1. Introduction
Bedding plants represent a quantitatively dominant segment within the global floriculture sector, accounting for approximately 30–47% of total production value across key markets such as the United States and Europe, thereby underscoring their critical economic and structural role in ornamental horticulture [
1,
2]. In the northern hemisphere (Europe and North America), the commercial production of bedding plants—particularly vegetatively propagated balcony species such as
Pelargonium,
Petunia, and
Calibrachoa—begins during the winter months with the import and rooting of unrooted herbaceous cuttings. These cuttings are typically derived from mother plants cultivated under optimal photothermal conditions in equatorial or subtropical regions (e.g., Kenya, Israel, Colombia, Ecuador) where year-round high irradiance and stable temperatures enable the continuous production of vigorous, high-quality vegetative material. Following harvesting, the cuttings are shipped via cold-chain logistics to propagation nurseries in temperate climates, where rooting usually starts around calendar weeks 1–5, depending on the species [
3,
4].
Propagation during winter presents considerable challenges due to limited natural light, suboptimal temperatures, and short photoperiods. In January and February, the day lengths in much of Europe and the northern USA range from 5 to 7 h while the greenhouse PPFD values often remain below 100 µmol·m
−2·s
−1. To overcome these constraints, growers implement supplemental lighting (LED or HPS) to extend the photoperiods to 14–16 h and increase the PPFD, promoting rooting and early vegetative development [
5]. Rooted cuttings, known in the professional horticulture world as liners, are typically produced over 8–10 weeks and then purchased by finishing growers who cultivate them to market maturity under increasingly favourable spring conditions [
3,
4]. The scheduling of flowering has become more precise in response to mass-market demands for uniform, flower-ready products. The limited natural light available during winter and early spring necessitates the use of supplemental lighting to ensure consistent plant quality [
6]. Photoperiod manipulation through artificial lighting not only enables year-round production but also enhances morphological traits such as height, stem strength, and flowering uniformity [
7,
8,
9]. The early application of extended photoperiods to rooted cuttings has been shown to promote flowering in ornamental long-day plants (LDPs), ensuring timely floral induction [
10,
11]. From a commercial perspective, the presence of visible flowers at the point of sale plays a critical role in consumer decision-making [
12]. Therefore, it is advisable to induce photoperiod-sensitive crops under long-day conditions from the earliest stages of propagation [
13,
14]. Alternatively, facultative LDPs may be cultivated under short-day conditions to stagger flowering and extend the sales window [
15].
Plant growth regulators (PGRs), especially growth retardants, are widely applied in bedding plant production to limit excessive vegetative growth and internode elongation, thus ensuring compact and aesthetically marketable plants [
16,
17]. Beyond size control, growth retardants influence architectural traits and flowering behaviour depending on the compound, method of application, and concentration used [
18]. Most commercial PGRs function by inhibiting gibberellin biosynthesis, and commonly include ancymidol, daminozide, chlormequat chloride, flurprimidol, and triazoles such as paclobutrazol and uniconazole [
19,
20]. While effective, some formulations—e.g., chlormequat chloride (Cycocel)—may cause phytotoxic responses. In contrast, ancymidol is noted for its safety in plug production, while paclobutrazol is widely used for height control in container-grown bedding plants [
20].
Environmental conditions influence PGR effectiveness. Plants grown under low light and cool temperatures may require lower doses than or reduced application frequencies from those cultivated under high-light and -temperature conditions. Accordingly, adjustments to PGR strategies are necessary between winter and summer production [
21]. However, the scientific data on these adaptations remains limited. Recent work by Collado and Hernández [
8] has demonstrated that supplemental lighting, alone or combined with paclobutrazol, can effectively modulate growth and morphology in seed-propagated bedding species such as
Petunia ×
hybrida,
Dianthus chinensis,
Pelargonium ×
hortorum, and
Viola ×
wittrockiana.
Among vegetatively propagated bedding plants,
Petunia and
Calibrachoa are of particular importance [
22].
Petunia is a dominant bedding plant, valued for its continuous flowering and broad adaptability [
23], while
Calibrachoa, with its vibrant and compact blooms, has gained widespread popularity in container plantings [
24]. Both genera hold significant ornamental and commercial value due to intensive breeding and the availability of diverse cultivars [
25,
26].
The present study aimed to evaluate the effects of supplemental lighting and growth retardants on the plant architecture, flowering time, mineral uptake, and overall visual quality of petunias (Petunia × atkinsiana Surfinia® ‘Purple’ and Surfinia® ‘Lime’) and calibrachoas (Calibrachoa × hybrida Superbells® ‘Unique Red’ and Superbells® ‘Unique Golden Yellow’). A key objective was to assess whether such combined treatments, initiated during the rooting phase of herbaceous cuttings, can improve the final market value of mature plants.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
This experiment included two species of balcony plants, petunias and calibrachoas (Solanaceae), with two cultivars of each: Petunia × atkinsiana Surfinia® ‘Purple’ and Surfinia® ‘Lime’ and Calibrachoa × hybrida Superbells® ‘Unique Red’ and Superbells® ‘Unique Golden Yellow’. Cuttings of these cultivars were obtained on 1 February 2023 (week 5 of the year) from the stock plants of the Plantpol company and were set for rooting on the same day. This research was conducted in the greenhouse facilities of Plantpol (ul. Jezioro 33–35, Zaborze, Poland), a bedding plant nursery in Central Europe.
