Effect of Compost Addition on Carbon Mineralization and Kinetic Characteristics in Three Typical Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Incubation Setting up
2.3. Soil Sample Analysis
2.4. Determination of Carbon Dioxide Release Rate
2.5. Statistics
3. Results
3.1. Differences in Basic Physicochemical Properties and Particle Composition of Soil Before Incubation
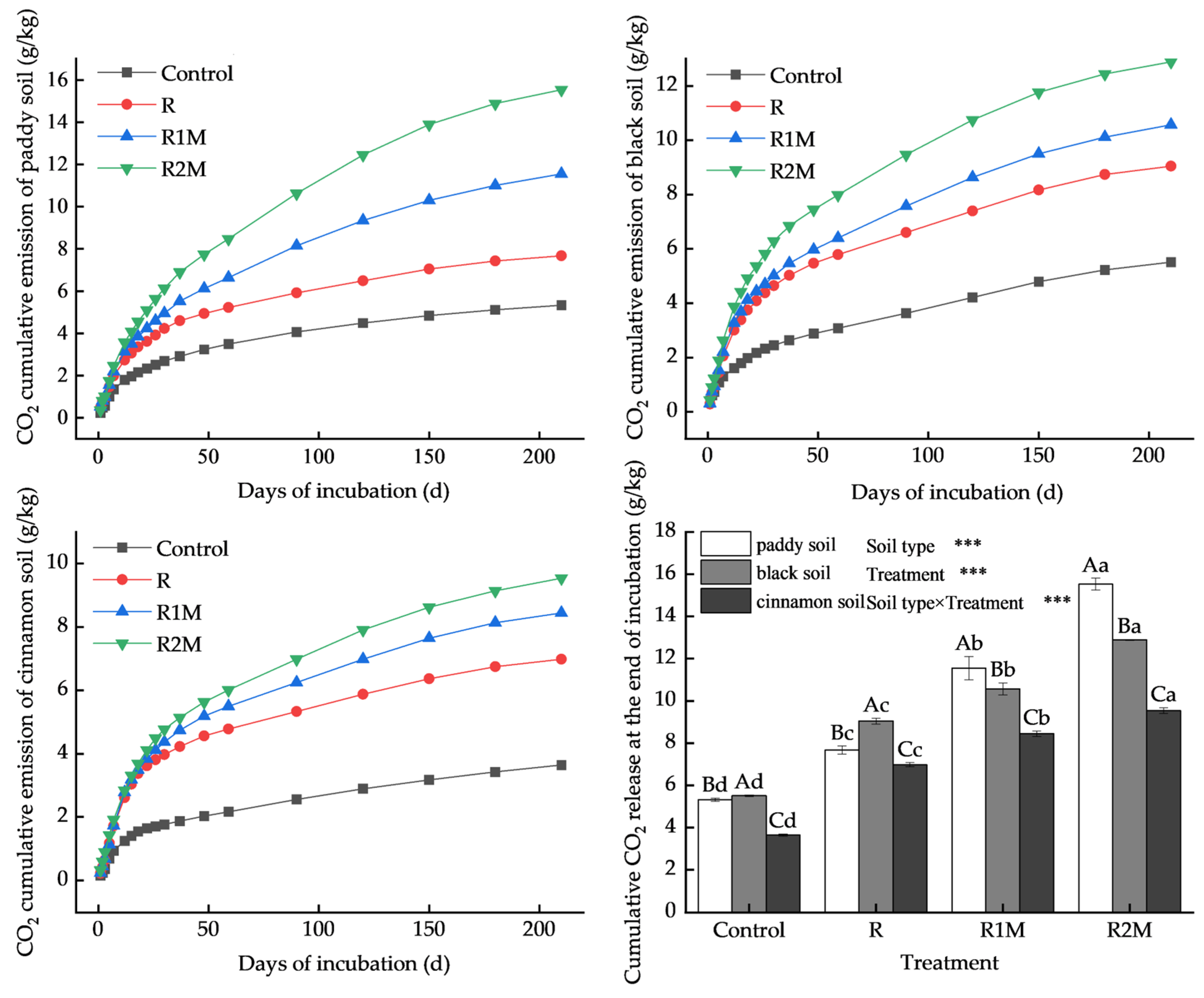
3.2. Characteristics of CO2 Emission Rates from Different Types of Soil
3.3. Cumulative CO2 Emissions from Different Types of Soil
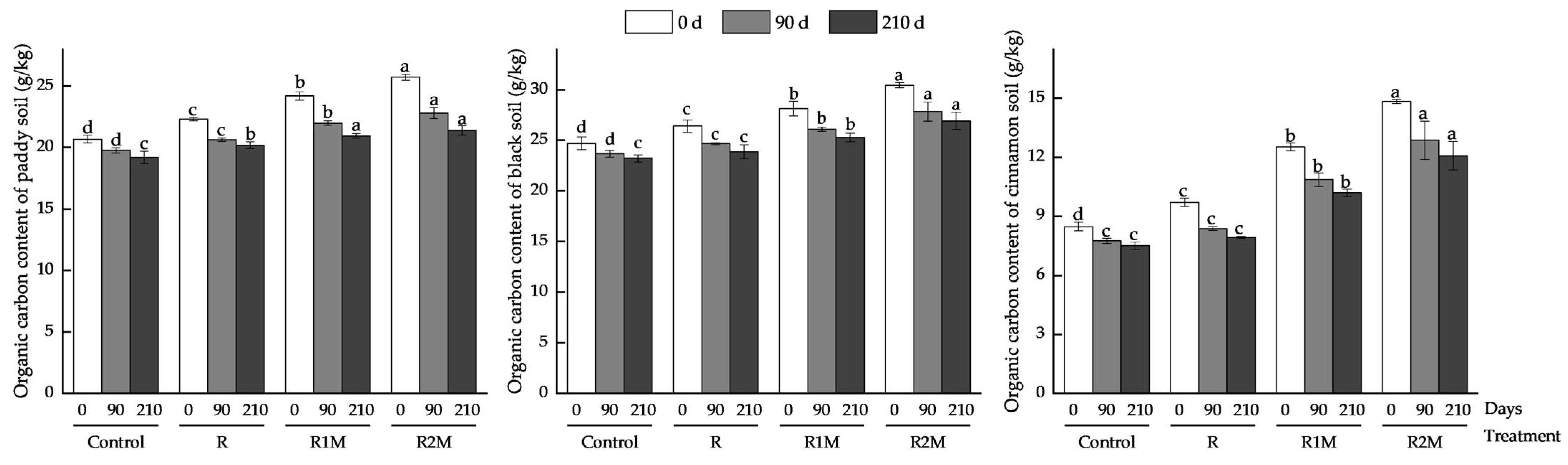
3.4. Organic Carbon Content of Different Types of Soil
3.5. Mineralization Parameters of Organic Carbon in Different Types of Soil
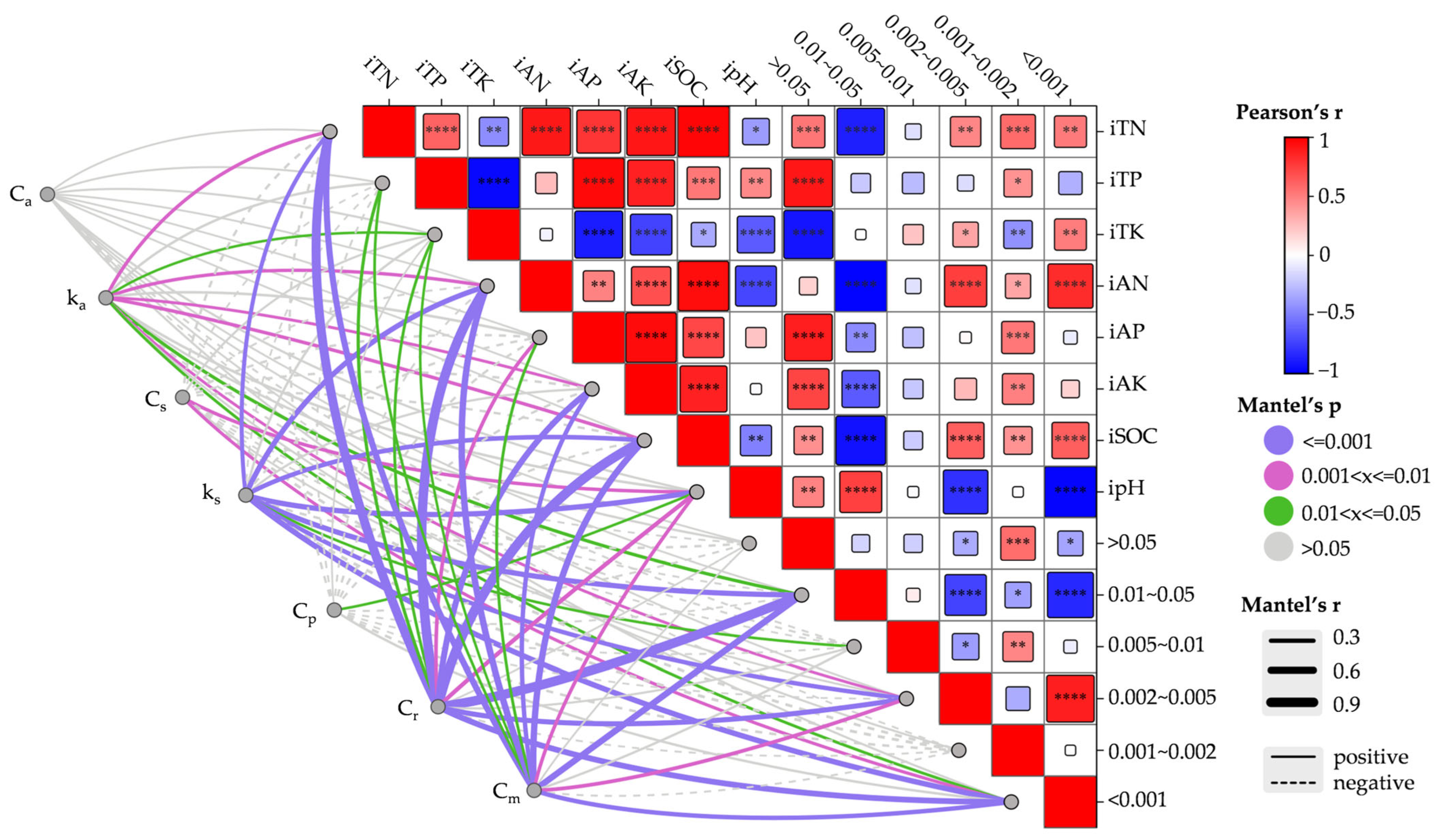
3.6. Correlation Between Organic Carbon and Mineralization Parameters at Different Incubation Times
3.7. The Relationship Between the Basic Physicochemical Properties and Particle Composition of the Initial Soil and the Mineralization Parameters
4. Discussions
4.1. Carbon Dioxide Emission Rates and Cumulative Emissions from Different Types of Soil
4.2. Dynamic Changes in Soil Organic Carbon Content of Different Types of Soil
4.3. Kinetic Characteristics of Organic Carbon Mineralization in Different Types of Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Guo, X.; Zhang, S.; Xiao, L.; Mishra, U.; Yang, Y.; Zhu, B.; Wang, G.; Mao, X.; Qian, T.; et al. Global soil profiles indicate depth-dependent soil carbon losses under a warmer climate. Nat. Commun. 2022, 13, 5514. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.; Koven, C.; Riley, W.; Torn, M. Toward improved model structures for analyzing priming: Potential pitfalls of using bulk turnover time. Glob. Change Biol. 2015, 21, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Caitlin, E.; Castanha, C.; Porras, R.; Torn, M. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar]
- Zhang, S.; Wang, M.; Xiao, L.; Guo, X.; Zheng, J.; Zhu, B.; Luo, Z. Reconciling carbon quality with availability predicts temperature sensitivity of global soil carbon mineralization. Proc. Natl. Acad. Sci. USA 2024, 121, e2313842121. [Google Scholar] [CrossRef]
- Lü, Z.; Liu, X.; Zhong, J.; Lan, X.; Hou, H.; Ji, J.; Feng, Z.; Liu, Y. Effects of Long-Term Fertilization on Mineralization of Soil Organic Carbon in Red Paddy Soil. Sci. Agric. Sin. 2019, 52, 2636–2645. [Google Scholar]
- Parton, W.; Stewart, W.; Cole, C. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1998, 5, 109–131. [Google Scholar] [CrossRef]
- Denis, C.; Campbell, C.; Abdul, J. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar]
