Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurements and Methods
2.3.1. Characterization of Biochar
2.3.2. Agronomic Traits and Yield Measurement
2.3.3. Soil Sampling
2.3.4. Soil Physical and Chemical Analysis
2.3.5. Soil Bacteria Analysis
2.4. Statistical Analysis
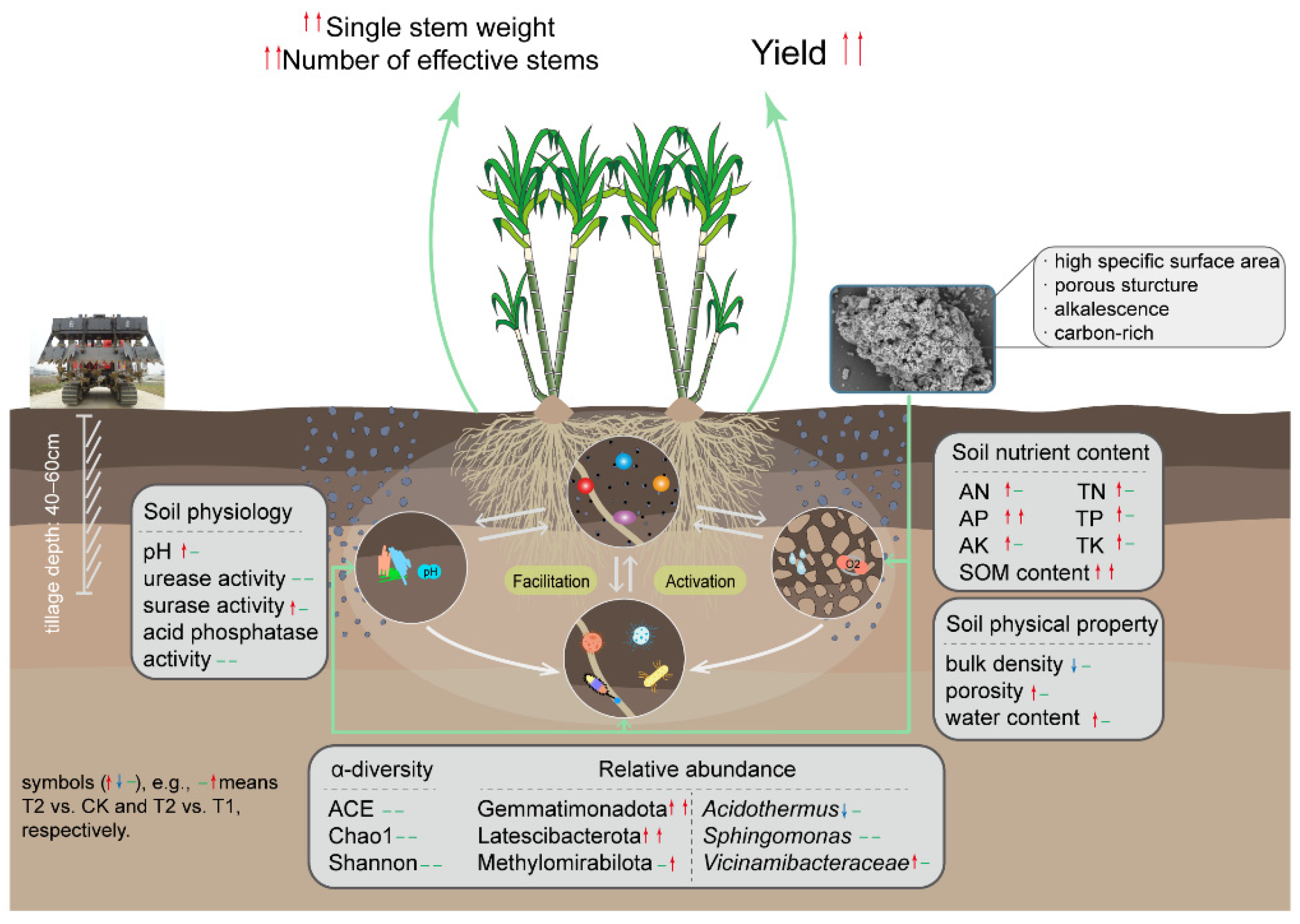
3. Results
3.1. Soil Bulk Density, Porosity, and Soil Water Content
3.2. Soil pH
3.3. Soil Available and Total Nutrients
3.4. Soil Enzyme Activity
3.5. Bacteria
3.5.1. Alpha Diversity
3.5.2. Relative Abundance of Major Bacterial Taxa
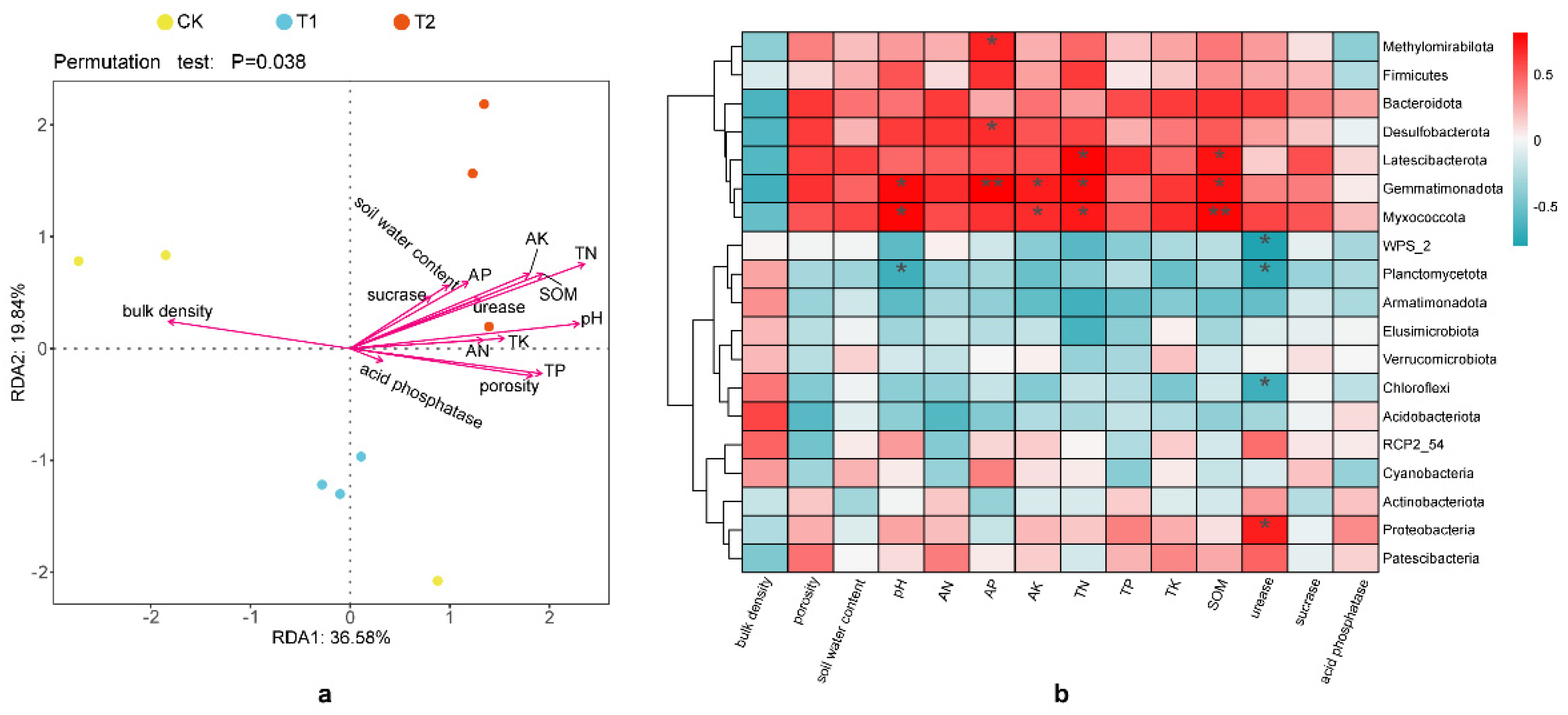
3.5.3. Associations Between Soil Bacterial Community and Environmental Factors
