The Development of a Micropropagation System for a Rare Variety of an Agricultural and Medicinal Elderberry Plant Sambucus nigra ‘Albida’
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Culture Initiation
2.2. Multiplication Experiment
2.3. In Vitro Rooting and Acclimatization to Ex Vitro Conditions
2.4. Statistical Evaluation of Multiplication and Rooting Experiments
3. Results
3.1. Initiation of Micropropagation and Multiplication
3.2. In Vitro Rooting and Acclimatization to Ex Vitro Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G. Bioactive Properties of Sambucus nigra L. As a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Sala, G.; Pasta, S.; Maggio, A.; La Mantia, T. Sambucus nigra L. (Fam. Viburnaceae) in Sicily: Distribution, Ecology, Traditional Use and Therapeutic Properties. Plants 2023, 12, 3457. [Google Scholar] [CrossRef]
- Atkinson, M.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Charlebois, D.; Byers, P.L.; Finn, C.E.; Thomas, A.L. Elderberry: Botany, Horticulture, Potential. In Horticultural Reviews, Volume 37; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-0-470-54367-2. [Google Scholar]
- Corrado, G.; Basile, B.; Mataffo, A.; Yousefi, S.; Salami, S.A.; Perrone, A.; Martinelli, F. Cultivation, Phytochemistry, Health Claims, and Genetic Diversity of Sambucus nigra, a Versatile Plant with Many Beneficial Properties. Horticulturae 2023, 9, 488. [Google Scholar] [CrossRef]
- Finn, C.; Thomas, A.; Byers, P.; Serce, S. Evaluation of American (Sambucus Canadensis) and European (S. nigra) Elderberry Genotypes Grown in Diverse Environments and Implications for Cultivar Development. HortScience 2008, 43, 1385–1391. [Google Scholar] [CrossRef]
- Svanberg, I.; de Vahl, E.; Olsen, N.; Ståhlberg, S. From Supernatural to Ornamental: Black Elder (Sambucus nigra L., Family Adoxaceae) in Sweden. Plants 2024, 13, 3068. [Google Scholar] [CrossRef]
- Jørgensen, U.; Hansen, M.; Christensen, L.P.; Jensen, K.; Kaack, K. Olfactory and Quantitative Analysis of Aroma Compounds in Elder Flower (Sambucus nigra L.) Drink Processed from Five Cultivars. J. Agric. Food Chem. 2000, 48, 2376–2383. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X. Selection of Elderberry (Sambucus nigra L.) Genotypes Best Suited for the Preparation of Elderflower Extracts Rich in Flavonoids and Phenolic Acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press Inc.: Boca Raton, FL, USA, 2012; ISBN 978-1-4200-8044-5. [Google Scholar]
- Diviš, P.; Pořízka, J.; Vespalcova, M.; Matějíček, A.; Kaplan, J. Elemental Composition of Fruits from Different Black Elder (Sambucus nigra L.) Cultivars Grown in the Czech Republic. J. Elem. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza Michalowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus nigra) in Food—A Review. J. Funct. Foods 2014, 18, 941–958. [Google Scholar] [CrossRef]