- Varney, R.; Chadburn, S.; Burke, E.; Cox, P. Evaluation of soil carbon simulation in CMIP6 Earth system models. Biogeosciences 2022, 19, 4671–4704. [Google Scholar] [CrossRef]
- Alejandro, D.; Andrew, J.; Ramón, E. Decomposing Temperature Extremes Errors in CMIP5 and CMIP6 Models. Geophys. Res. Lett. 2020, 47, e2020GL088031. [Google Scholar]
- Paul, E.; Van, V. The use of tracers to determine the dynamic nature of organic matter. In Proceedings of the 11th International Society of Soil Science, Edmonton, AB, USA, 19–27 June 1978; pp. 61–102. [Google Scholar]
- Li, Z.; Liu, M.; Wu, X.; Han, F.; Zhang, T. Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Tillage Res. 2009, 106, 268–274. [Google Scholar] [CrossRef]
- Zhang, B.; Dou, S.; Guo, D.; Guan, S. Straw Inputs Improve Soil Hydrophobicity and Enhance Organic Carbon Mineralization. Agronomy 2023, 13, 2618. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Hong, Y.; Zhao, J.; Xuan, Q.; Huo, L.; Tan, B.; Xiu, W.; Wang, L. Characteristics of soil organic carbon mineralization in the soil of maize fields with different soil fertility. J. Agro-Environ. Sci. 2019, 38, 590–599. [Google Scholar]
- Li, S.; Qiu, L.; Zhang, X. Mineralization of soil organic carbon and its relations with soil physical and chemical properties on the Loess Plateau. Acta Ecol. Sin. 2010, 30, 1217–1226. [Google Scholar]
- Wu, M.; Li, Z.; Feng, Y.; Chen, R.; Jiang, C.; Liu, M. Dynamic Differences of Organic Carbon Mineralization in Differentypes of Paddy Soil Under Long-Term Located Fertilization. Sci. Agric. Sin. 2016, 49, 1705–1714. [Google Scholar]
- Li, M.; Chen, T.; Hong, X.; Yu, T.; Hu, Y. Effects of adding exogenous carbon with different chemical structure on the dynamics of organic carbon mineralization in red and sandy soils. Chin. J. Ecol. 2021, 40, 1609–1617. [Google Scholar]
- Mamedov, I.; Huang, C.; Aliev, F.; Levy, G. Aggregate Stability and Water Retention Near Saturation Characteristics as Affected by Soil Texture, Aggregate Size and Polyacrylamide Application. Land Degrad. Dev. 2017, 28, 543–552. [Google Scholar] [CrossRef]
- Wu, D.; Mehmet, S.; Zang, H.; Ugurlar, F.; Aydemir, S.; Brüggemann, N.; Kuzyakov, Y.; Bol, R.; Evgenia, B. Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl. Soil Ecol. 2018, 129, 121–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, H.; Hu, X.; Xie, W.; Liu, Z.; Zhou, H.; Yang, Z. Characteristics and Driving Forces of Organic Carbon Mineralization in Brown Soil with Long-term Straw Returning. Environ. Sci. 2024, 45, 2353–2362. [Google Scholar]