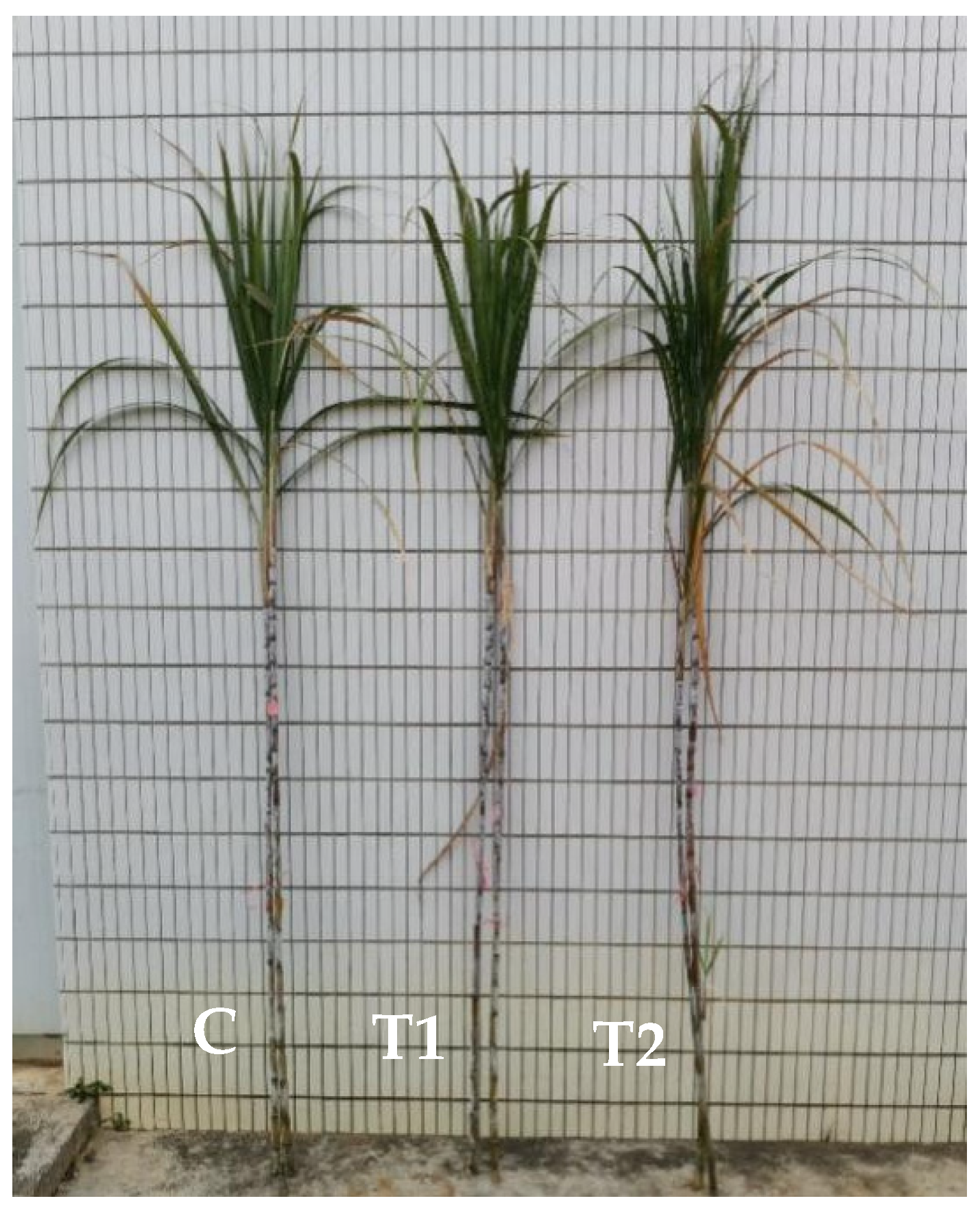
3.6. Agronomic Traits and Yield
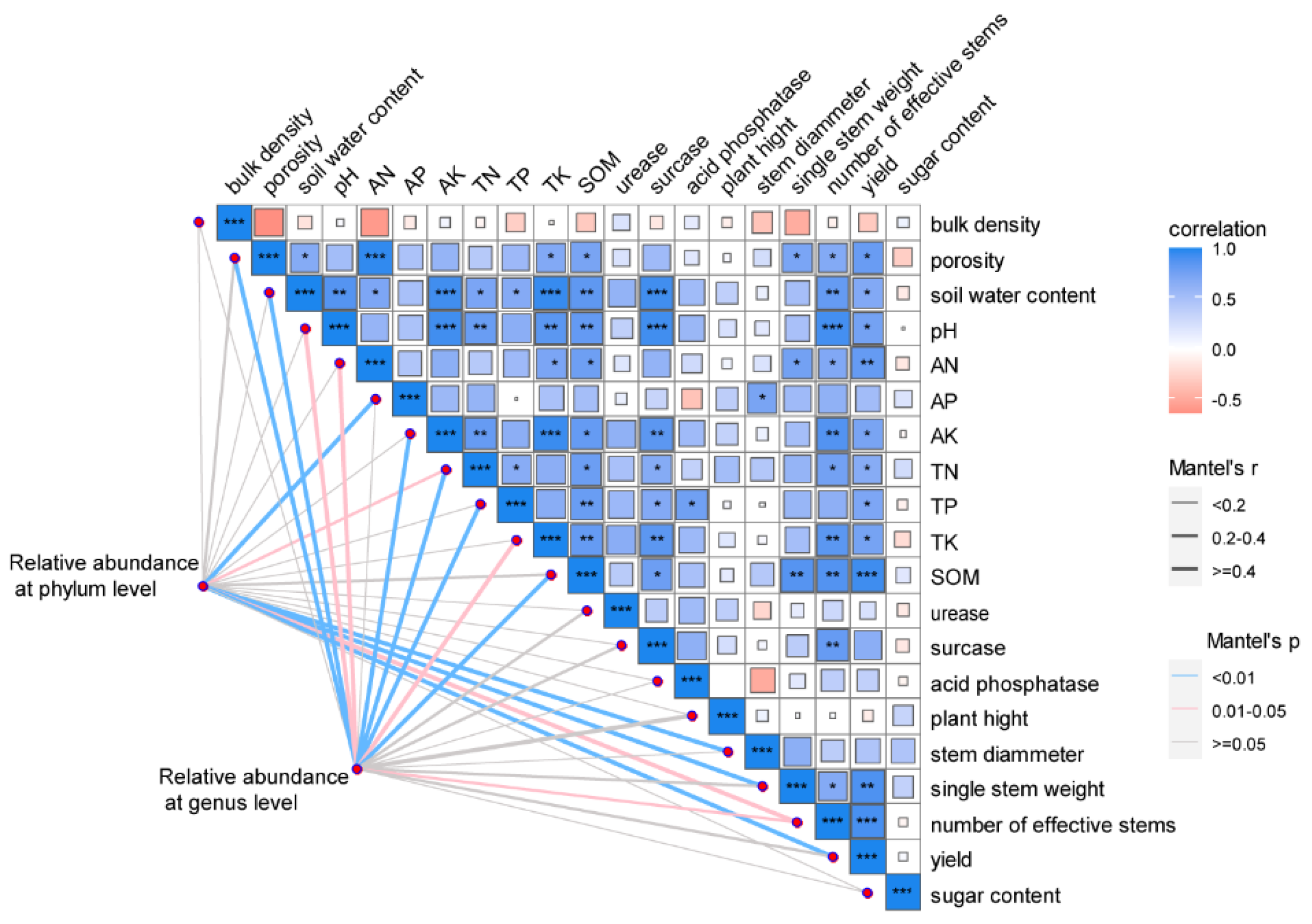
3.7. Correlation Analysis
4. Discussion
4.1. Biochar-Based Fertilizers Improved Soil More than Chemical Fertilizers
4.2. Sugar Filter Mud Biochar-Based Fertilizers Improved Soil More than Straw Biochar-Based Fertilizers
4.3. Sugar Filter Mud Biochar-Based Fertilizers Were the Most Beneficial in Improving Sugarcane Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Guo, D.-J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Diversity of Nitrogen-Fixing Rhizobacteria Associated with Sugarcane: A Comprehensive Study of Plant-Microbe Interactions for Growth Enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil Metagenomics Reveals Effects of Continuous Sugarcane Cropping on the Structure and Functional Pathway of Rhizospheric Microbial Community. Front. Microbiol. 2021, 12, 627569. [Google Scholar] [CrossRef]
- Thomaz, E.L.; Marcatto, F.S.; Antoneli, V. Soil Erosion on the Brazilian Sugarcane Cropping System: An Overview. Geogr. Sustain. 2022, 3, 129–138. [Google Scholar] [CrossRef]
- State Statistics Bureau. China Statistical Yearbook 2023; China Statistics Press: Beijing, China, 2023; ISBN 978-7-5230-0190-5.
