1. Introduction
In contemporary horticultural greenhouse production of vegetables, herbs, and ornamental plants, modern farms rely on soilless cultivation, where plants are grown in specialized substrates placed in containers or mats. Since, in this type of cultivation, the substrate is an artificially created and imposed environment for plant root growth and development, its physical, chemical, and microbiological properties are crucial for the success and profitability of production [
1].
To meet plant needs, horticultural substrates should have a stable and porous structure, a consistent chemical composition, high water and heat retention capacity [
2], and should be free from pathogens while allowing the growth of beneficial microorganisms [
3]. From the perspective of production management, the substrate should also be lightweight, cost-effective, accessible, and easy to dispose of [
4].
Peat is the primary component of horticultural substrates, as it meets most of these requirements [
5]. However, due to the unique and valuable characteristics of peatlands as ecosystems with high biodiversity, which serve as natural water retention reservoirs and store significant amounts of CO
2 in their biomass, most peatlands are now legally protected, making their exploitation increasingly restricted [
6]. Historically, peat was primarily harvested as fuel, and the near-total destruction and depletion of natural peatlands in the Netherlands and the United Kingdom were driven by industrial demands. Today, peat for horticultural use is primarily extracted in Europe from the Baltic countries (Lithuania, Latvia, Finland), Ireland, and Poland. Canada and Russia are also significant global producers of peat [
7].
Due to the need to protect unique peatland ecosystems and reduce CO
2 emissions into the atmosphere, increasingly strict regulations are being introduced in Europe and North America to limit the use of peat in horticultural production [
8]. The United Kingdom has announced a complete ban on peat use in the amateur gardening market by 2026 and in the professional sector by 2030. However, recognizing the technical challenges faced by professional growers, the transition for this sector is expected to take longer [
9]. Other European countries, including the Netherlands and Germany, have committed to gradually increasing the share of renewable substrates in growing media, aiming for 90% by 2050 [
10]. Consequently, finding suitable alternatives to peat is currently a major challenge for both horticultural substrate producers and plant growers, who will have to modify their cultivation systems to accommodate new substrates [
8].
Researchers continue to explore substances that could serve as growing media, partially or completely replacing peat. Peat substitutes must meet modern horticultural substrate requirements: they should be biodegradable, sourced from renewable materials, and possess appropriate physicochemical properties, including good structural integrity, high porosity, capillarity, and heat retention capacity. Coconut fiber (coir), derived from coconut husks, meets most of these criteria, has excellent aeration and water retention properties, but it may have unsuitable pH, excessive salinity expressed as high electrical conductivity (EC), and lack a sorption complex [
11]. Although coir has been noted also for its lower environmental impact than peat in terms of its extraction, yet one of the downsides of coir utilization is that it demands great distance transportation [
12].
Therefore, another important feature of a plant growth medium is its availability in the close distance to its place of destination and application. This is fulfilled by other alternatives as peat substitutes, such as composted agricultural and forestry wastes, garden waste and mushroom residue, which demonstrated comparable growth performance and improved root characteristics for New Guinea impatiens [
13]. Similarly, biochar from pruning wood waste activated with wood vinegar, was also investigated as a sustainable peat substitute in horticulture: it enhances plant growth at low concentrations but exhibits phytotoxicity at higher levels [
14]. Another candidate for peat substitute, vermicompost, can improve nitrogen supply in growing media [
15]. Recently, procedures were elaborated to identify peat substitutes and evaluate materials like composted heather, alder, cattail, and reed, which are plant originated products [
16,
17]. More attention is also being paid to assessing the chemical, physical, and biological characteristics of substrates to address environmental impacts and greenhouse gas emissions in horticulture [
16,
18]. Nonetheless, novel ideas for replacing peat with plant-based organic by-products often face limiting factors such as the need for pre-processing and restricted availability. These obstacles do not apply to the material investigated in this study—coffee silverskin—which is ready to use and widely available wherever coffee is roasted.
Our research investigates whether peat-based substrates used in nursery production for cuttings and ready-for-sale pot plants can be partially replaced with coffee silverskin (CS), a by-product of coffee bean roasting. Coffee silverskin (
Figure 1) accounts for 4.2% of the bean’s weight before roasting, is lightweight, formed with delicate flake-like particles. It consists of 54% fiber and separates from green coffee beans under high temperatures [
19]. Approximately 10 L of coffee silverskin can be obtained from 50 to 60 kg of green coffee beans, what calculates as one ton of the CS from 120 tons of roasted coffee beans. Due to its content of valuable compounds like fibers, sugars, and polyphenols, it was studied as a food component [
20] or in cosmetic products [
21,
22]. Coffee silverskin was tested in the field plant production as an organic fertilizer, contributing to sustainable agricultural practices by enriching soil quality [
23]. Nevertheless, CS utility in soilless, horticultural production in containers has never been explored before.
Applying coffee silverskin in horticultural substrates could offer multiple benefits: it is a cost-effective, lightweight, biodegradable, and renewable material, available wherever coffee roasting takes place. Additionally, it is free from pathogens and could provide added value to the substrate as a carrier of antioxidant compounds. According to the International Coffee Organization, global coffee consumption in the 2022/2023 coffee year was 173.1 million 60 kg bags, with an increasing trend [
24]. This amounts to a total of 10,386.0 million kilograms of coffee consumed and an estimated 2077.2 million liters of coffee silverskin waste. The vast potential of this renewable resource as a substrate for plant production has yet to be explored.
For plants nursery production, the first step is the rooting of cuttings, which is performed usually in multitrays, and the second step is the cultivation of mature plants, often up to flowering, to offer them for sale for individual or commercial consumers [
25]. Both stages of nursery production rely on high-quality substrate which directly influences the efficiency of rooting, cuttings performance, and the quality of the final product, which is a flowering plant [
26]. A good horticultural substrate is essential for healthy plant growth at all production stages in containers and soilless systems. It should balance water retention, air capacity, and nutrient availability, while maintaining physical stability and sustainability. These features together create an optimal environment for plant roots, supporting healthy and vigorous plant growth [
27].
The aim of our experiment was to evaluate the effect of horticultural peat-based substrate modified by the addition of 25%, 50%, and 75% coffee silverskin (CS) on cuttings growth and rooting, as well as on overall mature plants performance in greenhouse production. The plants used in the study were three popular perennial species commonly propagated vegetatively by shoot-tip cuttings and offered in retail centers in containers: Buddleja davidii, Lythrum salicaria, and Veronica longifolia.
3. Results and Discussion
In the present study, three commonly cultivated ornamental species—Buddleja, Lythrum, and Veronica—were used to evaluate the effects of coffee silverskin (CS) as a horticultural substrate component. These species are widely propagated vegetatively from shoot cuttings and grown in pots for retail sale, making them representative models for assessing substrate performance under nursery conditions.