- Li, N.; Teng, P.; Lei, W.; Long, J.; Li, L. Effects and mechanisms of addition of different types of exogenous organic materials on priming effect of organic carbon in arable black soils. Chin. J. Eco-Agric. 2023, 31, 1588–1601. [Google Scholar]
- Hu, S.; Nan, Q.; Wen, L.; Zhao, L. Effects of adding microplastics and rice straw on emission of CO2 from typical farmland soils. J. Huazhong Agric. Univ. 2024, 43, 219–228. [Google Scholar]
- Xi, W.; Xu, X.; Zhao, S.; Wei, D.; Zhou, B.; Huang, S.; Yu, X.-C.; Qiu, S.; He, P.; Zhou, W. Comparison of organic carbon content and its mineralization potential in three dryland soils under long-term fertilization. J. Plant Nutr. Fertil. 2021, 27, 2094–2104. [Google Scholar]
- Liu, S.; Chen, F.; Wang, C.; Kong, F.; Jiang, Z. Effects of polyethylene microplastics with different particle sizes on soil organic carbon characteristics and mineralization in agricultural soil. Environ. Sci. 2025, 46, 3161–3170. [Google Scholar]
- Shi, Y.; Zeng, H.; Fang, L.; Deng, Y.; Xiao, R. Mineralization and humification of chicken manure and composted kitchen waste in soils based on an in situ litter-bag experiment: Impacts of organic inputs and microbial community. Front. Agr. Sci. Eng. 2024, 11, 602–614. [Google Scholar]
- Guo, L.; Wu, G.; Li, Y.; Li, C.; Liu, W.; Meng, J.; Liu, H.; Yu, X.; Jiang, G. Effects of cattle manure compost combined with chemical fertilizer on topsoil organic matter, bulk density and earthworm activity in a wheat–maize rotation system in Eastern China. Soil Tillage Res. 2016, 156, 140–147. [Google Scholar] [CrossRef]
- Ahmed, H.; Adel, R.; Abdulrasoul, A.; Yong, S.; Mahtab, A.; Mohammad, I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar]
- Liu, Y.; Ge, T.; Zhu, Z.; Liu, S.; Luo, Y.; Li, Y.; Wang, P.; Gavrichkova, O.; Xu, X.; Wang, J.; et al. Carbon input and allocation by rice into paddy soils: A review. Soil Biol. Biochem. 2019, 133, 97–107. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, J.; Sun, Z. Study on the Factors Affecting the Humus Horizon Thickness in the Black Soil Region of Liaoning Province, China. Agronomy 2024, 14, 2106. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, H.; Wen, Y.; Xiang, Y.; Cheng, M. Ecological stoichiometric characteristics of nutrients and enzyme activities in brown soil under long-term fertilization. J. Plant Nutr. Fertil. 2022, 28, 972–983. [Google Scholar]
- Zhang, P.; Hu, W.; Guan, Z.; Wang, C.; Ma, X.; Zou, Y. Effects of grass on soil nutrients, enzyme activities and bacterial community in apple orchard. Northwest A&F Univ. Nat. Sci. Ed. 2024, 52, 96. [Google Scholar]
