Abstract
Anthropogenic nitrogen (N) enrichment alters soil biotic (e.g., microbial metabolism) and abiotic (e.g., pH and mineralogy) properties, substantially affecting the persistence and storage of soil organic carbon (SOC). However, the response of relatively persistent mineral-associated organic carbon (MAOC) to N enrichment and the underlying mechanisms are not well understood, leading to significant uncertainties regarding SOC stability under continuous N input. Based on a 15-year field N fertilisation experiment (0, 28.5, 60.0, 72.0 g N m−2 yr−1), we studied the responses of MAOC to N input and the associated changes in soil mineralogy and microbiology. N fertilisation significantly reduced MAOC content by 16.0%. The loss of MAOC was primarily attributed to soil acidification (pH decreased from 6.4 to 4.2), leading to exchangeable calcium (Ca) leaching and loss of Ca-bound organic carbon by 37.9% on average. Furthermore, N-induced shifts in dominant microbial keystone taxa from K-strategists (e.g., Actinobacteriota and Sordariomycetes) to r-strategists (e.g., Subgroups 4 and 6 Acidobacteriota) impeded the formation of MAOC through the reduction of microbial carbon use efficiency and oxidase activity (e.g., phenol oxidases and peroxidases). These results suggest that keystone taxa play crucial roles in regulating carbon metabolism and are responsible for MAOC reduction. Moreover, our data pinpoint the importance of Ca leaching for SOC destabilisation, particularly in near-neutral and neutral soils.
1. Introduction
Since the Industrial Revolution, excessive reactive nitrogen (N) inputs (such as N fertilizer) have significantly altered the global N cycle [1,2]. The influx of anthropogenic reactive nitrogen (N) into land ecosystems has surged nearly threefold compared with natural sources, resulting in global soil N enrichment [2,3]. This enrichment can influence soil acidity, plant growth, and microbial community structure [3,4,5], thereby affecting the global carbon (C) cycle by altering microbial respiration and biomass yield. Generally, soils harbor three to four times as much carbon (C) as the atmosphere [6,7]; thus, alterations in soil organic carbon (SOC) resulting from N addition can profoundly impact soil fertility and terrestrial C stability. Previous studies have attributed SOC accumulation under N enrichment to reduced microbial catabolism or increased belowground biomass [8,9,10]. However, N-induced acidification may also negatively affect SOC accumulation by inhibiting microbial activity or biomass production [8,9,10]. Therefore, the net effect of N enrichment on soil C stocks remains uncertain. Such discrepancies may be attributed to the different responses of soil C fractions to N addition [11]. Globally, N enrichment leads to SOC accumulation mainly as partly decomposed plant debris or particulate organic C (POC) [10]. Mineral-associated organic C (MAOC) constitutes a more enduring SOC reservoir with an extended turnover period compared to POC [12]. However, the response of MAOC to N application remains controversial [13]. Thus, clarifying the response mechanism of MAOC to N enrichment is imperative for uncovering the fate of SOC under increased anthropogenic N input.
Specifically, MAOC arises from microbial assimilation and depolymerisation of complex macromolecules, followed by bonding with the soil mineral matrix [12,14]. Therefore, the effects of N enrichment on MAOC formation mainly depend on the specific changes in substrate quality and microbial metabolic activity and efficiency [15,16]. According to the theory of stoichiometric homeostasis, N enrichment is anticipated to promote the conversion of POC into MAOC, only when decomposition is N-limited and the soil is not acidic enough to impair microbial physiology [17]. However, N enrichment is generally associated with soil acidification [5], which inhibits microbial metabolism [18]. Such inhibition might hinder the formation of MAOC because microbial metabolic products are considered important precursors of MAOC [14,19]. Concomitantly, N enrichment may negatively affect MAOC by directly suppressing ligninolytic activity [20], which is associated with increased substrate recalcitrance [21]. These microbial-driven changes in MAOC dynamics are closely linked to microbial C metabolism [22]. Minority microbes have been demonstrated to play a pivotal role in regulating microbial C metabolism and are recognised as keystone taxa [23]. However, the role of keystone taxa in regulating MAOC reduction through their impact on C metabolism has not been studied.
Beyond the effects of N enrichment on microbial metabolism and its cascading effects on MAOC reduction, decline in the soil MAOC pool may be directly triggered by alterations in abiotic factors such as soil pH and mineralogy [9,11]. Thus, for example, the stabilisation and size of the soil MAOC pool are largely controlled by the availability of sites for adsorbing organic C [24]. In particular, in dryland soils, polyvalent cations like calcium (Ca) control MAOC stabilization by reducing SOC bioavailability by bridging or binding to organic molecules [25]. Indeed, N enrichment can destabilise Ca-bridges and MAOC via Ca-leaching induced by acidification [26]. Concurrently, the increased solubility of iron (Fe) and aluminium (Al) phases upon acidification can reportedly influence MAOC dynamics through co-precipitation [13]. Therefore, a thorough understanding of the fundamental mechanisms regulating the mineral protection of MAOC under N-induced acidification is pivotal for SOC destabilisation under N enrichment. All of these mechanisms can individually and interactively influence MAOC dynamics under N input. Hence, there is an urgent need to understand the underlying mechanisms by integrating potential biotic and abiotic controlling factors [9].
Based on a 15-year N addition experiment, we aimed (1) to reveal the response of the MAOC pool to continuous N fertilisation and its key drivers, and (2) to ascertain the roles of microbial keystone taxa in regulating MAOC reduction by influencing extracellular enzyme activity and CUE. Given that long-term N fertilisation resulted in soil acidification, we hypothesised that (1) N fertilisation reduces MAOC content by decreasing microbial biomass and increasing Ca leaching, and (2) N fertilisation reduces microbial CUE and ligninolytic activity by shifting microbial keystone taxa, which in turn decreases microbial biomass and enhances substrate recalcitrance. Our findings provide mechanistic in-sights into how N-induced acidification reduces SOC stability and highlight the critical role of keystone taxa in regulating MAOC destabilisation.
2. Materials and Methods
2.1. Experimental Design and Soil Sampling
The study site is located at the Experimental Station of Southwest University in Chongqing, China (29°48′ N, 106°26′ E, at an elevation of 266.3 m a.s.l.). The region is characterised by a subtropical monsoon climate with a mean annual temperature of 18.3 °C and a mean annual precipitation of 1115 mm. According to the USDA Soil Taxonomy, the soils at the study site are classified as Entisols, with a sandy loam soil texture in the 0–20 cm topsoil layer (51.8% sand, 21.0% silt, and 27.2% clay). Typical agricultural soil managed under long-term dryland farming was used to establish microplots 1.5 m long, 1.0 m wide, and 1.5 m deep. These microplots were filled with soil in layers, reaching a depth of 1 m, to maintain the integrity of the soil matrix. Each microplot was surrounded by 15-cm cement mortar brick walls, and the base was layered with 20-cm quartz sand. Detailed information about the study site is shown in Figure S1 and can be reviewed in a previous report by Xie et al. (2023) [27].