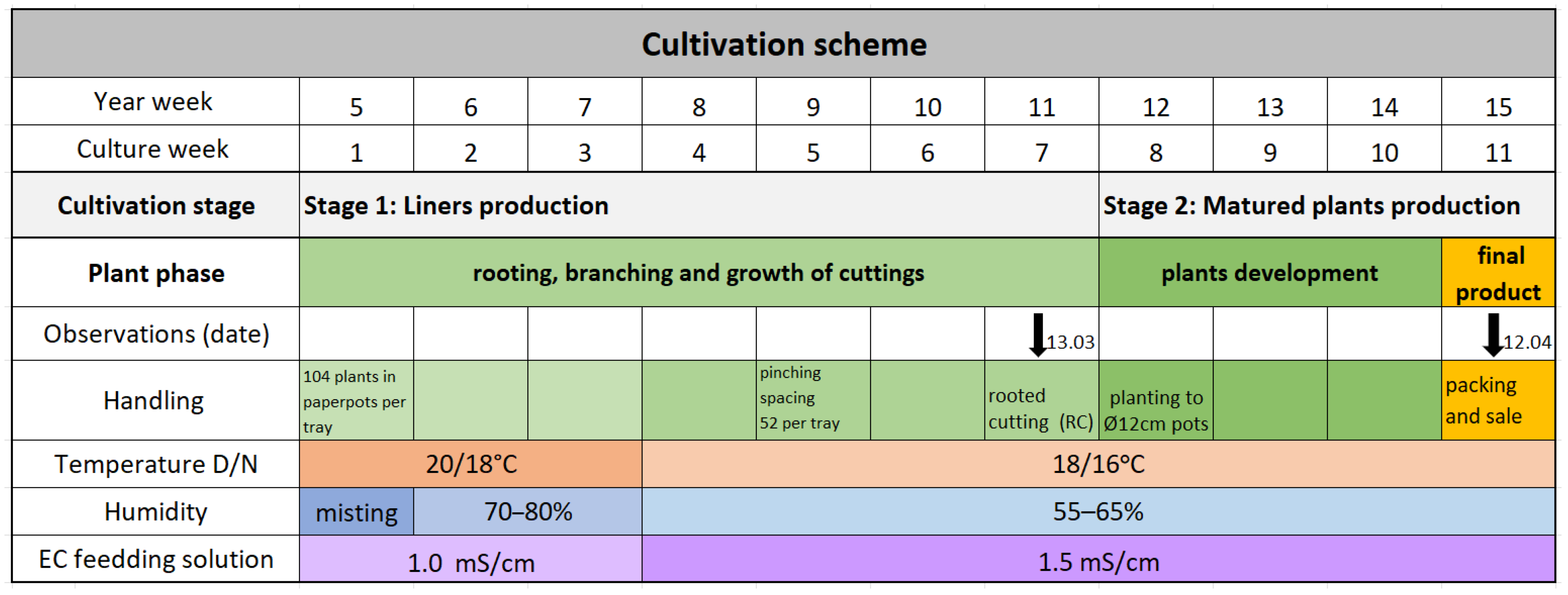
The culturing consisted of two stages: (1) vegetative propagation with liner production (rooted cuttings for further cultivation) and (2) production of matured plants (final product for sale). The stages of production and environmental greenhouse conditions are described in
Figure 1.
Stage 1—liner production: This stage lasted 7 weeks and began with a three-week rooting phase, followed by a 4-week branching phase. Top leafy cuttings (approx. 2.5 cm long) were taken from the stock plants and planted singly in paper pots filled with a substrate of 30% coconut fibre, 40% fine sod peat, 15% polystyrene, 15% perlite, and a wetting agent (substrate code: SoMi 537; producer: Hawita, Vechta, Germany), with a pH of 5.3. The paper pots with the cuttings were placed in 104 plastic trays for stabilisation(HerkuPlant, Ering, Germany) placed on cultivation benches in the greenhouse. During the first week, the cuttings were misted by a misting system and maintained in an environment with 70–80% relative humidity, which was later reduced to 55–65%. The temperature during the first three weeks was maintained at 20 °C during the day and 18 °C at night, with an allowance of ± 2 °C variation. After this period, the temperature was lowered to 18 °C/16 °C day/night ± 2 °C. The relative humidity was actively controlled by professional greenhouse ventilation fans (Ziehl-Abegg SE, Künzelsau, Germany) and passively regulated through heating adjustments and roof windows. In the fourth week of the culturing, the rooted cuttings were trimmed to a height of about 3–4 cm and spaced at half the original density, resulting in 52 cuttings per tray. They remained on the same tray and continued to grow under the same environmental conditions for an additional three weeks.