In nursery production, rooting and subsequent cultivation are both critically dependent on substrate quality, which affects rooting efficiency, plant development, and the final product’s marketability [
27,
28,
30]. An optimal substrate should ensure adequate water retention, aeration, nutrient availability, and structural stability to support healthy root growth. After three weeks of cultivation, with the primary goal to regenerate the rooting system, the number of living, rooted cuttings were recorded for each substrate and species (
Figure 2 and
Figure 3).
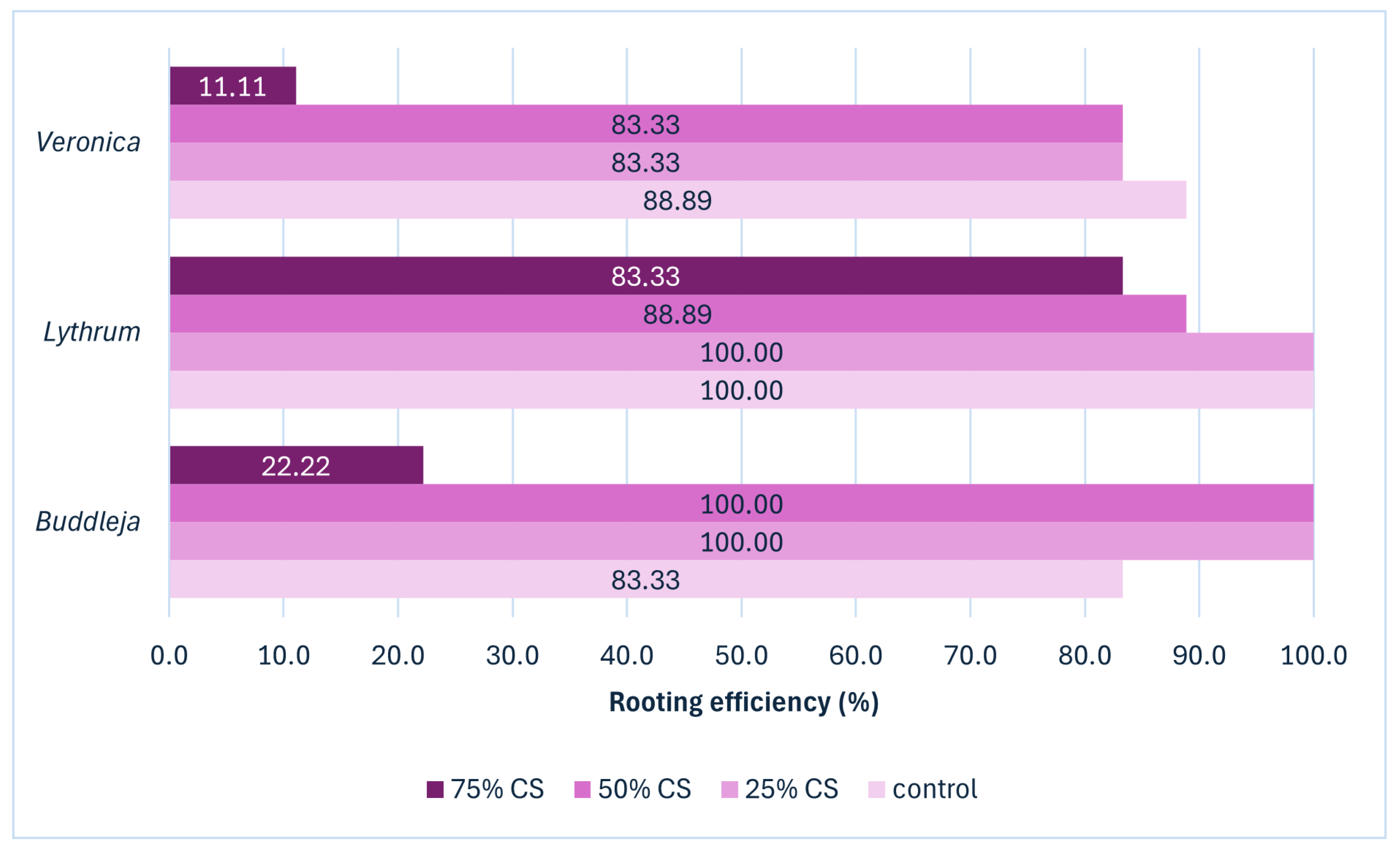
Rooting efficiency varied depending on the content of CS added and on the plant species. The highest percentage of rooted cuttings was obtained in the substrate with 25% CS addition (94,4% in average for all the species), which is a typical ratio for flowering shrubs [
33]. Rooting efficiency in the control substrate was on average the same as in the substrate with 50% CS addition (90.7%). The lowest average rooting efficiency was observed in cuttings placed in the substrate with 75% CS addition (38.6%). The detailed analysis of root system parameters revealed the 25% CS generally enhanced root development or maintained it at levels similar to control across all species. The addition of 50% CS consistently reduced root growth, especially in
Buddleja and
Veronica, while mean root diameter was not significantly affected by CS levels for any species (
Table 1).
Figure 2.
The rooting efficiency (%) of Buddleja, Lythrum, and Veronica shoot apical cuttings depending on the coffee silverskin (CS) content in the peat-based substrate.
Figure 2.
The rooting efficiency (%) of Buddleja, Lythrum, and Veronica shoot apical cuttings depending on the coffee silverskin (CS) content in the peat-based substrate.
Cuttings of the
Lythrum rooted well in all the substrates, with efficiency ranging from 83.3% in the substrate with the highest CS content to 100% in the control substrate and the substrate containing 25% CS. The lowest rooting percentage across all the substrates tested was recorded for
Veronica. The 75% addition of CS to the substrate at the rooting stage caused only 11% of Veronica cuttings to produce root. Since the number of rooted cuttings in
Veronica and
Buddleja in 75% CS were extremely low and the overall quality of the survived plantlets was poor as seen in
Figure 3, we decided to exclude this treatment from the further statistical analyses for rooting stage plants.