The N fertilisation experiment was initiated in September 2007. The trial included four N fertilisation treatments: no N (N0, 0 g N m−2 year−1 for Chinese cabbage and maize), low N (N1, 15.0 for Chinese cabbage and 13.5 g N m−2 yr−1 for maize), conventional N (N2, 37.5 for Chinese cabbage and 22.5 g N m−2 yr−1 for maize), and high N (N3, 45.0 for Chinese cabbage and 27.0 g N m−2 yr−1 for maize). Each treatment consisted of three replicates. All plots were randomly arranged and cultivated under a winter vegetable (Brassica pekinensis Rupr.)—summer maize (Zea mays L.) rotation scheme. Nitrogen, phosphorous (P) and potassium (K) inputs and fertilisation periods for cabbage and maize under the different treatments are shown in Table S1. The commercial sources of N, P, and K were urea (46% N), potassium dihydrogen phosphate (52% P2O5 and 34% K2O), and potassium sulphate (50% K2O), respectively.
Soil sampling was conducted in February and August 2022 during the harvest of cabbage and maize. Five top-soil (0–20 cm) cores were randomly selected from each block (n = 12) using a soil probe (diameter, 3.5 cm) and immediately mixed into a composite soil sample. Two sampling time points were used during the experimental period, namely, February and August 2022. A total of 24 composite soil samples were prepared. These soil samples were passed through a 2-mm sieve in the field to remove roots and stones. Subsequently, they were stored at −80 °C for microbial analysis and −20 °C for geochemical analysis. The rest of each soil sample was air-dried for soil carbon (C) and exchangeable cation analysis. The indicator measurements were taken from soil samples after fifteen years of N fertilisation.
2.2. Soil Organic Carbon Fractionation
Particulate OC and MAOC were separated from bulk soil by wet sieving [28]. Briefly, 20 g of air-dried soil was added to a 100-mL plastic bottle, and 50 mL of 5% (mass/volume) sodium hexametaphosphate solution was added as a dispersant. The bottle was then placed on a shaker for 18 h at 180 rpm to disperse the soil aggregates. Then, deionised water was used to wash the contents through a 53-μm sieve. The two fractions separated by the sieve were dried to constant mass at 60 °C. Finally, we obtained two particle-size classes: particulate organic matter (POM, >53 μm) and mineral-associated organic matter (MAOM, <53 μm). POC and MAOC contents were determined using an elemental analyser (Vario EL III, Elementar, Hanau, Germany).
2.3. Measurement of Mineral Protection
To identify the mineral protection of SOC through different forms of association, we determined the content of soil exchangeable cations, including Fe3+, Al3+, Ca2+, and Mg2+. Specifically, exchangeable Fe3+, Al3+, Ca2+, and Mg2+ were extracted with 1.0 M ammonium acetate at pH 7.0 and then determined using an inductively coupled plasma optical emission spectrometer (ICP-OES, PerkinElmer, Waltham, MA, USA).
To further explore the MAOC fractions protected by different minerals, we measured the concentrations of calcium-bound organic C (Ca-OC) and iron/aluminium-bound organic C (Fe/Al-OC) using a sequential extraction method [29]. First, 2 g of MAOM was mixed with 20 mL of 0.5 M Na2SO4 and shaken at 180 rpm for 2 h, followed by centrifugation at 3000× g for 10 min. The extraction procedure was repeated multiple times until Ca was no longer detected in the extraction solution. The amount of C released during Na2SO4 extraction was measured using a TOC analyser (TOC-VCPH, Shimadzu, Kyoto, Japan) and interpreted as Ca-OC. Subsequently, the residual soil was extracted with 20 mL of a mixed solution of NaOH (0.1 M NaOH and 0.1 M Na4P2O7). The suspension was then vortexed for 1 min and shaken for 16 h. After centrifugation at 3000× g for 20 min, the C concentration in the supernatant was measured using a total TOC analyser to obtain Fe/Al-OC.
2.4. Edaphic Properties
Soil pH was determined with a glass electrode using a water-to-soil ratio of 2.5:1 (v/w). Soil available N (NH4+-N and NO3−-N) was extracted with 50 mL of 2 M KCl and then quantified with a Continuous Flow Analyser (Skalar San++, Breda, North Brabant, The Netherlands). Soil available P was determined using sodium bicarbonate extraction and analysed using the molybdenum blue method. In turn, available potassium was extracted using ammonium acetate and detected using atomic absorption spectrophotometry. Soil cation exchange capacity (CEC) was measured using the buffered ammonium acetate method at pH 7.0. These indicators were measured using the method described by Lu et al. (2021) [30]. Total N was determined by micro–Kjeldahl digestion [31]. Bulk density was measured using the ring–knife method [32]. Soil organic C was determined using the Walkley and Black dichromate oxidation method [33]. SOC storage was calculated using the following formula [34]:
where, SOCS is SOC storage (Mg C ha−1), SOCC is SOC concentration (g kg−1), BD is the soil bulk density (g cm−3), and D is the measured soil depth (cm).
SOCs = SOCs × BD × D × 10−1
2.5. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
The functional groups of SOC in bulk soil were analysed using an FTIR spectrometer (ThermoFisher Nicolet iS50, Waltham, MA, USA) [35]. Briefly, 1 mg of air-dried soil was manually mixed with 80 mg of spectral-grade KBr using an agate mortar and pestle. The mixture was then kept in an oven at 40 °C for 24 h to minimise moisture in the sample. The mixture was then pressed into pellets before scanning. Spectra were collected in the 4000–400 cm−1 wavenumber range with a resolution of 4 cm−1 and 32 scans per sample. Each sample was scanned three times separately to ensure reproducibility of the analysis. Raw spectral data were baseline-corrected using OMNIC 8.2 software. The absorption peaks in the 1000–4000 cm−1 wavenumber range were used for data analysis, covering important C functional groups, including chemically labile C and chemically recalcitrant C. Chemically labile C functional groups were represented by the carbonylic C (wavenumber 1030 cm−1) of polysaccharides [36]. Chemically recalcitrant C functional groups included aromatic C (wavenumber 1630 cm−1), aliphatic C of methyl and methylene groups (wavenumber 2930 cm−1), and carboxylic C (wavenumber 3420 cm−1) [35]. Absorbance values reflected the abundance of functional groups, and the ratios of the absorbance values of recalcitrant C to labile C were calculated to better characterise substrate quality [36]. A higher ratio indicated a higher SOC chemical recalcitrance.
2.6. Microbial Biomass Carbon and Carbon Use Efficiency (CUE)
Microbial CUE in bulk soil was determined using the 13C-glucose tracing method [37]. Specifically, 20 g of fresh soil samples were supplemented with 0.05 mg C g−1 dry soil using 5 at% 13C-labeled glucose. Universally labelled 99 at% 13C-glucose was mixed with unlabelled glucose to attain a total glucose enrichment of 5 at%. The samples were incubated in airtight 500 mL glass jars at 60% water holding capacity for 24 h at 25 °C. Control samples were treated with deionised water instead of the labelled solution. During the 24-h incubation, increased CO2 concentrations inside the jars were monitored at 0 and 24 h. CO2 concentration and 13C at% abundance were determined using a gas chromatograph (Agilent 7890, Santa Clara, CA, USA) coupled with an isotope ratio mass spectrometer (IRMS; MAT 253, Thermo Finnigan, Bremen, Germany). Cumulative CO2–C accumulated in the jars during the 24 h incubation and its 13C content were used to calculate the cumulative respiration derived from added glucose (13CO2; μg 13CO2–C g−1 dry soil) using the two-pool mixing model expressed by Equation (2) [38]:
where, at% CLabel denotes the 13C at% of labelled soil or CO2 sample, at% label denotes the 13C at% of label solution (5 at%), at% Cref indicates the 13C at% of natural abundance control soil or CO2 sample, and cumulative 12CO2 represents the measured total respiration over the incubation period (μg CO2–C g−1 dry soil).
