Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Experimental Design and Treatments
2.3. Growth Parameters
2.4. Spectral Vegetation Indices
2.5. Nutritional Parameters
2.6. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Spectral Vegetation Indices
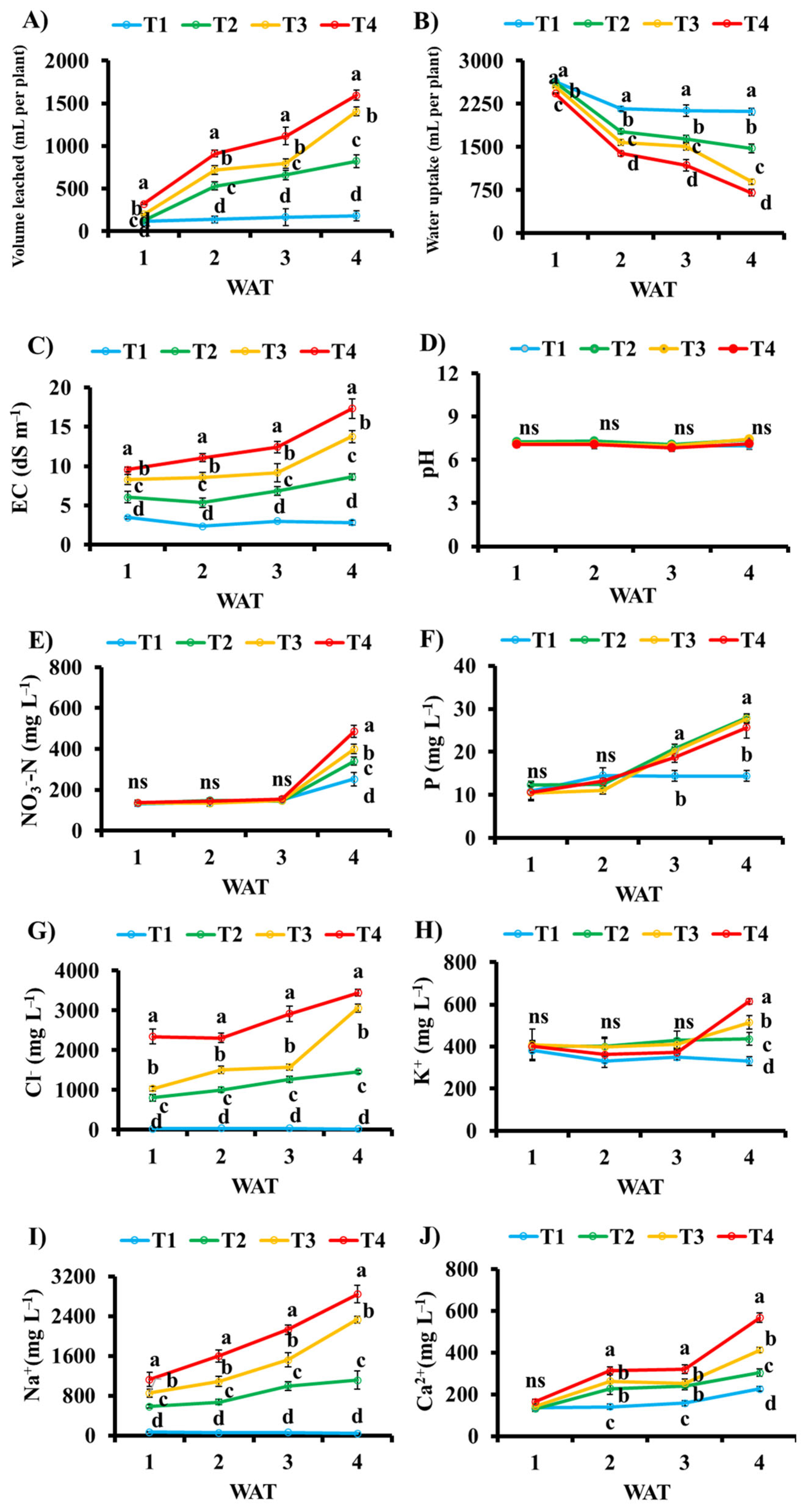
3.3. Nutritional Parameters
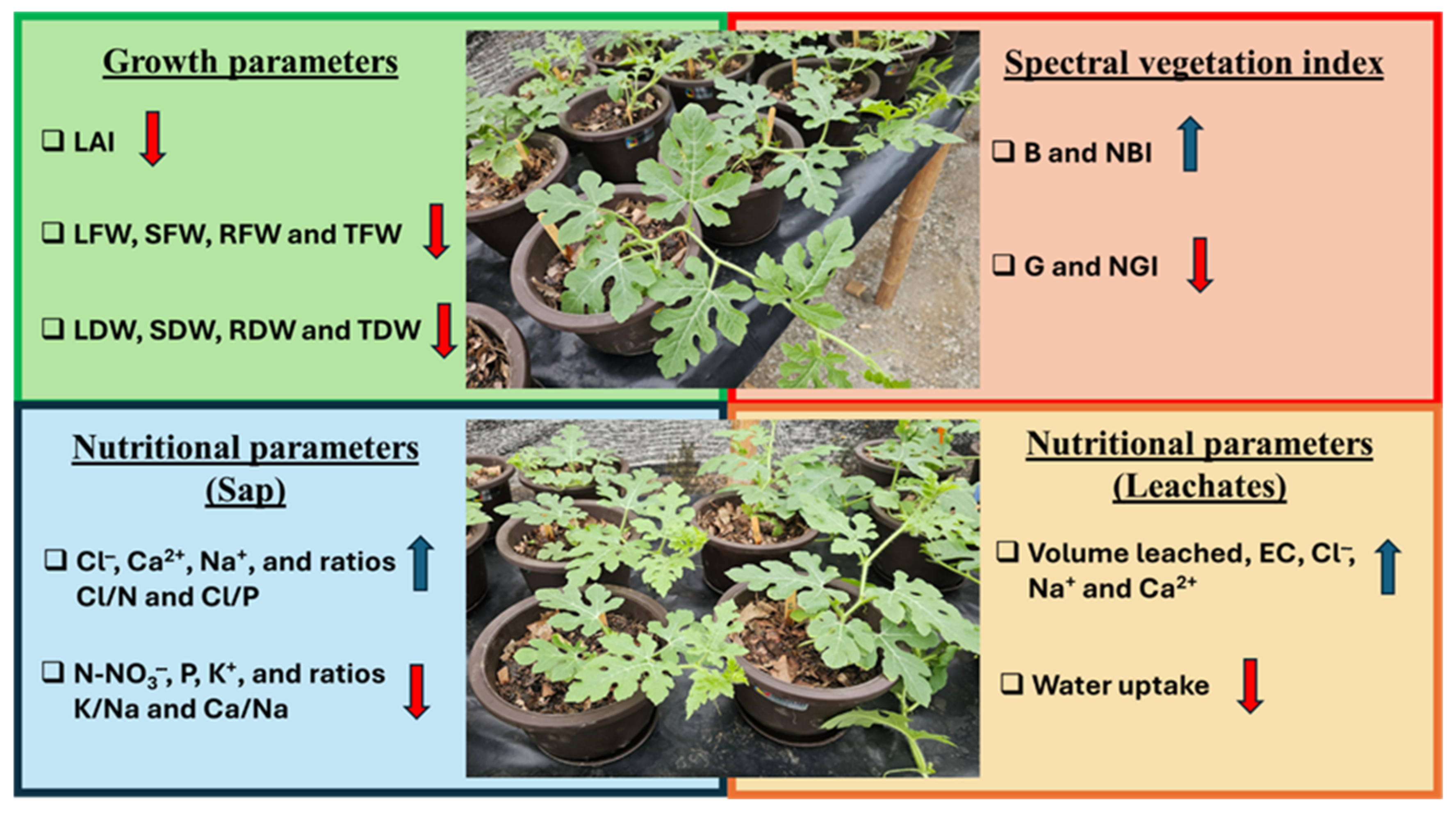
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef] [PubMed]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-scale soil salinization dynamics from global to pore scale: A review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Al-Azzawi, M.J.; Flowers, T.J. Economic uses of salt-tolerant plants. Plants 2023, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [PubMed]
- Ashraf, M.; Munns, R. Evolution of approaches to increase the salt tolerance of crops. Crit. Rev. Plant Sci. 2022, 41, 128–160. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubińska-Mielińska, S.; Piernik, A. Osmotic stress or ionic composition: Which affects the early growth of crop species more? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion homeostasis for salinity tolerance in plants: A molecular approach. Physiol. Plant. 2021, 171, 578–594. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Verma, O.; Sharma, S.; Kumar, V.; Singh, T.; Kumar, R.; Auji, R. Salinity stress effect on staple food crops and novel mitigation strategies. Biologia 2024, 79, 2359–2374. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, C.; Zhang, Q.; Qiu, Z.; Zhang, X.; Hou, Y.; Zang, J. Salinity survival: Molecular mechanisms and adaptive strategies in plants. Front. Plant Sci. 2025, 16, 1527952. [Google Scholar] [CrossRef]