- Wei, S.; Chen, X.; Zhao, G.; Li, Y.; Dong, Y.; Jiao, R.; Zhang, X. Effects of close-to-nature transformation of Chinese fir plantation on soil chemical properties and enzyme activities. Acta Ecol. Sin. 2024, 44, 4277–4287. [Google Scholar]
- Zhang, R.; Yuan, D.; Qin, S.; Hu, C. Effects of carbon, nitrogen, and phosphorus stoichiometry on the priming of soil carbon mineralization. Chin. J. Eco-Agric. 2023, 31, 1311–1321. [Google Scholar]
- Börger, M.; Bublitz, T.; Dyckmans, J.; Wachendorf, C.; Jørgensen, R.G. Microbial carbon use efficiency of litter with distinct C/N ratios in soil at different temperatures, including microbial necromass as growth component. Biol. Fertil. Soils 2022, 58, 761–770. [Google Scholar] [CrossRef]
- Guenet, B.; Camino-Serrano, M.; Ciais, P.; Tifafi, M.; Maignan, F.; Soong, J.L.; Janssens, I.A. Impact of priming on global soil carbon stocks. Glob. Change Biol. 2018, 24, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Mercer, G.D.; Mickan, B.S.; Gleeson, D.B.; Walker, E.; Krohn, C.; Bühlmann, C.H.; Ryan, M.H. Probing the pump: Soil carbon dynamics, microbial carbon use efficiency and community composition in response to stoichiometrically-balanced compost and biochar. Soil Biol. Biochem. 2025, 205, 109770. [Google Scholar] [CrossRef]
- Zhou, G.; Li, G.; Liang, H.; Liu, R.; Ma, Z.; Gao, S.; Chang, D.; Liu, J.; Chadwick, D.R.; Jones, D.L.; et al. Green Manure Coupled With Straw Returning Increases Soil Organic Carbon via Decreased Priming Effect and Enhanced Microbial Carbon Pump. Glob. Change Biol. 2025, 31, e70232. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, P.; Wu, Y.; Wu, X.; Ni, H.; Lu, Q.; Zang, S. Long-term surface composts application enhances saline-alkali soil carbon sequestration and increases bacterial community stability and complexity. Environ. Res. 2024, 240, 117425. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Liang, B.; Wang, W.; Liu, J. The impact of green waste on carbon sequestration and emission reduction in coastal saline soil and its effect on microbial community structure. Environ. Eng. 2024, 42, 95–101. [Google Scholar]
- Paterson, E.; Sim, A. Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob. Change Biol. 2013, 19, 1562–1571. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Kou, T.; Xu, P.; Tan, X.; Guo, D. Transport of straw-derived carbon in black soil and cinnamon soil and its response to nitrogen fertilization. Acta Pedol. Sin. 2022, 59, 1248–1257. [Google Scholar]
- Song, W.; Liang, Y.; Tao, Z.; Zhong, Q.; He, Y. Advances on soil organic carbon dynamics mediated by microorganisms. Adv. Earth Sci. 2023, 38, 1213–1223. [Google Scholar]
