AMV proportions of 1:3, 2:3, 3:1, 2:1, and 1:1 on the same days. The different capital letters indicate significant differences at the 0.01 level between AMV CP relative expression in AMV single infection and co-infections (WCMV CP: AMV) at different treatments for the same day post inoculation. (B) The relative expression of AMV CP at 5 dpi for each treatment was normalized to 1.0. The AMV CP relative expression at 10 dpi and 15 dpi was calculated for co-infections at WCMV CP: AMV proportions of 1:3, 2:3, 3:1, 2:1, and 1:1. The different lowercase letters indicate significant differences at the 0.05 level between AMV CP relative expression in AMV single infection and co-infections (WCMV CP: AMV) at the same treatment for different days post inoculation.
3.6. Inhibitory Effects on AMV Infection of the WCMV CP and AMV Different Proportions of Infection
The infection of N. benthamiana with different proportions of WCMV CP and AMV showed that the WCMV CP: AMV was 1:3 and 2:3 and had a synergistic effect on AMV infection. The rate of promotion of synergism on AMV was −196.72% and −166.30%. WCMV CP: AMV was 3:1, 2:1, and 1:1, which had antagonistic effects on AMV infection. The antagonistic effect of 2:1 on AMV infection gradually increased with the increasing dpi; at 10 dpi, the antagonism inhibition on AMV infection reached 60.60%. At 15 dpi, when the WCMV CP: AMV was 3:1, AMV infection antagonism inhibition reached the maximum value, which was 44.23%, and at 10 dpi, when the WCMV CP: AMV was 1:1, AMV infection antagonism inhibition reached the maximum value, which was 31.72%. However, at 5 dpi, WCMV CP: AMV was 3:1 and 1:1, which no antagonistic effect on AMV infection (Figure 10). This indicates that a high proportion of WCMV CP has an antagonistic effect on AMV infection.
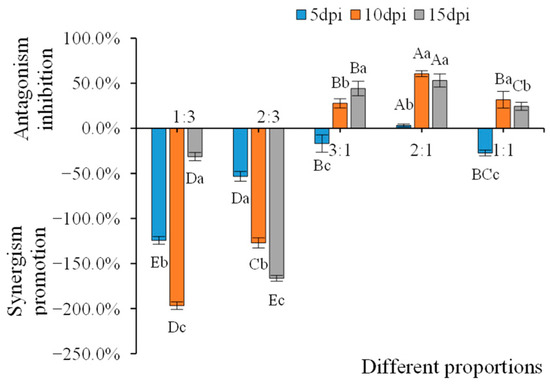
Figure 10.
Inhibitory effects of WCMV CP and AMV co-infection proportions on AMV. Synergistic promotion effects are denoted by (-), and antagonistic inhibitory effects are denoted by (+). Different capital letters indicate significant differences at the 0.01 level among treatments (WCMV CP: AMV) at the same time. The different lowercase letters indicate significant differences at the 0.05 level within the same treatment (WCMV CP: AMV) across different days post inoculation.
4. Discussion
Plant viral disease occurrence results from the interplay of host, viral, and environmental conditions, all of which exhibit spatiotemporal variation [42,43]. Our previous research found that when AMV and WCMV were co-inoculated at a ratio of 3:1 (AMV: WCMV), it led to more severe symptoms of N. benthamiana, and the relative accumulation of both AMV CP and WCMV CP increased [13]. Meanwhile, we inoculated a mixture of AMV and WCMV (3:1) on different hosts of the Leguminosae, Solanaceae, Poaceae, Cucurbitaceae, Asteraceae, Brassicaceae, and Chenopodiacea. Under these conditions, the co-infection of these two viruses would enhance the disease symptoms of the host plants, and the damage was higher than that caused by single infection with the viruses [17]. Gene mutations are crucial for viral infection, because they can cause changes in the replication, movement, and aggregation of the virus; vector transmission; and symptoms [44,45,46]. The CP is the shell that comprises the virus and, thus, the surface antigen [47]. The unenveloped virus CPs were involved in the adsorption and invasion of the virus and determined its host tropism. Therefore, this study sought to determine whether the synergistic phenomenon of AMV and WCMV (3:1) co-infection is related to the mutation of CPs in both viruses [48,49].