Stage 2—mature plant production: In the 11th week of the year, the rooted cuttings were individually planted into 12 cm pots (1 L volume) filled with a potting substrate composed of 70% white peat, 15% coconut fibre, and 15% clay, with pH 5.8 (substrate code EP 340, producer Hawita, Vechta, Germany). The plants were cultivated for an additional three weeks until they matured into final plants ready for retail sale (
Figure 1).
In both stages of production, fertilisation (fertigation with nutrient water) was conducted on an ebb-and-flow system on the growing benches. The climate computer SERCOM SC800 and SercoVision 8 software (Regeltechniek BV, Lisse, The Netherlands) maintained consistent environmental conditions during cultivation for each batch being tested.
2.2. Treatments in This Experiment
This study assessed the effects of day length extension in control plants without supplemental lighting (NL) vs. plants exposed to 16 h days (L) and growth retardation in untreated control plants (NR) vs. plants treated with a growth retardant (R) on cutting growth and development, ultimately on the quality of market-ready plants.
Four treatment combinations were applied to each of the four tested varieties:
NL-NR—no lighting, no retardant;
NL-R—no lighting, with retardant;
L-NR—extended lighting, no retardant;
L-R—extended lighting, with retardant.
This experiment was conducted in commercial greenhouses. In the first stage (liner production), each treatment was replicated four times (one pallet of 104 liners per replication). The same setup was used in the second stage (mature plant production), where each replication included 52 plants. The cultivation conditions were uniform, and the plants retained their treatment origins from stage one. This experiment was conducted separately for each species. Within each species, two varieties were analysed and compared. The experimental design included three factors: variety, light conditions, and the application of the growth retardant.
2.2.1. Prolonged Day with Supplemental Light Treatment
Supplemental lighting was applied by extending the day to a 16 h photoperiod from 5:00 AM to 9:00 PM. The prolonged day treatment (L) was implemented from the beginning of stage 1 until the planting of the rooted liners into pots (week 11). The plants under the no-light treatment (NL) were exposed solely to natural daylight. The supplemental lighting regime followed a 16 h light/8 h dark cycle. The natural day length during this experiment ranged from 8 to 11.5 h, depending on the calendar week (
Figure 2). High-pressure sodium (HPS) lamps, OSRAM Plantastar 600 W, 87,000 lumens, 2000 K (OSRAM GmbH, Munich, Germany), were used for lighting. They were suspended 1 m above the plants, providing a photosynthetic photon flux density (PPFD) of 100 µmol·m
−2·s
−1. To avoid overlap with natural sunlight, the lamps were switched off 20 min. after sunrise and turned on 20 min. before sunset.
2.2.2. Growth Retardant Treatment
Growth retardant (PGR) treatments were applied to the designated plant batches once per week (approximately every 10 days) from week 2 to week 7 of cultivation. The plants, at the beginning of the cultivation, were sprayed every 7–10 days with Dazide Enhance 85 SG (Fine Agrochemicals BV, Breda, The Netherlands) containing 850 g/kg (85%) daminozide—a hydrazide-class compound—at a concentration of 0.2%. The working solution volume was 100 L per 1000 m2. Additionally, a single foliar spray at week 7 of the culture was made with BONZI (Syngenta, Basel, Switzerland), containing 4 g/L paclobutrazol—a triazole-class compound—and was applied at a concentration of 0.3%. The plants were thoroughly sprayed to ensure complete coverage of the leaves and shoots, with no runoff into the pots. The working solution volume was 100 L per 1000 m2.
2.3. Biometric Observations and Biochemical Analyses
After completing Stage 1, we recorded the number of shoots (branching) and the length of the shoots. We also collected plant material to analyse the dry mass of the above-ground part, the contents of photosynthetic pigments (chlorophyll a and b and carotenoids), and the levels of macro- and microelements. At the end of Stage 2, which concluded the experiment and production, we made observations on the branching, specifically counting the number of shoots exceeding 5 cm, and measured the lengths of these shoots. Additionally, we counted the numbers of flowers and flower buds. Plant material was collected again to determine the dry mass, photosynthetic pigment content, and levels of macro- and microelements.
2.3.1. Photosynthetically Active Pigment Determination
At the ends of Stages 1 and 2, the samples collected from the leaf blades of 30 plants were lyophilised and then homogenised. A total of 3 mg of the homogenate was mixed with 1 mL of 96% ethanol and macerated overnight in a refrigerator. The resulting supernatants were analysed using a spectrophotometer, and the absorbance was measured at 470, 649, and 664 nm. The concentrations (μg/mL) of chlorophyll a (Ch a), chlorophyll b (Ch b), and carotenoids (C c) were calculated using the following formulas: Ch a = 13.36 A 664–5.19 A 649; Ch b = 27.43 A 649–8.12 A 664; and C c = (1000 A 470–2.13 C a–97.63 C b)/209. In these formulas, A denotes absorbance [
27].