- Liu, S.; Wang, J.; Pu, S.; Evgenia, B.; Yakov, K.; Bahar, S. Impact of manure on soil biochemical properties: A global synthesis. Sci. Total Environ. 2020, 745, 141003. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Sayre, J.; Schmidt, R.; Fonte, S.; Rodrigues, J.; Scow, K. Compost amendment maintains soil structure and carbon storage by increasing available carbon and microbial biomass in agricultural soil-A six-year field study. Geoderma 2022, 427, 116117. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, H.; Zhu, Q.; Wang, X.; Zhang, Y.; Yu, X.; Peng, X. Carbon sequestration efficiency in paddy soil and upland soil under long-termfertilization in southern China. Soil Tillage Res. 2013, 130, 42–51. [Google Scholar] [CrossRef]
- Zuijdeest, A.; Baumgarther, S.; Wehrli, B. Hysteresis effects in organic matter turnover in a tropical floodplain during a flood cycle. Biogeochemistry 2016, 131, 49–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, S.; Bol, R.; Mansoor, A.; Wu, W.; Hu, Y.; Meng, F. Intensive organic vegetable production increases soil organic carbon but with a lower carbon conversion efficiency than integrated management. J. Plant Nutr. Soil Sci. 2020, 183, 155–168. [Google Scholar] [CrossRef]
- Pan, J.; Dai, W.; Shang, Z.; Guo, R. Review of research progress on the influence and mechanism of filed straw residue incorporation on soil organic matter and nitrogen availability. Chin. J. Eco-Agric. 2013, 21, 526–535. [Google Scholar] [CrossRef]
- Chen, Y.; Marta, C.; Shen, Q.; Singh, B.; Maria, L. The long-term role of organic amendments in building soil nutrient fertility: A meta analysis and review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, L.; Zhao, Y.; Ru, S.; Zhao, O.; Hou, L.; Sun, S.; Wang, L.; Wang, C. Effects of organic materials input on soil organic carbon in China based on Meta-analysis. Soil Fertil. Sci. China 2023, 8, 23–32. [Google Scholar]
- Ding, J.; Chen, L.; Zhang, B.; Liu, L.; Yang, G.; Fang, K.; Chen, Y.; Li, F.; Kou, D.; Ji, C.; et al. Linking temperature sensitivity of soil CO2 release to substrate, environmental, and microbial properties across alpine ecosystems. Glob. Biogeochem. Cycles 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Frostegard, A.; Vick, S.; Lim, N.; Bakken, L.; Shapleigh, J. Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J. 2021, 16, 26–37. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2004, 124, 3–22. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, B.; Zhao, Q. A review on relationship between soil organic carbon pools and soil structure stability. Acta Pedol. Sin. 2004, 41, 618–623. [Google Scholar]