In order to further reveal the interaction during the co-infection of AMV and WCMV (3:1), our analysis revealed nucleotide and amino acid mutations in the AMV CP and WCMV CP following combined infection. Mutations were observed in the CPs of both viruses upon AMV and WCMV co-infection compared to single infections with either virus. The Co-AMV CP had a mutation rate of 7.66% for its nucleotide bases and 9.05% for its amino acids. The nucleotide base C mutated to T, and the amino acid Val mutated to Ala; Glu mutated to Asp, Tyr mutated to Ser, and His mutated to Tyr with higher frequencies of mutations, with rates of 22.00% and 10.53%, respectively. The Co-WCMV CP had a mutation rate of 3.37% for its nucleotide bases and 5.77% for its amino acids. Nucleotide base A mutated to T, and amino acid Ala mutated to Gly at higher frequencies of mutations with rates of 33.33% and 16.67%, respectively. This indicates that the synergistic effect between AMV and WCMV during co-infection correlates with CPs mutations. However, the amino acid mutation rule of AMV CP and AMV CP and the function of amino acid mutation frequencies with low and high symbiotic or antagonistic effects of AMV and WCMV co-infection merit further study. Moreover, the Co-AMV CP had higher rates of mutation of the nucleotide bases and amino acids than the Co-WCMV CP, and they were all located in the nucleus or cytoplasm. In addition, Co-AMV CP is highly homologous with the French (AF0157161.1) and UK (AJ130709.1) isolates. It is a member of group I. The Co-WCMV CP is highly homologous with the New Zealand isolate (X16636.1), which is a member of group II [50].
The analyses of secondary and tertiary structures of the AMV CP and WCMV CP amino acids in co-infection showed that the Co-AMV CP and Co-WCMV CP have 36 and 88 α-helices, 41 and 16 extended chains, and 144 and 104 random coils, respectively. The number of α-helices decreased by 6, and the number of random coils increased by 6 compared with those of the Si-AMV CP. The number of α-helices was reduced by 3, and the numbers of extended chains and random coils were increased by 1 and 2, respectively, compared with the Si-WCMV CP. Furthermore, amino acid molecular docking studies of the two viral CP sequences showed that hydrogen bonds formed between the residues Arg95 and Ala135 of Co-AMV CP and residues Gln208 and Gln168 of Co-WCMV CP, respectively. This interaction is essential for stable binding. In addition, salt bridges were formed between residues Arg95 and Glu145 of Co-AMV CP and residues Gln208 and His176 of Co-WCMV CP, which further enhanced the stability of the interaction. Moreover, the docking fraction of Co-AMV CP and Co-WCMV CP was −67.44, the contact area was 1158.7 Å2, and the free energy of binding was −12.7 kcal/mol. These results indicate that the CP sequences of the two viruses interact stably with a large binding interface [51,52]. These findings lay a theoretical foundation to more deeply understand the mechanisms and functions of protein interactions.
Viral CPs are also involved in the replication and translation of RNA, as well as in the motility, symptom formation, and pathogenesis of viruses [36]. It has been reported that the TMV CP protein wraps genomic RNA to form viral particles, protects the RNA from degradation by foreign enzymes, and modifies the CP gene to affect the replication, movement, and symptom formation of that virus [53]. The cucumber green mottle mosaic virus CP interacts with the movement protein to inhibit host antiviral activities and help facilitate the systemic invasion of viruses [54]. It is apparent that the CP plays an important role in the infection of plant viruses. Thus, the in-depth studies of its function provide a basis to analyze the pathogenic mechanism of plant viruses [55].
Therefore, we studied the effect of differing proportions of WCMV CP and AMV on AMV infection. It was found that the concentration of WCMV CP is a key influence on its synergistic or antagonistic effects with AMV. When its concentration was lower than that of AMV, with WCMV CP: AMV proportions of 1:3 and 2:3, it exhibited a synergistic effect and enhanced the symptoms of disease in N. benthamiana. The AMV CP relative expression was significantly higher than that of the AMV single infection by 4.13- and 2.66-fold, respectively. The synergistic effect of the promotion of AMV was -196.72% and -166.30%, respectively. In contrast, when its concentration was higher than or equal to that of AMV, and the WCMV CP: AMV was 3:1, 2:1, and 1:1, and it showed an antagonistic effect and inhibited the replication of AMV in N. benthamiana. In addition, it reduced the relative expression of AMV CP by 0.56-, 0.47-, and 0.76-fold, respectively. The antagonistic effects inhibited the AMV by 44.23%, 60.60%, and 31.72%, respectively. The antagonistic effect on AMV infection was maximal under the 2:1 (WCMV CP: AMV) treatment. This shows that the WCMV CP and AMV CP interact and inhibit the replication of AMV CP, which, in turn, inhibits infection with AMV and alleviates symptoms in N. benthamiana.