- Pang, Z.; Tayyab, M.; Kong, C.; Liu, Q.; Liu, Y.; Hu, C.; Huang, J.; Weng, P.; Islam, W.; Lin, W.; et al. Continuous Sugarcane Planting Negatively Impacts Soil Microbial Community Structure, Soil Fertility, and Sugarcane Agronomic Parameters. Microorganisms 2021, 9, 2008. [Google Scholar] [CrossRef]
- Tian, L.; Shi, S.; Ma, L.; Lam-Son, P.T.; Tian, C. Community Structures of the Rhizomicrobiomes of Cultivated and Wild Soybeans in Their Continuous Cropping. Microbiol. Res. 2020, 232, 126390. [Google Scholar] [CrossRef]
- Lei, H.; Liu, A.; Hou, Q.; Zhao, Q.; Guo, J.; Wang, Z. Diversity Patterns of Soil Microbial Communities in the Sophora Flavescens Rhizosphere in Response to Continuous Monocropping. BMC Microbiol. 2020, 20, 272. [Google Scholar] [CrossRef]
- Gui, Y.; Li, H.; Wei, J.; Mao, L.; Zhang, R.; Ou, H.; Zhu, K.; Zhao, P.; Zhou, H.; Liu, X. Effects of biochar on soil nutrients, enzyme activities and microbial diversity of ratoon sugarcane in dry slope land. J. South Agric. 2022, 53, 776–784. [Google Scholar]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does Biochar Improve Soil Water Retention? A Systematic Review and Meta-Analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Kamau, S.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Short-Term Influence of Biochar and Fertilizer-Biochar Blends on Soil Nutrients, Fauna and Maize Growth. Biol. Fertil. Soils 2019, 55, 661–673. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Khan, E.; Yip, A.C.K.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised Fabrication of Nitrogen-Doped Biochar for Environmental and Energy Applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, R. Pyrolysis of Agroforestry Bio-Wastes with Calcium/Magnesium Oxides or Carbonates—Focusing on Biochar as Soil Conditioner. Biomass Bioenergy 2021, 155, 106277. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Tang, X.; Yan, L.; El-Desouki, Z.; Li, Y.; Wang, X.; Cuncang, J. Insight into Mechanisms of Biochar-Fertilizer Induced of Microbial Community and Microbiology of Nitrogen Cycle in Acidic Soil. J. Environ. Manag. 2023, 336, 117602. [Google Scholar] [CrossRef]
- Lima, I.; Marshall, W.E. Utilization of Turkey Manure as Granular Activated Carbon: Physical, Chemical and Adsorptive Properties. Waste Manag. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, W.; Meng, J. Advances and prospects in research of biochar utilization in agriculture. Sci. Agric. Sin. 2013, 46, 3324–3333. [Google Scholar]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of Biochar on the Physical and Structural Properties of a Sandy Soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Fallah, N.; Yang, Z.; Tayyab, M.; Zhang, C.; Abubakar, A.Y.; Lin, Z.; Pang, Z.; Allison, A.; Zhang, H. Depth-Dependent Influence of Biochar Application on the Abundance and Community Structure of Diazotrophic under Sugarcane Growth. PLoS ONE 2021, 16, e0253970. [Google Scholar] [CrossRef]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus Biochar Application Enhances Ca Uptake of Upland Rice, Soil Available P, Exchangeable K, Yield, and N Use Efficiency of Sugarcane in a Crop Rotation System. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Todd, J.; Glaz, B.; Irey, M.S.; Zhao, D.; Hu, C.-J.; El-Hout, N. Sugarcane Genotype Selection on a Sand Soil with and without Added Mill Mud. Agron. J. 2014, 106, 315–323. [Google Scholar] [CrossRef]
- Li, L.; You, X.; Sun, J.; Li, Z.; He, X. Review on utilization of sugarcane field wastes and sugar refining byproducts. Food Ind. 2013, 34, 170–173. [Google Scholar]
- Lin, Q.; Wei, F.; Lin, H.; Chen, T.; Zhou, Z. Present situation and countermeasure research of filter mud disposal of raw sugar mill in Guangxi. Yunnan Chem. Technol. 2019, 46, 69–70. [Google Scholar]
- Liu, H.; Zhang, W.; Li-Jiang, G.; Wang, Q.; Cheng, R.; Zhang, S. Effects of different biochars on phosphorus adsorption and desorption in brown soil. Soils 2023, 55, 1198–1206. [Google Scholar]