- Xu, P.; Liu, Y.; Zhu, J.; Shi, L.; Fu, Q.; Chen, J.; Hu, H.; Huang, Q. Influence mechanisms of long-term fertilizations on the mineralization of organic matter in Ultisol. Soil Tillage Res. 2020, 201, 104594. [Google Scholar] [CrossRef]
- Marinari, S.; Lagomarsino, A.; Moscatellim, C.; Tizio, A.; Campiglia, E. Soil carbon and nitrogen mineralization kinetics in organic and conventional three years cropping systems. Soil Tillage Res. 2010, 109, 161–168. [Google Scholar] [CrossRef]
- Li, X.; Bei, Q.; Rabiei Nematabad, M.; Peng, J.; Liesack, W. Time-shifted expression of acetoclastic and methylotrophic methanogenesis by a single Methanosarcina genomospecies predominates the methanogen dynamics in Philippine rice field soil. Microbiome 2024, 12, 39. [Google Scholar] [CrossRef]
- Song, X.; Liu, S.; Liu, Q.; Zhang, W.; Hu, C. Carbon sequestration in soil humic substances under long-term fertilization in a wheat-maize system from north China. J. Integr. Agric. 2014, 13, 562–569. [Google Scholar] [CrossRef]
- Groenigen, K.; Xuan, Q.; Osenberg, C.; Luo, Y.; Hungate, B. Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 2014, 344, 508–509. [Google Scholar] [CrossRef]
- Malik, A.; Puissant, J.; Buckeridge, K.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.; Peyton, J.; Mason, K.; Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Das, S.R.; Chatterjee, D.; Saha, S.; Sarkar, D.; Alam, R.; Dey, S.; Ghosh, S.; Nayak, B.K.; Smith, P.; Pathak, H. Enhancing carbon sequestration potential of lowland rice agroecosystems for environmentally clean production system: A review. Clim. Smart Agric. 2025, 2, 100054. [Google Scholar] [CrossRef]
- Song, X.; Hei, R.; Yao, Y.; Guo, D.; Luo, J.; Ma, Y. Effects of compost products of different Chinese medicinal residues on soil carbon mineralization. Soils 2023, 55, 45–52. [Google Scholar]


| Soil Type | Chinese Soil Classification System | FAO Soil Classification System | Soil Characteristics | Pedogenesis Environment | Diagnostic Horizons/Features |
|---|---|---|---|---|---|
| paddy soil | Soil order: Anthrosols (man-made soils) Soil class: paddy soil | Reference soil group: anthrosols (man-made soils) | Long-term paddy cultivation, with distinct plow layer and gleyed horizon, soil appears grayish-black or black, with heavy texture and high organic matter content. | Long-term paddy field environment, mainly found in plains or low-lying areas. | Plow layer (Aq), gleyed horizon (Gleyic horizon) |