- Tola, E.; Al-Gaadi, K.A.; Madugundu, R.; Zeyada, A.M.; Edrris, M.K.; Edrees, H.F.; Mahjoop, O. The use of spectral vegetation indices to evaluate the effect of grafting and salt concentration on the growth performance of different tomato varieties grown hydroponically. Horticulturae 2025, 11, 368. [Google Scholar] [CrossRef]
- Taha, M.F.; Mao, H.; Wang, Y.; ElManawy, A.I.; Elmasry, G.; Wu, L.; Menon, M.S.; Niu, Z.; Huang, T.; Qiu, Z. High-throughput analysis of leaf chlorophyll content in aquaponically grown lettuce using hyperspectral reflectance and RGB images. Plants 2024, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Maoto, M.M.; Beswa, D.; Jideani, A.I. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- FAO Statistics. 2023. Available online: https://www.fao.org/statistics/en (accessed on 23 June 2025).
- Yan, Y.; Wang, S.; Wei, M.; Gong, B.; Shi, Q. Effect of different rootstocks on the salt stress tolerance in watermelon seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- do Ó, L.M.G.; Cova, A.M.W.; de Azevedo Neto, A.D.; da Silva, N.D.; Silva, P.C.C.; Santos, A.L.; Gheyi, H.R.; da Silva, L.L. Osmotic adjustment, production, and post-harvest quality of mini watermelon genotypes differing in salt tolerance. Sci. Hortic. 2022, 306, 111463. [Google Scholar] [CrossRef]
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; dos Anjos Soares, L.A.; Moreira, R.C.L.; Fernandes, P.D.; Andrade, E.M.G.A.; Pinheiro, F.W.A. Salinity management strategies and potassium fertilization in watermelon (‘Citrullus lanatus’) cultivation. Aust. J. Crop Sci. 2020, 14, 1601–1607. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and silicon-based biostimulants differentially affect the physiology and growth of watermelon transplants under abiotic stress factors: The case of salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef]