2.3.2. Plant Nutritional Status Assessment
At the ends of Stages 1 and 2, the above-ground parts of the plants, excluding the flowers and flower buds, were harvested. A mixed sample consisted of 30 plants from the replicate. The water content of the plant material was determined using the dry-mass content method. The plants were dried in a laboratory oven with forced air circulation at a temperature of 105 °C ± 5 °C until they reached a constant weight, following the PN-ISO 6496:2002 [
28] and PN-ISO 712:2002 [
29] standards. To analyse the mineral composition, the plants were crushed with a knife, dried at 60 °C, and then ground into a finer material. The protein nitrogen content was measured in the crushed plant material using the Kjeldahl method [
30]. The total macro- and microelement contents were determined after microwave mineralisation with the MARS 2 Microwave Digestion System (CEM Corporation, Matthews, NC, USA) of the samples in concentrated nitric acid (HNO3). The elements were analysed using the ICP-OES method on a Prodigy Plus instrument (Teledyne Leeman Labs, Hudson, NH, USA).
2.4. Statistical Analysis
The results were analysed statistically with a two-way analysis of variance (ANOVA) using Statistica 13.3 software (licence StatSoft Polska, Kraków, Poland; TIBCO Software Inc., Palo Alto, CA, USA). A post hoc multiple-range Tukey test was used. Significantly different means were separated at p ≤ 0.05.
3. Results
At the end of Stage 1 (
Figure 3), during which the plants were subjected to four different treatments, biometric observations revealed that differences in the plant architecture were already noticeable among the treatments in both species and their cultivars (
Table 1). The shoot elongation was influenced by day length extension, cultivars, and growth retardant application. Regarding shoot numbers, Surfinia ‘Purple’ generally produced more shoots compared to ‘Lime’, indicating a better branching tendency in ‘Purple’. In this cultivar, the highest shoot number was recorded in the rooted cuttings treated with both extended day length and growth retardant (L-R), although not significantly different from NL-R for ‘Purple’ (
Table 1). However, the light treatment alone had no significant effect on the shoot length at this stage. In ‘Lime’, the longest shoots were observed in the non-retarded plants regardless of light treatment, similar to ‘Purple’. In both cultivars, the retardant-treated plants consistently exhibited shorter shoots irrespective of the lighting conditions. As for the content of the photosynthetically active pigments, both light extension and retardant application had a notable effect. The chlorophyll a under the combined treatment of supplemental lighting and growth retardant for ‘Lime’ was significantly high, followed by the L-R ‘Purple’, whereas for chlorophyll b, it was also the highest but the opposite in terms of cultivars. This combination also resulted in the highest carotenoid content, though only in ‘Lime’. Furthermore, the dry weight was significantly high in the ‘Purple’ grown under the prolonged day length combined with retardant application, followed by the L-NR ‘Purple’, which was not significantly different from the L-R ‘Lime’ (
Table 1).
The untreated Surfinia cuttings (NL-NR) had the lowest sulphur (S) content (in ‘Purple’) but the highest magnesium (Mg) levels (in ‘Lime’) (
Table 2). The individually applied treatments—either light extension (L-NR) or growth retardation (NL-R)—significantly reduced the phosphorus (P) content in the biomass of this cultivar. Analyses conducted after the rooting stage showed that the untreated (NL-NR) and treated (LR) Surfinia ‘Lime’ contained the highest levels of phosphorus (P) (
Table 2). When analysing the main experimental factors in relation to the mineral composition after the rooting stage, it was found that supplemental lighting generally increased the average dry mass and calcium (Ca) contents in the plant tissue. In contrast, the biomasses of the non-illuminated plants had higher levels of potassium (K) and magnesium (Mg) (
Table 2). The growth retardant application led to decreases in the dry mass, calcium, and sulphur contents while increasing the potassium accumulation. The mineral profiles differed between the cultivars. Surfinia ‘Lime’ had significantly higher concentrations of K, Mg, P, and S, whereas the biomass of the red-flowered Surfinia ‘Purple’ was characterised by higher nitrogen (N) and calcium (Ca) contents. When comparing the mineral nutrient status of the petunia plants with the sufficiency ranges proposed by Bryson and Mills [
31], it was found that the cuttings exhibited optimal concentrations of N, Ca, Mg, and S; optimal-to-high levels of K; and elevated levels of P (
Table 2).
