Abstract
The apple tree (Malus domestica), a member of the Rosaceae family, holds significant economic value but faces postharvest challenges, like blue mold caused by Penicillium expansum and gray mold caused by Botrytis cinerea. While synthetic fungicides are widely used, their limitations highlight the need for sustainable alternatives. This study explores the antifungal properties of extracts from Celtis australis, Olea europea var. sylvestris, Chamaerops humilis, and Asparagus albus against these pathogens. In vitro tests assessed mycelial growth inhibition, whereas in vivo trials consisted of measurement of weight loss, firmness, total soluble solids, titratable acidity, and maturity index. Moreover, the phytochemical traits of the extracts were determined using the Folin–Ciocalteu method and HPLC. The results revealed notable antifungal activity, particularly for Celtis australis extract at a concentration of 300 g L−1, which led to significant mycelial growth inhibition (61% for P. expansum and 41% for B. cinerea), a reduction in diseases’ severity (39% and 50%), and a notable decrease in diseases’ incidence (43% and 48%), respectively. Phytochemical analysis reflected the presence of phenols and flavonoids in the tested extracts. Importantly, the natural treatments helped preserve the apples’ quality during storage. Molecular docking studies further revealed that major compounds in Celtis australis extract inhibit the 14α-demethylase enzyme, a key target in fungal sterols biosynthesis.
1. Introduction
The global cultivation of apple trees (Malus domestica) plays a crucial role in the international food system, covering approximately 4.8 million hectares and generating around 95 million tons annually. China dominates global apple production with 45.98 million tons, followed by Turkey (4.49 million tons) and the United States (4.46 million tons). In Africa, Morocco is the second-largest apple producer after South Africa, with an annual yield of 889.736 tons cultivated across 52.550 hectares, averaging 16.93 tons per hectare [1].
The increasing incidence of fungal diseases in apple orchards has become a pressing concern for fruit producers worldwide, significantly impacting both quality and yield. Among the most damaging postharvest pathogens are Penicillium expansum and Botrytis cinerea, responsible for blue mold and grey mold, respectively [2,3,4]. These fungi not only result in substantial economic losses and reduced marketability but also compromise food safety. P. expansum is particularly concerning due to its production of patulin, a mycotoxin with genotoxic, immunosuppressive, and cytotoxic effects, commonly detected in infected apples and derived products [5,6]. Meanwhile B. cinera is considered as the second most important plant pathogen globally [7], and it produces two major phytotoxins, namely the sesquiterpene botrydial and the polyketide botcinic acid [8].
Although conventional fungicides provide some control, their use in increasingly limited by environmental concerns, the development of resistant fungal strains, and threats to food security [9,10]. These challenges highlight the urgent need for sustainable and effective alternatives. Promising strategies include biological control agents such as yeasts and bacteria, as well as the use of nanomaterials [11,12,13]. Recent research has shifted focus towards plant-derived compounds for their potent antifungal properties. Extracts from various plants have shown promise against P. expansum and B. cinerea [14,15,16,17].
However, most works have been carried out on common or popular medicinal plants, often without analyzing a combination of species native to the Mediterranean or North African regions. This work investigates for the first time the antifungal activity of the ethanolic extracts of four less investigated Mediterranean species, namely Celtis australis, Olea europaea subsp. sylvestris (O. oleaster), Chamaerops humilis (C. humilis), and Asparagus albus (A. albus). Indeed, these species have been used in traditional medicinal uses [18,19]. However, they have never been explored for their potential to control these postharvest pathogens of apple. Additionally, this research combines both in vitro and in vivo assays, alongside chemical profiling by High-Performance Liquid Chromatography (HPLC), providing a comprehensive approach that bridges bioactivity assessments with chemical characterization for the development of natural fungicides.
Ultimately, this research aspires to contribute to sustainable agriculture by promoting plant-based disease management practices that are both effective in disease management and environmentally sound. As the agricultural sector grapples with the dual challenges of increasing production and minimizing environmental impacts [20], our findings offer potential pathways toward a safer, more sustainable protection of apple crops that benefits both producers and consumers.
2. Materials and Methods
2.1. Plant Material
The four plant species studied, A. albus, C. australis, O. oleaster and C. humilis, are angiosperms belonging to the families Amaranthaceae, Cannabaceae, Oleaceae, and Arecaceae, respectively [21,22]. The plant material was sampled from the Fez-Meknes region, from the city of Fes (34°2′36″ N, 5°0′12″ W, altitude 479 m), characterized by a mild and sunny Mediterranean climate, with highest and lowest temperatures of 46.7 °C and −8.2 °C, respectively.
2.2. Extraction Mode
Leaves from mature growing plants were shade-dried and ground into powder. The powder was passed through a 1 mm mesh sieve to ensure homogeneity of particle size. Ethanolic extracts were prepared in January 2024, and they were obtained via maceration in ethanol: 10 g of powdered leaves was mixed with 100 mL of 70:30 (v/v) ethanol/distilled water and agitated for 24 h [23]. The solvent was removed using a rotary evaporator, and the extracts were then stored at 4 °C for further use. Since the ethanol/water solvent was completely evaporated before testing, a separate solvent control was not included in the antifungal assays, as no residual solvent remained to affect the results.
2.3. Fungal Pathogens Preparation
Fungal strains P. expansum (strain Aby4, OR426630) and B. cinerea (strain PR1, OQ691642) were isolated from decayed apples and selected based on pathogenicity evaluations. They were identified through the sequencing of the internal transcribed spacer (ITS) genomic region. The fungal strains were stored at 4 °C on Potato Dextrose Agar (PDA). Prior to experiments, cultures were incubated on PDA at 25 °C for 7 d. Spore suspensions were prepared by gently scraping colonies into sterile distilled water and filtering them through Whatman No. 1 paper. Spore concentrations were adjusted to 1 × 104 spores mL−1 using a hemocytometer [24]. The antifungal assays were carried out between February and June 2024.
2.4. In Vitro Effect of the Studied Ethanolic Extracts on Mycelial Growth
The antifungal activity of the extracts was studied by measuring mycelial growth inhibition at three concentrations (100 g L−1, 200 g L−1, 300 g L−1). The present test was performed as follows: 9 mm wells were made in the center of Petri dishes and filled with 100 µL of each extract at the tested concentrations, mixed with 100 µL of fungal suspension (104 spore mL−1). The positive control was made by mixing, in the wells, 100 µL of the spore suspension with 100 µL of sterile distilled water. The plates were incubated at 25 °C for one week, and the percentage inhibition of mycelial growth (MGI) was calculated as
where DC = diameter of mycelium in control and DT = diameter of mycelium in treatment [25].