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; dos Anjos Soares, L.A.; Oliveira, J.P.M.; Araujo, C.A.; Gomes, J.A. Production and quality of watermelon fruits under salinity mangement strategies and nitrogen fertilization. Semin. Cienc. Agrar. 2020, 41, 2923–2936. [Google Scholar] [CrossRef]
- Lucena, R.R.M.; Negreiros, M.Z.; Medeiros, J.F.; Grangeiro, L.C.; Marrocos, S.T.P. Crescimento e acúmulo de macronutrientes em melancia ‘Quetzale’ cultivada sob diferentes níveis de salinidade da água de irrigação. Rev. Caatinga 2011, 24, 34–42. [Google Scholar]
- Martins, D.C.; da Saúde de Sousa Ribeiro, M.; de Souza Neta, M.L.; da Silva, R.T.; Gomes, L.P.; Guedes, R.A.A.; de Assis de Oliveira, F. Desenvolvimento inicial de cultivares de melancia sob estresse salino. Agropecu. Cient. No SemiÁrido 2013, 8, 62–68. [Google Scholar]
- Ferre, F.C.; Rodríguez, E.J.F. El cultivo de sandía apirena injertada, bajo invernadero, en el litoral mediterráneo español; Caja Rural de Almería: Almeria, Spain, 2000; 315p. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; Circular 347; Agricultural Experiment Station: Berkeley, CA, USA, 1938; pp. 1–35. [Google Scholar]
- Vaesen, K.; Gilliams, S.; Nackaerts, K.; Coppin, P. Ground-measured spectral signatures as indicators of ground cover and leaf area index: The case of paddy rice. Field Crops Res. 2001, 69, 13–25. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Tolerance mechanisms of three potted ornamental plants grown under moderate salinity. Sci. Hortic. 2016, 201, 84–91. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, Y.; Ma, J.; Huang, L.; Zhuang, H. Detection and dynamic variation characteristics of rice nitrogen status after anthesis based on the RGB color index. Agronomy 2021, 11, 1739. [Google Scholar] [CrossRef]
- Cadahía, C. El análisis de la savia como índice de fertilización; Manuales de la Ciencia Actual; CSIC: Madrid, Spain, 1973.