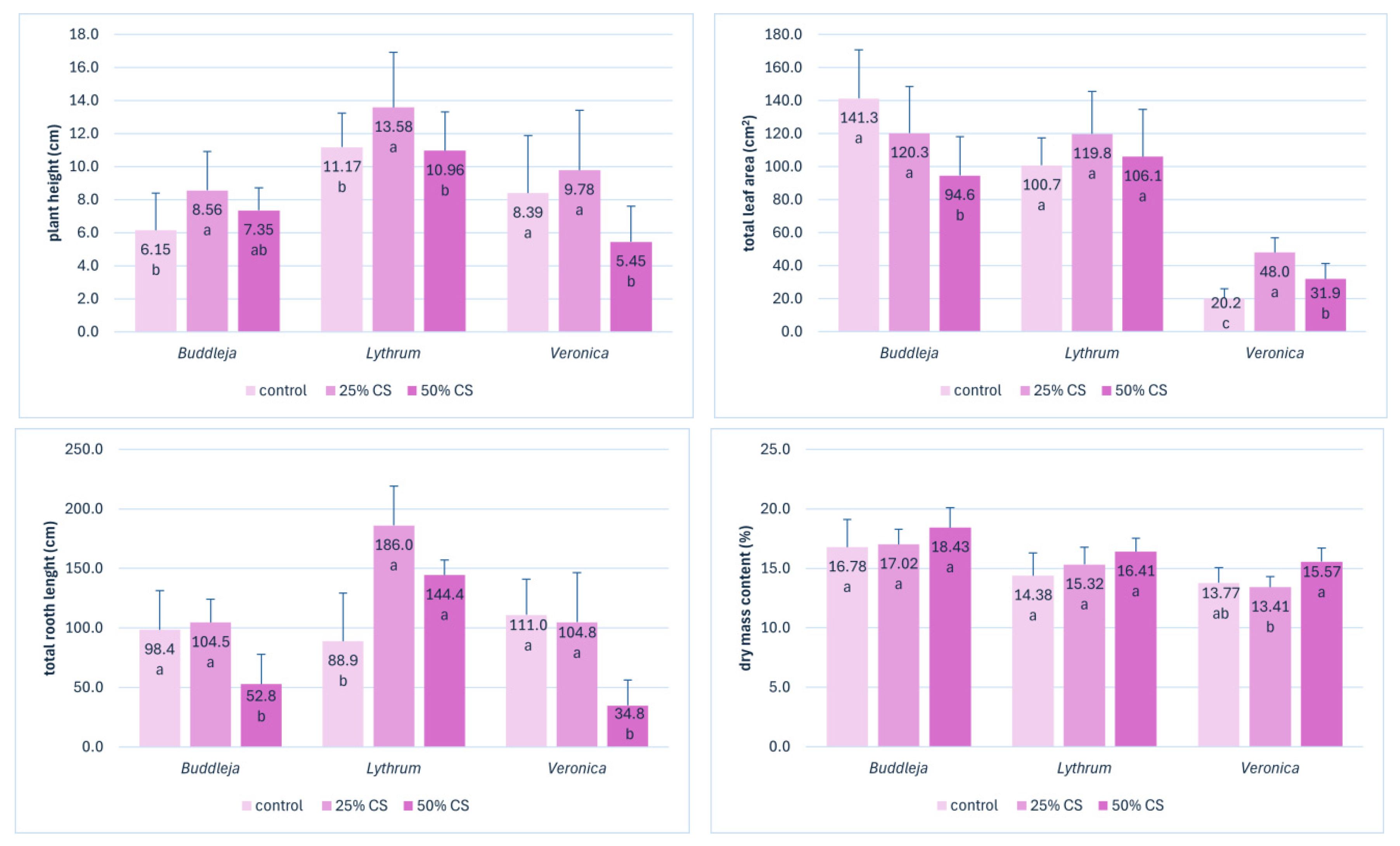
The detailed analysis of the root system parameters showed that in
Buddleja and
Veronica, the addition of 50% silver skin resulted in a shortening of the total root system length by half and 70%, respectively (
Figure 4). On the contrary, in
Lythrum, the addition of CS at 25% and 50% led to an elongation of the root system compared to control plants. This may be due to the fact that
Lythrum is a species that thrives in wet, water-rich environments, and the addition of CS in peat-based substrate helps retain more water and increase substrate moisture, which acts beneficial for this species [
34].
Moreover,
Lythrum, as a plant growing in marshy habitats, is adapted to conditions with reduced air access to the root system [
35]. There are many internal and external factors affecting efficient rooting [
36], but since all the cuttings were subjected to the same environmental conditions and were taken from similar mother plants, the effect of inhibited rooting is contributed to the substrate used in compared treatments. Physical and chemical properties of the substrates used in the experiment and their influence on cuttings performance are discussed further.
Figure 3.
Buddleja, Lythrum, and Veronica (from the top) shoot apical cuttings after three weeks of rooting in substrates supplemented with different levels of coffee silverskin (CS): (from the left: control, 25% CS, 50% CS, 75% CS). The bar stands for 10 cm.
Figure 3.
Buddleja, Lythrum, and Veronica (from the top) shoot apical cuttings after three weeks of rooting in substrates supplemented with different levels of coffee silverskin (CS): (from the left: control, 25% CS, 50% CS, 75% CS). The bar stands for 10 cm.
Figure 4.
The effect of substrate composition on the most important traits directly related to cuttings performance: plant height (cm), total leaf area (cm3), total length of root system (cm), and dry mass content (%) of Buddleja, Lythrum, and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS). Means followed by different letters (within the trait and the species) differ significantly according to Tukey’s HSD test at p < 0.05, whiskers stand for SD.
Figure 4.
The effect of substrate composition on the most important traits directly related to cuttings performance: plant height (cm), total leaf area (cm3), total length of root system (cm), and dry mass content (%) of Buddleja, Lythrum, and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS). Means followed by different letters (within the trait and the species) differ significantly according to Tukey’s HSD test at p < 0.05, whiskers stand for SD.
Table 1.
Root system parameters of Buddleja, Lythrum, and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 1.
Root system parameters of Buddleja, Lythrum, and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
| Plant Species | Substrate | Root Net Density (mm/cm3) | Sum of Roots Area (cm2) | Sum of Roots Volume (cm3) | Mean Root Diameter (mm) |
|---|
| Buddleja davidii | control | 8.48 ± 2.85 a | 14.46 ± 5.25 ab | 0.17 ± 0.07 ab | 0.47 ± 0.04 a |
| 25% CS | 9.01 ± 1.67 a | 17.02 ± 4.08 a | 0.22 ± 0.07 a | 0.51 ± 0.04 a |
| 50% CS | 4.56 ± 2.14 b | 8.14 ± 2.88 b | 0.10 ± 0.02 b | 0.52 ± 0.07 a |
| Lythrum salicaria | control | 7.66 ± 3.46 b | 12.93 ± 5.93 b | 0.15 ± 0.07 a | 0.46 ± 0.03 a |
| 25% CS | 16.04 ± 2.84 a | 27.92 ± 6.45 a | 0.34 ± 0.10 a | 0.47 ± 0.05 a |
| 50% CS | 12.45 ± 1.09 a | 18.44 ± 2.11 b | 0.19 ± 0.04 a | 0.41 ± 0.04 a |
| Veronica longifolia | control | 9.57 ± 2.57 a | 20.82 ± 4.87 a | 0.31 ± 0.06 a | 0.60 ± 0.04 a |
| 25% CS | 9.03 ± 3.58 a | 18.56 ± 7.67 a | 0.26 ± 0.12 a | 0.56 ± 0.04 a |
| 50% CS | 3.00 ± 1.83 b | 6.60 ± 2.81 b | 0.10 ± 0.03 b | 0.64 ± 0.09 a |
For the appropriate growth and rooting of plants, the well-developed leaves considered in sum as an assimilation organ are fundamental. For cuttings, which are primarily just small shoot fragments with no roots and a few leaves, photosynthesis efficiency is important for the rooting performance, as a source of substrates and energy for developing root system. Photosynthesis efficiency and rooting are closely linked through the plant’s source–sink relationship, where roots act as a major sink for assimilates produced in the leaves [
37]. The cuttings grown in different CS content substrates had different length three weeks post planting (
Figure 4). Shoots longer than in the control plants were produced in
Buddleja and
Lythrum in 25% CS substrate. The cuttings of
Veronica grown in 50% CS were significantly shorter. The dry mass of shoots was not affected by the CS content in the shoots of
Buddleja and
Lythrum. However, in
Veronica, the highest dry mass content was recorded in 50% CS. The length of the shoots was related with the number of leaves. The planimetric analyses of leaves revealed no significant impact of the CS content on the leaves’ characteristics, which remained particularly stable regardless of the CS content in substrate for
Lythrum (
Table 2).