MGI (%) = [(DC − DT)/DC] × 100
Ethanolic extracts are comprised of different phytochemicals, although their specific role in the antifungal activity of the extracts has not been studied. Further experiments involving the fractionation and investigation of their individual bioactivity are intended in further studies.
2.5. Determination of Median Inhibitory Concentration of Mycelial Growth
The median inhibitory concentration (IC50) of mycelial growth was determined only for C. australis extract against P. expansum, as it was the only treatment achieving more than a 50% inhibition at 300 g L−1. The IC50 was calculated from the linear regression equation obtained after plotting inhibition percentages of P. expansum mycelial growth against the concentrations used of C. australis (100 g L−1, 200 g L−1, 300 g L−1). The median inhibitory concentration corresponds to the concentration that allows a 50% inhibition of P. expansum mycelial growth.
2.6. In Vivo Antifungal Effect of the Studied Ethanolic Extracts
2.6.1. In Vivo Effect of the Studied Ethanolic Extracts on Apples Diseases’ Severity
Golden Delicious apples were purchased locally and stored at 4 °C. Golden Delicious has been reported to be very susceptible to postharvest fungal pathogens, such as Penicillium expansum and Botrytis cinerea, and can be considered as a model cultivar to assess the effectiveness of antifungal treatments according to its pathogenic susceptibility. Healthy, undamaged apples were sanitized by immersing them in a 1% sodium hypochlorite solution, then rinsing them three times with sterile distilled water. They were subsequently allowed to air-dry for one hour in a sterile airflow environment at ambient temperature. After drying, two equal-sized wounds, each measuring 6 mm in diameter and 4 mm deep, were created on the equatorial sides of the apples [26,27]. In the obtained wounds, 50 µL of each plant extract at a concentration of 300 g L−1 was introduced. For the untreated control, 50 µL of sterile distilled water (SDW) was applied, while the fungicide treatment consisted of the same volume of Difenoconazole at 0.001 g L−1.
After 4 h of incubation at ambient temperature in a hood with a laminar flow, each wound was infected with 10 µL of a spore suspension of 104 spores mL−1 of P. expansum or B. cinerea. Negative control apples were inoculated solely with the same volume of sterile distilled water [28]. All apples were then placed in aseptic boxes and incubated in a growth chamber for 7 d at 25 °C. Each treatment was conducted on three apples (six wounds in total), and the experiment was repeated twice [29]. After the 7 d incubation, disease severity was assessed using the following formula [28]:
Disease severity = (mean lesion diameter of treated apples/mean lesion diameter of untreated controls) × 100
2.6.2. Effect on Apple Quality Parameters
To assess the effects of treatments on the apples’ quality, we measured several quality parameters after 7 d of incubation at 25 °C. These parameters included weight loss, firmness, total soluble solids, titratable acidity and maturity index.
Weight Loss
The weight of the apples was recorded prior to treatment and again 7 d afterward. Weight loss (WL) was determined using the formula [30]
WL = [(initial weight − final weight)/initial weight] × 100
Firmness
The firmness of each apple was assessed individually after 7 d of incubation using a penetrometer (Agrosta®100 USB Digital Firmness Instrument, [Agrosta, Serqueux, France]). Measurements were taken at four evenly spaced points around the equatorial section of each fruit [31]. Firmness values were reported in Newtons (with the instrument calibrated to 100% corresponding to 8.06 N).
Total Soluble Solids
The total soluble solids content was assessed by measuring the refractive index of the apples following a 7 d incubation period, using a digital refractometer (model PAL-1, Atago, Tokyo Tech, Tokyo, Japan). The results are presented as a percentage [32].
Maturity Index
The maturity index (MI) was calculated by taking the ratio of total soluble solids (TSS) to titratable acidity (TA), as described by El Khetabi et al. (2021) [33].
2.7. Semi-Commercial Test Using C. australis Ethanolic Extract
Based on prior results, C. australis extracts were tested under semi-commercial conditions. Apples were disinfected and dried as before. Four equidistant wounds, each measuring 1 mm in diameter and 2 mm in depth, were created on each fruit. Afterward, the apples received sprays of ethanolic extracts from C. australis at a concentration of 300 g L−1. Approximately 2 mL of the extract was sprayed per apple, using a hand-held atomizer to ensure uniform coverage. A similar application was conducted using the fungicide Difenoconazole at a concentration of 0.001 g L−1. The control group consisted of apples treated with SDW. Twenty-four hours later, the apples were sprayed with a spore suspension (104 spores mL−1) of P. expansum and B. cinerea, using approximately 2 mL per apple to ensure complete coverage of the wounded surface. After a 2 h period for air-drying at room temperature, all the apples were placed in plastic bags, with 5 apples per bag, in triplicate, and then incubated at 4 °C in the dark. The number of infected wounds was monitored after 15 d of incubation. In total 90 apples were used in this experiment, with 30 apples for each treatment. This bioassay was repeated twice throughout the study [28]. The incidence of disease was calculated by dividing the number of rotten wounds by the total number of wounds, and the result was multiplied by 100 [34].
2.8. Total Polyphenols Content
The determination of total polyphenols content (TPC) was performed by the Folin–ciocalteu method [35]. An amount of 100 µL of extract was added to 900 µL of distilled water; then, 100 µL of Folin–Ciocalteu reagent was added to the mixture. After 5 min, 1 mL of Na2CO3 (7%) was added. The obtained solution was completed to a volume of 2.5 mL by distilled water. After incubation at room temperature for 90 min, the absorbance was measured at 750 nm. The calibration curve was plotted using gallic acid. The results are expressed as g gallic acid equivalent per kg extract (g kg−1).
2.9. Phytochemical Profile Analysis of Extracts via HPLC-DAD
The identification of key polyphenols was performed based on the methodology outlined by the International Olive Oil Council (COI/T.20/Doc. Nº 29/Rev.1, 2017), with modifications. This analysis utilized a SHIMADZU PROMINANCE HPLC-DAD system (Shimadzu Corporation, Kyoto, Japan), which included a SPD-M30A DAD detector, a DGU-20A5 degasser, an LC A20 quaternary pump, and a SIL-20A thermostated autosampler. Polyphenol separation was achieved using a Supelco® C18 Ascentis® C18 analytical column (Supelco Inc., Bellefonte, PA, USA) (150 mm × 4.6 mm) featuring a 5 µm particle size and 100 Åm porosity. The flow rate was set at 0.5 mL min−1, employing a mobile phase consisting of a ternary gradient of acetonitrile, methanol, and acidified water (0.2% phosphate buffer). The column was maintained at a temperature of 45 °C, with an injection volume of 10 µL. Standard solutions of the target analytes were also injected under these conditions to determine the response factors, identify various phenolic compounds, and establish quantification ranges. Quantification was performed based on three replicates of each extract derived from the same sample, and the results are presented for each depicted phenolic compound in g per kg extract (g kg−1).
