Processing Tomato Crop Benefits from Flowering Plants in Field Margins That Support Pollinators and Natural Enemies
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Plant Species and Mixture Composition
2.2. Seed Rate Calculation
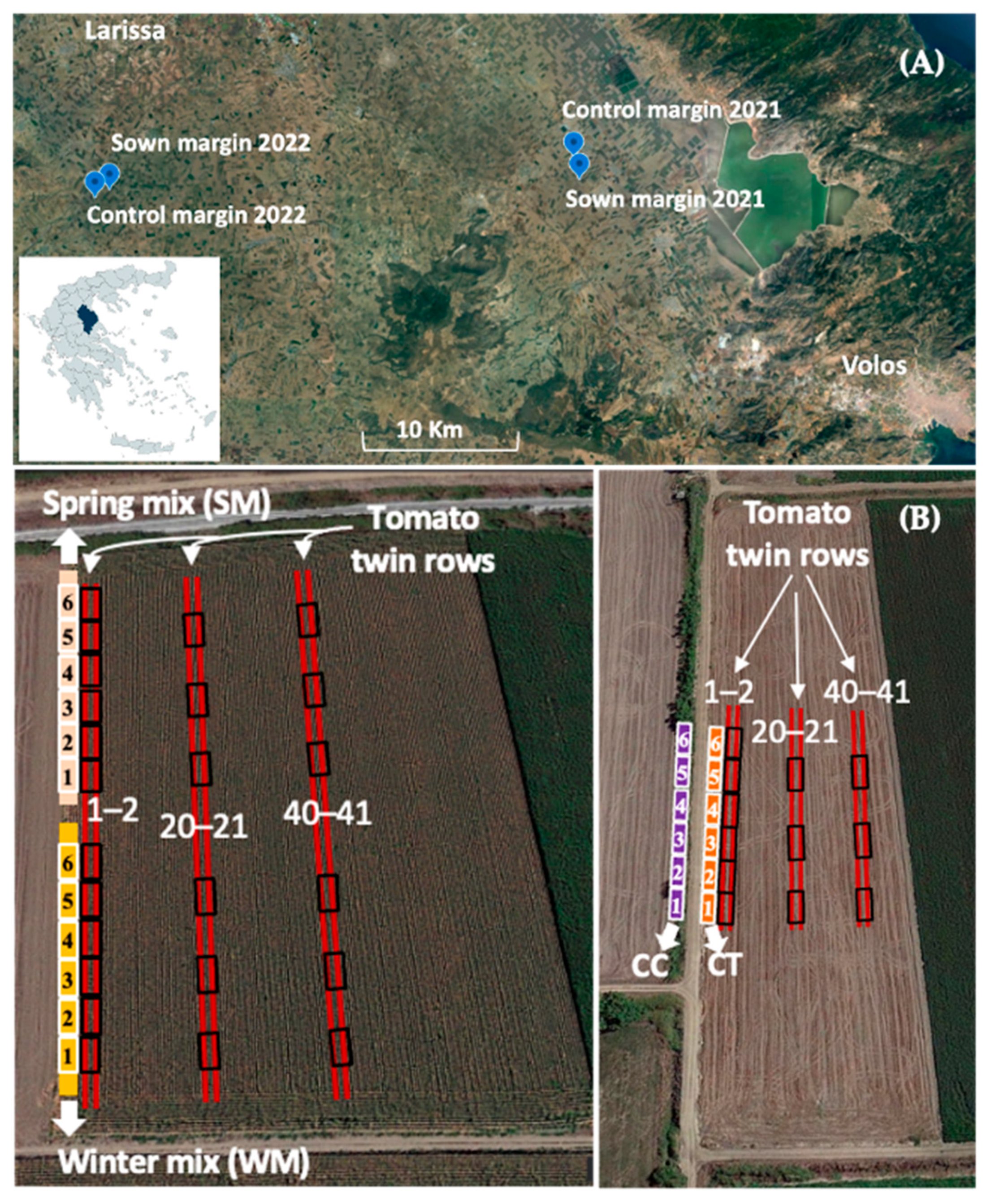
2.3. Experimentation Site
2.4. Arthropod Measurements
2.5. Crop Yield Parameters
2.6. Statistical Analysis
3. Results
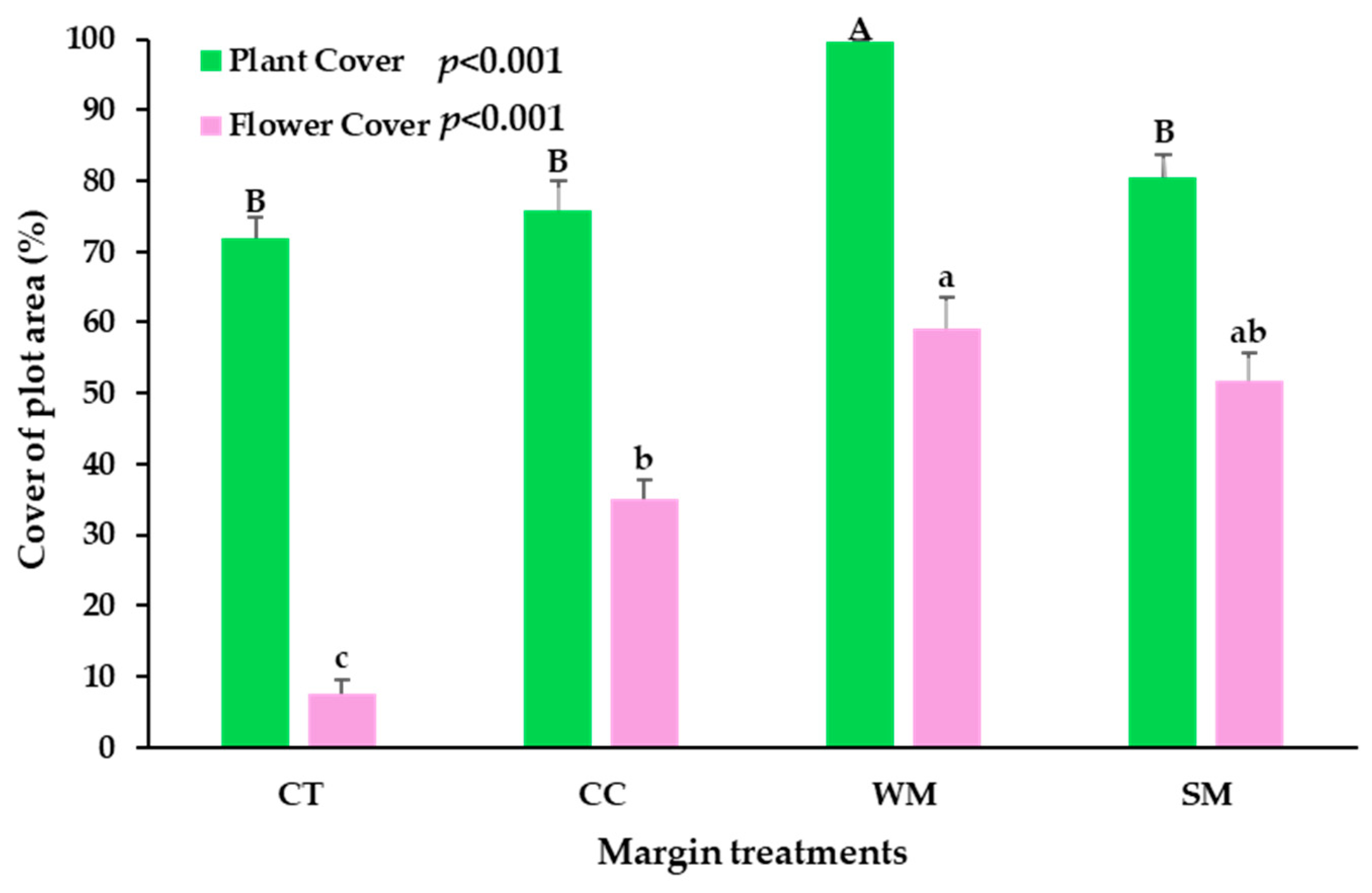
3.1. Plant Cover
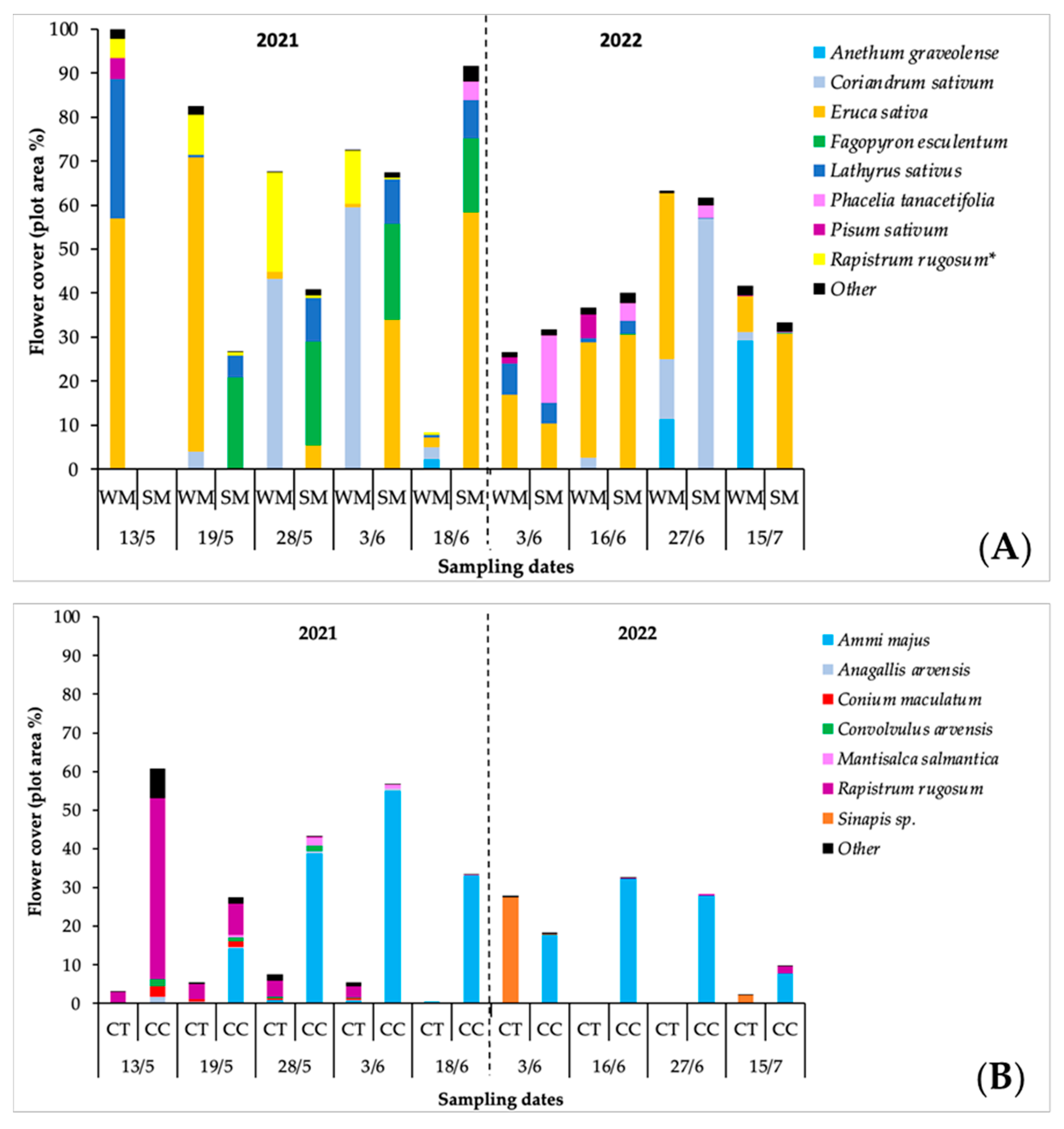
3.2. Flower Cover
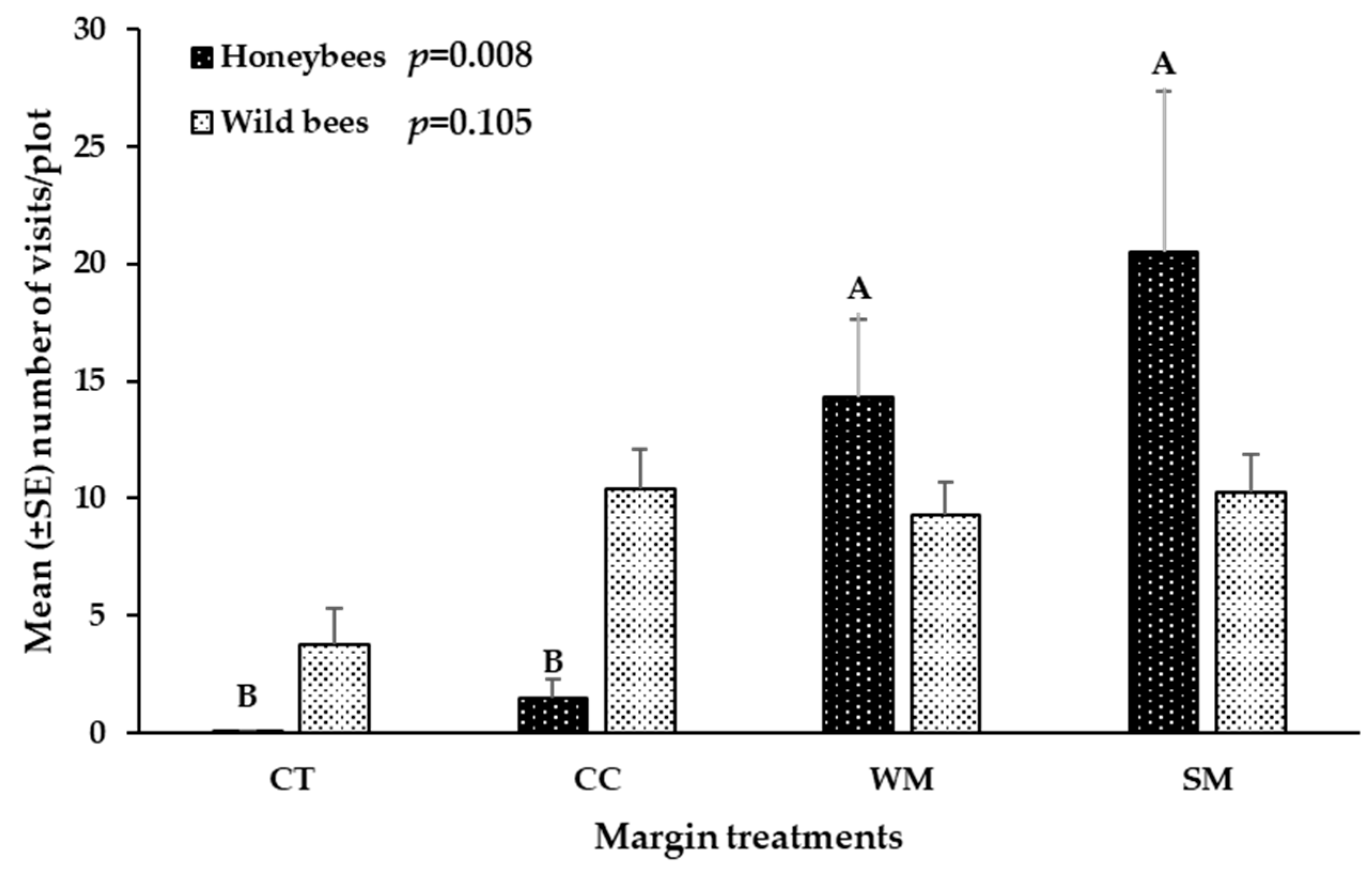
3.3. Hymenoptera Pollinators
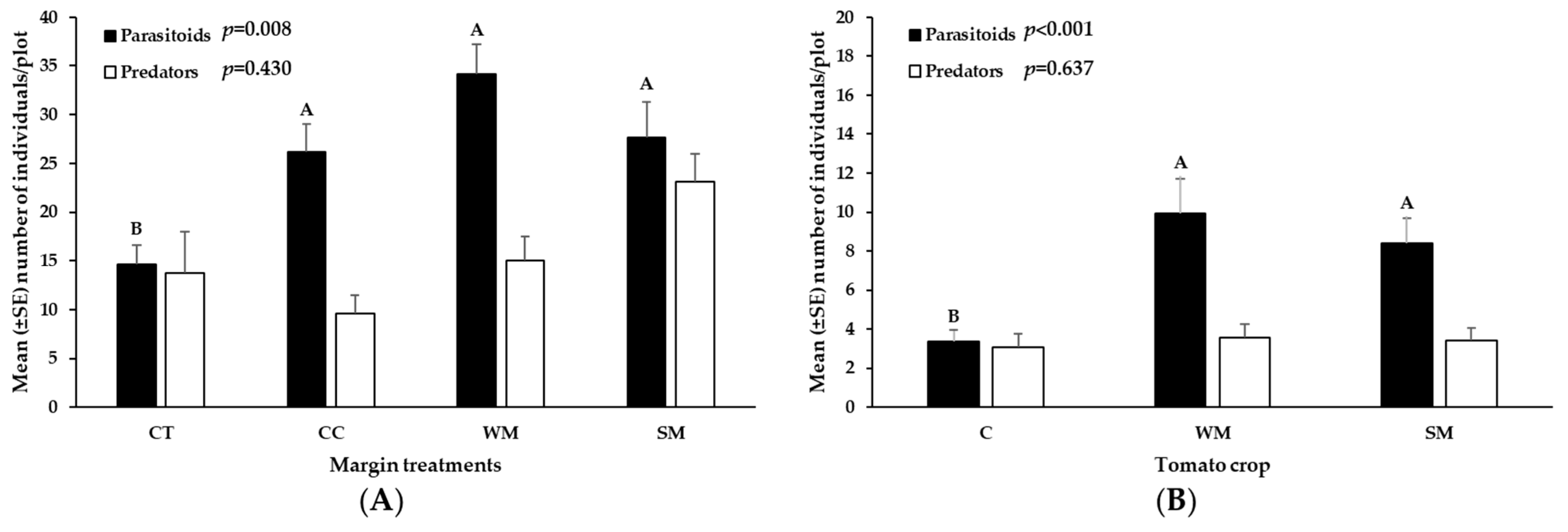
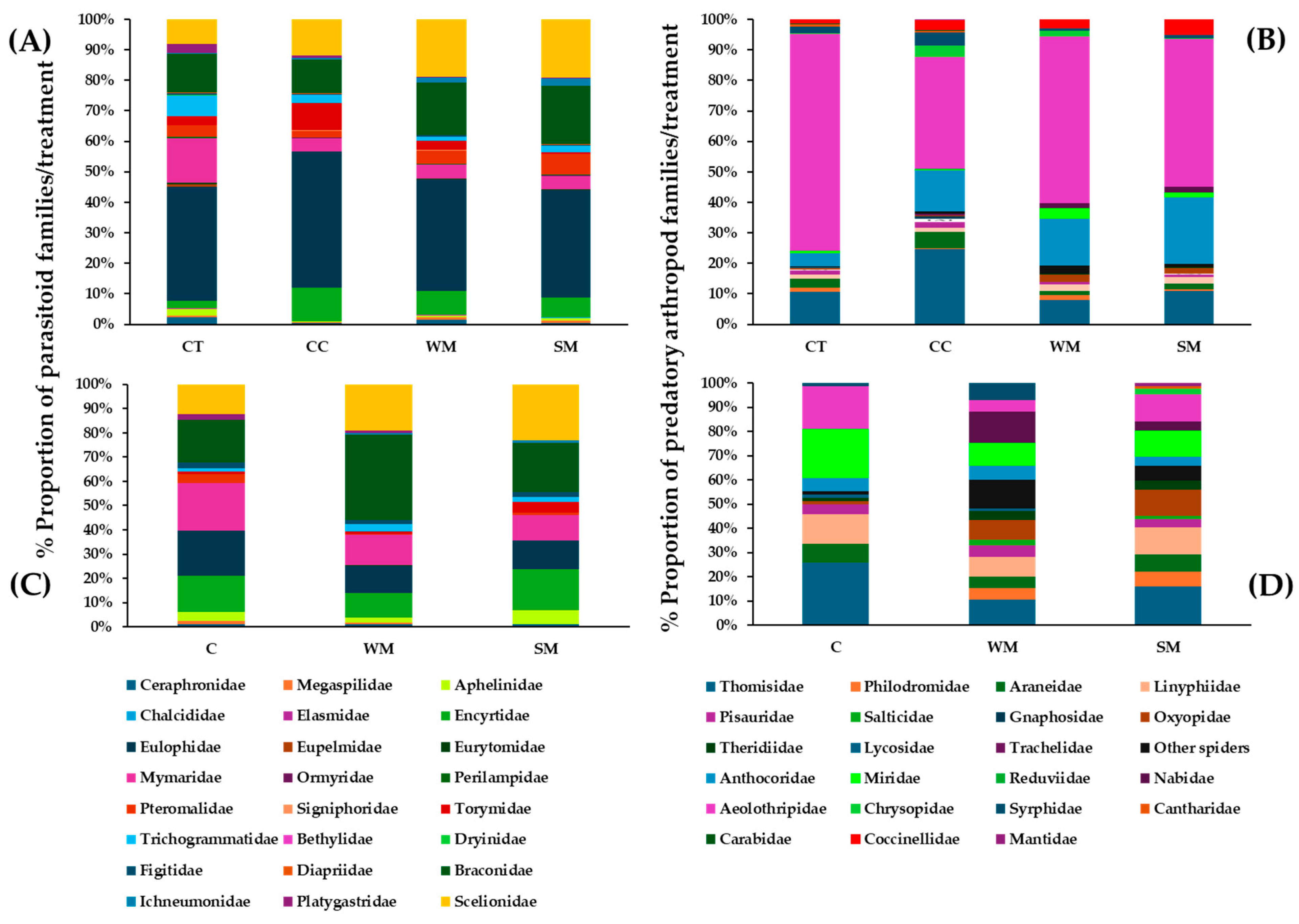
3.4. Natural Enemies
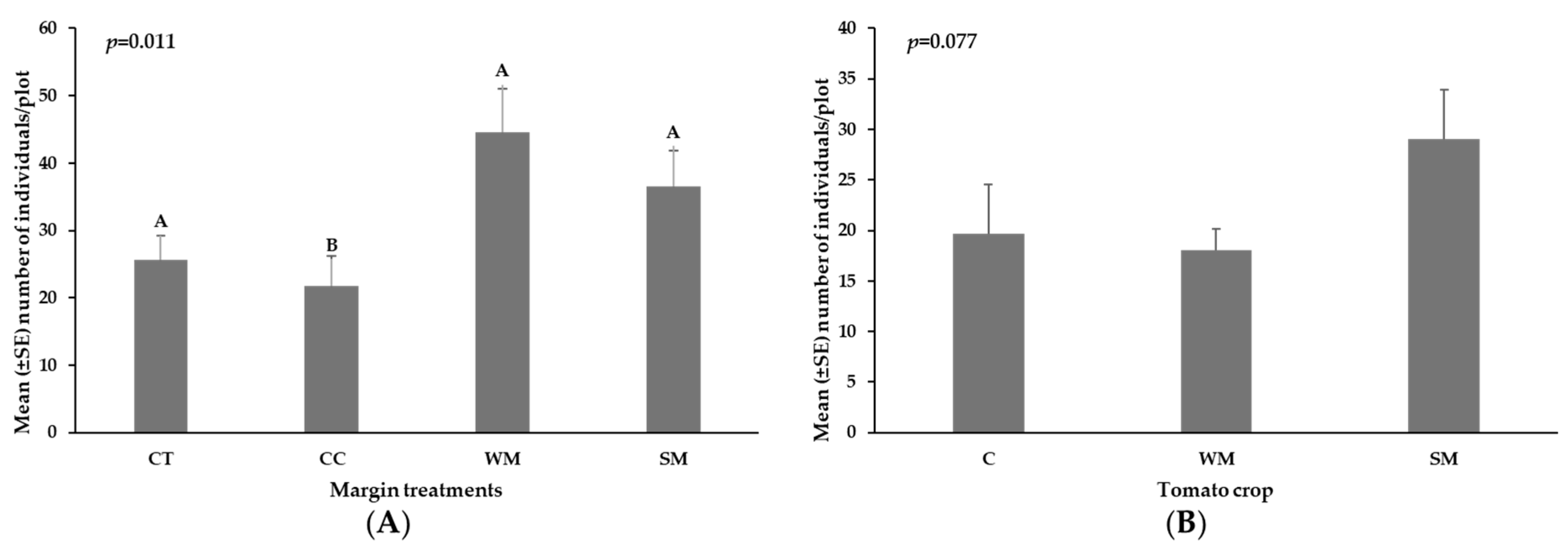
3.5. Insect Pests
3.6. Crop Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeanneret, P.; Lüscher, G.; Schneider, M.K.; Pointereau, P.; Arndorfer, M.; Bailey, D.; Balázs, K.; Báldi, A.; Choisis, J.-P.; Dennis, P.; et al. An increase in food production in Europe could dramatically affect farmland biodiversity. Commun. Earth Environ. 2021, 2, 183. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Ives, A.R. Effects of experimental shifts in flowering phenology on plant–pollinator interactions. Ecol. Lett. 2011, 14, 69–74. [Google Scholar] [CrossRef]
- Memmott, J.; Carvell, C.; Pywell, R.F.; Craze, P.G. The potential impact of global warming on the efficacy of field margins sown for the conservation of bumble-bees. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Elias, M.A.S.; Borges, F.J.A.; Bergamini, L.L.; Franceschinelli, E.V.; Sujii, E.R. Climate change threatens pollination services in tomato crops in Brazil. Agric. Ecosyst. Environ. 2017, 239, 257–264. [Google Scholar] [CrossRef]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Guzmán, C.; Aguilar-Fenollosa, E.; Sahún, R.M.; Boyero, J.R.; Vela, J.M.; Wong, E.; Jaques, J.A.; Montserrat, M. Temperature-specific competition in predatory mites: Implications for biological pest control in a changing climate. Agric. Ecosyst. Environ. 2016, 216, 89–97. [Google Scholar] [CrossRef]
- Naylor, R.; Ehrlich, P.R. Natural pest control services and agriculture. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997; pp. 151–174. [Google Scholar]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2006, 274, 303–313. [Google Scholar] [CrossRef]
- Emmerson, M.; Morales, M.B.; Oñate, J.J.; Batáry, P.; Berendse, F.; Liira, J.; Aavik, T.; Guerrero, I.; Bommarco, R.; Eggers, S.; et al. Chapter Two—How agricultural intensification affects biodiversity and ecosystem services. In Advances in Ecological Research; Large-Scale Ecology: Model Systems to Global Perspectives; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 5, pp. 43–97. [Google Scholar]
- Dudley, N.; Alexander, S. Agriculture and Biodiversity: A Review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Peeters, A.; Lefebvre, O.; Balogh, L.; Barberi, P.; Batello, C.; Bellon, S.; Gaifami, T.; Gkisakis, V.; Lana, M.; Migliorini, P.; et al. A green deal for implementing agroecological systems—Reforming the common agricultural policy of the European Union. J. Sustain. Agric. Syst. 2020, 70, 83–93. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Wäckers, F.L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol. Control 2008, 46, 400–408. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F. Getting more power from your flowers: Multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kati, V.; Volakakis, N.; Varikou, K.; Garantonakis, N.; Economou, L.; Birouraki, A.; Markellou, E.; Liberopoulou, S.; Edwards, M. Ground cover management with mixtures of flowering plants to enhance insect pollinators and natural enemies of pests in olive groves. Agric. Ecosyst. Environ. 2019, 274, 76–89. [Google Scholar] [CrossRef]
- Kati, V.; Karamaouna, F.; Economou, L.; Mylona, P.V.; Samara, M.; Mitroiu, M.-D.; Barda, M.; Edwards, M.; Liberopoulou, S. Sown wildflowers enhance habitats of pollinators and beneficial arthropods in a tomato field margin. Plants 2021, 10, 1003. [Google Scholar] [CrossRef]
- Barda, M.; Karamaouna, F.; Kati, V.; Perdikis, D. Do patches of flowering plants enhance insect pollinators in apple orchards? Insects 2023, 14, 208. [Google Scholar] [CrossRef]
- Scheper, J.; Badenhausser, I.; Kantelhardt, J.; Kirchweger, S.; Bartomeus, I.; Bretagnolle, V.; Clough, Y.; Gross, N.; Raemakers, I.; Vilà, M.; et al. Biodiversity and pollination benefits trade off against profit in an intensive farming system. Proc. Natl. Acad. Sci. USA 2023, 120, e2212124120. [Google Scholar] [CrossRef]
- Sanchez, J.A.; de Pedro, L.; López-Gallego, E.; Pérez-Marcos, M.; Ramírez-Soria, M.J.; Perera-Fernández, L.G.; Atenza, J.F. How plant composition in margins influences the assemblage of pests and predators and its effect on biocontrol in melon fields. Sci. Rep. 2024, 14, 13094. [Google Scholar] [CrossRef]
- Jachowicz, N.; Sigsgaard, L. Highly diverse flower strips promote natural enemies more in annual field crops: A review and meta-analysis. Agric. Ecosyst. Environ. 2025, 381, 109412. [Google Scholar] [CrossRef]
- Pérez-Méndez, N.; Alcaraz, C.; Catala-Forner, M. Ecological restoration of field margins enhances biodiversity and multiple ecosystem services in rice agroecosystems. Agric. Ecosyst. Environ. 2025, 382, 109484. [Google Scholar] [CrossRef]
- Crowther, L.I.; Wilson, K.; Wilby, A. The impact of field margins on biological pest control: A meta-analysis. BioControl 2023, 68, 387–396. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-Herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, S.S.; Kremen, C. Wild bee species increase tomato production and respond differently to surrounding land use in Northern California. Biol. Conserv. 2006, 133, 81–87. [Google Scholar] [CrossRef]
- Teppner, H. Pollinators of tomato, Solanum lycopersicum (Solanaceae), in Central Europe. Phyton Ann. Rei Bot. 2005, 45, 217–235. [Google Scholar]
- Barda, M.S.; Karamaouna, F.; Kati, V.; Stathakis, T.I.; Economou, L.P.; Perdikis, D.C. Flowering plant patches to support the conservation of natural enemies of pests in apple orchards. Agric. Ecosyst. Environ. 2025, 381, 109405. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964; Volumes 1–5. [Google Scholar]
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MA, USA, 2007. [Google Scholar]
- Collins, A.G. Key to the Genera of British Bees. 2012. Available online: https://bwars.com/sites/default/files/diary_downloads/Britain%27s_Bees_Chapter_4_Keys_to_Genera.pdf (accessed on 30 May 2025).