In Veronica, the addition of 25% CS resulted even in higher values of certain leaves traits, like the total number of leaves, single and total leaf area, and leaf perimeter and length. In contrast, the addition of 50% CS affected adversely leaf area, perimeter, and length in Buddleja cuttings.
Pigment analysis in leaves serves for preliminary evaluation of stress and photosynthetic capacity in plants. The increase in flavonoids and anthocyanins contents indicate stress and the necessity of photoprotection, while chlorophyll content is a proxy indicator of photosynthetic performance [
38]. In our experiment,
Lythrum salicaria showed the most notable response, with significant increases in all pigments, especially chlorophyll at higher CS levels.
Buddleja and
Veronica showed no significant changes in pigment levels with CS supplementation (
Table 3). The addition of 50% CS did not induce pigment accumulation as stress response in most cases, except for
Lythrum. This suggests moderate stress response of cuttings under different CS addition to the substrate.
Table 2.
Leaves characteristics of Buddleja, Lythrum and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 2.
Leaves characteristics of Buddleja, Lythrum and Veronica shoot apical cuttings after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
| Plant Species | Substrate | Total Number of Leaves | Leaf Area (cm2) | Leaf Perimeter (cm) | Leaf Width (cm) | Leaf Length (cm) |
|---|
| Buddleja davidii | control | 11.33 ± 2.35 a | 6.24 ± 0.88 a | 12.47 ± 0.76 a | 2.13 ± 0.33 a | 4.62 ± 0.26 a |
| 25% CS | 10.13 ± 2.36 a | 5.45 ± 0.74 ab | 12.16 ± 0.57 a | 2.18 ± 0.18 a | 4.50 ± 0.33 a |
| 50% CS | 9.73 ± 2.25 a | 4.26 ± 1.18 b | 9.83 ±1.51 b | 1.75 ± 0.36 a | 3.69 ± 0.53 b |
| Lythrum salicaria | control | 12.00 ± 2.07 a | 3.70 ± 0.49 a | 8.32 ± 0.59 a | 1.53 ± 0.13 a | 3.45 ± 0.24 a |
| 25% CS | 12.25 ± 2.52 a | 5.06 ± 1.36 a | 9.89 ± 1.45 a | 1.69 ± 0.19 a | 4.14 ± 0.72 a |
| 50% CS | 10.67 ± 2.89 a | 4.46 ± 0.92 a | 9.52 ± 1.06 a | 1.64 ± 0.17 a | 3.89 ± 0.56 a |
| Veronica longifolia | control | 7.19 ± 2.04 ab | 2.81 ± 0.92 b | 11.25 ± 2.33 b | 4.94 ± 1.20 a | 0.97 ± 0.14 b |
| 25% CS | 8.63 ± 1.59 a | 5.57 ± 0.92 a | 17.12 ± 1.29 a | 7.68 ± 0.76 a | 1.23 ± 0.07 a |
| 50% CS | 6.27 ± 1.83 b | 5.31 ± 1.34 a | 16.84 ± 1.53 a | 7.65 ± 0.83 a | 1.19 ± 0.28 a |
One of the important measures, which indicates the photosynthetic apparatus status is the maximum quantum efficiency Fv/Fm index, which refers to the stress level in plants and is calculated based on variant (Fv) and maximum (Fm) fluorescence of chlorophyll representing photosystem II [
39]. The Fv/Fm coefficient values for all cuttings of the tested species across all tested substrates with different CS content ranged from 0.811 to 0.833 (
Table 4), which corresponds to the absence of physiological stress, which is indicated by the Fv/Fm value less than 0.74–0.85 [
40].
The SPAD index is another non-invasive measure used to monitor the nutritional status of plants and their exposure to stress. It is based on measuring light transmission at wavelengths of 650 and 940 nm [
41]. In an experiment on blueberry plants, a significant correlation was evidenced between the measurement of the SPAD index and the concentration of nitrogen and magnesium quantified in leaves [
42].
The SPAD values in our experiment fall within the average range for both rooting-stage cuttings (
Table 4) and plants grown in pots (Table 7) when CS content was up to 50%, thus we concluded that the addition of CS did not negatively affect the overall physiological condition of the plants, and most importantly, did not impact chlorophyll content (
Table 3).
Table 3.
The pigment content in leaves of shoot apical cuttings of Buddleja, Lythrum, and Veronica after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. The contents of the pigments were measured based on the optical fluorescence (F) and transmittance (T) at specific light wavelengths (nm) and thus expressed in relative units. Means ± SD followed by different letters within the trait and the species differ significantly according to the Tukey’s HSD test at α < 0.05.
Table 3.
The pigment content in leaves of shoot apical cuttings of Buddleja, Lythrum, and Veronica after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. The contents of the pigments were measured based on the optical fluorescence (F) and transmittance (T) at specific light wavelengths (nm) and thus expressed in relative units. Means ± SD followed by different letters within the trait and the species differ significantly according to the Tukey’s HSD test at α < 0.05.
| Plant Species | Substrate | Flavonoids
(F660 nm/F325 nm) | Anthocyanins
(F660 nm/F525 nm) | Chlorophyll
(T850 nm/T720 nm) |
|---|
| Buddleja davidii | control | 0.158 ± 0.06 a | 0.016 ± 0.02 a | 8.450 ± 3.21 a |
| 25% CS | 0.146 ± 0.06 a | 0.017 ± 0.03 a | 7.522 ± 2.28 a |
| 50% CS | 0.164 ± 0.07 a | 0.039 ± 0.03 a | 7.738 ± 3.76 a |
| Lythrum salicaria | control | 0.091 ± 0.02 b | 0.012 ± 0.01 b | 9.088 ± 0.92 b |
| 25% CS | 0.105 ± 0.03 a | 0.025 ± 0.01 ab | 11.511 ± 1.41 a |
| 50% CS | 0.145 ± 0.02 a | 0.027 ± 0.01 a | 13.271 ± 1.81 a |
| Veronica longifolia | control | 0.118 ± 0.03 a | 0.035 ± 0.01 a | 15.200 ± 3.09 a |
| 25% CS | 0.101 ± 0.02 a | 0.033 ± 0.01 a | 15.538 ± 3.66 a |
| 50% CS | 0.168 ± 0.10 a | 0.037 ± 0.01 a | 17.483 ± 7.06 a |
Some fluctuations in SPAD values can be observed—for example, in Lythrum during the rooting stage, the value is higher in cuttings grown in substrate with 25% CS. However, in the same species grown in substrate with 75% CS during the pot cultivation stage, the SPAD value is significantly lower compared to plants in other substrates. Nevertheless, in both cases, the values remain within the range of 30 to 45, which is considered an optimal SPAD index indicating favorable growing conditions for plants. The optimal values of SPAD differs in various species and the locations of the leaves that were subjected to the measurements. For instance, the optimal range of SPAD values for oregano plants under optimal environmental conditions was classified as high, which corresponded to values greater than 46, indicating productivity greater than 90% of maximum productivity [
43]. In contrast, the optimal SPAD value ranges for flue-cured tobacco leaves under ideal conditions differed according to the location of measurements: 3rd leaves (10.9–15.0), 6th leaves (13.7–17.3), 9th leaves (16.9–20.6), and 12th leaves (20.7–24.0), indicating good maturity and high yield quality [
44]. Based on the above results it could be stated that 25% CS content in the substrate did not affect adversely the important photosynthetic organs. The records of flavonols, anthocyanins, and chlorophyll content revealed differences only in cuttings of Lythrum, in which with the increase in CS content, the pigment content also increased.