| black soil | Soil order: chernozems (black soils) Soil class: black soil | Reference soil group: phaeozems (black soils) | Thick humus layer (ah), dark black or dark brown in color, high soil fertility, heavy texture, rich in humus and clay minerals. | Temperate semi-humid climate zone, meadow steppe vegetation, mainly found in the Northeast China Plain. | Humus layer (Ah), illuvial layer (Bts) |
| cinnamon soil | soil order: luvisols (luvic soils) soil class: cinnamon soil | Reference soil group: lixisols (low-activity lixisols) | Distinct leaching layer and illuvial layer, soil body appears brown, clay minerals mainly montmorillonite and illite, with moderate fertility. | Temperate humid climate zone, forest or grassland vegetation, mainly found in the Loess Plateau and North China Plain. | Leaching layer (E), illuvial layer (Bts), redox layer (gleyic features) |
| Soil Types | Total Nitrogen (iTN, g/kg) | Total Phosphorus (iTP, g/kg) | Total Potassium (iTK, g/kg) | Alkaline Hydrolyzable Nitrogen (iAN, mg/kg) | Available Phosphorus (iAP, mg/kg) | Available Potassium (iAK, mg/kg) | Organic Carbon (iSOC, g/kg) | pH (ipH) |
|---|---|---|---|---|---|---|---|---|
| Paddy soil | 0.25 ± 0.02 b | 4.27 ± 0.04 c | 8.43 ± 0.01 a | 166.11 ± 5.80 a | 23.83 ± 1.01 b | 177.42 ± 13.07 b | 20.68 ± 1.07 b | 5.35 ± 0.02 c |
| Black soil | 0.33 ± 0.02 a | 12.08 ± 0.17 a | 7.12 ± 0.09 c | 158.47 ± 1.31 a | 202.79 ± 11.34 a | 278.44 ± 7.08 a | 24.68 ± 0.09 a | 6.89 ± 0.02 b |
| Cinnamon soil | 0.11 ± 0.00 c | 5.90 ± 0.00 b | 7.90 ± 0.06 b | 49.17 ± 3.70 b | 13.04 ± 1.38 b | 132.35 ± 2.28 c | 8.48 ± 0.81 c | 7.38 ± 0.09 a |
| Soil Types | Proportion of Different Soil Particle Size Fractions (%) | |||||
|---|---|---|---|---|---|---|
| >0.05 mm | 0.01~0.05 mm | 0.005~0.01 mm | 0.002~0.005 mm | 0.001~0.002 mm | <0.001 mm | |
| Paddy soil | 16.71 ± 2.03 b | 35.55 ± 1.02 b | 12.19 ± 2.03 a | 16.25 ± 2.03 a | 4.06 ± 2.03 a | 15.24 ± 1.02 a |
| Black soil | 26.56 ± 3.10 a | 38.27 ± 1.03 b | 11.38 ± 1.03 a | 10.34 ± 6.21 ab | 6.21 ± 4.14 a | 7.24 ± 1.03 b |
| Cinnamon soil | 19.37 ± 1.02 b | 59.20 ± 2.04 a | 12.25 ± 2.04 a | 4.08 ± 2.04 b | 3.06 ± 1.02 a | 2.04 ± 0.00 c |
| Soil Types | Treatment | Ca (g/kg) | ka (×10−1 d) | Cs (g/kg) | ks (×10−2 d) | Cp (g/kg) | Cr (g/kg) | Cm (%) |
|---|---|---|---|---|---|---|---|---|
| Paddy soil | Control | 0.46 ± 0.06 Ab | 1.28 ± 0.06 Ba | 1.16 ± 0.02 Bd | 0.91 ± 0.14 Aa | 1.62 ± 0.08 Bd | 19.06 ± 0.26 Bb | 7.85 ± 0.32 Bd |
| R | 0.88 ± 0.07 Ba | 1.01 ± 0.12 Ab | 1.58 ± 0.05 Bc | 0.72 ± 0.15 Aab | 2.46 ± 0.08 Bc | 19.83 ± 0.21 Ba | 11.02 ± 0.42 Bc | |
| R1M | 0.79 ± 0.03 Ba | 1.14 ± 0.06 Aab | 3.08 ± 0.20 Ab | 0.70 ± 0.021 Ab | 3.86 ± 0.18 Ab | 20.32 ± 0.43 Ba | 15.98 ± 0.84 Bb | |