In the calibrachoas (
Table 3,
Figure 4), the treatments had no significant effect on the branching of the cultivars, which formed between 4.2 and 5.4 shoots. Although ‘Unique Red’ exhibited slightly better branching, the differences were not statistically significant. Statistical analysis confirmed that the cultivars were the only factor with a significant effect on the shoot number, with ‘Unique Red’ producing the highest number of shoots. the shoot length followed a similar pattern to that observed in petunias: the longest shoots were recorded in plants subjected to light extension but not treated with a growth retardant, albeit the results were not statistically significant. However, in ‘Unique Golden Yellow’, the plants that were both illuminated and retarded also developed long shoots under combined light and retardant treatment. Regarding the chlorophyll a and b contents, generally, a similar trend was observed for both chlorophyll a and carotenoids, with the highest concentrations detected in the plants exposed to prolonged day lengths—regardless of whether they were treated with a growth retardant. Precisely, the levels of chlorophyll a in the plants from L-NR and L-R were significantly higher than in all other treatments but not in each other. In the case of the chlorophyll b concentration in ‘Unique Red’, there was no significant difference between the L-R, L-NR, and NL-R treatments and NL-NR, NL-R, and L-NR. The results were similar in ‘Unique Golden Yellow’, with the exception that the L-NR and L-R treatments had no significant difference. The carotenoids in L-NR and L-R were significantly higher than in the other two treatments in both cultivars. The lengthening of the light exposure proved to be the key factor contributing to the rise in the photosynthetically active pigments in the calibrachoas. In general, the calibrachoas accumulated higher levels of these pigments than the petunias. The dry-mass content across all calibrachoa treatments remained relatively stable. The highest value was recorded in the ‘Unique Red’ plants subjected to the light extension without growth retardation (L-NR). Overall, the dry-mass content in the calibrachoas was higher than in the petunias (
Table 3).
In the calibrachoa cuttings, the light extension without growth retardation resulted in a decrease in the phosphorus (P) content in the biomass (
Table 4). The plants subjected to the growth retardation without supplemental lighting (NL-R) exhibited significantly higher levels of potassium (K) and phosphorus (P) while showing the lowest levels of calcium (Ca) and sulphur (S). The untreated cuttings (NL-NR) of the cultivar ‘Unique Golden Yellow’ contained significantly more P and S. In this same cultivar, the plants that were not illuminated but treated with a growth retardant (NL-R) showed the lowest K content.
Regardless of the application of a growth retardant, the light extension increased the nitrogen (N) content in the ‘Unique Golden Yellow’ plants (
Table 4). Analysis of the main experimental factors affecting the mineral composition of the
Calibrachoa ×
hybrida Superbells after the rooting stage revealed that the light extension generally increased the average concentrations of N, Ca, and magnesium (Mg) in the plants. In contrast, the biomass of the non-illuminated plants was richer in phosphorus (P). The growth retardation treatments reduced the Mg and S contents in the plants.