Based on rooting efficiency as well as qualitative and quantitative parameters of rooted cuttings, it can be concluded that for all species the addition of 25% CS either has a beneficial effect or does not negatively affect the quality of the obtained cuttings.
Table 4.
Physiological parameters of the condition of shoot apical cuttings of Buddleja, Lythrum and Veronica after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 4.
Physiological parameters of the condition of shoot apical cuttings of Buddleja, Lythrum and Veronica after three weeks of rooting in substrates supplemented with 25% and 50% of coffee silverskin (CS) compared to control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Plant
Species | Substrate | NFI | SPAD | Fv/Fm |
|---|
| Buddleja davidii | control | 29.1 ± 7.22 a | 58.1 ± 17.71 a | 0.833 ± 0.02 a |
| 25% CS | 27.4 ± 7.54 a | 61.4 ± 30.01 a | 0.833 ± 0.01 a |
| 50% CS | 26.8 ± 9.52 a | 49.5 ± 19.74 a | 0.830 ± 0.02 a |
| Lythrum salicaria | control | 103.29 ± 20.33 a | 31.46 ± 1.58 b | 0.818 ± 0.01 a |
| 25% CS | 114.31 ± 28.14 a | 35.16 ± 2.00 a | 0.819 ± 0.01 a |
| 50% CS | 92.00 ± 14.70 a | 37.44 ± 2.13 a | 0.814 ± 0.01 a |
| Veronica longifolia | control | 131.94 ± 28.39 ab | 39.42 ± 3.49 a | 0.817 ± 0.01 a |
| 25% CS | 156.36 ± 31.46 a | 39.68 ± 3.89 a | 0.811 ± 0.01 a |
| 50% CS | 115.29 ± 26.59 b | 40.98 ± 5.90 a | 0.818 ± 0.01 a |
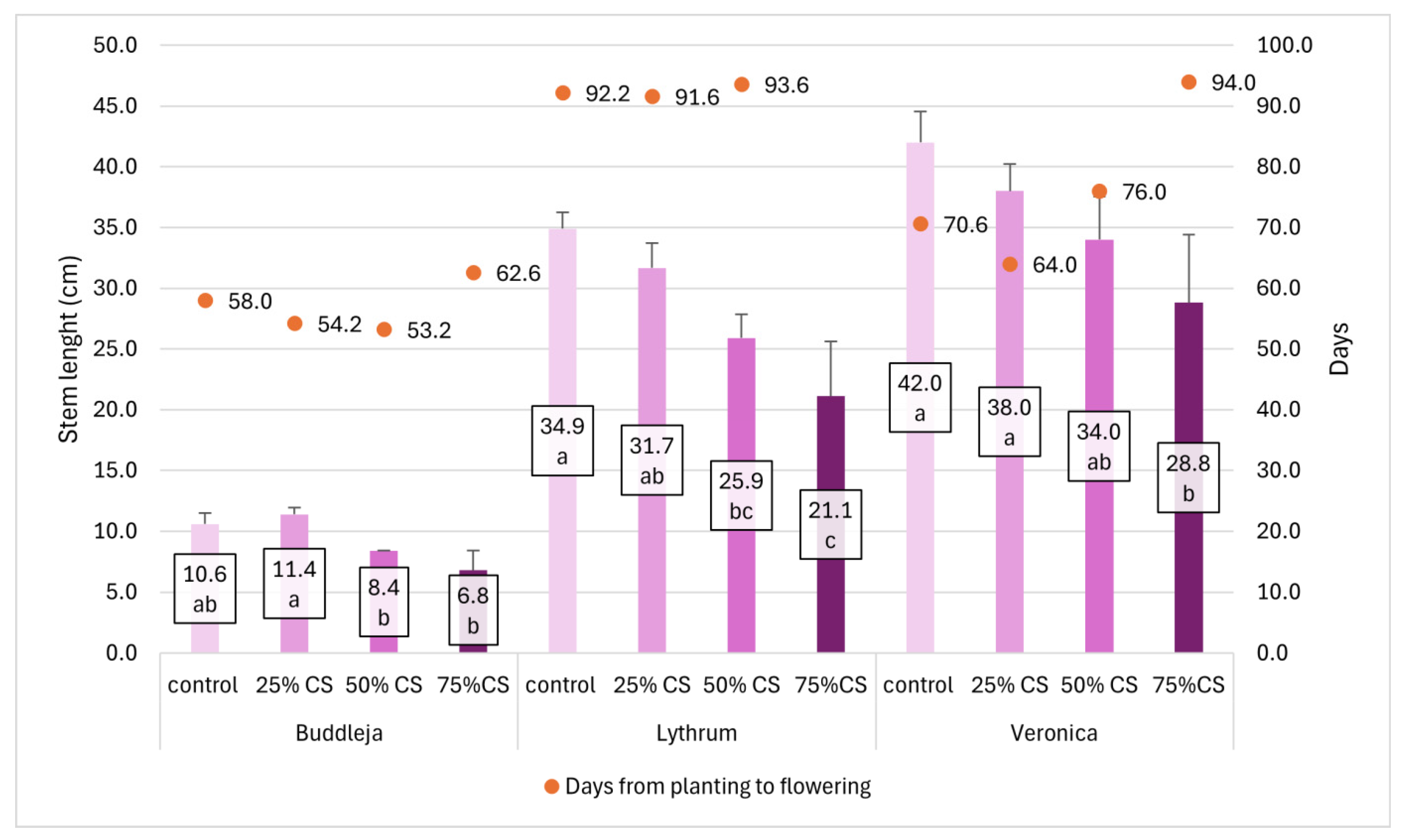
The response of mature plants grown in pots in substrates with different CS content varied between species, but generally plants were shorter with the increased content of CS, with particularly poor growth of shoots in 75% CS substrate (
Figure 5). The number of days from planting to flowering was highest in plants cultivated in 75% CS, with
Lythrum not even producing flowers before the end of experiment in the highest CS content. On the contrary, the period required to flowering was shorter in 25% CS than in control plants in all species:
Veronica flowered six days earlier;
Buddleja, four days earlier; and
Lythrum, less than one day earlier than the control plants. The enhanced earliness could be beneficial in terms of production organization (
Figure 6).