Soil samples were analysed for microbial biomass C using the chloroform fumigation–extraction method following incubation [39]. The δ13C of microbial biomass was determined by a continuous-flow isotope ratio mass spectrometer (IRMS; Thermo Finnigan DELTA XPPlus, Bremen, Germany) interfaced with an elemental analyser (Flash EA 1112 Series, Thermo Finnigan, Bremen, Germany) via the open split interface (Conflow III, Thermo Finnigan, Bremen, Germany). Microbial CUE was calculated using Equation (3) [38]:
where MB13C is total microbial growth (μg C g−1 soil) and 13CO2 is the cumulative respiration derived from added glucose (μg 13CO2–C g−1 soil) during the 24 h incubation period.
2.7. Extracellular Enzyme Activity (EEA) Analysis
The activities of C-hydrolases in bulk soil, including α-glucosidase (AG), β-glucosidase (BG), and cellobiohydrolase (CBH), and C-oxidases, including phenol oxidases (PPO) and peroxidases (POD), were measured using 96-well micro-plates [40]. We added 2.75 g fresh soil to 91 mL 50 mM Tris buffer (pH 7.5) in each soil sample. For the three hydrolytic enzymes (AG, BG and CBH), a soil slurry of 200 μL was added to 96-well plates, along with 50 μL of 200 μM enzyme substrate. As for the two oxidative enzymes (PPO and POD), 50 μL of 20 mM DOPA was added to each well along with 200 μL of soil slurry. The microplates were incubated for 4 h in the dark at 25 °C for hydrolytic enzyme activities and 18 h for oxidative enzyme activities [41]. Enzyme activity was assayed at 360 nm excitation for hydrolytic enzymes and 450 nm emission for oxidative enzymes using a microplate fluorometer reader (Tecan Infinite 200, Männedorf, Switzerland). Enzyme activities were expressed in nmol g−1 h−1.
2.8. Microbial Community and Co-Occurrence Network Analysis
To assess the composition of the fungal and bacterial communities in bulk soil, we obtained amplicon sequence-variant abundance tables for both kingdoms via high-throughput sequencing on an Illumina HiSeq2500 platform (San Diego, CA, USA). Subsequently, principal coordinate analysis (PCoA) and analysis of similarities (ANOSIM) were used to evaluate the impact of N fertilisation on bacterial and fungal community composition. These analyses were performed using the vegan package. To visualise the influence of the pH gradient, a trend surface was plotted onto the ordination space of PCoA using a generalised additive model through the ordisurf function of the vegan package. The score along the first PCoA axis was adopted as an indicator of general changes in the microbial community structure resulting from N fertilisation [42]. The non-parametric Kruskal–Wallis test was used to further reveal any differences in microbial community composition among N treatments.
Co-occurrence network analysis at the bacterial and fungal genus levels was performed using CoNet in Cytoscape 3.7.2, based on Pearson’s and Spearman’s correlation scores (Pearson’s and Spearman’s r > 0.8 or r <−0.8, p < 0.01) [43]. Both bacterial and fungal genera present in all samples were retained for network analysis. The p-values were adjusted using the Benjamini–Hochberg procedure to minimise false-positive signals [44]. Gephi 0.10.1 was then used to visualise the final network and calculate topological characteristics, such as positive and negative correlations, and the average clustering coefficient [45]. The value importance in projection (VIP) was used as a predictor to identify potential microbial keystone taxa that exert control over carbon dynamics. The mixOmics package (R 4.0.5) was used for the partial least squares regression analysis to estimate the importance of genera in the network with respect to ecosystem enzyme activity and microbial CUE [46]. Predictors were ranked according to their VIP. Higher VIP values indicate a higher contribution to the PLS regression. Those with VIP > 1 were recognised as relevant predictors [47]. Genera with a high degree in the network (top 10) and high VIP values (VIP >1) were recognised as keystone taxa [48]. The Mantel test was performed using the vegan package in R 4.0.5 to determine the correlations between microbial keystone taxa and edaphic properties, SOC fractions and chemistry, and microbial CUE and EEA.
2.9. Statistical Analysis
All data analyses were performed using R 4.0.5 (R Core Team, Vienna, Austria, 2021) and SPSS AMOS software (AMOS IBM 20.0, USA). All data were tested for normality using the Shapiro–Wilk method and for variance homogeneity using the Levene test. Original data were normalised via log-transformation when necessary and then analysed using the following three steps. First, a linear mixed-effects model was used to examine the effects of N fertilisation on SOC and its fractions, and abiotic (mineral protection and SOC chemistry) and biotic variables (microbial CUE, biomass, and EEAs). This analysis used the lme function of the nlme package. In this model, the N fertilisation rate was treated as a fixed effect and each block was considered a random effect. Tukey’s post-hoc test was used to evaluate the differences between treatments across variables. As a second step, a linear regression model was used to explore the relationships between MAOC and mineral protection (i.e., exchangeable cations and their bound organic C), microbial C metabolic properties (i.e., microbial CUE and biomass), and enzyme activity with SOC chemistry. Random forest modelling was used to further screen the important predictors of MAOC using the randomForest package. The significance of the model was determined using the A3 package, and predictor importance was determined using the rfPermute package [23]. Lastly, as a third step, structural equation modelling was used to uncover the intricate regulatory pathways of various factors in MAOC under N fertilisation [49]. Only factors that were significantly affected by N fertilisation and significantly correlated with MAOC were included in the initial model. Therefore, the model was constructed based on the assumption that soil N availability and acidification primarily influence MAOC content by regulating the microbial community, C metabolic properties (i.e., microbial CUE, enzyme activity, and SOC chemistry), and mineral protection (i.e., reactive cations and their bound organic C). Structural equation modelling analysis was conducted using the robust maximum-likelihood evaluation method in SPSS AMOS software (AMOS IBM 20.0, USA). The main criteria used to evaluate the model were as follows [50]: (1) a chi-square value-to-degrees of freedom ratio (chi-square/df) less than or equal to 2 and a chi-square test p-value greater than 0.05; (2) a goodness-of-fit index value greater than 0.90; and (3) a root mean square error of approximation less than 0.05.
3. Results
3.1. Soil Organic Carbon Content in Bulk Soil and Soil Fractions
After 15 years of continuous N fertilisation, SOC content and storage in the bulk soil increased by an average of 20.0% (Figure 1a) and 18.1% (Figure 2a), respectively, compared to the unfertilised control treatment. Meanwhile, POC content significantly increased by an average of 62.1% (Figure 1c), whereas the MAOC content decreased by an average of 16.0% under N fertilisation (p < 0.05) (Figure 1b). Additionally, soil pH decreased significantly from 6.4 ± 0.1 to 4.2 ± 0.2 along the N addition gradient (Figure 2b), indicating pronounced soil acidification.
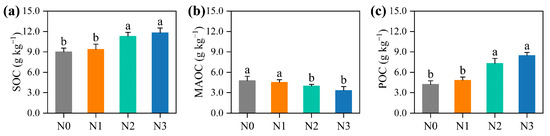
Figure 1.
Effects of nitrogen (N) fertilisation on (a) soil organic carbon (SOC), (b) mineral-associated organic carbon (MAOC), and (c) particulate organic carbon (POC) contents. Different lower-case letters denote significant differences among different N fertilisation rates (p < 0.05). Values are mean ± standard errors (n = 6). N0, no N treatment; N1, low N treatment; N2: conventional N treatment; N3, high N treatment.