In summary, after the compound infection of AMV and WCMV, the CPs of both viruses were mutated, and the rates of mutation of the nucleotide bases and amino acids of Co-AMV CP were higher than those of Co-WCMV CP by 7.69% and 9.05% and by 3.37%, 5.77%, respectively. Val and Lys had the highest rates of mutation. The concentration magnitude of WCMV CP is a key influence on its synergistic or antagonistic effects with AMV. At 15 dpi, when the WCMV CP: AMV was 1:3 and 2:3, it showed a synergistic effect and enhanced the disease symptoms of N. benthamiana, with a 4.13- and 2.66-fold increase in the relative expression of AMV CP, respectively. A WCMV CP: AMV of 3:1, 2:1, and 1:1 showed antagonistic effects and inhibited the replication of AMV on infection in N. benthamiana, and the relative expression of AMV CP was reduced by 0.56-, 0.47-, and 0.76-fold, respectively. The highest antagonism inhibition rate on AMV infection occurred when the WCMV CP: AMV was 2:1. These results lay the foundation for further studies on the synergy and antagonism of these two viruses. Furthermore, the antagonistic effect can be utilized. A preparation made with WCMV CP as the effective component can be applied in the field to effectively prevent and control the occurrence and severity of virus diseases caused by co-infection. However, the questionS of whether recombination occurs between the genes that encode the CPs of the two viruses after the co-infection with AMV and WCMV, the reasons for the synergistic effects and antagonistic effects of different concentrations of WCMV CP with AMV, and the effects of AMV CP on the infection of WCMV and the mutualistic relationship have not yet been addressed. These all merit further study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071646/s1, S1. Mutation analysis of AMV CP during the AMV+WCMV co-infection and AMV single infection. (A) nucleotide sequence, (B) amino acid sequence. S2. Mutation analysis of WCMV CP during the AMV+WCMV co-infection and WCMV single infection. (A) nucleotide sequence, (B) amino acid sequence. S3. Amino acid mutations of AMV CP and WCMV CP during the co-infection and single infection. S4. Physicochemical properties of AMV CP and WCMV CP during the co-infection and single infection. S5. Biological information of AMV CP and WCMV CP during the co-infection and single infection.
Author Contributions
Conceptualization, Y.C. and Q.L.; data curation, Y.C. and L.W.; formal analysis, Y.C.; investigation, X.Z. and Y.C.; methodology, visualization, Y.C., X.Z. and Q.L.; writing—original draft Y.C.; conceptualization L.W., X.Z. and Q.L.; funding acquisition, X.Z. and Q.L.; supervision Y.C.; writing—review and editing, Y.C. and Q.L.; writing—review and editing Y.C., X.Z. and S.L.; funding acquisition, Y.C. and S.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Key Research and Development Plan Project of Gansu Province, grant number 25YFNA031.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
We deeply thank Qiaolan Liang for providing technical supports, and the High-Performance Computing Center of Gansu Agricultural University for providing computing resources.
Conflicts of Interest
All authors declare no competing interests.