The morphological traits of leaves in mature plants showed that 25% and 50% CS content generally improved leaf morphology, especially in
Veronica and
Buddleja. Highest CS content (75%) consistently reduced leaf area, width, and length across all species, indicating stress and growth inhibition (
Table 5).
Veronica demonstrated the strongest positive response to CS, particularly at 50%, while
Lythrum was the most sensitive to high CS levels, showing consistent declines. Analyzing the pigment contents in leaves, no differences in flavonoids, anthocyanins, and chlorophyll contents were stated in
Buddleja regardless the CS content, while significant reduction in chlorophyll content was recorded for
Lythrum and
Veronica at 75% CS addition; in the case of the latter one, it decreased by more than half (
Table 6).
Figure 6.
Stem length (without inflorescence) after 12 weeks of growth (cm, column graph, left y-axis) and number of days from planting to first flower’s full development (days, line graph, right y-axis) of Buddleja, Lythrum, and Veronica plants in pots filled with substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to control. Lythrum plants did not flower in 75% CS before the end of the experiment. For shoot length, means followed by different letters within the species differ significantly according to Tukey’s HSD test at α < 0.05; whiskers stand for SD. For days to flowering no significant differences were indicated.
Figure 6.
Stem length (without inflorescence) after 12 weeks of growth (cm, column graph, left y-axis) and number of days from planting to first flower’s full development (days, line graph, right y-axis) of Buddleja, Lythrum, and Veronica plants in pots filled with substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to control. Lythrum plants did not flower in 75% CS before the end of the experiment. For shoot length, means followed by different letters within the species differ significantly according to Tukey’s HSD test at α < 0.05; whiskers stand for SD. For days to flowering no significant differences were indicated.
For mature plants, unlike in cuttings, physiological response to cultivation in substrate containing CS, expressed as differences in the NFI, SPAD, and Fv/Fm values, were more differentiated (
Table 7). The results showed clearly that 75% CS induces stress in all species, reflected by lower SPAD and Fv/Fm values compared to control. Species-specific responses suggest
Veronica may be the most CS-tolerant, while
Lythrum is the most sensitive.
Table 5.
Leave characteristics of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in pots filled with substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to control. Means followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 5.
Leave characteristics of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in pots filled with substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to control. Means followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
| Plant Species | Substrate | Leaf Area (cm2) | Leaf Perimeter (cm) | Leaf Width (cm) | Leaf Length (cm) |
|---|
| Buddleja davidii | control | 4.54 ± 1.06 ab | 13.11 ± 1.63 a | 5.92 ± 0.76 a | 1.27 ± 0.19 ab |
| 25% CS | 4.24 ± 0.37 b | 12.85 ± 0.83 ab | 5.82 ± 0.46 a | 1.19 ± 0.11 a |
| 50% CS | 5.79 ± 1.38 a | 14.45 ± 1.93 a | 6.35 ± 0.76 a | 1.45 ± 0.17 b |
| 75% CS | 3.77 ± 1.41 b | 11.04 ± 1.93 b | 4.88 ± 0.86 b | 1.15 ± 0.28 a |
| Lythrum salicaria | control | 6.40 ± 1.02 a | 12.12 ± 1.24 a | 5.15 ± 0.54 a | 1.69 ± 0.11 b |
| 25% CS | 7.23 ± 2.29 a | 11.74 ± 2.13 ab | 4.76 ± 0.83 ab | 2.02 ± 0.30 a |
| 50% CS | 5.64 ± 1.54 ab | 11.09 ± 1.77 ab | 4.67 ± 0.80 ab | 1.67 ± 0.26 b |
| 75% CS | 4.41 ± 0.89 b | 9.95 ± 1.35 b | 4.17 ± 0.55 b | 1.41 ± 0.11 c |
| Veronica longifolia | control | 4.44 ± 0.93 c | 9.66 ± 1.11 b | 3.81 ± 0.45 b | 1.63 ± 0.23 c |
| 25% CS | 7.40 ± 1.16 b | 13.88 ± 0.82 a | 5.61 ± 0.32 a | 1.92 ± 0.25 b |
| 50% CS | 9.38 ± 1.67 a | 14.79 ± 1.25 a | 5.77 ± 0.38 a | 2.37 ± 0.28 a |
| 75% CS | 4.37 ± 0.72 c | 10.38 ± 1.29 b | 4.05 ± 0.48 b | 1.63 ± 0.18 c |
Table 6.
The pigment content in leaves of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to the control. The contents of the pigments were measured based on the optical fluorescence (F) and transmittance (T) at specific light wavelengths (nm) and thus expressed in relative units. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 6.
The pigment content in leaves of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to the control. The contents of the pigments were measured based on the optical fluorescence (F) and transmittance (T) at specific light wavelengths (nm) and thus expressed in relative units. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Plant
Species | Substrate | Flavonols
(F660 nm/F325 nm) | Anthocyanins
(F660 nm/F525 nm) | Chlorophyll
(T850 nm/T720 nm) |
|---|
| Buddleja davidii | control | 0.400 ± 0.04 a | 0.014 ± 0.01 a | 29.080 ± 26.05 a |
| 25% CS | 0.550 ± 0.15 a | 0.010 ± 0.01 a | 14.600 ± 2.86 a |
| 50% CS | 0.536 ± 0.11 a | 0.014 ± 0.01 a | 15.060 ± 1.78 a |
| 75% CS | 0.524 ± 0.06 a | 0.020 ± 0.01 a | 14.120 ± 2.92 a |
| Lythrum salicaria | control | 0.538 ± 0.12 a | 0.020 ± 0.02 ab | 15.920 ± 3.26 a |
| 25% CS | 0.534 ± 0.12 a | 0.012 ± 0.01 b | 14.520 ± 4.03 a |
| 50% CS | 0.602 ± 0.04 a | 0.012 ± 0.01 b | 15.360 ± 1.49 a |
| 75% CS | 0.508 ± 0.10 a | 0.058 ± 0.04 a | 8.000 ± 3.17 b |
| Veronica longifolia | control | 1.150 ± 0.05 b | 0.012 ± 0.00 a | 52.300 ± 11.17 a |
| 25% CS | 0.770 ± 0.04 a | 0.024 ± 0.01 a | 57.100 ± 18.54 a |
| 50% CS | 0.830 ± 0.10 ab | 0.014 ± 0.01 a | 48.680 ± 8.43 a |
| 75% CS | 0.728 ± 0.34 a | 0.016 ± 0.01 a | 20.440 ± 12.24 b |
By comparing Fv/Fm values, which reflect maximum quantum yield of PSII photochemistry, indicating photosynthetic efficiency, in cuttings and mature plants, one can notice that no stress and uniform data are indicated in cuttings, regardless of the CS content, while results for mature plants were more differentiated. In research concerning light intensity and daily light integral (DLI) influence on
Veronica plants’ performance, it was found that maximum quantum efficiency (Fv/Fm) was strongly affected by the irradiance [
40], with the lower values at higher DLI, but not exceeding the safe 0.74 to 0.85 range [
39].