- Ho, G.; Kase, E.; Wangensteen, H.; Barsett, H. Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells. Molecules 2017, 22, 90. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Terzić, M.; Majkić, T.; Zengin, G.; Beara, I.; Cvetanovic, A.; Mahomoodally, F.; Radojković, M. Advantages of Contemporary Extraction Techniques for the Extraction of Bioactive Constituents from Black Elderberry (Sambucus nigra L.) Flowers. Ind. Crops Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Nikolova, K.; Buhalova, D.; Andonova, V.; Gugleva, V.; Petkova, N.; Yotkovska, I.; Ivanova, N. Antioxidant Activity and Chemical Characteristics of Sambucus nigra L. Blossom from Different Regions in Bulgaria. Horticulturae 2022, 8, 309. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial Activity of Elder (Sambucus nigra L.) Flower or Berry against Hospital Pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar]
- Sánchez-Hernández, E.; Balduque-Gil, J.; González-García, V.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical Profiling of Sambucus nigra L. Flower and Leaf Extracts and Their Antimicrobial Potential against Almond Tree Pathogens. Int. J. Mol. Sci. 2023, 24, 1154. [Google Scholar] [CrossRef]
- Schunko, C.; Vogl, C.R. Is the Commercialization of Wild Plants by Organic Producers in Austria Neglected or Irrelevant? Sustainability 2018, 10, 3989. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Oziembłowski, M.; Brandova, P.; Czaplicka, M. Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry. Molecules 2022, 27, 5050. [Google Scholar] [CrossRef]
- Papagrigoriou, T.; Iliadi, P.; Mitić, M.N.; Mrmošanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N.; et al. Wild-Growing and Conventionally or Organically Cultivated Sambucus nigra Germplasm: Fruit Phytochemical Profile, Total Phenolic Content, Antioxidant Activity, and Leaf Elements. Plants 2023, 12, 1701. [Google Scholar] [CrossRef]
- Borodušķe, A.; Jekabsons, K.; Riekstina, U.; Muceniece, R.; Rostoks, N.; Nakurte, I. Wild Sambucus nigra L. from North-East Edge of the Species Range: A Valuable Germplasm with Inhibitory Capacity against SARS-CoV2 S-Protein RBD and hACE2 Binding In Vitro. Ind. Crops Prod. 2021, 165, 113438. [Google Scholar] [CrossRef]
- Kiprovski, B.; Malenčić, Đ.; Ljubojevic, M.; Ognjanov, V.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. Quality Parameters Change during Ripening in Leaves and Fruits of Wild Growing and Cultivated Elderberry (Sambucus nigra) Genotypes. Sci. Hortic. 2020, 277, 109792. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants In Vitro Propagation with Its Applications in Food, Pharmaceuticals and Cosmetic Industries; Current Scenario and Future Approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, J.C.; Wang, M.-R.; Wang, Q.-C. In Vitro Regeneration, Micropropagation and Germplasm Conservation of Horticultural Plants. Horticulturae 2024, 10, 45. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Putri, A.I.; Kartikawati, N.K.; Nirsatmanto, A.; Sunarti, S.; Haryjanto, L.; Herawan, T.; Santosa, P.B.; Wahyuningtyas, R.S.; Lestari, F.; Rimbawanto, A. In Vitro Multiplication of Lophostemon Suaveolens (Sol.Ex Gaertn.) Peter G.Wilson & J.T. Waterh): Peatland Tree Species for Rehabilitation. Sustainability 2022, 14, 14720. [Google Scholar] [CrossRef]