2.10. Molecular Docking Protocol
In order to investigate the mechanism of the antifungal activity of the tested ethanolic extracts, we have performed a molecular docking analysis. This allows one to determine the types of interactions between ligands (phenolic compounds of the extracts) and the fungal enzyme 14 α-demethylase, which represents the enzyme playing a key role in the synthesis of sterols (the main components of cell membrane eukaryotic cells).
The major phenolic compounds in the tested extracts were selected to assess their interaction with 14 α-demethylase, namely, isovitexin, kaempferol, chlorogenic acid, and oleuropein, in the extracts of C. australis, A. albus, C. humilis, and O. oleaster, respectively. The protein referred as CYP51 was downloaded from the Protein Data Bank Website. Protein and phenolic compounds were prepared for the analysis using AutoDock Tools software. Then, each phenolic compound was docked with the enzyme 14 α-demethylase, and analyzed using Pymol (version 3.1.6.1, Schrödinger, Inc., New York, NY, USA) and Discovery Studio (version 2025 SP1, Dassault Systèmes, Vélizy-Villacoublay, France) to understand types of interactions.
2.11. Data Presentation and Statistical Analysis
The obtained results from each test are presented as the mean values ± standard deviation. One-way ANOVA was performed using XLSTAT software, with each parameter analyzed separately. Following the ANOVA, Student’s t-tests were utilized to determine the least significant differences at p < 0.05 among the four plant extracts and controls, which are represented by superscript letters. Values assigned with the same letters are not significantly different from each other, whereas values assigned with different letters indicate statistically significant differences (p < 0.05). Triplicate measurements were used for mycelial growth inhibition, disease severity, and quality parameters, while disease incidence values are based on two independent repetitions.
3. Results
3.1. In Vitro Effect of the Tested Plants Extracts on Mycelial Growth Inhibition
The in vitro antifungal ability of ethanolic extracts from the tested plant species against P. expansum and B. cinerea is presented in Figure 1A–C. In the case of P. expansum, mycelial growth inhibition (MGI) showed a positive correlation with the increase in extracts’ concentration; moreover, the highest inhibition was obtained at the maximal concentration used with C. australis extract, followed by O. oleaster, C. humilis, and A. albus extracts: 61%, 48%, 46.00%, and 37% MGI respectively. Statistically, C. australis extract at 300 g L−1 (letter “x”) was significantly more effective than all the other treatments.
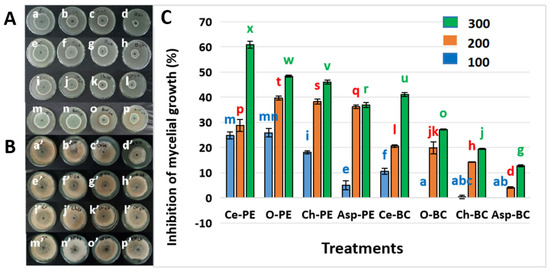
Figure 1.
(A) Effect of the tested extracts on P. expansum mycelial growth inhibition; a, b, c, and d are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of C. australis extract against P. expansum; e, f, g and h are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of O. oleaster extract against P. expansum; i, j, k, and l are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of C. humilis extract against P. expansum; m, n, o, and p are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of A. albus against P. expansum. (B) Effect of the tested extracts on B. cinerea mycelial growth inhibition; a′, b′, c′, and d′ are respectively the untreated control and treatments with concentrations of 100, 200 and 300 g L−1 of C. australis extract against B. cinerea; e′, f′, g′, and h′ are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of O. oleaster extract against B. cinerea; i′, j′, k′, and l′ are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of C. humilis extract against B. cinerea; m′, n′, o′, and p′ are respectively the untreated control and treatments with concentrations of 100, 200, and 300 g L−1 of A. albus against B. cinerea. (C) Percentages of mycelial growth inhibition; Ce: C. australis; O: O. oleaster; Ch: C. humilis; Asp: A. albus; Pe: P. expansum; BC: B. cinerea (means not differing significantly are assigned a common superscript letter). The results are presented as the mean of three replicates ± standard deviation. Bars labeled with different letters are significantly different at p < 0.05 (one-way ANOVA), while treatments sharing the same letter are not significantly different at this level.
As regards B. cinerea, O. oleaster, C. humilis, and A. albus did not show any effect against this fungus at a concentration of 100 g L−1, whereas a weak MGI was obtained by these extracts at the higher concentrations of 200 g L−1 and 300 g L−1, not exceeding 30% (Figure 1C). Using C. australis extract, B. cinerea’s MGI was more important: 11%, 21%, and 41% MGI at concentrations of 100 g L−1, 200 g L−1 and 300 g L−1, respectively.
Moreover, statistical comparisons confirmed that the antifungal effect of C. australis extract at 300 g L−1 (letter “u”) was significantly higher than all other treatments against B. cinerea. Conversely, treatments assigned the same letter, such as “ab” or “abc”, did not differ significantly from one another.
3.2. Determination of Median Concentration of Mycelial Growth Inhibition
The results of the determination of the median inhibitory concentration of P. expansum mycelial growth by C. australis extract are presented in Figure 2. Indeed, we notice that a concentration of 263.01 g L−1 of this extract is required to inhibit the mycelial growth of P. expansum.
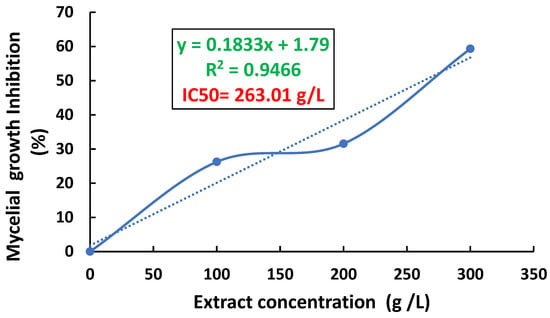
Figure 2.
Median inhibitory concentration (IC50) of P. expansum mycelial growth by C. australis extract.
3.3. In Vivo Effect of the Tested Plants Extracts
3.3.1. Effect of the Extracts on the Severity of Apple Rot
The in vivo tests reflected a variation in the antifungal activity against P. expansum and B. cinerea (Table 1 and Table 2 and Figure 3). Against both fungi, C. australis extract was the most effective in reducing disease severity (39% against P. expansum and 49% against B. cinerea, followed by O. oleaster (70% against P. expansum and 73% against B. cinerea), C. humilis (75% against P. expansum and 88% against B. cinerea), and A. albus (87% against P. expansum, and 80% against B. cinerea).

Table 1.