The cultivars differed significantly in their mineral profiles. The ‘Unique Golden Yellow’ plants contained significantly more N, Ca, P, and S, while the red cultivar ‘Unique Red’ accumulated more K in its biomass. When the mineral nutrient statuses of the calibrachoa plants after the rooting stage were compared with the threshold values [
31], high concentrations of N, Ca, K, and P were found, while the sulphur (S) levels were within the optimal range.
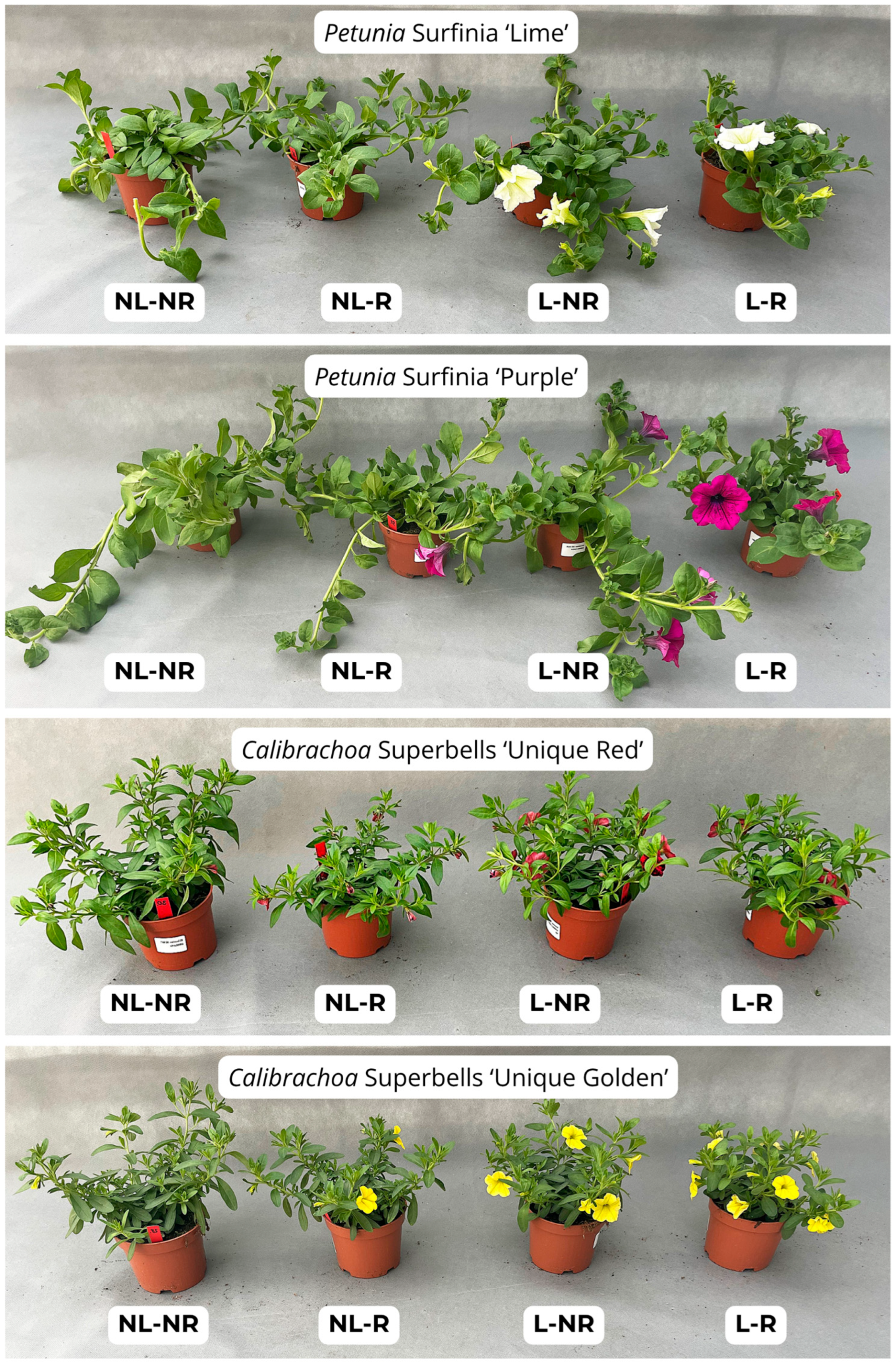
All petunia and calibrachoa plants developed flowers and flower buds. In both species and across all cultivars, the highest total numbers of flowers and flower buds were observed under prolonged lighting, regardless of whether growth retardants were applied. Specifically, for the ‘Lime’ petunia, the plants produced 23.9 and 31.2 flowers and buds under prolonged light without and with retardants, respectively; for ‘Purple’, the values were 34.0 and 35.0. In the calibrachoas, ‘Unique Red’ produced 30.7 and 30.6, while ‘Unique Golden Yellow’ had 35.2 and 36.8 flowers and buds, respectively (
Figure 4A,B and
Figure 5). The ‘Lime’ petunia did not flower under the non-illuminated conditions, whereas ‘Purple’ formed only a few open flowers. At the commercial stage, both cultivars grown under prolonged light had more than five open flowers per plant, and in the ‘Purple’ subjected to growth retardants, this number exceeded six. In the calibrachoas, open flowers were observed in all treatment combinations on the final assessment day. However, a similar trend to the petunias was observed: the highest flower count was recorded in the plants exposed to prolonged lighting, and the retardant treatment had no effect on this trait (
Figure 4A,B and
Figure 5).
The petunia plants exposed to light extension during the rooting phase exhibited significantly more shoots compared with the non-illuminated plants, indicating enhanced branching.(
Figure 5) Statistical analysis confirmed that each main factor—light extension and growth retardation—independently affected the shoot numbers, whereas their interactions were not statistically significant. In the retardant-treated plants, the shoot numbers were slightly lower and the differences were not statistically significant, suggesting that the growth retardation did not affect the branching. Regarding the shoot length, the longest shoots were recorded in the combination without light extension and without growth retardants. Conversely, the shortest shoots were observed in the L-R combination, where the plants were illuminated and treated during the rooting phase (
Table 5).
In the petunias at the mature stage, compared to the rooting stage, general decreases were observed in the contents of all analysed macronutrients except for magnesium (Mg), while the dry-mass (DW) content increased. Analysis of the plant material at the mature stage revealed that untreated ‘Lime’ petunias (NL-NR) contained the lowest nitrogen content compared to the other treatment combinations (
Table 6). A similar trend was noted for sulphur (S). Light supplementation, regardless of growth retardant application, increased the calcium (Ca) and sulphur (S) contents in the plants. The combination of light supplementation with growth retardation (L-R) significantly increased the potassium (K) and sulphur (S) contents.