- Wang, J.; Xia, H.; Li, Y.; Wang, X.; Jiang, C. Effects of biochar from different feedstocks on soil nitrogen transformation and physicochemical properties in acid red soil. J. Huazhong Agric. Univ. 2022, 41, 61–70. [Google Scholar]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- He, L.; Zhong, Z.; Yang, H. Effects on Soil Quality of Biochar and Straw Amendment in Conjunction with Chemical Fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Zhang, H.; Guo, L.; Chen, Y.; Heiling, M.; Zhou, B.; Mao, Y. Biochar Interaction with Chemical Fertilizer Regulates Soil Organic Carbon Mineralization and the Abundance of Key C-Cycling-Related Bacteria in Rhizosphere Soil. Eur. J. Soil Biol. 2021, 106, 103350. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility. A Review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Chen, W.; Li, P.; Li, F.; Xi, J.; Han, Y. Effects of Tillage and Biochar on Soil Physiochemical and Microbial Properties and Its Linkage with Crop Yield. Front. Microbiol. 2022, 13, 929725. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Zhang, L.; Liu, T.; Ji, Y. Effects of biochar combined with subsoiling on soil aggregates and organic carbon. Bull. Soil Water Conserv. 2022, 42, 368–375+385. [Google Scholar]
- Wang, M.; Tong, H.; Han, Y.; Li, P.; Chen, W.; Bi, Q. Effects of Deep Tillage and Fertilization on Wheat Yield and Physicochemical Properties of Lime Concretion Black Soil. Chin. J. Soil Sci. 2022, 53, 1431–1439. [Google Scholar]
- Jiang, B.; Xu, X.; Zhang, D.; Li, W.; Liang, S.; Xu, H.; Wang, Y.; Bai, X. Effect of biochar and tillage on nitrogen absorption and yield of maize under reduced fertilizer. J. Northeast Agric. Univ. 2019, 50, 23–31. [Google Scholar]
- Lu, H.; Zhang, X.; Li, L.; Fan, Y.; Zhang, M.; Zhang, Y.; Yan, X.; Liang, T.; Zhai, Z.; Daai, H. Effects of biochar on tobacco yield, quality and soil nutrients under deep tillage and green manure treatments. Tob. Sci. Technol. 2021, 54, 14–22. [Google Scholar]
- Xiao, J.; Zhu, S.; Han, S.; Wei, B.; He, Z.; Li, Z.; Li, R.; Wang, L.; Chen, L.; Li, S. Fenlong-Ridging Deep Tillage Integrated with Biochar and Fertilization to Improve Sugarcane Growth and Yield. Agronomy 2023, 13, 2395. [Google Scholar] [CrossRef]
- Wei, B. Fenlong cultivation-the fourth set of farming methods invented in China. Agric. Sci. Technol. 2017, 18, 2045–2048+2052. [Google Scholar]
- Wei, B.; Gan, X.; Li, Y.; Shen, Z.; Zhou, L.; Zhou, J.; Liu, B.; Lao, C.; Hu, P. Effects of once Fenlong cultivation on soil properties and rice yield and quality for 7 consecutive years. Agric. Sci. Technol. 2017, 18, 2365–2371. [Google Scholar]
- He, J.; Shi, W.; Liu, F.; Ma, Q.; Yang, Y.; Yang, J.; Cui, Y.; Chen, Y. Effects of Fenlong cultivation on soil key physical properties in arid areas. Agric. Res. Arid Areas 2023, 41, 195–201+228. [Google Scholar]
- Zhu, S.; Xiao, J.; Han, S.; Li, X.; Li, Z.; Wei, B.; Zhang, D.; Wang, R.; Li, R.; Yang, L.; et al. Transcriptomics Combined with Photosynthetic Physiology and Leaf Structure Analysis Revealed Increased Sugarcane Yield by Fenlong-Ridging. Agronomy 2023, 13, 1196. [Google Scholar] [CrossRef]
- Dao, J.; Fan, X.; Li, F.; Deng, J. Sugarcane Nutrition Management and Detection Technology; China Agriculture Press: Beijing, China, 2018; ISBN 978-7-109-24555-6. [Google Scholar]
- Bao, K. Soil Mineralogy; Science Press: Beijing, China, 2000. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Guan, S. Soil Enzyme and Its Research Method; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Bluman, A.G. Elementary Statistics: A Step by Step Approach, 6th ed.; McGraw Hill Higher Education: New York, NY, USA, 2007. [Google Scholar]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of Biochar and Biochar-Based Fertilizer on Yield, Quality of Tea and Microbial Community in an Acid Tea Orchard Soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Wu, F.; Tong, C.; Zhang, W.; Liu, D.; He, Z.; Mao, Y. Effects of tobacco stalk biochar- and bamboo biochar-based fertilizers on carbon, nitrogen, and microbes in soil of tobacco field. Fujian J. Agric. Sci. 2023, 38, 109–115. [Google Scholar]