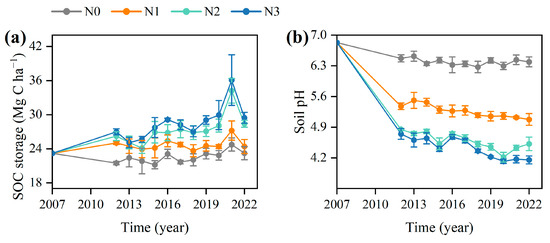
Figure 2.
Dynamics of (a) soil pH and (b) soil organic carbon (SOC) storage under different nitrogen (N) fertilisation rates over ten years. Values are mean ± standard errors (n = 6). N0, no N treatment; N1, low N treatment; N2, conventional N treatment; N3, high N treatment.
3.2. Microbial Carbon Metabolic Properties and Keystone Taxa
On average, N fertilisation significantly suppressed microbial CUE by 35.4% and microbial biomass C (MBC) content by 43.8%, relative to the N0 control (p < 0.05; Figure 3a,b). Further, linear regression analysis indicated that the MBC content increased with microbial CUE (p < 0.01; Figure 3c). Concomitantly, hydrolases activity (AG, BG, and CBH) increased significantly along the N addition gradient, whereas oxidases activity (POD and PPO) decreased (Figure 3d,e).
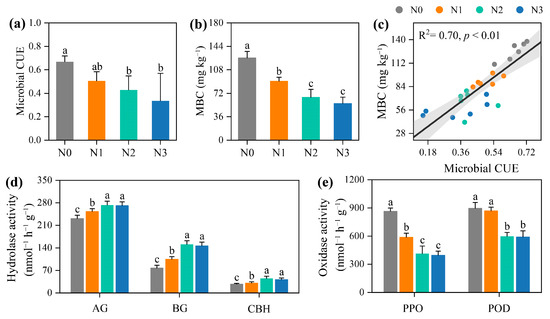
Figure 3.
Effect of nitrogen (N) fertilisation on microbial carbon metabolic properties. (a) Microbial carbon use efficiency (CUE), (b) microbial biomass carbon (MBC), (d) hydrolase and (e) oxidase activities under different N fertilisation rates, and (c) the relationships between microbial CUE and MBC. AG, α-glucosidase; BG, β-glucosidase; CBH, cellobiohydrolase; PPO, phenol oxidases; POD, peroxidases. Different lower-case letters denote significant differences among different N fertilisation rates (p < 0.05). Values are mean ± standard errors (n = 6). N0, no N treatment; N1, low N treatment; N2, conventional N treatment; N3, high N treatment.
Analysis through FTIR spectroscopy showed that N fertilisation enhanced the chemical recalcitrance of SOC. Specifically, N fertilisation significantly increased the ratio of aromatic C: carbonylic C by 12.3% and aliphatic C: carbonylic C by 43.1%, on average (p < 0.05; Figure S2). Additionally, the ratio of carboxylic C: carbonylic C significantly increased by 8% under the N2 treatment, compared to the N0 control. Lastly, the ratio of recalcitrant C to labile C increased with hydrolase activity but decreased with oxidase activity (p < 0.01; Figure S3).
As for the microbial community, N fertilisation induced significant changes in the bacterial and fungal community compositions (ANOSIM test, p = 0.001; Figure 4a,b). The gam model showed that the distribution of the bacterial and fungal communities along the pH gradient was significant (p < 0.001). Further, variations in the bacterial and fungal communities with N fertilisation followed a similar pattern in the maize and cabbage cropping phases. Specifically, N fertilisation significantly increased the relative abundances of Ktedonobacteria, Actinobacteria, Planctomycetes, and Chaetothyriales, while reducing the relative abundances of Alphaproteobacteria and Sordariales (p < 0.05; Figure 4c,d). Co-occurrence networks showed that N fertilisation significantly altered the microbial keystone taxa (Figure 5). Specifically, the dominant bacterial keystone taxa shifted from Actinobacteriota and Chloroflexi under N0 to Acidobacteriota under N addition treatments. Concurrently, the dominant fungal keystone taxa changed from Sordariomycetes and Glomeromycetes under N0 to Dothideomycetes under N addition. These bacterial and fungal keystone taxa significantly mediated microbial C metabolic processes (microbial CUE, oxidase and hydrolase activities, and SOC chemistry), as well as the MAOC/POC ratio and MAOC content (p < 0.05; Figure S4). Moreover, these keystone taxa were significantly affected by soil pH and N availability.
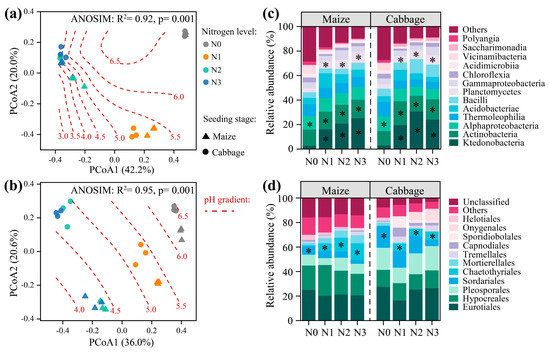
Figure 4.
Effect of nitrogen (N) fertilisation on (a,c) bacterial and (b,d) fungal community composition and distribution. Principal coordinate analysis (PCoA) and similarity test (ANOSIM) based on the Bray–Curtis distances show (a) bacterial and (b) fungal communities under different N fertilisation rates. Relative abundance of major (c) bacterial (class) and (d) fungal (order) communities under different N fertilisation rates. Maize, maize-planted stage; Cabbage, cabbage-planted stage. Asterisks denote significant difference between fertilisations with and without N by LSD post hoc tests (p < 0.05). Values are mean ± standard errors (n = 6). N0, no N treatment; N1, low N treatment; N2, conventional N treatment; N3, high N treatment.
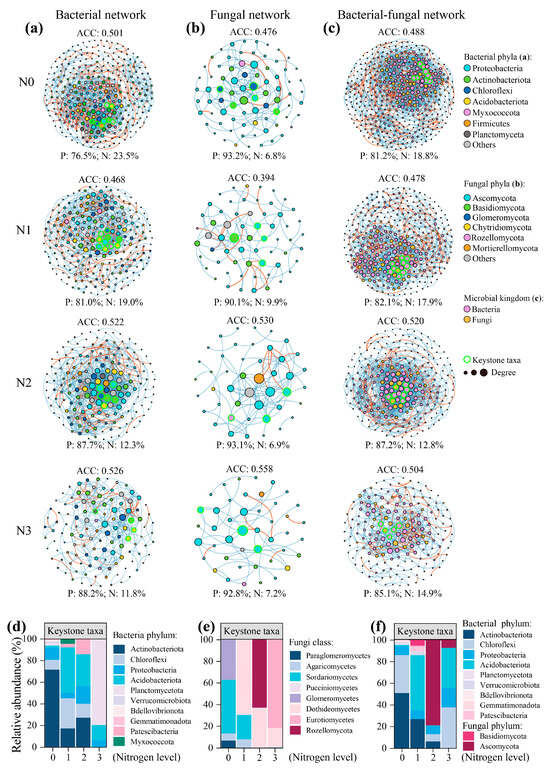
Figure 5.