Effect of the tested extracts at a concentration of 300 g L−1 and the synthetic fungicide at a concentration of 0.001 g L−1 on the severity (%) of blue mold after a 7 d incubation at 25 °C.

Table 2.
Effect of the tested extracts at a concentration of 300 g L−1 and the synthetic fungicide at a concentration of 0.001 g L−1 on the severity (%) of gray apple rot after a 7 d incubation at 25 °C.
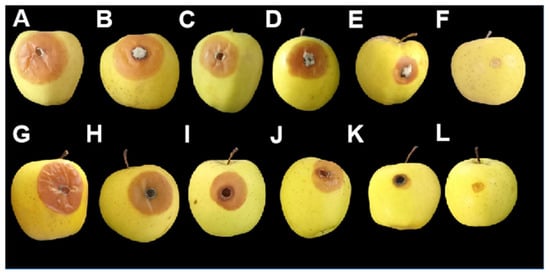
Figure 3.
Lesions of blue and gray mold on apples after 7 days of incubation at 25°C. (A) Untreated control infected by B. cinerea; (B) treatment with C. humilis extract against B. cinerea; (C) treatment with A. albus extract against B. cinerea; (D) treatment with O. oleaster extract against B. cinerea; (E) treatment with C. australis extract against B. cinerea; (F) control treated with the fungicide against B. cinerea; (G) untreated control infected by P. expansum; (H) treatment with A. albus extract against P. expansum; (I) treatment with C. humilis extract against P. expansum (J) treatment with O. oleaster extract against P. expansum; (K) treatment with C. australis extract against P. expansum; (L) control treated with the fungicide against P. expansum.
Indeed, it can be noted that the antifungal activity of the different plant species extracts is distinct; the disease severity observed with C. australis was significantly lower than that of the other extracts (letter “c”), indicating a more pronounced protective effect (Table 1 and Table 2).
However, for the same plant species, similar results were observed against both fungi. Regarding treatment with the synthetic fungicide Difenoconazole, it allowed the total suppression of disease severity related to infection by P. expansum or B. cinerea.
3.3.2. Effect of Plant Extracts Treatment on the Quality of Apples
The results presented in Table 3 and Table 4 show the important effect of the tested extracts on the preservation of apples quality parameters, especially in the case of the treatment with C. australis extract. Indeed, with a focus on C. australis extract, which was chosen for the semi-commercial test since it allowed the best reduction in diseases’ severity, we note that this treatment enabled one to maintain the weight of apples infected by P. expansum and B. cinerea in comparison with untreated controls. Statistically, this difference was significant, as indicated by distinct superscript letters. Moreover, their firmness (6 N; 7 N, respectively) was close to that of the negative control and significantly higher to that of the untreated control at p < 0.05. Moreover, the total soluble solids values of C. australis extract-treated apples (12%; 13%, respectively) were significantly (p < 0.05) higher than those of untreated controls (10%; 11%, respectively), confirming the effectiveness of the treatment in preserving sugar content. Furthermore, especially in the case of P. expansum infection, the acidity and maturity index of apples treated with C. australis extract were close to those of the negative control apples.

Table 3.
Effect of tested extracts on apple quality parameters after infection by P. expansum and 7 d incubation at 25 °C.

Table 4.
Effect of the tested extracts on apple quality parameters after infection by B. cinerea and 7 d incubation at 25 °C.
3.3.3. Effectiveness of the Studied Plant Species Extracts Under Semi-Commercial Conditions
The results of the semi-commercial test (Figure 4) showed that C. australis extract significantly reduced (p < 0.05) the incidence of both fungi diseases. Indeed, after 15 d of incubation at 4 °C, disease incidence was decreased from 100% to 43% and 48% in the cases of P. expansum and B. cinerea infection, respectively, after treatment with C. australis extract.
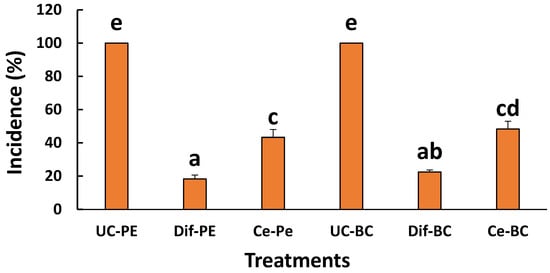
Figure 4.
Effect of ethanolic extracts from C. australis on the incidence of molds caused by P. expansum and B. cinerea; UC-PE: untreated control fruit infected by P. expansum; Dif-PE: fruit treated by Difenoconazole and infected by P. expansum; Ce-Pe: fruit treated by C. australis extract and infected by P. expansum; UC-BC: untreated control fruit infected by B. cinerea; Dif-BC: fruit treated by Difenoconazole and infected by B. cinerea; Ce-BC: fruit treated by C. australis extract and infected by B. cinerea. Data are expressed as the mean of two independent repetitions ± standard deviation. Different lowercase letters above bars indicate statistically significant differences between treatments (p < 0.05, one-way ANOVA).
Concerning the chemical treatment with the synthetic fungicide Difenoconazole (0.001 g L−1), the antifungal efficacy was significantly (p < 0.05) more important than the treatment with C. australis extract, characterized by an incidence reduction from 100% to 18% and to 23% in the cases of blue mold and gray mold, respectively.
3.4. Phytochemical Analysis
3.4.1. Extracts’ Total Polyphenols Content
The results of total polyphenols content assessment (Table 5) show that all the studied extracts are rich in polyphenols, with the highest content in C. humilis (137 g kg−1), followed by O. oleaster (121 g kg−1), then C. australis (86 g kg−1) and A. albus (72 g kg−1).

Table 5.
Total polyphenols content of C. australis, O. oleaster, C. humilis, and A. albus ethanolic extracts.
3.4.2. Phenolic Compounds in the Studied Extracts
Table 6 summarizes their phytochemical composition. In addition, Table 7 provides data on the limit of detection (LOD), limit of quantification (LOQ), and recovery rates for each identified compound. The LOD values varied between 1.8 and 23.2 µg/mL, whereas LOQ values ranged from 6.1 to 77.3 µg/mL, depending on the compound. Furthermore, the recovery rates for the quantified phenolic compounds were between 94.5% and 103.5%.

Table 6.
Phytochemical composition of the analyzed extracts based on HPLC assessment.

Table 7.
Limit of detection (LOD), limit of quantification (LOQ), and recovery for each individual analyte.
Furthermore, it appears that the studied extracts contain a variety of phenolic compounds (Table 6).