- Garcia-Caparros, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622–627. [Google Scholar] [CrossRef]
- Silva, E.G.D.; Silva, A.F.D.; Lima, J.D.S.; Silva, M.D.F.C.D.; Maia, J.M. Vegetative development and content of calcium, potassium, and sodium in watermelon under salinity stress on organic substrates. Pesqui. Agropecu. Bras. 2017, 52, 1149–1157. [Google Scholar] [CrossRef]
- Valenzuela, F.J.; Reineke, D.; Leventini, D.; Chen, C.C.L.; Barrett-Lennard, E.G.; Colmer, T.D.; Dodd, I.; Shabala, S.; Brown, P.; Bazihizina, N. Plant responses to heterogeneous salinity: Agronomic relevance and research priorities. Ann. Bot. 2022, 129, 499–518. [Google Scholar] [CrossRef]
- Joshi, S.; Nath, J.; Singh, A.K.; Pareek, A.; Joshi, R. Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol. Plant. 2022, 174, e13702. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Verstraeten, W.W.; Van Aardt, J.A.N.; Keulemans, W.; Coppin, P. Hyperspectral indices to diagnose leaf biotic stress of apple plants, considering leaf phenology. Int. J. Remote Sens. 2009, 30, 1887–1912. [Google Scholar] [CrossRef]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. The assessment of leaf water content using leaf reflectance ratios in the visible, near-, and short-wave-infrared. Int. J. Remote Sens. 2008, 29, 3701–3713. [Google Scholar] [CrossRef]
- Junttila, S.; Hölttä, T.; Saarinen, N.; Kankare, V.; Yrttimaa, T.; Hyyppä, J.; Vastaranta, M. Close-range hyperspectral spectroscopy reveals leaf water content dynamics. Remote Sens. Environ. 2022, 277, 113071. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Pokovai, K.; Mészáros, J.; Balog, K.; Koós, S.; Árvai, M.; Fodor, N. Optical leaf area assessment supports chlorophyll estimation from UAV images. Smart Agric. Technol. 2025, 11, 100894. [Google Scholar] [CrossRef]
- Devadas, R.; Lamb, D.W.; Backhouse, D.; Simpfendorfer, S. Sequential application of hyperspectral indices for delineation of stripe rust infection and nitrogen deficiency in wheat. Precis. Agric. 2015, 16, 477–491. [Google Scholar] [CrossRef]
- Burns, B.W.; Green, V.S.; Hashem, A.A.; Massey, J.H.; Shew, A.M.; Adviento-Borbe, M.A.A.; Milad, M. Determining nitrogen deficiencies for maize using various remote sensing indices. Precis. Agric. 2022, 23, 791–811. [Google Scholar] [CrossRef]
- Qiao, B.; He, X.; Liu, Y.; Zhang, H.; Zhang, L.; Liu, L.; Reineke, A.-J.; Liu, W.; Müller, J. Maize characteristics estimation and classification by spectral data under two soil phosphorus levels. Remote Sens. 2022, 14, 493. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Zhou, Z.; Zhang, Z.; Xu, C.; Zha, Q.; Wang, F.; Hu, X. Prediction of the nitrogen, phosphorus and potassium contents in grape leaves at different growth stages based on UAV multispectral remote sensing. Remote Sens. 2022, 14, 2659. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Contreras, J.I.; Segura, M.L.; Lao, M.T. Evaluation of the nutrients variability in sap of different petiole samples in tomato plant. Commun. Soil Sci. Plant Anal. 2018, 49, 745–750. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Segura, M.L.; Contreras, J.I.; Lao, M.T. Nutritional changes in petiole sap over space and time in a tomato crop greenhouse. J. Plant Nutr. 2019, 42, 1205–1217. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Pérez-Alonso, J.; Contreras, J.I.; Segura, M.L.; Reca, J.; Lao, M.T. Approach to petiole sap nutritional diagnosis method by empirical model based on climatic and growth parameters. Agronomy 2020, 10, 188. [Google Scholar] [CrossRef]
- Hochmuth, G.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. Plant tissue analysis and interpretation for vegetable crops in Florida. In Nutrient Management of Vegetable and Row Crops Handbook; University of Florida Press: Gainesville, FL, USA, 2012; pp. 45–92. [Google Scholar]
- Santos, M.G.; Moreira, G.S.; Pereira, R.; Carvalho, S.M. Assessing the potential use of drainage from open soilless production systems: A case study from an agronomic and ecotoxicological perspective. Agric. Water Manag. 2022, 273, 107906. [Google Scholar] [CrossRef]
- Jasik, M.; Kormanek, M.; Staszel-Szlachta, K.; Małek, S. Leachate tables as a tool for monitoring changes in physical and chemical parameters of the peat substrate in the cells of nursery containers. Forests 2023, 14, 2398. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Rodríguez, J.C.; Maksimovic, I.; Urrestarazu, M.; Lao, M.T. Rosemary growth and nutrient balance: Leachate fertigation with leachates versus conventional fertigation. Sci. Hortic. 2018, 242, 62–68. [Google Scholar] [CrossRef]
- Ehtaiwesh, A.F. The effect of salinity on nutrient availability and uptake in crop plants. Sci. J. Appl. Sci. Sabratha Univ. 2022, 9, 55–73. [Google Scholar]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
| Temperature (°C) | Relative Humidity (%) | |
|---|---|---|
| Average | 25.58 | 71.19 |
| Minimum | 19.80 | 48.80 |
| Maximum | 35.40 | 89.40 |
| LN (n°) | LAI (cm2) | LFW (g) | SFW (g) | RFW (g) | TFW (g) | LDW (g) | SDW (g) | RDW (g) | TDW (g) | LWC (g/g) | LWR (-) | SWR (-) | RWR (-) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 53.25 ± 5.06 a | 5348.33 ± 566.96 a | 121.6 ± 9.50 a | 97.50 ± 6.66 a | 18.50 ± 3.87 a | 236.40 ± 19.32 a | 11.64 ± 1.09 a | 8.96 ± 2.22 a | 5.31 ± 0.58 a | 25.91 ± 2.68 a | 0.90 ± 0.03 a | 0.45 ± 0.06 a | 0.36 ± 0.05 a | 0.19 ± 0.03 a |
| T2 | 51.20 ± 4.49 a | 3831.06 ± 239.05 b | 70.60 ± 4.87 b | 83.13 ± 2.77 b | 10.38 ± 1.14 b | 164.25 ± 13.31 b | 6.23 ± 0.59 b | 7.49 ± 0.95 a | 2.70 ± 0.34 b | 16.42 ± 1.22 b | 0.92 ± 0.04 a | 0.38 ± 0.07 a | 0.46 ± 0.04 a | 0.16 ± 0.02 a |