In Surfinia ‘Purple’, light supplementation—regardless of retardant use—resulted in significantly higher Ca and K levels (
Table 6). The highest N content was recorded in the plants treated with retardant but not illuminated (NL-R), while the lowest was found in the untreated petunias (NL-NR). The L-R combination increased the sulphur accumulation in the biomass of Surfinia ‘Purple’.
Overall, the light supplementation increased the average contents of all macronutrients in the petunias at the mature stage except nitrogen (
Table 6). The growth retardation significantly decreased the average levels of N, K, P, and S while increasing the Ca and Mg contents. The biomass of Surfinia ‘Lime’ at the mature stage contained significantly more Ca, K, P, and S, whereas ‘Purple’ accumulated more dry mass. When compared with the threshold values [
31] for petunia, the mineral composition of the plant biomass was characterised by optimal levels of N, Ca, K, Mg, and S and elevated phosphorus.
The trends observed in the petunia cultivars were also found in both calibrachoa cultivars (
Figure 5). The plants exposed to the light supplementation—regardless of growth retardation—developed the highest number of shoots. The longest shoots were observed in the plants subjected to both the supplemental lighting and retardation. The calibrachoas showed a similar pattern to the petunias, with more shoots produced under the supplemental lighting. The dry-mass content was statistically similar across all treatments in both the petunias (
Table 5) and calibrachoas (
Table 7), but the calibrachoas exhibited higher DW values compared to the petunias. The contents of chlorophyll a and b were highest in the light treatments.
In the mature stage, compared to the rooted cutting stage, the contents of the N, P, K, and S in the calibrachoa plants decreased while the dry-mass and Mg contents increased. Plant material analyses at this developmental stage revealed that the ‘Unique Red’ calibrachoa plants subjected to both light supplementation and retardation (L-R) contained significantly more nitrogen compared with the untreated plants (NL-NR) (
Table 8). A similar response was observed for sulphur. Light supplementation, regardless of growth retardation, increased the phosphorus content. Retardation without lighting (NL-R) significantly reduced the calcium levels in the plant biomass. In the ‘Unique Golden Yellow’ cultivar, the lowest N and Ca contents were recorded in plants that were retarded but not illuminated (NL-R). A similar response to the light and retardant treatments as in the red cultivar was observed for the phosphorus and sulphur accumulation in ‘Unique Golden Yellow’.
When analysing the effects of the main experimental factors on the mineral composition of
Calibrachoa ×
hybrida Superbells at the mature stage, the light supplementation generally increased the average contents of N, Ca, P, and S (
Table 8). The growth retardation increased the Ca and Mg contents, whereas the non-retarded plants had higher dry-mass and K levels. The biomass of ‘Unique Golden Yellow’ at the mature stage contained significantly more dry mass as well as nitrogen and phosphorus. In contrast, the biomass of the red cultivar ‘Unique Red’ had higher levels of Ca, Mg, and S. When compared to the threshold nutrient values [
31] for calibrachoas at this growth stage, the plant material was characterised by high contents of Ca, Mg, and P and optimal levels of N, K, and S.
4. Discussion
The vegetative propagation of Petunia and Calibrachoa cuttings during winter is inherently challenged by short photoperiods, low light intensities, and reduced temperatures—factors that limit photosynthesis, delay rooting, and compromise both vegetative growth and flowering capacity. These limitations are especially critical during the early propagation phase, when plants rely on stored resources and environmental cues to initiate development. Our study demonstrated that extending the photoperiod to 16 h using high-pressure sodium (HPS) lamps and applying growth retardants during the rooting stage significantly improved multiple aspects of plant performance. These improvements included enhanced shoot proliferation, increased dry matter accumulation, better chlorophyll synthesis, and more synchronised and abundant flowering.
The photoperiod is one of the most influential environmental signals controlling plant growth and development. In long-day species such as
Petunia ×
atkinsiana and
Calibrachoa ×
hybrida, floral induction is triggered when the day length exceeds a genetically determined threshold, activating a cascade of photoreceptor- and hormone-mediated signalling events [
32,
33]. These responses are governed by the activity of phytochromes and cryptochromes, which perceive changes in red/far-red and blue light, respectively, and initiate transcriptional programs that control vegetative and reproductive transitions [
34].
In our experiment, supplemental lighting from the beginning of the rooting of the cuttings increased the shoot numbers and branching in both the petunia and calibrachoa cultivars. This improvement in the vegetative growth likely stemmed from enhanced axillary meristem activity and increased photosynthetic duration, which collectively promote assimilate availability and support structural development. Similar responses have been reported in other bedding plants, where an increased daily light integral (DLI) improved liner quality and growth rate [
14,
35]. More notably, the photoperiod extension significantly advanced the flowering time across all cultivars. By initiating long-day conditions early—during the rooting phase—we accelerated the transition to the generative phase. These results confirm previous work by Craver et al. [
13] and Oh et al. [
36] showing that the early application of extended photoperiods facilitates uniform floral induction and enhances flowering quality. From a commercial perspective, early flowering not only shortens production cycles but also ensures that finished plants reach retail markets in a visually appealing, flower-ready state—an essential criterion for consumer acceptance [
35,
37].