Indeed, C. australis extract contains the polyphenol resveratrol (6.8 ± 0.78 g kg−1), in addition to several phenolic acids, namely caffeic acid (5.03 ± 0.42 g kg−1), gallic acid (3.16 ± 0.09 g kg−1), ferulic acid (4.5 ± 0.3 g kg−1), vanillic acid (8.19 ± 0.7 g kg−1), p-coumaric acid (9.66 ± 0.2 g kg−1), sinapic acid (2.07 ± 0.098 g kg−1), Rosmarinic acid (4.22 ± 0.57 g kg−1), and cenamic acid (4.41 ± 0.31 g kg−1). Importantly, the flavonoid isivitexin is dominant in this extract (47.3 ± 0.96 g kg−1).
A. albus extract also contains resveratrol (4.01 ± 0.1 g kg−1) and different phenolic acids, including caffeic acid (4.01 ± 0.1 g kg−1), ferulic acid (6.75 ± 0.9 g kg−1), protocatechuic acid (4.08 ± 0.2 g kg−1), vanillic acid (11.21 ± 0.57 g kg−1), syringic acid (10.04 ± 0.18 g kg−1), and chlorogenic acid (8.5 ± 0.41 g kg−1). Moreover, flavonoids were also revealed in this extract in different amounts; kaempferol (18.19 ± 1.52 g kg−1), quercetin (2.11 ± 0.84 g kg−1), rutin (4.012 ± 0.9 g kg−1), hyperoside (8.9 ± 0.14 g kg−1), nicotiflorin (12.06 ± 0.99 g kg−1), and narcissin (6.11 ± 0.4 g kg−1).
Regarding C. humilis extract, different phenolic acids have been revealed, namely gallic acid (19.72 ± 0.15 g kg−1), ferulic acid (26.10 ± 0.09 g kg−1), ellagic acid (12.19 ± 0.4 g kg−1), and chlorogenic acid (39.6 ± 0.17 g kg−1). It also has various flavonoids present in varying quantities; apigenin (8.04 ± 0. 01 g kg−1), luteolin (4.12 ± 0.2 g kg−1), kaempferol (15.55 ± 0.13 g kg−1), quercetin (10. 21 ± 0.7 g kg−1), and rutin (8.82 ± 0.04 g kg−1).
As for O. oleaster, the dominant compound is the polyphenol glycoside ‘Oleuropein’ (96.71 ± 1.31 g kg−1), followed by the polyphenol hydroxytyrosol (27.88 ± 0.42 g kg−1), then caffeic acid (19.3 ± 0.11 g kg−1), rutin (8.2 ± 0.47 g kg−1), quercetin (7.9 ± 0.062 g kg−1), and verbascoside (6.6 ± 0.5 g kg−1).
3.5. Molecular Docking Analysis
The interactions between the active sites of the enzyme 14α-demethylase, and the major compounds of the studied plant species ethanolic extracts, namely, isovitexin, kaempferol, chlorogenic acid, and oleuropein (in C. australis, A. albus, C. humilis, and O. oleaster extracts, respectively) are illustrated in Figure 5. Indeed, results from the molecular docking analysis showed that isovitexin (A) has two types of Pi-Pi interactions (PHE 78 and PHE 83), three Pi alkyl interactions (LEU 321, MET 79, and ALA 256), Pi cation interaction (HIS259), and just one unfavorable donor–donor interaction.
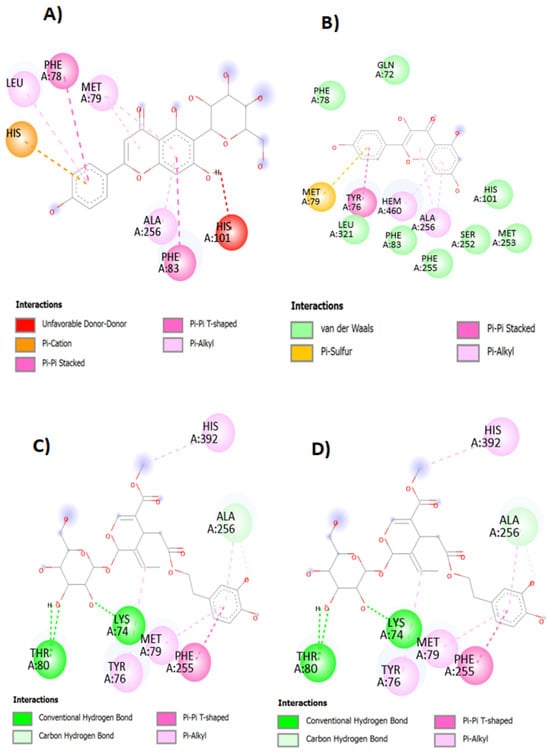
Figure 5.
The representation (2D view) of the interactions between the ligands (isovitexin) (A), kaempferol (B), chlorogenic acid (C), oleuropein (D), and the 14 α-demethylase enzyme.
As for kaempferol, it represents eight van der Waals interactions with targeted enzyme active sites (PHE 78, GLN 72, LEU 321, PHE 83, PHE 255, SER 252, HIS 101, and MET 253). It also exhibited two Pi alkyl interactions (HEM 460 and ALA 256) and one Pi-Pi stacked (TYR76) and one Pi–sulfur interactions (MET 79).
Regarding chlorogenic acid, it showed two conventional hydrogen bonds (MET 433 and ALA 75), one Pi–alkyl (LEU 321) and one Pi-Pi (PHE 78) interaction with the enzyme’s active sites.
Concerning oleuropein, it exhibited three Pi–alkyl interactions (TYR 76, MET 79, and His 392), two conventional hydrogen bonds (ALA 256), and one Pi-Pi T-shaped interaction with the enzyme’s bonding sites. Overall, it can be concluded that the major compounds of the tested ethanolic extracts display different interactions with the enzyme, suggesting that they are potential inhibitors of 14α-demethylase, exerting therefore an antifungal activity against P. expansum and B. cinerea.
4. Discussion
The primary method for managing postharvest pathogens is the application of fungicides. However, this strategy has several disadvantages. A major concern is the potential for fungicide residues to remain on fruit, posing risks to both human health and the environment. Additionally, the continuous use of fungicides may lead to the development of fungicide-resistant pathogenic strains [36,37].
Considering the abundance of naturally bioactive compounds in plants with antimicrobial potential, numerous research efforts have focused on identifying such molecules as effective and safer alternatives to synthetic fungicides [38,39]. These natural compounds can inhibit fungal growth and spread through direct or indirect mechanisms, contributing to the suppression of plant diseases and promoting the development of resistance in host plants [40].