- Chmielarz, P.; Kotlarski, S.; Kalemba, E.M.; Martins, J.P.; Michalak, M. Successful In Vitro Shoot Multiplication of Quercus robur L. Trees Aged up to 800 Years. Plants 2023, 12, 2230. [Google Scholar] [CrossRef]
- Saensouk, S.; Sonthongphithak, P.; Chumroenphat, T.; Muangsan, N.; Souladeth, P.; Saensouk, P. In Vitro Multiplication, Antioxidant Activity, and Phytochemical Profiling of Wild and In Vitro-Cultured Plants of Kaempferia larsenii Sirirugsa—A Rare Plant Species in Thailand. Horticulturae 2025, 11, 281. [Google Scholar] [CrossRef]
- Cooper, D.W. Applying a Simple Statistical Test to Compare Two Different Particle Counts. J. Aerosol Sci. 1991, 22, 773–777. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Oxford & IBH Publishing Co.: New Delhi, India, 1967. [Google Scholar]
- Cochran, W.G.; Cox, G.M. Experimental Designs, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1992; ISBN 978-0-471-54567-5. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Charlebois, D.; Brassard, N. Micropropagation of American Elderberry: Culture Medium Optimization and Field Performance. Acta Hortic. 2015, 1061, 175–182. [Google Scholar] [CrossRef]
- Kopper, E.; Granilshchikova, M.; Leichtfried, T.; Reisenzein, H. Micropropagation and Pathogen Elimination in Elderberry (Sambucus nigra L.). Plant Cell Tissue Organ Cult. 2020, 142, 647–652. [Google Scholar] [CrossRef]
- Wang GuiQing, W.G.; Zhang Tao, Z.T.; Sun Hua, S.H. Tissue culture and mass propagation of Sambucus williamsii Hance. J. Shenyang Agric. Univ. 2012, 43, 284–288. [Google Scholar]
- Hildebrandt, V.; Harney, P.M. In Vitro Propagation of Viburnum opulus ‘Nanum’. J. Environ. Hortic. 1985, 3, 41–45. [Google Scholar] [CrossRef]
- Yaseen, S.A. Micropropagation of Viburnum opulus (Roseum) by Using Single Nodes. Iraqi J. Agric. Sci. 2022, 53, 1388–1396. [Google Scholar] [CrossRef]
- Ning, Y.; Dong, H.; Zhang, X.; Li, Y.; Cui, C.; Li, S. The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’. Plants 2025, 14, 374. [Google Scholar] [CrossRef]
- Moura, M.; Candeias, M.I.; Silva, L. In Vitro Propagation of Viburnum treleasei Gand., an Azorean Endemic with High Ornamental Interest. HortScience 2009, 44, 1668–1671. [Google Scholar] [CrossRef]
- Schoene, G.; Yeager, T. Micropropagation of Sweet Viburnum (Viburnum odoratissimum). Plant Cell Tissue Organ Cult. 2005, 83, 271–277. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical methods for Research Workers; Oliver & Boyd: London, UK, 1934. [Google Scholar]