Effect of nitrogen (N) fertilisation on microbial co-occurrence patterns. (a) Bacterial, (b) fungal, and (c) Bacterial–fungal co-occurrence networks under different N fertilisation rates. The relative abundance of the keystone taxa in (d) bacterial, (e) fungal, and (f) Bacterial–fungal co-occurrence networks under different N fertilisation rates. The size of each node reflects the number of connections (degree), and the connection (edge) between two nodes reflects the value of Pearson and Spearman correlation scores (|r| > 0.8, p < 0.01). The blue edges indicate positive associations between two microbial nodes, while the red edges indicate negative associations. ACC, average clustering coefficients. N0, no N treatment; N1, low N treatment; N2, conventional N treatment; N3, high N treatment.
3.3. Mineral Protection
Nitrogen fertilisation increased exchangeable Al by 232.2–617.2%, while decreasing exchangeable Ca by 13.7–75.3% along the N fertilisation gradient (p < 0.05; Figure 6). Meanwhile, N addition significantly increased exchangeable Fe by an average of 66.6%. Correspondingly, Fe/Al-OC increased by 35.5–59.0%, whereas Ca-OC decreased by 13.3–53.0% along the N addition gradient.
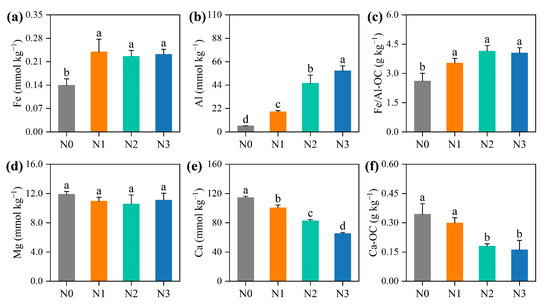
Figure 6.
Effects of nitrogen (N) fertilisation on mineral protection of soil organic carbon. The contents of exchangeable (a) Fe, (b) Al, (d) Mg and (e) Ca, and (c) Fe/Al- and (f) Ca-bound organic carbon under different N fertilisation rates. Different lower-case letters denote significant differences among different N fertilisation rates (p < 0.05). Values are mean ± standard errors (n = 6). N0, no N treatment; N1, low N treatment; N2, conventional N treatment; N3, high N treatment.
3.4. Relationships Between Biotic and Abiotic Factors and Mineral-Associated Organic Carbon Under Nitrogen Fertilisation
Regression analysis showed that MAOC increased with MBC, exchangeable Ca, and bound organic C (Ca-OC) (p < 0.05; Figure S5). In contrast, MAOC decreased with exchangeable Fe, Al, and their bound organic C (Fe/Al-OC). Further, the random forest model suggested that soil pH, available N, the aliphatic C: carbonylic C ratio, and bacterial and fungal keystone taxa had pronounced effects on MAOC (p < 0.05, Figure S6).
Structural equation modelling analysis showed that the increase in chemical recalcitrance of OC and the reduced NBC were the biotic variables responsible for the reduction in MAOC, with standardised coefficients of −0.70 and 0.71, respectively (Figure 7). Notably, N-induced repression of microbial C metabolic processes, including decreased microbial CUE and hydrolase activity, is controlled by bacterial and fungal keystone taxa. In addition to the biotic variables abovementioned, weakened mineral protection resulting from increased leaching of exchangeable Ca emerged as an abiotic variable responsible for the reduction in MAOC, with a standardised coefficient of 0.63.
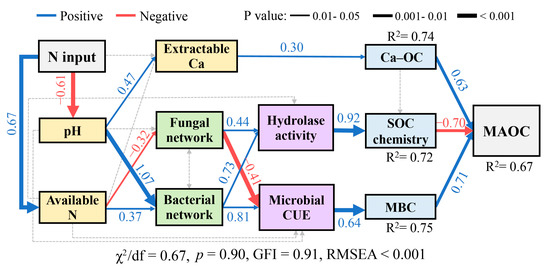
Figure 7.
Effects of soil properties (pH, available N, extractable Ca and Ca-OC), microbial parameters (bacterial and fungal networks, hydrolase activity, microbial CUE, and MBC), and SOC chemistry (the ratio of 2930/1030) on MAOC content under N fertilisation, as estimated using the structural equation model (SEM). Blue and red arrows indicate positive and negative relationships, respectively, while grey arrows represent nonsignificant paths. Numbers adjacent to the arrows are standardised path coefficients, and path width indicates the strength of significant standardised path coefficients. Bacterial and fungal networks are represented by the first principal component (PC1) scores from principal component analysis. Ca-OC, calcium-bound organic C; MBC, microbial biomass carbon; MAOC, mineral-associated organic carbon; Microbial CUE, microbial carbon use efficiency; GFI, goodness-of-fit index; RMSEA, root mean square error of approximation.
4. Discussion
4.1. Regulation of Nitrogen Application Rates in SOC Storage
After 15 years of N fertilisation, there was a significant increase in SOC content in bulk soil (Figure 1a), which is consistent with results from N addition studies across various ecosystems [10,18]. The net amount of SOC is determined by the balance between plant-derived C input and the loss of existing C [11]. Previous studies have attributed increases in SOC under N enrichment to greater aboveground and belowground C inputs [13]. Supporting this explanation, our previous work confirmed that N fertilisation enhances maize litter and root biomass [27]. Notably, SOC content significantly increased when the N application rate was elevated from the N1 to N2 level (Figure 1a). This is likely due to maize’s sensitivity to N application rates, with biomass significantly increasing at N2 (22.4 g m−2) [27], in line with the results of Wang et al. (2023) [23]. However, the promoting effect did not further increase when the N application rate was raised from N2 to N3. This may be attributed to excessive N fertilisation, which reduces the C/N ratio of photosynthetic products, thereby increasing microbial C demand and potential C loss [20]. Additionally, excessive N inputs exacerbate soil acidification and increase the risk of plant physiological stress (e.g., aluminium toxicity), directly inhibiting biomass production [18]. Overall, the effect of N fertilisation on SOC storage exhibited a dosage-dependent effect, emphasizing that appropriate N dosages are crucial for SOC sequestration.
4.2. Nitrogen-Induced Acidification Reduces MAOC Content
Our study found that prolonged N fertilisation significantly reduced MAOC content (Figure 1b). This aligns with the findings of long-term (>5 years) N addition studies conducted in various ecosystems [9,13,18]. The reported decline in MAOC was associated with increased loss and/or decreased formation owing to shifts in microbial physiology, substrate quality, and soil acidification [11,21,51]. In agreement with this notion, we found that the reduction in MAOC under N addition was driven by changes in microbial biomass, substrate recalcitrance, and mineral protection (Figure 7). Therefore, we propose the following three possible mechanisms.
According to the first possible mechanism, the decrease in MAOC content was highly related to the N-fertilisation induced decrease in microbial biomass (Figure 3c and Figure 7). This is consistent with the importance of soil microbial products as the dominant sources of the MAOC pool [12,14]. A reduction in microbial biomass under N enrichment has been previously reported [13,18] and is attributed to the inhibition of microbial anabolism due to N-induced stress (e.g., aluminium/ammonium toxicity) [5]. This interpretation is supported by the lower microbial CUE under N fertilisation (Figure 3a,c). Microbial CUE, which is the ratio of C used for microbial growth to the total C uptake by microbes [17], is positively correlated with microbial biomass [52]. Hence, the observed decrease in microbial CUE with N addition suggests a reduction in newly formed MAOC due to a reduction in microbial products.