| R2M | 0.86 ± 0.04 Ca | 1.01 ± 0.04 Ab | 4.45 ± 0.13 Aa | 0.69 ± 0.03 Ab | 5.31 ± 0.11 Aa | 20.40 ±0.32 Ba | 20.66 ±0.55 Ba | |
| Black soil | Control | 0.43 ± 0.02 Ac | 1.71 ± 0.12 Aa | 1.84 ± 0.10 Ac | 0.42 ± 0.02 Bb | 2.28 ± 0.08 Ac | 22.41 ± 0.61 Ac | 9.23 ± 0.34 Bb |
| R | 1.06 ± 0.06 Ab | 0.86 ± 0.04 ABb | 2.59 ± 0.32 Ab | 0.41 ± 0.13 Bb | 3.65 ± 0.38 Ab | 22.74 ± 0.23 Ac | 13.80 ± 1.13 Ba | |
| R1M | 1.01 ± 0.06 Bb | 0.96 ± 0.05 Bb | 2.83 ± 0.27 Aab | 0.53 ± 0.08 Bab | 3.84 ± 0.31 Aab | 24.29 ± 0.70 Ab | 13.64 ± 1.08 Ba | |
| R2M | 1.20 ± 0.06 Aa | 0.92 ± 0.05 Ab | 3.10 ± 0.10 Ba | 0.67 ± 0.03 Aa | 4.30 ± 0.07 Ba | 26.13 ± 0.21 Aa | 14.12 ± 0.19 Ca | |
| Cinnamon soil | Control | 0.39 ± 0.01 Ab | 1.15 ± 0.03 Ba | 1.37 ± 0.26 Bb | 0.29 ± 0.07 Bb | 1.76 ± 0.27 Bb | 6.72 ± 0.06 Cc | 20.72 ± 2.60 Ab |
| R | 1.03 ± 0.04 Aa | 0.79 ± 0.04 Bb | 2.43 ± 0.63 Aa | 0.24 ± 0.10 Bb | 3.46 ± 0.67 Aa | 6.25 ± 0.81 Cc | 35.65 ± 7.27 Aa | |
| R1M | 1.08 ± 0.10 Aa | 0.72 ± 0.02 Cc | 2.40 ± 0.34 Ba | 0.35 ± 0.09 Cb | 3.54 ± 0.43 Aa | 8.99 ± 0.53 Cb | 28.26 ± 3.62 Aab | |
| R2M | 1.04 ± 0.04 Ba | 0.79 ± 0.04 Bb | 2.46 ± 0.18 Ca | 0.52 ± 0.10 Ba | 3.44 ± 0.22 Ca | 11.39 ± 0.10 Ca | 23.21 ± 1.28 Ab | |
| Treatment | *** | *** | *** | ** | *** | *** | *** | |
| Soil type | *** | *** | ** | *** | ** | *** | *** | |
| Treatment × soil type | *** | *** | *** | ** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, X.; Shi, A.; Xu, M.; Zhang, F.; Zhang, L.; Zang, J.; Xu, X.; Gao, J. Effect of Compost Addition on Carbon Mineralization and Kinetic Characteristics in Three Typical Agricultural Soils. Agronomy 2025, 15, 1559. https://doi.org/10.3390/agronomy15071559
Zhang S, Chen X, Shi A, Xu M, Zhang F, Zhang L, Zang J, Xu X, Gao J. Effect of Compost Addition on Carbon Mineralization and Kinetic Characteristics in Three Typical Agricultural Soils. Agronomy. 2025; 15(7):1559. https://doi.org/10.3390/agronomy15071559
Chicago/Turabian StyleZhang, Shanglong, Xianni Chen, Aoxue Shi, Minggang Xu, Fenggang Zhang, Lu Zhang, Jiaojiao Zang, Xiaofeng Xu, and Jiakai Gao. 2025. "Effect of Compost Addition on Carbon Mineralization and Kinetic Characteristics in Three Typical Agricultural Soils" Agronomy 15, no. 7: 1559. https://doi.org/10.3390/agronomy15071559
APA StyleZhang, S., Chen, X., Shi, A., Xu, M., Zhang, F., Zhang, L., Zang, J., Xu, X., & Gao, J. (2025). Effect of Compost Addition on Carbon Mineralization and Kinetic Characteristics in Three Typical Agricultural Soils. Agronomy, 15(7), 1559. https://doi.org/10.3390/agronomy15071559