- Lehmann, E.L. Elements of Large Sample Theory; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Wang, M.R.; Cui, Z.-H.; Jingwei, L.; Hao, X.-Y.; Zhao, L.; Wang, Q.-C. In Vitro Thermotherapy-Based Methods for Plant Virus Eradication. Plant Methods 2018, 14, 87. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A.; et al. Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.M.; Engelmann, F.; Dulloo, M.; Engels, J. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections. In IPGRI Handbooks for Genebanks No. 7; International Plant Genetic Resources Institute: Rome, Italy, 2004; ISBN 92-9043-640-9. [Google Scholar]







| Compound | mg/L | Compound | mg/L |
|---|---|---|---|
| KNO3 | 1900 | Na2MoO4·2H2O | 0.25 |
| NH4NO3 | 1650 | CoCl2·6H2O | 0.025 |
| CaCl2·2H2O | 440 | CuSO4·5H2O | 0.025 |
| MgSO4·7H2O | 370 | Inositol | 100 |
| KH2PO4 | 170 | Glycine | 2 |
| Na2EDTA·2H2O | 37.3 | Nicotinic acid | 0.5 |
| FeSO4·7H2O | 27.8 | Pyridoxine | 0.5 |
| MnSO4·H2O | 16.9 | Thiamine | 0.1 |
| ZnSO4·7H2O | 8.6 | Ascorbic acid | 4 |
| H3BO3 | 6.2 | Sucrose | 30,000 |
| KI | 0.83 | Agar (Difco Bacto) | 7000 |
| Treatment | Shoot Number/Explant | HSD | LSD | Duncan | K-W |
|---|---|---|---|---|---|
| BAP 1 mg/L | 2.04 | a | a | a | a |
| BAP 2 mg/L | 2.02 | a | a | a | a |
| BAP 4 mg/L | 1.84 | ab | ab | ab | ab |
| TDZ 0.5 mg/L | 1.64 | b | b | b | b |
| TDZ 1 mg/L | 1.71 | ab | b | b | b |
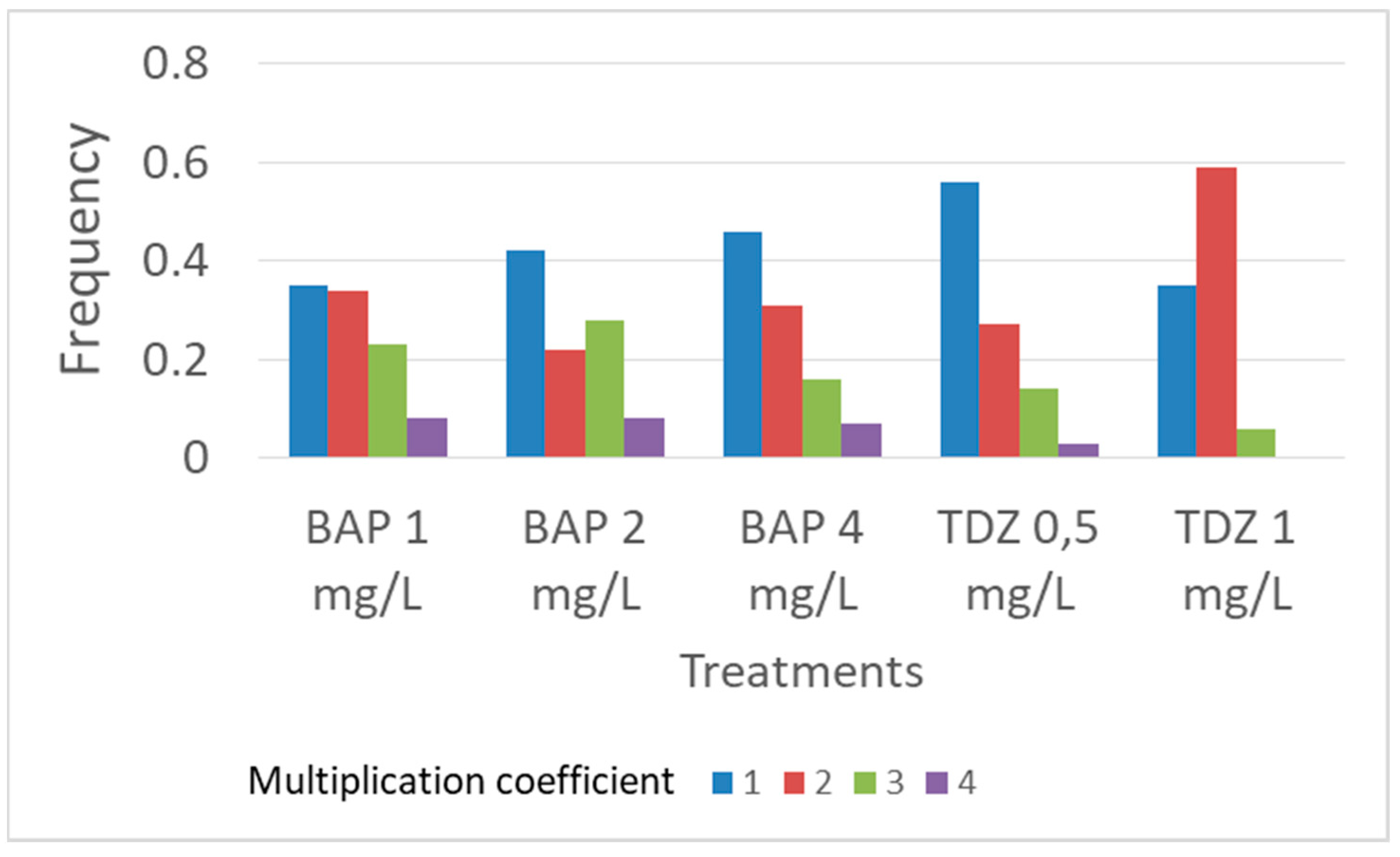
| Treatment | Shoot Number/Explant | All Classes | Class 1 | Class 2 | Class 3 | Class 4 |
|---|---|---|---|---|---|---|
| BAP 1 mg/L | 2.04 | a | 35 b | 34 b | 23 ab | 8 a |
| BAP 2 mg/L | 2.02 | a | 42 ab | 22 b | 28 a | 8 a |
| BAP 4 mg/L | 1.84 | ab | 46 ab | 31 b | 16 ab | 7 a |
| TDZ 0.5 mg/L | 1.64 | b | 56 a | 27 b | 14 bc | 3 ab |
| TDZ 1 mg/L | 1.71 | c | 35 b | 59 a | 6 c | 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlák, J.; Mészáros, M.; Semerák, M.; Pech, P. The Development of a Micropropagation System for a Rare Variety of an Agricultural and Medicinal Elderberry Plant Sambucus nigra ‘Albida’. Agronomy 2025, 15, 1588. https://doi.org/10.3390/agronomy15071588
Sedlák J, Mészáros M, Semerák M, Pech P. The Development of a Micropropagation System for a Rare Variety of an Agricultural and Medicinal Elderberry Plant Sambucus nigra ‘Albida’. Agronomy. 2025; 15(7):1588. https://doi.org/10.3390/agronomy15071588
Chicago/Turabian StyleSedlák, Jiří, Martin Mészáros, Matěj Semerák, and Pavel Pech. 2025. "The Development of a Micropropagation System for a Rare Variety of an Agricultural and Medicinal Elderberry Plant Sambucus nigra ‘Albida’" Agronomy 15, no. 7: 1588. https://doi.org/10.3390/agronomy15071588
APA StyleSedlák, J., Mészáros, M., Semerák, M., & Pech, P. (2025). The Development of a Micropropagation System for a Rare Variety of an Agricultural and Medicinal Elderberry Plant Sambucus nigra ‘Albida’. Agronomy, 15(7), 1588. https://doi.org/10.3390/agronomy15071588