Table 7.
Physiological parameters of physiological condition of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to the control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 7.
Physiological parameters of physiological condition of Buddleja, Lythrum, and Veronica plants after 12 weeks of growth in substrates supplemented with 25%, 50%, and 75% of coffee silverskin (CS) compared to the control. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Plant
Species | Substrate | NFI | SPAD | Fv/Fm |
|---|
| Buddleja davidii | control | 42.10 ± 5.53 a | 46.54 ± 10.68 a | 0.845 ± 0.01 a |
| 25% CS | 29.06 ± 12.40 a | 38.84 ± 3.05 a | 0.818 ± 0.03 ab |
| 50% CS | 28.69 ± 5.08 a | 39.50 ± 1.88 a | 0.822 ± 0.03 ab |
| 75% CS | 27.23 ± 6.88 a | 38.28 ± 3.20 a | 0.783 ± 0.02 b |
| Lythrum salicaria | control | 30.50 ± 7.15 a | 40.24 ± 3.24 a | 0.791 ± 0.02 a |
| 25% CS | 28.69 ± 11.88 a | 38.54 ± 4.14 a | 0.797 ± 0.02 a |
| 50% CS | 25.52 ± 1.68 a | 39.84 ± 1.48 a | 0.770 ± 0.02 ab |
| 75% CS | 16.87 ± 9.17 a | 28.50 ± 6.35 b | 0.755 ± 0.02 b |
| Veronica longifolia | control | 45.66 ± 10.40 bc | 59.16 ± 3.34 a | 0.831 ± 0.00 a |
| 25% CS | 74.27 ± 24.19 a | 60.34 ± 4.44 a | 0.841 ± 0.01 a |
| 50% CS | 59.77 ± 14.23 ab | 58.14 ± 2.86 a | 0.811 ± 0.01 b |
| 75% CS | 26.96 ± 6.18 c | 42.08 ± 9.81 b | 0.805 ± 0.00 b |
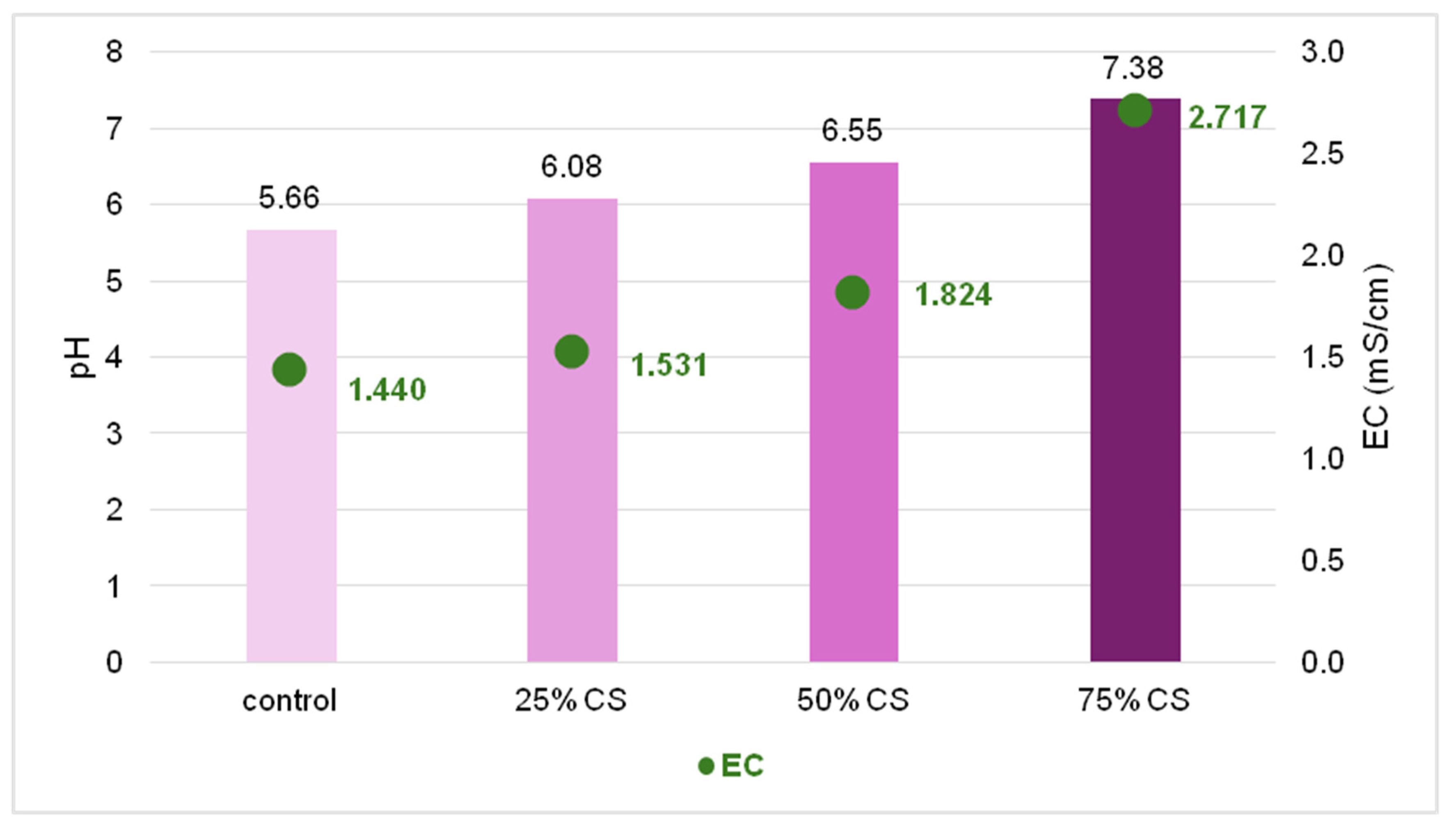
Physical and chemical characteristics of the substrates used in our experiments were affected by the amount of the coffee silverskin addition. Both, pH and electrical conductivity (EC) increased with the increased volume of CS: in the 75% CS substrate the values were about two units higher as compared to control reaching 7.38 (pH) and 2.717 mS/cm, respectively (
Figure 7).