References
- Fang, Q.E.; Li, Y.B. Classification and distribution of domestic species of genus Medicago in China. Grassl. Prataculture 2019, 31, 1–7. [Google Scholar]
- Wang, W.X. Countermeasures for the development of alfalfa planting industry in China. J. Beijing Univ. Agric. 2022, 37, 117–120. [Google Scholar]
- Carrasco, J.L.; Sánchez-Navarro, J.A.; Elena, S.F. Exploring the role of cellular homologous of the 30K-superfamily of plant virus movement proteins. Virus Res. 2019, 262, 54–61. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, B.Y.; Aziguli, M.; Li, K.M. Harm and control of alfalfa virus disease. Rural. Sci. Technol. 2018, 10, 25–28. [Google Scholar]
- Li, J.; Shang, Q.; Liu, Y.; Dai, W.; Li, X.; Wei, S.; Hu, G.; McNeill, M.R.; Ban, L. Occurrence, distribution, and transmission of alfalfa viruses in China. Viruses 2022, 14, 1519. [Google Scholar] [CrossRef]
- Guo, Z.P.; Feng, C.S.; Zhang, J.X.; Wang, M.L.; Qu, G.; Liu, J.Y.; Guan, Y.Z.; Zhang, X.T.; Guo, Y.X.; Yan, X.B. Field resistance to alfalfa mosaic virus among 30 alfalfa varieties. Acta Prataculturae Sin. 2019, 28, 157–167. [Google Scholar]
- McLaughlin, A.A.; Hanley-Bowdoin, L.; Kennedy, G.G.; Jacobson, A.L. Vector acquisition and co-inoculation of two plant viruses influences transmission, infection, and replication in new hosts. Sci. Rep. 2022, 12, 20355. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Congdon, B.S. Australian cool-season pulse seed-borne virus research: 1. alfalfa and cucumber mosaic vi-ruses and less important viruses. Viruses 2024, 16, 144. [Google Scholar] [CrossRef]
- Bellah, H.; Seiler, N.F.; Croll, D. Divergent outcomes of direct conspecific pathogen strain interaction and plant co-infection suggest consequences for disease dynamics. Microbiol. Spectr. 2023, 11, e0444322. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, T.; Chen, Z.; Niu, J.; Cui, X.; Mao, Y.; Hassan, M.U. Occurrence, distribution, and genetic diversity of alfalfa (Medicago sativa L.) viruses in four major alfalfa producing provinces of China. Front. Microbiol. 2022, 12, 771361. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, G.; Cheng, S.; Zhang, W.; Bai, Y. Evolutionary history and global spatiotemporal pattern of alfalfa mosaic virus. Front. Microbiol. 2022, 13, 1051834. [Google Scholar] [CrossRef]
- Amin, H.A.; Younes, H.A.; Shafie, R.M.; Fathallah, M.M. Molecular characterization and evolution of the resident population of some alfalfa mosaic virus (AMV) isolates in Egypt. BMC Microbiol. 2023, 23, 261. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liang, Q.L.; Wei, L.X.; Wang, D.; Tian, L.; Tong, F.Y.; Zhang, G.Y. Study on the epidemic factors of alfalfa mosaic virus and white clover mosaic virus co-infection Nicotiana benthamiana. Pratacultural Sci. 2023, 40, 90–100. [Google Scholar]
- Liang, Q.L.; Wei, L.X.; Xu, B.L.; Calderón-Urrea, A.; Xiang, D. Study of viruses co-infecting white clover (Trifolium repens) in China. J. Integr. Agric. 2017, 16, 1990–1998. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Wang, J.Y.; Xie, K.Z.; Wang, R.Y.; Yang, G.; Guo, Z.H.; Qiu, Y. A study of viral coat protein accumulation in lily chloroplasts from mixed virus infections of Lily mottle virus and cucumber mosaic virus. Plant Pathol. 2019, 68, 261–268. [Google Scholar] [CrossRef]
- Cheng, S.F.; Liang, Q.L.; Wei, L.X.; Sang, X.W.; Jiang, Y.L. Detection of alfalfa mosaic virus and white clover mosaic virus in alfalfa and their effects on physiological and biochemical characteristics of alfalfa plants. Acta Prataculturae Sin. 2020, 29, 140–149. [Google Scholar]
- Chen, Y.E.; Liang, Q.L.; Wei, L.X.; Zhou, X. Double infection of Nicotiana benthamiana with AMV and WCMV increases both virus concentrations and synergistically changes both host organelle ultrastructure and chlorophyll content. Microb. Pathog. 2024, 196, 106956. [Google Scholar] [CrossRef]
- Simón, D.; Cristina, J.; Musto, H. Nucleotide composition and codon usage across viruses and their respective hosts. Front. Microbiol. 2021, 12, 646300. [Google Scholar] [CrossRef]