According to the second possible mechanism, N fertilisation led to an increase in substrate recalcitrance, with a negative correlation observed between MAOC content and substrate recalcitrance (Figure S2 and Figure 7). Indeed, chemically recalcitrant plant-derived C has been widely reported to hamper the depolymerisation of POC or the formation of MAOC compared to labile C [16,21]. Several studies have shown that prolonged N fertilisation restricts the decomposition of complex and recalcitrant macromolecules by inhibiting the production of ligninolytic enzymes [20,53]. In our study, N fertilisation significantly reduced the activity of oxidase (Figure 3e), a typical ligninolytic enzyme. These findings were consistent with those of previous studies [20,54]. The observed reduction in oxidase activity directly prevented the depolymerisation of complex macromolecules in POC into smaller molecules that are more easily adsorbed by minerals. Altogether, these results suggest that N enrichment may hinder the transformation of POC into MAOC by suppressing ligninolytic activity.
Finally, according to the third possible mechanism, N-induced acidification significantly reduced exchangeable Ca and its bound organic C (Figure 6), and MAOC content was positively correlated with their contents (Figure S5). Our results suggest that acidification-induced Ca leaching was the primary abiotic cause for MAOC loss at the study site. This is consistent with results observed in neutral and near-neutral soils across diverse ecosystems [9,26,55]. Furthermore, these results demonstrate that Ca plays a crucial role in bridging negatively charged C compounds on mineral surfaces with negative charges. In contrast, acidification can reduce the existing MAOC pool through the desorption of the originally Ca-bridged organic C [13]. This process either enhances SOC bioavailability or contributes to its loss as dissolved organic C [56]. Therefore, these results suggest that the abiotic loss of MAOC under N enrichment is primarily attributed to the acidification-induced destabilisation of organo-Ca associations, particularly in neutral and near-neutral soils.
4.3. Microbial Keystone Taxa-Mediated Carbon Metabolism Is Responsible for MAOC Reduction
Consistent with previous reports [48,57], our study indicated that extracellular enzyme activity was primarily controlled by keystone taxa within the microbial networks (Figure 7 and Figure S4). Several nutrient-addition experiments have found that the dominant keystone taxa shift from K-strategists to r-strategists, resulting in reduced ligninolytic activity and subsequent accumulation of more recalcitrant C [42,58]. These findings may explain the reduced oxidase activity observed under N fertilisation, which is linked to shifts in keystone taxa. In this study, Sordariomycetes was identified as the dominant keystone taxon in the absence of N fertilisation (Figure 4). However, N fertilisation reduced the relative abundance of Sordariomycetes, which is consistent with previous reports [59]. Sordariomycetes are the major producers of lignocellulosic enzymes [60]. Additionally, they exhibit N sensitivity, and their growth is suppressed by an elevated N supply [59]. In contrast, we identified Subgroups 4 and 6 of Acidobacteriota as the dominant keystone taxa under N fertilisation. These species are r-strategists that prefer labile to recalcitrant C and are promoted by sufficient N [61]. These findings confirm that higher substrate recalcitrance under N addition is linked to enzyme activity regulated by r-strategists as keystone taxa.
Short-term (24-h incubation) microbial CUE is largely regulated by the microbial community and its physiological response [38]. Our experiment showed that microbial CUE was significantly regulated by keystone taxa in the microbial networks (Figure 7 and Figure S4). Similarly, a soil transplanting trial indicated that dominant keystone taxa might be major contributors to microbial anabolism [48]. In our study, we found that the dominant keystone taxa changed from K-strategists to r-strategists under N fertilisation (Figure 5) [62]. The latter are characterised by rapid growth and low CUE compared with K-strategists [63]. Indeed, N fertilisation delivers more photosynthates (e.g., exudates, roots, and litter) and reduces the C/N ratio of these photosynthates [54]. Additionally, acidification causes the release of labile C previously protected by calcium, as discussed earlier. These changes promote the growth of r-strategists [64]. Correspondingly, a significant increase in the relative abundance of r-strategists such as Ktedonobacteria and Planctomycetes was observed in the entire microbial community under N addition (Figure 4). Altogether, our results suggest that under N addition, the microbial community, especially the keystone taxa, undergoes a progressive shift from K-strategists to r-strategists. This shift led to a reduction in microbial CUE, consequently reducing the formation of new MAOC.
4.4. Implications, Limitations, and Future Research Prospects
A reduction in MAOC entails a decrease in the persistence or stabilisation of the SOC pool [21,65]. Continuous N fertilisation for 15 years resulted in soil acidification and MAOC loss (Figure 1b and Figure 2b), suggesting that prolonged reactive N input may increase the risk of a future decline in C storage in soils prone to acidification. Notably, this MAOC loss is mainly attributed to acidification-induced Ca and concurrent Ca-bound OC losses in drylands with a pH above 6.5, which are dominated by phyllosilicates [26,55]. These findings suggest that Ca associations constitute an important fraction of C stabilisation in near-neutral and neutral soils [55]. Interestingly, despite the observed MAOC loss, there was no decrease in SOC content in the bulk soil; instead, there was an increase in SOC and POC contents. It is possible that more SOC is occluded within soil aggregates, leading to an extension of the time of residence of hundreds of years [66]. Alternatively, long-term N fertilisation may facilitate the formation of occluded POC in the soil. Therefore, future studies should consider physical protection from aggregates to elucidate the mechanisms of soil C storage under N enrichment.
Microbial processes directly determine the formation of MAOC [14]. Our study emphasised the dominant role of keystone taxa in regulating MAOC formation via their effects on extracellular enzyme activity and CUE. Our findings are based on a long-term field experiment with a limited number of observations. Further research is necessary to assess the generalisability of these findings to other environmental conditions. Additionally, it is necessary to further explore the functional connections between potential keystone species, extracellular enzymes, and functional genes involved in C metabolism. State-of-the-art techniques, such as metagenomic analysis and Kyoto Encyclopedia of Genes and Genomes pathways, should be used for a more in-depth exploration of these relationships. Furthermore, a recent study found that exchangeable Ca not only mediates SOC bioavailability but directly influences microbial community structure and C metabolism as well [25,65]. However, the short-term 13C-isotope labeled incubation used herein did not fully represent in situ microbial CUE, as it overlooked Ca–microbe interactions [38]. In addition, although shifts in enzyme activity are presumed to account for changes in POC transformation, direct evidence linking changes in plant-derived C turnover is lacking. Thus, future research should consider longer incubation periods to comprehensively evaluate microbial CUE and C turnover across various soil C fractions. This approach should help to elucidate the intricate microbial mechanisms that contribute to MAOC formation under soil N enrichment.