In parallel with photoperiod manipulation, chemical growth regulation is an essential tool in modern ornamental production. Our study showed that daminozide (a hydrazide) and paclobutrazol (a triazole) effectively reduced the shoot elongation and produced more compact plants in both genera without adversely affecting the shoot numbers. These effects are consistent with the known action of these compounds in inhibiting gibberellin biosynthesis—thereby reducing cell elongation and internode length [
18]. Such compact morphology is not only aesthetically preferred by consumers but also facilitates shipping, reduces the risk of mechanical damage, and ensures uniformity across batches [
8]. Interestingly, in our study, while the growth retardants did not significantly influence the branching, they enhanced the plant compactness, which is a critical trait in high-value bedding plants. This complements the branching promoted by light extension, confirming the synergistic nature of both treatments. Together, they produced plants with improved form, pigment content, and earlier flowering traits that align closely with market demand. Moreover, by applying these treatments during the early rooting stage, we achieved long-lasting effects visible even at the mature, retail-ready phase. This finding underscores the importance of early intervention and suggests that the rooting phase is a critical window of opportunity for shaping both the physiological and commercial traits of bedding plants [
35].
In both species, the light extension generally increased the contents of Ca, P, and S in mature plants, underscoring its role in promoting nutrient uptake and allocation. Similar effects have been described by Hurt et al. [
38], who noted that supplemental lighting enhanced seedling quality and mineral nutrition. The growth retardants, by contrast, tended to decrease the N, P, K, and S contents while increasing the Ca and Mg. These shifts may be due to altered assimilation rates and partitioning patterns in slower-growing compact plants [
39]. The combined application of lighting and retardants resulted in particularly high P and S levels in the petunias: nutrients closely linked to floral initiation and photosynthetic function [
40].
Consistent differences were observed between the cultivars, particularly in nutrient accumulation and growth habits. For example, the ‘Unique Golden Yellow’ calibrachoa had higher levels of N and P, while ‘Unique Red’ was richer in Ca and Mg, a finding in line with the cultivar-level distinctions reported by Collado and Hernández [
8]. These differences suggest that breeding history and genetic background influence responsiveness to environmental and chemical stimuli [
41,
42]. Tailoring light regimes and growth-regulator strategies to specific cultivars may therefore further optimise resource use and improve commercial outcomes.
Our findings offer actionable insights for commercial growers. By initiating photoperiod extension during the early rooting phase, it is possible to synchronise flowering, improve shoot structure, and reduce time to market. Earlier flowering reduces production costs and allows growers to meet peak seasonal demand with superior-quality plants [
6,
10]. Integrating PGRs enhances morphological appeal and nutrient balance, further aligning plant traits with market expectations. Moreover, combining lighting and PGRs maximises pigment content and flowering uniformity, directly enhancing retail value and customer satisfaction. As greenhouse production moves towards precision horticulture, integrating data-driven lighting schedules and controlled PGR use—possibly assisted by machine learning models—may further improve efficiency, sustainability, and profitability [
43].
5. Conclusions
We investigated the effects of retardants and extended lighting (up to 16 h) during the rooting stage of cuttings to determine how these factors influence quality, plant architecture, and flowering in mature plants. Our results after the rooting stage indicated that the application of retardant stimulates branching in petunias and significantly inhibits shoot elongation in both petunias and calibrachoas. The combination of extended light and retardants resulted in a higher concentration of photosynthetically active pigments in the cuttings and increased the dry-mass and Ca levels. In the ‘Unique Golden Yellow’ calibrachoa, the supplementary lighting resulted in increased concentrations of nitrogen and calcium, while the potassium content decreased in response to the growth retardant application. Observations of mature plants rooted under diverse conditions indicated that prolonged light exposure enhances the number of shoots, flower buds, and flowers compared with cuttings that do not receive additional light. The presence of visible flowers is important for practical purposes, as it ensures the plants maintain commercial value and are market-ready while allowing for easy identification of the variety at the point of sale. The mature petunias exposed to additional light during the rooting phase contained significantly more of the macronutrients Ca, K, and S. In contrast, the mature calibrachoas had higher N, P, Ca, and S levels.
Overall, the prolonged light exposure, regardless of the use of growth retardant, improved the plants’ nutritional statuses, particularly in terms of phosphorus. The treatment with retardants, however, increased the Ca and Mg contents in both species. The combined application of retardants and extended lighting resulted in compact, well-branched plants at the flowering stage with desirable commercial traits. Our findings support precision agriculture principles by demonstrating how optimising lighting and retardant treatments can enhance plant architecture, flowering, and nutrient uptake in controlled environments. Integrating these insights with a machine learning model can improve efficiency, reduce resource waste, and enhance crop quality in commercial greenhouse production.