Ethanolic extracts are considered eco-friendly or “green” solvents [41]. They are frequently reported to exhibit greater antimicrobial activity than aqueous extracts and are generally regarded as safely for use in protecting apples from fungal diseases without posing harmful side effects [42,43,44]. To the best of our knowledge, no studies have been conducted on the ability of ethanolic extracts from C. australis, O. oleaster, C. humilis, and A. albus to manage postharvest apple diseases. In the present work, in vitro tests showed that the best inhibition of fungi mycelial growth was obtained at 300 g L−1, and the inhibition rate was positively correlated with the increase of extracts’ concentration, especially in the case of P. expansum. Our findings are in agreement with other studies indicating that the antifungal activity generally increases with extract concentration [16,17,45]. However, the comparative analysis revealed that the tested plant extracts did not exhibit the same efficacy. Indeed, C. australis extract showed superior performance. O. oleaster extract revealed moderate activity, while C. humilis and A. albus extracts exhibited less antifungal efficacy. These differences may be related to the distinct phytochemical profile of the plant extracts and to specific interactions with the bioactive compounds and fungal pathogens.
Moreover, C. australis extract showed the most promising results either in in vitro or in vivo assays. The importance of in vivo trials must be emphasized, as they are essential to confirm in vitro results [46] and to account for factors like compound degradation, hydrolysis, and polymerization that may impact the effectiveness of certain compounds of plant extracts [42].
The outcomes obtained from the in vivo assays we performed reveal that the disease severity caused by B. cinerea and P. expansum was significantly decreased by all the studied plants extracts, with C. australis extract being the most effective, lowering disease severity to 50% and to 39%, respectively. The effectiveness of plant extracts in postharvest disease control has been corroborated by several studies [43,44]. The mechanism of antifungal action of the plant extracts in fruit was associated with the activation of defense pathways, like the increase in the production of defense enzymes, including peroxidase, polyphenol oxidase, phenylalanine ammonia-lyase, and β-1,3-glucanase, which are involved in apple’s resistance against fungal attacks [47,48].
Moreover, plant extract-based treatments are reported to regulate phytohormone production, thereby maintaining the integrity of the fruit cell wall, improving their resistance, and extending their shelf life [46].
As for quality parameters, our results highlight the impact of biological extracts in the preservation of apples’ quality, especially with C. australis extract. This extract preserved apples’ weight compared to untreated fruit. Moreover, C. australis extract maintained fruits’ firmness, soluble solids content, and acidity, as well as maturity levels, achieving effects similar to those of the chemical fungicide Difenoconazole. Similar findings have been reported in studies evaluating the effectiveness of plant extracts in apple quality preservation [24,43].
In semi-commercial tests, C. australis extract significantly decreased disease incidence, with infection rates dropping to 43% for P. expansum and 48% for B. cinerea. However, the synthetic fungicide Difenoconazole, which was used as a positive control at 0.001 g L−1, was more effective, with disease incidence being reduced to 18% and 23% for P. expansum and B. cinerea, respectively. The efficacy of plant extracts against postharvest fungi under semi-commercial conditions was also proved in other works [28,49].
The assessment of total phenols content using the Folin–Ciocalteu method has demonstrated to us the richness of the studied extracts in phenols. Many studied have linked the abundance of phenols to increased antifungal activity [23,50,51]. The antimicrobial activities of phenolic compounds are related to their ability to disturb fungal hyphae, destabilize microbial structures, and increase cellular permeability [28].
Additionally, our HPLC analysis have unveiled the chemical composition of our studied extracts. All the extracts were revealed to contain different phenolic compounds, with isovitexin, kaempferol, chlorogenic acid, and oleuropein being the major compounds of C. australis, A. albus, C. humilis, and O. oleaster, respectively. These compounds have all been reported to exhibit strong antimicrobial activities [52,53,54,55]. Molecular docking analysis showed that these molecules interact with the active site of the 14-alpha demethylase enzyme, a key enzyme in fungal sterol biosynthesis. Inhibition of this enzyme compromises the integrity of the fungal cell membrane, contributing to the antifungal effects observed in this study.
Furthermore, from a practical point of view, the use of ethanolic plant extracts represents a more sustainable and cost-effective alternative to synthetic pesticides, thanks to the simplicity of their preparation and the accessibility of plant materials. This strategy contributes to a reduction in the cost of postharvest disease management, as well as to the mitigation of environmental and health risks. However, for real-world applications, several limitations need to be considered. The short storage life of plant-based extracts stands among the major constraints. Indeed, plant extracts may be degraded over time. Therefore, they can lose their bioactivity. In addition, the process of extraction and formulation needs to be optimized in order to meet industrial demands.
Furthermore, the use of extracts at 300 g L−1 in practical applications at commercial scale could present limitations. Indeed, the preparation of 1 L of extract may require approximately 3000 g of dry plant material, since the yield is about 10% w/w. Spraying large batches of apples would therefore require high quantities of plant biomass. Therefore, this method will be a useful as a complementary or integrated approach rather than a complete replacement for synthetic fungicides
Finally, an important aspect to be further studied is whether the use of these ethanolic plant extracts has any influence on patulin synthesis by P. expansum. Indeed, plant-derived compounds may induce the inhibition or, conversely, the production of mycotoxins such as patulin. Hence, the investigation of the potential of these extracts to modulate patulin’s biosynthesis would provide an essential insight into their safety and suitability for postharvest disease management.
5. Conclusions
In this study, we investigated the antifungal properties of ethanolic extracts from C. australis, O. europea, C. humilis, and A. albus. Our results demonstrated that these extracts exhibited significant antifungal activity against Penicillium expansum and Botrytis cinerea, the causal agents of blue mold and gray mold, respectively. This bioactivity is attributed to their rich phytochemical composition, particularly the high content of phenolic compounds, such as isovitexin, kaempferol, chlorogenic acid, and oleuropein, in the extracts of C. australis, A. albus, C. humilis and O. oleaster, respectively. Molecular docking analysis confirmed that these compounds effectively interact with the active site of the fungal enzyme sterol 14α-demethylase, a key player in fungal membrane biosynthesis, thereby contributing to the inhibition of fungal growth. Moreover, the extracts effectively maintained apple quality parameters, highlighting their potential as natural fungicides instead of synthetic ones.
Future work should focus on the field-scale validation of these extracts and the development of appropriate formulations to enhance their stability, efficacy, and practical application in commercial apple production.