- González-Pérez, E.; Chiquito-Almanza, E.; Villalobos-Reyes, S.; Canul-Ku, J.; Anaya-López, J.L. Diagnosis and characterization of plant viruses using HTS to support virus management and tomato breeding. Viruses 2024, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- García-Arenal, F.; Fraile, A.; Malpica, J.M. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 2001, 39, 157–186. [Google Scholar] [CrossRef]
- Lagzian, A.; Ghorbani, A.; Tabein, S.; Riseh, R.S. Genetic variations and gene expression profiles of rice black-streaked dwarf virus (RBSDV) in different host plants and insect vectors: Insights from RNA-Seq analysis. BMC Genom. 2024, 25, 736. [Google Scholar] [CrossRef]
- Yoshida, T.; Ishikawa, M.; Toki, S.; Ishibashi, K. Heritable tissue-culture-free gene editing in Nicotiana benthamiana through viral delivery of spcas9 and SgRNA. Plant Cell Physiol. 2024, 65, 1743–1750. [Google Scholar] [CrossRef]
- Verdin, E.; Wipf-Scheibel, C.; Gognalons, P.; Aller, F.; Jacquemond, M.; Tepfer, M. Sequencing viral siRNAs to identify previously undescribed viruses and viroids in a panel of ornamental plant samples structured as a matrix of pools. Virus Res. 2017, 241, 19–28. [Google Scholar] [CrossRef]
- Gupta, T.; Raj, S.K.; Singhal, T.; Srivastava, A. Phylogenetic and recombination analysis of yellow mosaic disease in soybean plant. J. Plant Dis. Sci. 2022, 17, 28–38. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Mäkinen, K. Coat proteins, host factors and plant viral replication. Curr. Opin. Virol. 2012, 2, 712–718. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Gao, Q.; Wang, H.J.; Yue, J.Y.; An, D.R.; Li, B.; Yan, F.F.; Carmen, S.M.; Zhao, Y.Z.; Zhou, H.Y.; et al. Syn-tasiRNAs targeting the coat protein of potato virus Y confer antiviral resistance in Nicotiana benthamiana. Plant Signal. Behav. 2024, 19, 2358270. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Li, S.; Ji, Y.; Sun, F.; Zou, B. Dicer-like 2 plays a crucial role in rice stripe virus coat protein-mediated virus resistance in Arabidopsis. Viruses 2023, 15, 2239. [Google Scholar] [CrossRef]
- Martínez, C.; López, C.; Pallás, V.; Pallás, F.; Aparicio, L. Role of the coat (CP), movement (MP) and 2b proteins of parietaria motte virus (PMoV) as pathogen determinants in Nicotiana benthamiana plants. Eur. J. Plant Pathol. 2025, 172, 291–304. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, E.; Nihranz, C.T.; Prakash, V.; Varsani, S.; Casteel, C.L. Engineering aphid transmission of foxtail mosaic virus in the presence of potyvirus helper component proteinase through coat protein modifications. J. Gen. Virol. 2023, 104, 10. [Google Scholar] [CrossRef]
- Bendahmane, M.; Szecsi, J.; Chen, I.; Berg, R.H.; Beachy, R.N. Characterization of mutant tobacco mosaic virus coat protein that interferes with virus cell-to-cell movement. Proc. Natl. Acad. Sci. USA 2002, 99, 3645–3650. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef]
- Lukhovitskaya, N.; Brown, K.; Hua, L.; Pate, A.E.; Carr, J.P.; Firth, A.E. A novel ilarvirus protein CP-RT is expressed via stop codon readthrough and suppresses RDR6-dependent RNA silencing. PLoS Pathog. 2024, 20, e1012034. [Google Scholar] [CrossRef]
- Suman, R.; Rani, A.; Rishi, N.; Dhir, S.; Hallan, V.; Chandel, V. First report of apple stem grooving virus infection in loquat from India. Virus Dis. 2022, 33, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Asurmendi, S.; Berg, R.H.; Smith, T.J.; Bendahmane, M.; Beachy, R.N. Aggregation of TMV CP plays a role in CP functions and in coat-protein-mediated resistance. Virology 2007, 366, 98–106. [Google Scholar] [CrossRef]
- Hosseini, H.; Mehrvar, M.; Zakiaghl, M.; Siampour, M. Comparative genetic diversity of potato virus Y populations based on coat protein gene. Acta Virol. 2017, 61, 161–174. [Google Scholar] [CrossRef]
- Pinczés, D.; Sáray, R.; Nemes, K.; Palkovics, L.; Salánki, K. Viral coat proteins decrease the gene silencing activity of cognate and heterologous viral suppressors. Sci. Rep. 2024, 14, 31008. [Google Scholar] [CrossRef]