The most universal pH of substrate for the plants, suitable for many species growing in the medium without specific demands, covers values 5.5–6.5 [
45]. The optimal electrical conductivity for plant growth substrates varies depending on the specific cultivation method and plant species. For most of the horticultural plants, EC ranges between 1.5 and 2.5 mS/cm, which ensures adequate nutrient availability without causing toxicity or deficiencies [
46]. Specific requirements regarding optimal pH and EC values for the three tested species are not widely available in the scientific literature. However, according to grower guidelines,
Veronica performs best at an EC of approximately 1.0–1.25 mS/cm and a pH of 5.5–6.2 [
47]. As reported by the Clemson University Home and Garden Information Center,
Buddleja grows well in soils with a pH of 6.0–7.0 [
48]. For
Lythrum, only general information is available, suggesting that its requirements align with the typical ranges mentioned above. Based on this, we can state that both the EC and the pH value of the substrate with the highest (75%) addition of CS in our experiment exceeded the optimal universal levels, which could negatively affect both roots and shoots performance and led to general poor rooting efficiency. To decrease the adverse effect of unsuitable pH and EC values, CS could be washed with pure water before application [
49] and/or mixed with mineral components, e.g., agricultural lime [
50], which are common methods of adjusting the substrates such as peat or coir.
Figure 7.
The pH and electric conductivity (EC) values of substrates used in the experiment.
Figure 7.
The pH and electric conductivity (EC) values of substrates used in the experiment.
The substrates used in the experiment, regardless of the addition of CS, were characterized by high water absorption and a high carbon content of around 50% (
Table 8). These are typical properties of organic-rich horticultural substrates. The addition of CS also increased the organic matter content expressed as the loss on ignition (LOI) while decreasing the levels of mineral nutrients. The highest LOI values were present in the 50% and 75% CS and reached 71.4%. Such high LOI together with TC values are characteristic features of organic substrates like peat or coir, in contrast to mineral soils which may have LOI at 10% [
2]. Generally beneficial feature of being organic substrate (high water capacity, high sorption and water retention, high air content) has its downsides. A substrate with high organic matter content may adversely affect nitrogen availability due to nitrogen immobilization by microorganisms [
51]. Therefore, high carbon content carries the risk of nitrogen deficiency, and nitrogen fertilization must be carefully controlled and balanced. However, in our experiment substrates with higher CS content also showed higher total nitrogen content, which suggests that CS introduces nitrogen into the tested substrates. The question remains: is this nitrogen available to plants [
52]?
The C:N ratio value is one of the factors determining the intensity of decomposition of organic matter. The value of this ratio in the range of 20–30:1 indicates the balance of mineralization and humification processes. Lower C:N values indicate intensification of mineralization processes. Higher C:N values point to the intensification of the humification processes, which favors carbon sequestration [
53]
The C:N ratio in the analyzed substrates varied from 41.4 to 24.9. The highest C:N was noted in pure peat-based substrate (control) and its value decreased with the increase in CS addition (25% CS—36.6, 50% CS—29.0, and 75% CS—24.9).
Table 8.
The main physical and chemical characteristics of substrates used in the experiments. CS—coffee silverskin content in the peat-based substrate, LOI—loss on ignition, TC—total carbon, TN—total nitrogen. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
Table 8.
The main physical and chemical characteristics of substrates used in the experiments. CS—coffee silverskin content in the peat-based substrate, LOI—loss on ignition, TC—total carbon, TN—total nitrogen. Means ± SD followed by different letters within the trait and the species differ significantly according to Tukey’s HSD test at α < 0.05.
| Substrate | Water
Absorption | Bulk Density | LOI | Ash | TC | TN | Moisture * | Dry Mass * |
|---|
| (gH2O gsubstrate−1) | (g cm−3) | (%) | (%) | (%) | (%) | (%) | (%) |
|---|
| control | 2.604 ± 0.18 a | 0.190 ± 0.004 a | 67.5 ± 0.40 a | 32.5 ± 0.4 a | 51.41 ± 4.78 a | 1.243 ± 0.134 c | 77.3 ± 1.0 a | 22.7 ± 1.0 a |
| 25% CS | 2.893 ± 0.22 a | 0.176 ± 0.010 ab | 68.6 ± 0.28 ab | 31.4 ± 0.3 ab | 49.02 ± 1.09 a | 1.340 ± 0.047 c | 76.7 ± 1.0 a | 23.3 ± 1.0 a |
| 50% CS | 2.709 ± 0.13 a | 0.172 ± 0.004 b | 70.4 ± 0.82 bc | 29.6 ± 0.8 bc | 48.46 ± 0.70 a | 1.673 ± 0.045 b | 80.1 ± 0.5 b | 19.9 ± 0.5 b |
| 75% CS | 2.778 ± 0.16 a | 0.138 ± 0.008 c | 71.4 ± 1.53 c | 28.6 ± 1.5 c | 50.96 ± 2.30 a | 2.046 ± 0.095 a | 80.6 ± 0.5 b | 19.4 ± 0.5 b |
The analysis of nitrogen balance index—NFI (nitrogen flavonoid index)—which is a relative indicator of nitrogen nutrition status in plants [
54], revealed no significant differences between plants rooted in control and CS-enriched substrates in
Buddleja and
Lythrum cuttings. The exception were
Veronica cuttings rooted in 25% CS substrate, in which the NFI value was higher than in 50% CS grown plants. Therefore it can be concluded that nitrogen load from CS was probably not efficiently utilized by plants. By analyzing the NFI in mature plants grown in pots, we assume that the nitrogen nutrition status is not influenced by the CS content in
Buddleja and
Lythrum, while in
Veronica the amendment of 25% CS resulted in higher NFI value and considerable drop for 75% CS cultivated plants, suggesting a distinct nitrogen metabolism pathway in this species.
The additional nitrogen load from CS was not efficiently utilized by the plants, which could be attributed to microbial NO
3−–N immobilization (I
NO3). I
NO3 can be stimulated by the addition of high-organic-carbon materials such as CS. The extent of this stimulation depends on the type of organic material applied [
55]. In the case of CS, further investigation is needed to determine whether this is a result of microbial activity or chemical reactions.
The substrates enriched with CS were generally lighter than the control, as confirmed by the significant decrease in bulk density values with increasing CS content. Unfortunately, due to the specific structure of coffee silver skin, which forms flat, delicate flakes rather than three-dimensional structures (
Figure 1), such substrate did not exhibit improved water–air balance, but rather showed an increased tendency to compaction. Therefore, despite the low bulk density, the oxygen availability to the root zone in substrates with high CS content could be reduced, which could result in poor rooting efficiency in 75% CS rich substrates in
Buddleja and
Veronica cuttings [
56].
More experiments are needed to test CS performance as substrate component in combination with some mineral additives and/or other (than peat) structural compounds. The biggest downside of CS is its flat, delicate structure, which makes the substrate vulnerable to compression and thus restricting the oxygen availability to root system. The target horticultural substrate should have an air-filled porosity of 15–25% and a hydraulic conductivity of 0.01–0.05 cm/s [
57]. Non-peat components that are most effective in maintaining substrate structure and preventing compaction while retaining adequate moisture include coconut fiber (coir), composted wood, or bark. The blend can be supplemented with perlite or pumice to enhance structural stability [
58].
With enhancing the three-dimensional structure of CS-based substrate by addition of structural components one can obtain the inexpensive, environmental friendly, easily available, renewable component for horticultural production.