- Stewart, A.J.A.; Wright, A.F. A New inexpensive suction apparatus for sampling arthropods in grassland. Ecol. Entomol. 1995, 20, 98–102. [Google Scholar] [CrossRef]
- CAP 2023-27. Agriculture and Rural Development. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-2023-27_en (accessed on 25 February 2025).
- Cole, L.J.; Kleijn, D.; Dicks, L.V.; Stout, J.C.; Potts, S.G.; Albrecht, M.; Balzan, M.V.; Bartomeus, I.; Bebeli, P.J.; Bevk, D.; et al. A Critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 2020, 57, 681–694. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kati, V.; Economou, L.; Troyanos, G.; Samara, M.; Liberopoulou, S.; Barda, M.; Mitroiu, M.-D.; Edwards, M. Selected flowering plants as a habitat for pollinators and natural enemies in field margins of a watermelon crop—Implications for crop yield. Int. J. Pest Manag. 2024, 70, 920–936. [Google Scholar] [CrossRef]
- Egan, P.A.; Dicks, L.V.; Hokkanen, H.M.T.; Stenberg, J.A. Delivering Integrated Pest and Pollinator Management (IPPM). Trends Plant Sci. 2020, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Lundin, O.; Ward, K.L.; Williams, N.M. Identifying native plants for coordinated habitat management of arthropod pollinators, herbivores and natural enemies. J. Appl. Ecol. 2019, 56, 665–676. [Google Scholar] [CrossRef]
- Lundin, O.; Rundlöf, M.; Jonsson, M.; Bommarco, R.; Williams, N.M. Integrated pest and pollinator management–expanding the concept. Front. Ecol. Environ. 2021, 19, 283–291. [Google Scholar] [CrossRef]
- Decourtye, A.; Mader, E.; Desneux, N. Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 2010, 41, 264–277. [Google Scholar] [CrossRef]
- Liira, J.; Jürjendal, I. Are bees attracted by flower richness? implications for ecosystem service-based policy. Ecol. Indic. 2023, 154, 110927. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Lüdemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Rowe, L.; Gibson, D.; Bahlai, C.A.; Gibbs, J.; Landis, D.A.; Isaacs, R. Flower traits associated with the visitation patterns of bees. Oecologia 2020, 193, 511–522. [Google Scholar] [CrossRef]
- Cooley, H.; Vallejo-Marín, M. Buzz-Pollinated Crops: A global review and meta-analysis of the effects of supplemental bee pollination in tomato. J. Econ. Entomol. 2021, 114, 505–519. [Google Scholar] [CrossRef]
- Cardinal, S.; Buchmann, S.L.; Russell, A.L. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 2018, 72, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Amala, U.; Shivalingaswamy, T.M. Role of Native buzz pollinator bees in enhancing fruit and seed set in tomatoes under open field conditions. J. Entomol. Zool. Stud. 2017, 5, 1742–1744. [Google Scholar]
- Toni, H.C.; Djossa, B.A.; Ayenan, M.A.T.; Teka, O. Tomato (Solanum lycopersicum) pollinators and their effect on fruit set and quality. J. Hortic. Sci. Biotechnol. 2021, 96, 1–13. [Google Scholar] [CrossRef]
- El-Berry, A.A.; Moustafa, M.A.; Abdel-Gawaad, A.A.; El-Bialey, S. Pollinators other than honey bees visiting certain vegetable plants in Egypt. Z. Angew. Entomol. 1974, 77, 106–110. [Google Scholar] [CrossRef]
- Shakeel, M.; Ali, H.; Ahmad, S.; Said, F.; Khan, K.A.; Bashir, M.A.; Anjum, S.I.; Islam, W.; Ghramh, H.A.; Ansari, M.J.; et al. Insect pollinators diversity and abundance in Eruca sativa Mill. (arugula) and Brassica rapa L. (field mustard) crops. Saudi J. Biol. Sci. 2019, 26, 1704–1709. [Google Scholar] [CrossRef]
- Sihag, R.C. Pollination ecology of rocket (Eruca vesicaria (L.) Cav. ssp. sativa (Mill.) Thell) in the semi-arid environments of Northwest India: Native bees are the major pollinators. Ecologies 2023, 4, 580–594. [Google Scholar] [CrossRef]
- Bendifallah, L.; Louadi, K.; Doumandji, S. Bee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria). J. Apic. Sci. 2013, 57, 59–70. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower strips in wheat intercropping system: Effect on pollinator abundance and diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef]
- Mena, G.T.; Gospodarek, J. White mustard, sweet alyssum, and coriander as insectary plants in agricultural systems: Impacts on ecosystem services and yield of crops. Agriculture 2024, 14, 550. [Google Scholar] [CrossRef]
- Usman, M. Incidence of different insect visitors and their relative abundance associated with coriander (Coriandrum sativum) in district Charsadda. Pure Appl. Biol. 2018, 7, 539–546. [Google Scholar] [CrossRef]