Author Contributions
Conceptualization, K.B., K.F.-B., S.I.K., R.L., R.E., M.K., M.R., L.A.H., I.D., S.B. and A.B.; methodology, K.B., M.E.O., K.F.-B., R.E., M.K., M.R., I.D., R.L., L.A.H., S.B., E.A.B. and A.B.; software, S.B.; validation, R.L., K.F.-B., S.I.K. and A.B.; formal analysis, E.A.B., S.B. and M.E.O.; investigation, K.B., R.E., M.K. and M.R.; resources, K.F.-B., S.I.K. and R.L.; data curation, K.B., R.E., M.K., M.R. and I.D.; writing—original draft preparation, K.B., R.E., M.E.O., M.K. and I.D.; writing—review and editing, K.B., K.F.-B., R.L. and S.I.K.; visualization, E.A.B., R.L. and K.B.; supervision, K.F.-B., R.L. and S.I.K.; project administration, K.F.-B., S.I.K. and R.L.; funding acquisition, K.F.-B., S.I.K. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: The National Center for Scientific and Technical Research (CNRST), grant number 5USMBA2022, and The Phytopathology Unit of the Ecole Nationale d’Agriculture de Meknes.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
This study was conducted in the Phytopathology Unit, Department of Plant Protection, Ecole Nationale d’Agriculture de Meknes, Morocco. We would like to express our gratitude to all the members of this laboratory for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TPC | Total polyphenols content |
| DC | Diameter of mycelium in control |
| MGI | Mycelial growth inhibition |
| HPLC | High-Performance Liquid Chromatography |
| IC50 | Median inhibitory concentration |
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal Activity of Zinc Oxide Nanoparticles Against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Pathogens of Autotrophs. In Fungi, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 245–292. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium Expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Keller, N.P.; Atoui, A.; El Khoury, A.; Lteif, R.; Oswald, I.P.; Puel, O. Secondary Metabolism in Penicillium expansum: Emphasis on Recent Advances in Patulin Research. Crit. Rev. Food Sci. Nutr. 2018, 58, 2082–2098. [Google Scholar] [CrossRef]
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic Effects of Mycotoxins. Toxicon 2020, 185, 104–113. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea—Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pêcheur, P.; Tudzynski, B.; Collado, I.G.; Viaud, M. The Botrytis cinerea Phytotoxin Botcinic Acid Requires Two Polyketide Synthases for Production and Has a Redundant Role in Virulence with Botrydial. Mol. Plant Pathol. 2011, 12, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Capote, N.; Mara, A.; Aguado, A.; Snchez-Torres, P. Molecular Tools for Detection of Plant Pathogenic Fungi and Fungicide Resistance. Plant Pathol. 2012, 7, 151–202. [Google Scholar] [CrossRef]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef]
- Castelo Branco Melo, N.F.; de MendonçaSoares, B.L.; Marques Diniz, K.; Ferreira Leal, C.; Canto, D.; Flores, M.A.P.; Henrique da Costa Tavares-Filho, J.; Galembeck, A.; Montenegro Stamford, T.L.; Montenegro Stamford-Arnaud, T.; et al. Effects of Fungal Chitosan Nanoparticles as Eco-Friendly Edible Coatings on the Quality of Postharvest Table Grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Millan, A.F.S.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of New Antifungal Metabolites Produced by the Yeast Metschnikowia pulcherrima Involved in the Biocontrol of Postharvest Plant Pathogenic Fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Q.; Xin, Y.; Ngea, G.L.N.; Dhanasekaran, S.; Luo, R.; Li, J.; Zhao, L.; Zhang, H. The Biocontrol Potentiality of Bacillus amyloliquefaciens Against Postharvest Soft Rot of Tomatoes and Insights into the Underlying Mechanisms. Postharvest Biol. Technol. 2024, 214, 112983. [Google Scholar] [CrossRef]
- Ahmadu, T.; Ahmad, K.; Ismail, S.I.; Rashed, O.; Asib, N.; Omar, D. Antifungal Efficacy of Moringa Oleifera Leaf and Seed Extracts Against Botrytis cinerea Causing Gray Mold Disease of Tomato (Solanum lycopersicum L.). Braz. J. Biol. 2020, 81, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, J.; Espino, M.; de Los Ángeles Fernández, M.; Pizzuolo, P.; Silva, M.F. Eco-Friendly Postharvest Protection: Larrea cuneifolia-Nades Extract Against Botrytis cinerea. Rev. Fac. Ciencias Agrar. 2019, 51, 427–437. [Google Scholar]
- Daniel, C.K.; Lennox, C.L.; Vries, F.A. In-Vitro Effects of Garlic Extracts on Pathogenic Fungi Botrytis Cinerea, Penicillium Expansum and Neofabraea Alba. S. Afr. J. Sci. 2015, 111, 8. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.H.; Ganie, A.A.; Pala, S.A.; Mir, R.A. Antifungal Activity of Some Plant Extracts on Some Pathogenic Fungi. Arch. Phytopathol. Plant Prot. 2014, 47, 279–284. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Falek, W.; Miazzi, M.M.; Montemurro, C. Current status of biodiversity assessment and conservation of wild olive (Olea europaea L. subsp. europaea var. sylvestris). Plants 2022, 11, 480. [Google Scholar] [CrossRef]
- Khan, M.P.; Ahmad, M.; Zafar, M.; Sultana, S.; Ali, M.I.; Sun, H. Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pakistan. J. Ethnopharmacol. 2015, 173, 191–203. [Google Scholar] [CrossRef]
- Pretty, J. Intensification for Redesigned and Sustainable Agricultural Systems. Science 2018, 362, eaav0294. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Pratique Du Maroc: Manuel de Détermination Des Plantes Vasculaires; Institut scientifique, Université Mohammed V—Agdal, Ed.; Institut Scientifique: Rabat, Morocco, 1999; Volume 3, ISBN 9954-0-1456-X. [Google Scholar]
- Fennane, M.; IbnTattou, M.; Mathez, J.; El Oualidi, J. Flore Pratique Du Maroc: Manuel De Détermination Des Plantes Vasculaires Volume 3, Dicotyledones (p.p.), Monocotyledones; Institut Scientifique: Rabat, Morocco, 2014; p. 793. [Google Scholar]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, Antifungal and Antioxidant Activity of Total Polyphenols of Withania Frutescens. L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Yang, Z.; Xiong, X.; Song, Q.; Li, B.; Zou, H.; Zhang, L.; Liu, T. Antifungal Effect of Oregano Essential Oil Against Penicillium expansum on Pyrus sinkiangensis. J. Fungi 2024, 10, 752. [Google Scholar] [CrossRef]
- Cherrate, M.; Echchgadda, G.; Amiri, S.; Ezrari, S.; Radouane, N.; Oulad El Majdoub, Y.; El Hamss, H.; Maissour, A.; Makroum, K.; Cacciola, F.; et al. Biological Control of Major Postharvest Fungal Diseases of Apple Using Two Lamiaceae Extracts. Arch. Phytopathol. Plant Prot. 2022, 55, 2356–2381. [Google Scholar] [CrossRef]