5. Conclusions
Our results showed that prolonged N fertilisation significantly reduced MAOC, indicating diminished persistence of the SOC pool under N enrichment. The loss of MAOC, which was primarily attributed to N-induced acidification, leads to the loss and depletion of Ca and its bound organic C, thereby destabilising MAOC. These observations underscore the importance of Ca-stabilisation for SOC, especially in neutral and near-neutral soils. Additionally, N fertilisation significantly reduced microbial CUE and oxidase activity, resulting in reduced MBC and increased substrate recalcitrance, which ultimately hampered MAOC formation. Our results suggest that N-induced shifts in dominant keystone taxa play a crucial role in mediating C metabolism and are responsible for MAOC formation. Further research is needed to explore the roles of potential keystone species and the underlying mechanisms of C metabolism. Moreover, our study suggests that comprehensively considering the effects of N fertilisation on physicochemical protection of SOC and microbial metabolism, as well as their interactions, would better predict the fate of soil C under increased anthropogenic N input.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071586/s1, Figure S1: Location of (a) study site and an overview of (b) experimental design. Figure S2: Effect of nitrogen (N) fertilisation on soil organic carbon chemistry. (a) Average Fourier transform infrared spectroscopy (FTIR) spectra under different N fertilisation rates. The absorbance values reflect the abundance of functional groups. FTIR showing the abundance of the carbonylic C of polysaccharides (absorbance at 1030 cm−1), aromatic C (absorbance at 1630 cm−1), aliphatic C of methyl and methylene groups (absorbance at 2930 cm−1), and carboxylic C (absorbance at 3420 cm−1). (b-d) The ratio of absorbance values in recalcitrant C to labile C. Figure S3: Relationships of hydrolase and oxidase activities with soil organic carbon (SOC) chemistry. SOC chemistry was denoted by the ratios of absorbance values in recalcitrant C to labile C. Figure S4: Relationships of bacterial and fungal keystone taxa with soil abiotic properties, SOC chemistry and microbial parameters. Figure S5: Relationships of mineral-associated organic carbon with (a-d) exchangeable cations and (e-f) metal-bound organic carbon as well as (g) microbial biomass carbon. Figure S6: Mean predictor importance (% of increased mean square error, MSE) of soil properties, soil organic carbon (SOC) chemistry and microbial community on mineral-associated organic carbon across all N fertilisation rates. Table S1: Nitrogen, phosphorous, and potassium inputs (g m−2 yr−1) and fertilisation period for Chinese cabbage and maize. References [67,68,69,70] are cited in the Supplementary Materials.
Author Contributions
Conceptualisation, methodology, X.S., X.C. and J.C.; formal analysis, J.W. and Y.Z.; investigation, resources, funding acquisition, D.W., X.S., J.W. and Y.Z.; data curation, X.S., J.W. and Y.Z.; writing—original draft preparation, D.W. and J.C.; writing—review and editing, supervision, X.S. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants from the National Key Research and Development Program of China (2022YFD1901404 and 2023YFD1902805) and the Foundation of Graduate Research and Innovation in Chongqing (CYB23122).
Data Availability Statement
The data presented in this study are openly available in the Sequence Read Archive at the National Centre for Biotechnology Information under accession number PRJNA1002456.
Acknowledgments
We would also like to thank Editage (www.editage.cn (accessed on 2 February 2024)) for English language editing.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Bowles, T.M.; Atallah, S.S.; Campbell, E.E.; Gaudin, A.C.M.; Wieder, W.R.; Grandy, A.S. Addressing Agricultural Nitrogen Losses in a Changing Climate. Nat. Sustain. 2018, 1, 399–408. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-System Perspective of the Global Nitrogen Cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Peng, D.; Lu, H.; Shuai, T.; Quan, Y.; Zeng, C.; Xu, K. Determining the Critical Nitrogen Application Rate for Maximizing Yield While Minimizing NO3−-N Leaching and N2O Emissions in Maize Growing on Purple Soil. Agronomy 2025, 15, 1358. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Spracklen, D.V.; Batterman, S.A.; Arnold, S.R.; Gloor, M.; Buermann, W. Have Synergies Between Nitrogen Deposition and Atmospheric CO2 Driven the Recent Enhancement of the Terrestrial Carbon Sink? Glob. Biogeochem. Cycles 2019, 33, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Nature 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, K.; Song, Q.; Wang, L.; Li, R.; Wu, K.; Han, J.; Li, S. Long-Term Film Mulching with Manure Amendment Drives Trade-Offs Between Spring Maize Nutrient Uptake and Topsoil Carbon Stability on the Loess Plateau. Agronomy 2025, 15, 1352. [Google Scholar] [CrossRef]
- Mack, M.C.; Schuur, E.A.G.; Bret-Harte, M.S.; Shaver, G.R.; Chapin, F.S. Ecosystem Carbon Storage in Arctic Tundra Reduced by Long-Term Nutrient Fertilization. Nature 2004, 431, 440–443. [Google Scholar] [CrossRef]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling Multiple Impacts of Nitrogen Enrichment on Soil Carbon: Plant, Microbial and Geochemical Controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen Increases Soil Organic Carbon Accrual and Alters Its Functionality. Glob. Change Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen Limitation of Decomposition and Decay: How Can It Occur? Glob. Change Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, W.; Zheng, C.; Zhu, B. Nitrogen Addition Has Contrasting Effects on Particulate and Mineral-Associated Soil Organic Carbon in a Subtropical Forest. Soil Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating Plant Litter Quality, Soil Organic Matter Stabilization, and the Carbon Saturation Concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and Stoichiometric Controls on Microbial Carbon-use Efficiency in Soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Feng, X.; Qin, S.; Zhang, D.; Chen, P.; Hu, J.; Wang, G.; Liu, Y.; Wei, B.; Li, Q.; Yang, Y.; et al. Nitrogen Input Enhances Microbial Carbon Use Efficiency by Altering Plant–Microbe–Mineral Interactions. Glob. Change Biol. 2022, 28, 4845–4860. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Haddix, M.L.; Kroeger, M.E.; Stewart, C.E. The Role of Plant Input Physical-Chemical Properties, and Microbial and Soil Chemical Diversity on the Formation of Particulate and Mineral-Associated Organic Matter. Soil Biol. Biochem. 2022, 168, 108648. [Google Scholar] [CrossRef]
- Shen, D.; Ye, C.; Hu, Z.; Chen, X.; Guo, H.; Li, J.; Du, G.; Adl, S.; Liu, M. Increased Chemical Stability but Decreased Physical Protection of Soil Organic Carbon in Response to Nutrient Amendment in a Tibetan Alpine Meadow. Soil Biol. Biochem. 2018, 126, 11–21. [Google Scholar] [CrossRef]
- Córdova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant Litter Quality Affects the Accumulation Rate, Composition, and Stability of Mineral-Associated Soil Organic Matter. Soil Biol. Biochem. 2018, 125, 115–124. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing Uncertainty in Soil Carbon Feedbacks to Climate Change. Nat. Clim. Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Zhao, P.; Chen, L.; Xiang, R.; Jiang, Y.; Long, G. Maize-Potato Residue Mixing in Agricultural Soils Enhances Residue Decomposition and Stable Carbon Content by Modifying the Potential Keystone Microbial Taxa. Geoderma 2023, 437, 116581. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil Carbon Storage Informed by Particulate and Mineral-Associated Organic Matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-Mediated Stabilisation of Soil Organic Carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Püspök, J.F.; Zhao, S.; Calma, A.D.; Vourlitis, G.L.; Allison, S.D.; Aronson, E.L.; Schimel, J.P.; Hanan, E.J.; Homyak, P.M. Effects of Experimental Nitrogen Deposition on Soil Organic Carbon Storage in Southern California Drylands. Glob. Change Biol. 2023, 29, 1660–1679. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Hu, Q.; Zhang, Y.; Wan, Y.; Zhang, C.; Zhang, Y.; Shi, X. Optimal N Management Improves Crop Yields and Soil Carbon, Nitrogen Sequestration in Chinese Cabbage-Maize Rotation. Arch. Agron. Soil Sci. 2023, 69, 1071–1084. [Google Scholar] [CrossRef]