- Chen, Y.; Shinogi, Y.; Taira, M. Influence of Biochar Use on Sugarcane Growth, Soil Parameters, and Groundwater Quality. Aust. J. Soil Res. 2010, 48, 526–530. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The Role of Biochar and Biochar-Compost in Improving Soil Quality and Crop Performance: A Review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Liu, X.H.; Li, S.; Li, K.; Deng, H. Effects of Bagasse Biochar Application on Soil Organic Carbon Fixation in Manganese-Contaminated Sugarcane Fields. Chem. Biol. Technol. Agric. 2023, 10, 46. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of Biochar Application on Soil Hydrological Properties and Physical Quality of Sandy Soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Islam, M.U.; Jiang, F.; Guo, Z.; Peng, X. Does Biochar Application Improve Soil Aggregation? A Meta-Analysis. Soil Tillage Res. 2021, 209, 104926. [Google Scholar] [CrossRef]
- Berglund, L.M.; DeLuca, T.H.; Zackrisson, O. Activated Carbon Amendments to Soil Alters Nitrification Rates in Scots Pine Forests. Soil Biol. Biochem. 2004, 36, 2067–2073. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-Induced Concomitant Decrease in Ammonia Volatilization and Increase in Nitrogen Use Efficiency by Wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Yang, S.; Wang, Y. Impacts of Biochar Addition on Rice Yield and Soil Properties in a Cold Waterlogged Paddy for Two Crop Seasons. Field Crops Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Forján, R.; Asensio, V.; Rodríguez-Vila, A.; Covelo, E.F. Contribution of Waste and Biochar Amendment to the Sorption of Metals in a Copper Mine Tailing. Catena 2016, 137, 120–125. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water Extractable Organic Carbon in Untreated and Chemical Treated Biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Dunst, G.; Glaser, B. Positive Effects of Composted Biochar on Plant Growth and Soil Fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Niazi, N.K.; Bolan, N.; Zhao, L.; Zhang, S.; Xue, J.; Yao, B.; Li, Y. Biochar Addition Increased Soil Bacterial Diversity and Richness: Large-Scale Evidence of Field Experiments. Sci. Total Environ. 2023, 893, 164961. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R.; Sun, T.; Liang, Y.; Jiang, Y.; Sun, B. Organic Amendments Shift the Phosphorus-Correlated Microbial Co-Occurrence Pattern in the Peanut Rhizosphere Network during Long-Term Fertilization Regimes. Appl. Soil Ecol. 2018, 124, 229–239. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of Pyrolysis Temperature and Residence Time on Physicochemical Properties of Different Biochar Types. ACTA Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting Biochar Properties and Functions Based on Feedstock and Pyrolysis Temperature: A Review and Data Syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Yang, X.; Fu, Q.; Hu, H.; Huang, Q. Biochar Produced from the Straw of Common Crops Simultaneously Stabilizes Soil Organic Matter and Heavy Metals. Sci. Total Environ. 2022, 828, 154494. [Google Scholar] [CrossRef]
- Li, X.; Wei, B.; Xu, X.; Zhou, J. Effect of Deep Vertical Rotary Tillage on Soil Properties and Sugarcane Biomass in Rainfed Dry-Land Regions of Southern China. Sustainability 2020, 12, 10199. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Liu, J.; Xue, J. A review of research advances in the effects of biochar on soil nitrogen cycling and its functional microorganisms. J. Ecol. Rural Environ. 2022, 38, 689–701. [Google Scholar]
- Ge, T.; Nie, S.; Wu, J.; Shen, J.; Xiao, H.; Tong, C.; Huang, D.; Hong, Y.; Iwasaki, K. Chemical Properties, Microbial Biomass, and Activity Differ between Soils of Organic and Conventional Horticultural Systems under Greenhouse and Open Field Management: A Case Study. J. Soils Sediments 2011, 11, 25–36. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial Utilisation of Biochar-Derived Carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Fahad, S. Melatonin Application Enhances Biochar Efficiency for Drought Tolerance in Maize Varieties: Modifications in Physio-Biochemical Machinery. Agron. J. 2020, 112, 2826–2847. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought Stress Alleviation by ACC Deaminase Producing Achromobacter Xylosoxidans and Enterobacter Cloacae, with and without Timber Waste Biochar in Maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Liu, H.; Wei, B.; Dnag, K.; Peng, J.; Li, J.; Dong, Y. Effect of deep vertical rotary tillage on microbial community in sugar cane soil. Chin. J. Trop. Crops 2022, 43, 597–605. [Google Scholar]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global Meta-Analysis Reveals Positive Effects of Biochar on Soil Microbial Diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Tong, C.; Hu, K.; Zhou, B.; Xing, S.; Mao, Y. Biochar-Fertilizer Interaction Modifies N-Sorption, Enzyme Activities and Microbial Functional Abundance Regulating Nitrogen Retention in Rhizosphere Soil. Sci. Total Environ. 2020, 739, 140065. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Farag, I.F.; Youssef, N.H.; Elshahed, M.S. Global Distribution Patterns and Pangenomic Diversity of the Candidate Phylum “Latescibacteria” (WS3). Appl. Environ. Microbiol. 2017, 83, e00521-17. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Oshkin, I.Y.; Danilova, O.V.; Philippov, D.A.; Ravin, N.V.; Dedysh, S.N. Rokubacteria in Northern Peatlands: Habitat Preferences and Diversity Patterns. Microorganisms 2022, 10, 11. [Google Scholar] [CrossRef]
- Marschner, P.; Yang, C.-H.; Lieberei, R.; Crowley, D.E. Soil and Plant Specific Effects on Bacterial Community Composition in the Rhizosphere. Soil Biol. Biochem. 2001, 33, 1437–1445. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Prakash, B.; Sathya, A.; Vijayabharathi, R. Plant Growth-Promoting Traits of Pseudomonas geniculata Isolated from Chickpea Nodules. 3 Biotech 2015, 5, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, L.; Yang, J.; Zhang, Z.; Awasthi, M.K.; Li, H. Insight to Bacteria Community Response of Organic Management in Apple Orchard-Bagasse Fertilizer Combined with Biochar. Chemosphere 2022, 286, 131693. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhang, L.; Qin, C.; Lu, P.; Bai, H.; Han, X.; Yuan, S. Temporal Changes of Microbial Community Structure and Nitrogen Cycling Processes during the Aerobic Degradation of Phenanthrene. Chemosphere 2022, 286, 131709. [Google Scholar] [CrossRef]
- Yin, S.; Suo, F.; Zheng, Y.; You, X.; Li, H.; Wang, J.; Zhang, C.; Li, Y.; Cheng, Y. Biochar-Compost Amendment Enhanced Sorghum Growth and Yield by Improving Soil Physicochemical Properties and Shifting Soil Bacterial Community in a Coastal Soil. Front. Environ. Sci. 2022, 10, 1036837. [Google Scholar] [CrossRef]
- Vasantha, S.; Gupta, C.; Shekinah, D.E. Physiological Studies on Tiller Production and Its Senescence in Sugarcane–Response Comparison between Plant and Ratoon Crops. Indian J. Agric. Sci. 2014, 84, 24–27. [Google Scholar] [CrossRef]
- Meesilp, N.; Jaisil, P.; Milintawisamai, N.; Niamsanit, S. Effects of a Formulated Bio-Product Containing the Nitrogen Fixing Bacterial Strain Enterobacter Oryzae 3LSO1 on Sugarcane Growth. Sugar Tech 2016, 18, 242–248. [Google Scholar] [CrossRef]
- Wayne, D. Breeding for tolerance to biotic stress in sugarcane. Guangxi Agric. Sci. 2010, 41, 91–94. [Google Scholar]









| Soil Properties | Value | Unit |
|---|---|---|
| pH | 4.10 | |
| Bulk density | 1.01 | g·cm−3 |
| Soil porosity | 44.00 | % |
| Soil organic matter (SOM) | 16.21 | g·kg−1 |
| Total nitrogen (TN) | 0.58 | g·kg−1 |
| Total phosphorus (TP) | 0.44 | g·kg−1 |
| Total potassium (TK) | 6.43 | g·kg−1 |
| Available nitrogen (AN) | 31.68 | mg·kg−1 |
| Available phosphorus (AP) | 9.84 | mg·kg−1 |
| Available potassium (AK) | 99.15 | mg·kg−1 |
| Treatment * | Source of Biochar | Biochar Properties | |||||
|---|---|---|---|---|---|---|---|
| Water Content % | pH | TN % | TP % | TK % | SOM % | ||
| Chemical fertilizers | No biochar | − | − | − | − | − | − |
| Straw biochar-based fertilizers | Produced from the pyrolysis of straw at 250 °C for 12 h. | 31.1 | 7.21 | 45.7 | 0.86 | 0.19 | 3.96 |
| Sugar filter mud biochar-based fertilizers | Produced from the pyrolysis of the solid waste of the sugar-making process at 250 °C for 12 h. | 5.28 | 7.39 | 23.09 | 0.7 | 0.21 | 5.38 |