- Ambaw, A.; Dekeyser, D.; Vanwalleghem, T.; Van Hemelrijck, W.; Nuyttens, D.; Delele, M.A.; Ramon, H.; Nicolai, B.; Bylemans, D.; Opara, U.L.; et al. Experimental and numerical analysis of the spray application on apple fruit in a bin for postharvest treatments. J. Food Eng. 2017, 202, 34–45. [Google Scholar] [CrossRef]
- Holmes, G.; Cunningham, S.J.; Dela Rue, B.T.; Bollen, A.F. Predicting apple bruising using machine learning. Acta Hortic. 1998, 476, 289–296. [Google Scholar] [CrossRef]
- Ngugi, H.K.; Esker, P.D.; Scherm, H. Meta-analysis to determine the effects of plant disease management measures: Review and case studies on soybean and apple. Phytopathology 2011, 101, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; de Almeida, F.A.; André, C.; Vanetti, M.C.D.; Pinto, U.M.; Hassimotto, N.M.A.; Vieira, É.N.R.; de Andrade, N.J. Phenolic Extract of Eugenia uniflora L. and Furanone Reduce Biofilm Formation by Serratia liquefaciens and Increase Its Susceptibility to Antimicrobials. Biofouling 2020, 36, 1031–1048. [Google Scholar] [CrossRef]
- Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Blenzar, A.; Barka, E.A. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid Against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy 2021, 11, 209. [Google Scholar] [CrossRef]
- Hassani, A.; Fathi, Z.; Ghosta, Y.; Abdollahi, A.; Meshkatalsadat, M.H.; Marandi, R.J. Evaluation of Plant Essential Oils for Control of Postharvest Brown and Gray Mold Rots on Apricot. J. Food Saf. 2012, 32, 94–101. [Google Scholar] [CrossRef]
- Hamss, H.E.; Kajad, N.; Belabess, Z.; Lahlali, R. Enhancing Bioefficacy of Bacillus amyloliquefaciens SF14 with Salicylic Acid for the Control of the Postharvest Citrus Green Mould. Plant Stress 2023, 7, 100144. [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Haddou, L.A.; Belabess, Z.; Merah, O.; Lahlali, R. In Vitro and In Vivo Antifungal Activities of Nine Commercial Essential Oils Against Brown Rot in Apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal Activity of Moroccan Medicinal Plants Against Citrus Sour Rot Agent Geotrichum candidum. Lett. Appl. Microbiol. 2012, 55, 155–161. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Slam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Iqbal, N. Post-Harvest Pathogens and Disease Management of Horticultural Crop: A Brief Review. Plant Arch. 2020, 20, 2054–2058. [Google Scholar]
- Bukhari, S. Synthetic Approaches and Pharmacological Attributes of Benzosuberone Skeleton. Mini-Rev. Med. Chem. 2023, 23, 3–23. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant Extracts and Other Natural Compounds as Alternatives for Post-Harvest Management of Fruit Fungal Pathogens: A Review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Behiry, S.I.; Al-Askar, A.A.; Soliman, S.A.; Alotibi, F.O.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M.; Hafez, E.E.; Heflish, A.A. Plantago lagopus Extract as a Green Fungicide Induces Systemic Resistance Against Rhizoctonia Root Rot Disease in Tomato Plants. Front. Plant Sci. 2022, 13, 966929. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Gatto, M.A.; Ippolito, A.; Linsalata, V.; Cascarano, N.A.; Nigro, F.; Vanadia, S.; Di Venere, D. Activity of Extracts from Wild Edible Herbs Against Postharvest Fungal Diseases of Fruit and Vegetables. Postharvest Biol. Technol. 2011, 61, 72–82. [Google Scholar] [CrossRef]
- Soleimani, M.; Rezaie, S.; Nabizadeh Nodehi, R.; Jahed Khaniki, G.; Alimohammadi, M.; Alikord, M.; Noorbakhsh, F.; Molaee-Aghaee, E.; Ghanbari, R. Eco-Friendly Control of Licorice Aqueous Extract to Increase Quality and Resistance to Postharvest Decay in Apple and Tangerine Fruits. J. Environ. Health Sci. Eng. 2021, 19, 1107–1116. [Google Scholar] [CrossRef]
- Zatla, A.T.; Mami, I.; Dib, M.E.A.; Sifi, M.E.A. Efficacy of Essential Oil and Hydrosol Extract of Marrubium vulgare on Fungi Responsible for Apples Rot. Anti-Infect. Agents 2019, 18, 285–293. [Google Scholar] [CrossRef]
- Nabila, V.K.; Putra, I.B. The Effect of Aloe Vera Ethanol Extract on the Growth Inhibition of Candida albicans. Med. Glas. 2024, 17, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F.; et al. Natural Plant Extracts: An Update About Novel Spraying as an Alternative of Chemical Pesticides to Extend the Postharvest Shelf Life of Fruits and Vegetables. Molecules 2022, 27, 5152. [Google Scholar] [CrossRef] [PubMed]
- Gholamnezhad, J. Effect of Plant Extracts on Activity of Some Defense Enzymes of Apple Fruit in Interaction with Botrytis cinerea. J. Integr. Agric. 2019, 18, 115–123. [Google Scholar] [CrossRef]
- Gholamnezhad, J.; Sanjarian, F.; Mohammadi Goltapeh, E.; Safaie, N.; Razavi, K. Study of Defense Genes Expression Profile Pattern of Wheat in Response to Infection by Mycosphaerella graminicola. Iran. J. Plant Biol. 2016, 8, 43–54. [Google Scholar]
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of Postharvest Fungal Rots on Citrus Fruit and Sweet Cherries Using a Pomegranate Peel Extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Goussous, S.J.; Abu el-Samen, F.M.; Tahhan, R.A. Antifungal Activity of Several Medicinal Plants Extracts Against the Early Blight Pathogen (Alternaria solani). Arch. Phytopathol. Plant Prot. 2010, 43, 1745–1757. [Google Scholar] [CrossRef]
- Ikegbunam, M.; Ukamaka, M.; Emmanuel, O.; Ikegbunam, M.; Ukamaka, M.; Emmanuel, O. Evaluation of the Antifungal Activity of Aqueous and Alcoholic Extracts of Six Spices. Am. J. Plant Sci. 2016, 7, 118–125. [Google Scholar] [CrossRef]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and Anti-Biofilm Activity of Flavonoids and Triterpenes Isolated from the Extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) Extracts. African J. Tradit. Complement. Altern. Med. 2014, 11, 124–131. [Google Scholar] [CrossRef]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic Acid is a Fungicide Active Against Phytopathogenic Fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.D.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.D.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal Effects of the Flavonoids Kaempferol and Quercetin: A Possible Alternative for the Control of Fungal Biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Bobnjarić, I.; Horvat, I.; Tomić, S.; Kosalec, I. Antifungal Activity of Oleuropein Against Candida albicans-the In Vitro Study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).