- Villar-Álvarez, D.; Pallás, V.; Elena, S.F.; Sánchez-Navarro, J.A. An evolved 5’ untranslated region of alfalfa mosaic virus allows the RNA transport of movement-defective variants. J. Virol. 2022, 96, e0098822. [Google Scholar] [CrossRef]
- Jin, L.L. Complete genomic sequence and biological characteristics of a new alfalfa mosaic virus isolate. Zhejiang Univ. Technol. 2011. [Google Scholar]
- Wetter, C. Partial purification of some elongated plant viruses and their use as antigens in immunization by means of Freund’s adjuvant. Arch. Mikrobiol. 1960, 37, 278. [Google Scholar]
- Janeczko, A.; Dziurka, M.; Gullner, G.; Kocurek, M.; Rys, M.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Kornas, A.; Barna, B. Comparative studies of compatible and incompatible pepper tobamovirus interactions and the evaluation of effects of 24-epibrassinolide. Photosynthetica 2018, 56, 763–775. [Google Scholar] [CrossRef]
- Niu, E.B.; Liu, H.; Zhou, H.; Luo, L.; Wu, Y.; Andika, I.B.; Sun, L. Autophagy inhibits intercellular transport of citrus leaf blotch virus by targeting viral movement protein. Viruses 2021, 13, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, C.; Sardanyés, J.; Elena, S.F.; Gómez, P. Increasing temperature alters the within-host competition of viral strains and influences virus genetic variability. Virus Evol. 2021, 7, veab017. [Google Scholar] [CrossRef]
- Agüero, J.; Gómez-Aix, C.; Sempere, R.N.; García-Villalba, J.; García-Núñez, J.; Hernando, Y.; Aranda, M.A. Stable and broad spectrum cross-protection against pepino mosaic virus attained by mixed infection. Front. Plant Sci. 2018, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Millar, A.H. Proteomics in evolutionary ecology. J. Proteom. 2016, 135, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Dillenberger, M.S.; Wei, N.; Tennessen, J.A.; Ashman, T.L.; Liston, A. Plastid genomes reveal recurrent formation of allopolyploid Fragaria. Am. J. Bot. 2018, 105, 862–874. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Steuernagel, B.; Ghosh, S.; Herren, G.; Hurni, S.; Adamski, N.; Vrána, J. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016, 17, 221. [Google Scholar] [CrossRef]
- Weber, P.H.; Bujarski, J.J. Multiple functions of capsid proteins in (+) stranded RNA viruses during plant-virus interactions. Virus Res. 2015, 196, 140–149. [Google Scholar] [CrossRef]
- Agranovsky, A. Enhancing capsid proteins capacity in plant virus-vector interactions and virus transmission. Cells 2021, 10, 90. [Google Scholar] [CrossRef]
- He, C.; Xing, F.; Zhao, X.; Li, S.; Zhan, B.; Liu, Z.; Xu, T. The coat protein of the ilarvirus prunus necrotic ringspot virus mediates long-distance movement. J. Gen. Virol. 2023, 104, 1009. [Google Scholar] [CrossRef]
- Maachi, A.; Nagata, T.; Silva, J.M.F. Date palm virus A: First plant virus found in date palm trees. Virus Genes. 2020, 56, 792–795. [Google Scholar] [CrossRef]
- Vinaykumar, H.D.; Hiremath, S.; Nandan, M.; Muttappagol, M.; Reddy, M.; Venkataravanappa, V.; Shankarappa, K.S. Genome sequencing of cucumber mosaic virus (CMV) isolates infecting chilli and its interaction with host ferredoxin protein of different host for causing mosaic symptoms. 3 Biotech 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Kumar, S.; Hallan, V. Screening of potential inhibitor against coat protein of apple chlorotic leaf spot virus. Cell Biochem. Biophys. 2018, 76, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, C.; Aranda, M.A. Determinants of persistent patterns of pepino mosaic virus mixed infections. Front. Microbiol. 2021, 12, 694492. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Yang, X.; Yang, L.L.; Li, Q.L.; Liu, X.M.; Han, X.Y.; Gu, Q.S. Interaction between cucumber green mottle mosaic virus MP and CP promotes virus systemic infection. Mol. Plant Pathol. 2023, 24, 208–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Jiang, L.; Yang, Q.; Luo, L.; Jiang, J.; Malichan, S. Pitaya virus x coat protein acts as an RNA silencing suppressor and can be used as a specific target for detection using RT-LAMP. Plant Dis. 2023, 107, 3378–3382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).