| Biochar Type | Specific Surface Area of Biochar (m2 g−1) |
|---|---|
| Straw biochar | 4.43 |
| Sugar filter mud biochar | 18.79 |
| Treatment | Macropores (>50 μm) | Mesopores (0.5–50 μm) | Micropores (<0.5 μm) |
|---|---|---|---|
| CK | 15.2 ± 1.8 | 52.3 ± 3.1 | 32.5 ± 2.4 |
| T1 | 18.6 ± 2.1 * | 54.7 ± 2.9 | 26.7 ± 1.9 * |
| T2 | 21.4 ± 2.3 * | 56.2 ± 3.0 * | 22.4 ± 2.1 * |
| Crop Season | Treatment | TN g kg−1 | TP g kg−1 | TK g kg−1 | AN mg kg−1 | AP mg kg−1 | AK mg kg−1 | SOM g kg−1 |
|---|---|---|---|---|---|---|---|---|
| 2021 | CK | 0.80 ± 0.04 b | 0.40 ± 0.03 b | 7.49 ± 0.49 b | 60.59 ± 0.68 b | 14.29 ± 0.19 c | 129.70 ± 5.65 b | 17.96 ± 0.30 b |
| T1 | 0.79 ± 0.06 b | 0.45 ± 0.03 b | 7.58 ± 0.10 b | 64.27 ± 0.74 a | 15.28 ± 0.03 b | 184.18 ± 3.88 a | 18.37 ± 0.40 b | |
| T2 | 0.93 ± 0.05 a | 0.53 ± 0.02 a | 8.39 ± 0.34 a | 65.02 ± 0.71 a | 15.96 ± 0.14 a | 186.76 ± 6.41 a | 19.18 ± 0.35 a | |
| 2022 | CK | 0.75 ± 0.04 b | 0.56 ± 0.04 b | 3.86 ± 0.11 b | 73.11 ± 3.30 b | 121.47 ± 2.57 b | 138.67 ± 8.02 b | 18.22 ± 0.40 c |
| T1 | 0.80 ± 0.06 ab | 0.67 ± 0.02 a | 4.21 ± 0.09 a | 87.50 ± 3.05 a | 120.64 ± 5.20 b | 168.00 ± 17.78 a | 21.10 ± 0.14 b | |
| T2 | 0.86 ± 0.02 a | 0.67 ± 0.02 a | 4.26 ± 0.02 a | 88.90 ± 1.21 a | 146.69 ± 18.55 a | 184.33 ± 12.39 a | 22.36 ± 0.39 a |
| Treatment | Urease | Sucrase | Acid Phosphatase |
|---|---|---|---|
| CK | 0.4078 ± 0.0136 a | 5.6885 ± 0.1751 b | 0.2276 ± 0.0151 a |
| T1 | 0.4206 ± 0.0029 a | 6.6236 ± 0.2577 a | 0.2616 ± 0.0243 a |
| T2 | 0.4318 ± 0.0297 a | 6.6383 ± 0.5424 a | 0.2459 ± 0.0165 a |
| Crop Season | Treatment | ACE | Chao1 | Shannon |
|---|---|---|---|---|
| 2021 | CK | 1858.33 ± 55.82 a | 1812.18 ± 108.42 a | 9.61 ± 0.07 a |
| T1 | 1860.67 ± 35.85 a | 1513.07 ± 459.91 a | 8.59 ± 1.19 a | |
| T2 | 1900.33 ± 42.06 a | 1855.26 ± 257.25 a | 8.95 ± 0.73 a | |
| 2022 | CK | 4835.74 ± 480.93 a | 4746.50 ± 351.86 a | 10.36 ± 0.31 a |
| T1 | 5326.07 ± 122.50 a | 5165.92 ± 93.87 a | 10.60 ± 0.06 a | |
| T2 | 5958.16 ± 206.55 a | 5745.28 ± 233.35 a | 10.90 ± 0.03 a |
| Crop Season | Treatment | Plant Height (cm) | Stem Diameter (mm) | Single Stem Weight (kg plant−1) | Number of Effective Stems (plant ha−1) | Yield (t ha−1) | Sugar Content (%) |
|---|---|---|---|---|---|---|---|
| 2021 | CK | 308.58 ± 1.22 b | 31.10 ± 0.30 b | 2.35 ± 0.05 b | 56,949 ± 323 a | 133.47 ± 3.08 b | 20.01 ± 0.16 b |
| T1 | 311.10 ± 1.15 a | 31.21 ± 0.31 b | 2.37 ± 0.04 b | 57,128 ± 273 a | 135.47 ± 2.63 b | 21.3 ± 0.15 a | |
| T2 | 312.85 ± 1.06 a | 31.91 ± 0.20 a | 2.5 ± 0.04 a | 57,329 ± 407 a | 143.10 ± 2.44 a | 21.56 ± 0.01 a | |
| 2022 | CK | 313.33 ± 9.07 a | 27.56 ± 0.06 b | 1.93 ± 0.03 b | 4856 ± 950 c | 93.72 ± 1.64 c | 17.40 ± 0.15 a |
| T1 | 314.00 ± 3.61 a | 27.30 ± 0.11 b | 1.95 ± 0.03 b | 51,700 ± 520 b | 100.63 ± 0.69 b | 17.10 ± 0.24 a | |
| T2 | 315.67 ± 3.06 a | 28.90 ± 0.27 a | 2.05 ± 0.04 a | 5456 ± 1041 a | 111.86 ± 2.67 a | 17.43 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liang, P.; Yang, L.; Wei, B.; Han, S.; Wu, M.; He, X.; Zeng, W.; He, Z.; Xiao, J.; et al. Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield. Agronomy 2025, 15, 1594. https://doi.org/10.3390/agronomy15071594
Zhu S, Liang P, Yang L, Wei B, Han S, Wu M, He X, Zeng W, He Z, Xiao J, et al. Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield. Agronomy. 2025; 15(7):1594. https://doi.org/10.3390/agronomy15071594
Chicago/Turabian StyleZhu, Shuifang, Penglian Liang, Lipei Yang, Benhui Wei, Shijian Han, Meiyan Wu, Xiangyi He, Weicong Zeng, Zhenli He, Jiming Xiao, and et al. 2025. "Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield" Agronomy 15, no. 7: 1594. https://doi.org/10.3390/agronomy15071594
APA StyleZhu, S., Liang, P., Yang, L., Wei, B., Han, S., Wu, M., He, X., Zeng, W., He, Z., Xiao, J., Li, S., & Li, Z. (2025). Effects of Biochar-Based Fertilizers on Fenlong-Ridging Soil Physical Properties, Nutrient Activation, Enzyme Activity, Bacterial Diversity, and Sugarcane Yield. Agronomy, 15(7), 1594. https://doi.org/10.3390/agronomy15071594