- Bradford, M.A.; Fierer, N.; Reynolds, J.F. Soil Carbon Stocks in Experimental Mesocosms Are Dependent on the Rate of Labile Carbon, Nitrogen and Phosphorus Inputs to Soils. Funct. Ecol. 2008, 22, 964–974. [Google Scholar] [CrossRef]
- Coward, E.K.; Thompson, A.T.; Plante, A.F. Iron-Mediated Mineralogical Control of Organic Matter Accumulation in Tropical Soils. Geoderma 2017, 306, 206–216. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen Addition Stimulates Soil Aggregation and Enhances Carbon Storage in Terrestrial Ecosystems of China: A Meta-analysis. Glob. Change Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef]
- Meng, L.; Jiang, C.; Huang, M.; Lu, Q.; Wan, Y.; Yang, A.; Tang, S.; Wu, Y.; Dan, X.; Zhu, Q.; et al. Effects of Three Years of Biochar Application on Soil Organic Nitrogen Fraction in Tropical Soil. Agronomy, 2025; 15, 1357. [Google Scholar] [CrossRef]
- Vaezi, A.R.; Ahmadi, M.; Cerdà, A. Contribution of Raindrop Impact to the Change of Soil Physical Properties and Water Erosion under Semi-Arid Rainfalls. Sci. Total Environ. 2017, 583, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Sedlář, O.; Balík, J.; Černý, J.; Suran, P.; Kulhánek, M.; Bihun, T. Soil Organic Matter Quality and Carbon Sequestration Potential Affected by Straw Return in 11-Year On-Farm Trials in the Czech Republic. Agronomy 2025, 15, 1277. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of Long-term Straw Return on Soil Organic Carbon Storage and Sequestration Rate in North China Upland Crops: A Meta-analysis. Glob. Change Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Müller, T.; Cadisch, G. Use of Specific Peaks Obtained by Diffuse Reflectance Fourier Transform Mid-Infrared Spectroscopy to Study the Composition of Organic Matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.H. Peat Decomposition Records in Three Pristine Ombrotrophic Bogs in Southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms Driving the Soil Organic Matter Decomposition Response to Nitrogen Enrichment in Grassland Soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Geyer, K.M.; Dijkstra, P.; Sinsabaugh, R.; Frey, S.D. Clarifying the Interpretation of Carbon Use Efficiency in Soil through Methods Comparison. Soil Biol. Biochem. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-Throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013, 81, 50961. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of Extracellular Enzymes in Physically Isolated Fractions of Restored Grassland Soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Bian, Q.; Wang, X.; Bao, X.; Zhu, L.; Xie, Z.; Che, Z.; Sun, B. Exogenous Substrate Quality Determines the Dominant Keystone Taxa Linked to Carbon Mineralization: Evidence from a 30-Year Experiment. Soil Biol. Biochem. 2022, 169, 108683. [Google Scholar] [CrossRef]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial Co-Occurrence Relationships in the Human Microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.R.; et al. Plankton Networks Driving Carbon Export in the Oligotrophic Ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.-H. Performance of Some Variable Selection Methods When Multicollinearity Is Present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Mao, J.; Jiang, Y.; Bian, Q.; Liang, Y.; Chen, Y.; Sun, B. Microbial Keystone Taxa Drive Succession of Plant Residue Chemistry. ISME J. 2023, 17, 748–757. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2006; ISBN 978-0-521-83742-2. [Google Scholar]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the Fit of Structural Equation Models: Tests of Significance and Descriptive Goodness-of-Fit Measures. Methods Psychol. Res. 2003, 8, 23–27. [Google Scholar] [CrossRef]
- Qi, P.; Chen, J.; Wang, X.; Zhang, R.; Cai, L.; Jiao, Y.; Li, Z.; Han, G. Changes in Soil Particulate and Mineral-Associated Organic Carbon Concentrations under Nitrogen Addition in China—a Meta-Analysis. Plant Soil 2023, 489, 439–452. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Sietiö, O.-M.; Meyer, N.; Poeplau, C.; Adamczyk, S.; Biasi, C.; Kalu, S.; Räsänen, M.; Ambus, P.; Fritze, H.; et al. Microbial Carbon Use Efficiency along an Altitudinal Gradient. Soil Biol. Biochem. 2022, 173, 108799. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic Nitrogen Enrichment Affects the Structure and Function of the Soil Microbial Community in Temperate Hardwood and Pine Forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Liu, D.; Fan, J.; Luo, J.; Lindsey, S.; He, J.-S.; Ding, W. Nutrient Addition Reduces Carbon Sequestration in a Tibetan Grassland Soil: Disentangling Microbial and Physical Controls. Soil Biol. Biochem. 2020, 144, 107764. [Google Scholar] [CrossRef]
- Keller, A.B.; Borer, E.T.; Collins, S.L.; DeLancey, L.C.; Fay, P.A.; Hofmockel, K.S.; Leakey, A.D.B.; Mayes, M.A.; Seabloom, E.W.; Walter, C.A.; et al. Soil Carbon Stocks in Temperate Grasslands Differ Strongly across Sites but Are Insensitive to Decade-long Fertilization. Glob. Change Biol. 2022, 28, 1659–1677. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What Do We Know about Soil Carbon Destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network Analysis Reveals Functional Redundancy and Keystone Taxa amongst Bacterial and Fungal Communities during Organic Matter Decomposition in an Arable Soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic Amendments Drive Shifts in Microbial Community Structure and Keystone Taxa Which Increase C Mineralization across Aggregate Size Classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Xing, W.; Lu, X.; Ying, J.; Lan, Z.; Chen, D.; Bai, Y. Disentangling the Effects of Nitrogen Availability and Soil Acidification on Microbial Taxa and Soil Carbon Dynamics in Natural Grasslands. Soil Biol. Biochem. 2022, 164, 108495. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; Singh, R.; Eltis, L.D.; Mohn, W.W. Bacterial Contributions to Delignification and Lignocellulose Degradation in Forest Soils with Metagenomic and Quantitative Stable Isotope Probing. ISME J. 2019, 13, 413–429. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward An Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Grandy, A.S.; Frey, S.D.; Diefendorf, A.F. Microbial Physiology and Necromass Regulate Agricultural Soil Carbon Accumulation. Soil Biol. Biochem. 2015, 91, 279–290. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent Responses of Soil Microbial Communities to Elevated Nutrient Inputs in Grasslands across the Globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Hättenschwiler, S.; Schimann, H.; Fromin, N. Interactive Effects of C, N and P Fertilization on Soil Microbial Community Structure and Function in an A Mazonian Rain Forest. Funct. Ecol. 2015, 29, 140–150. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium Promotes Persistent Soil Organic Matter by Altering Microbial Transformation of Plant Litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef]
- Mueller, C.W.; Koegel-Knabner, I. Soil Organic Carbon Stocks, Distribution, and Composition Affected by Historic Land Use Changes on Adjacent Sites. Biol. Fertil. Soils 2009, 45, 347–359. [Google Scholar] [CrossRef]
- Lee, C.K.; Barbier, B.A.; Bottos, E.M.; McDonald, I.R.; Cary, S.C. The Inter-Valley Soil Comparative Survey: The Ecology of Dry Valley Edaphic Microbial Communities. ISME J. 2012, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Ochman, H. Illumina-Based Analysis of Microbial Community Diversity. ISME J. 2012, 6, 183–194. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glockner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).