| T3 | 30.80 ± 3.03 b | 1950.31 ± 190.82 c | 47.33 ± 1.53 c | 42.60 ± 5.39 c | 8.67 ± 1.43 c | 98.59 ± 4.66 c | 4.27 ± 0.31 c | 4.09 ± 0.35 b | 1.17 ± 0.20 c | 9.53 ± 0.56 c | 0.91 ± 0.03 a | 0.45 ± 0.07 a | 0.43 ± 0.04 a | 0.12 ± 0.02 b |
| T4 | 25.75 ± 2.55 b | 1469.91 ± 214.38 d | 41.40 ± 2.63 c | 33.75 ± 1.15 d | 6.88 ± 1.63 d | 82.03 ± 4.09 d | 3.59 ± 0.15 c | 3.59 ± 0.62 b | 1.00 ± 0.24 c | 7.4 ± 0.87 d | 0.91 ± 0.04 a | 0.50 ± 0.08 a | 0.38 ± 0.04 a | 0.12 ± 0.02 b |
| Red (-) | Green (-) | Blue (-) | NRI (-) | NGI (-) | NBI (-) | |
|---|---|---|---|---|---|---|
| T1 | 33.72 ± 1.78 a | 55.20 ± 0.84 a | 13.00 ± 0.75 d | 0.34 ± 0.01 a | 0.53 ± 0.01 a | 0.13 ± 0.01 d |
| T2 | 35.25 ±1.01 a | 55.48 ± 0.83 a | 15.24 ± 0.65 c | 0.33 ± 0.01 a | 0.52 ± 0.01 b | 0.14 ± 0.01 c |
| T3 | 36.83 ± 2.70 a | 56.24 ± 1.99 a | 17.64 ± 0.39 b | 0.33 ± 0.01 a | 0.50 ± 0.01 b | 0.16 ± 0.01 b |
| T4 | 32.45 ± 0.64 a | 50.19 ± 1.93 b | 19.34 ± 0.34 a | 0.31 ± 0.01 b | 0.49 ± 0.01 c | 0.19 ± 0.01 a |
| pH | N-NO3− (ppm) | P (ppm) | Cl− (ppm) | K+ (ppm) | Ca2+ (ppm) | Na+ (ppm) | Cl/N | Cl/P | K/Na | Ca/Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 6.32 ± 0.11 a | 1300.61 ± 79.19 a | 359.90 ± 42.12 a | 743.35 ± 182.98 d | 4730.11 ± 183.67 a | 221.05 ± 43.28 c | 160.00 ± 15.59 d | 0.56 ± 0.05 d | 2.06 ± 0.40 d | 30.43 ± 3.98 a | 1.23 ± 0.16 a |
| T2 | 6.18 ± 0.08 a | 858.02 ± 45.16 b | 255.42 ± 32.38 b | 2040.00 ± 219.70 c | 4107.50 ± 145.77 b | 417.50 ± 15.00 b | 320.00 ± 18.61 c | 2.41 ± 0.35 c | 7.65 ± 0.77 c | 12.77 ± 0.69 b | 1.31 ± 0.13 a |
| T3 | 6.22 ± 0.04 a | 711.27 ± 42.24 c | 132.66 ± 25.82 c | 5387.50 ± 687.23 b | 3700.29 ± 105.77 c | 438.40 ± 33.16 b | 465.35 ± 36.93 b | 7.48 ± 0.78 b | 40.21 ± 12.71 b | 7.99 ± 0.67 c | 0.96 ± 0.08 b |
| T4 | 6.49 ± 0.12 a | 592.73 ± 38.56 d | 59.40 ± 15.02 d | 7162.50 ± 381.61 a | 3550.22 ± 100.00 d | 695.00 ± 67.58 a | 788.12 ± 126.77 a | 12.50 ± 0.84 a | 126.05 ± 26.05 a | 4.85 ± 0.45 d | 0.95 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanderal, A.; Vasquez Muñoz, G.; Pincay-Solorzano, M.S.; Ceasar, S.A.; García-Caparros, P. Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels. Agronomy 2025, 15, 1620. https://doi.org/10.3390/agronomy15071620
Llanderal A, Vasquez Muñoz G, Pincay-Solorzano MS, Ceasar SA, García-Caparros P. Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels. Agronomy. 2025; 15(7):1620. https://doi.org/10.3390/agronomy15071620
Chicago/Turabian StyleLlanderal, Alfonso, Gabriela Vasquez Muñoz, Malena Suleika Pincay-Solorzano, Stanislaus Antony Ceasar, and Pedro García-Caparros. 2025. "Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels" Agronomy 15, no. 7: 1620. https://doi.org/10.3390/agronomy15071620
APA StyleLlanderal, A., Vasquez Muñoz, G., Pincay-Solorzano, M. S., Ceasar, S. A., & García-Caparros, P. (2025). Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels. Agronomy, 15(7), 1620. https://doi.org/10.3390/agronomy15071620