- Sasaki, H.; Wagatsuma, T. Bumblebees (Apidae: Hymenoptera) are the main pollinators of common buckwheat, Fagopyrum esculentum, in Hokkaido, Japan. Appl. Entomol. Zool. 2007, 42, 659–661. [Google Scholar] [CrossRef][Green Version]
- Campbell, J.W.; Irvin, A.; Irvin, H.; Stanley-Stahr, C.; Ellis, J.D. Insect visitors to flowering buckwheat, Fagopyrum esculentum (Polygonales: Polygonaceae), in North-Central Florida. Fla. Entomol. 2016, 99, 264–268. [Google Scholar] [CrossRef]
- Denys, C.; Tscharntke, T. Plant-insect communities and predator-prey ratios in field margin strips, adjacent crop fields, and fallows. Oecologia 2002, 130, 315–324. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.-C. Augmenting flower trait diversity in wildflower strips to optimise the conservation of arthropod functional groups for multiple agroecosystem services. J. Insect Conserv. 2014, 18, 713–728. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing plants for bee-keeping at any cost?—Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2016, 238, 155–168. [Google Scholar] [CrossRef]
- Sowmya, K.S. Effect of number of Apis cerana visits on quantitative parameters in nectar plant, Ammi majus L. Insect Environ. 2012, 18, 15. [Google Scholar]
- Mavromoustakis, G.A. The bees (Hymenoptera, Apoidea) of Cyprus. Ann. Mag. Nat. Hist. 1957, 10, 321–337. [Google Scholar] [CrossRef]
- Balzan, M.V.; Moonen, A.-C. Field margin vegetation enhances biological control and crop damage suppression from multiple pests in organic tomato fields. Entomol. Exp. Appl. 2014, 150, 45–65. [Google Scholar] [CrossRef]
- Pellissier, M.E.; Jabbour, R. Herbivore and parasitoid insects respond differently to annual and perennial floral strips in an alfalfa ecosystem. Biol. Control 2018, 123, 28–35. [Google Scholar] [CrossRef]
- Rizzo, M.C.; Massa, B. Ecology of the Eulophid parasitoid community living on hosts of spontaneous flora linked to citrus grove (Hymenoptera: Chalcidoidea: Eulophidae). In Parasitic Wasps: Evolution, Systematics, Biodiversity and Biological Control; Melika, G., Thuroczy, C., Eds.; Agroinform: Budapest, Hungary, 2002; pp. 351–361. [Google Scholar]
- Denis, C.; Riudavets, J.; Gabarra, R.; Molina, P.; Arnó, J. Selection of insectary plants for the conservation of biological control agents of aphids and thrips in fruit orchards. Bull. Entomol. Res. 2021, 111, 517–527. [Google Scholar] [CrossRef]
- Stathakis, T.; Economou, L.; Barda, M.; Angelioudakis, T.; Kati, V.; Karamaouna, F. Potential of hedgerows with aromatic plants as reservoirs of natural enemies of pests in orange orchards. Insects 2023, 14, 391. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T.; Bar-Massada, A. Parasitoid abundance on plants: Effects of host abundance, plant species, and plant flowering state. Arthropod Plant Interact. 2017, 11, 155–161. [Google Scholar] [CrossRef]
- Yefremova, Z. Order Hymenoptera, family Eulophidae. Arthr. Fauna UAE 2008, 1, 345–360. [Google Scholar]
- Gebiola, M.; Bernardo, U.; Ribes, A.; Gibson, G.A.P. An integrative study of Necremnus Thomson (Hymenoptera: Eulophidae) associated with invasive pests in Europe and North America: Taxonomic and ecological implications. Zool. J. Linn. Soc. 2015, 173, 352–423. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.J.; Solans, P.; Vitalle, J. Parasitoides y depradadores de Helicoverpa armigera (Hübner) en cultivos de tomate para consume en fresco. Bol. San. Veg. Plagas 1994, 20, 521–530. [Google Scholar]
- Denis, C.; Riudavets, J.; Alomar, O.; Agustí, N.; Gonzalez-Valero, H.; Cubí, M.; Matas, M.; Rodríguez, D.; van Achterberg, K.; Arnó, J. Naturalized Dolichogenidea gelechiidivoris complement the resident parasitoid complex of Tuta Absoluta in North-Eastern Spain. J. Appl. Entomol. 2022, 146, 461–464. [Google Scholar] [CrossRef]
- Ivezić, A.; Popović, T.; Trudić, B.; Krndija, J.; Barošević, T.; Sarajlić, A.; Stojačić, I.; Kuzmanović, B. Biological control agents in greenhouse tomato production (Solanum lycopersicum L.): Possibilities, challenges and policy insights for Western Balkan region. Horticulturae 2025, 11, 155. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Yao, Y.-X.; Qiu, L.-F.; Li, Z.-X. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Gard, B.; Bout, A.; Pierre, P. Release strategies of Trissolcus basalis (Scelionidae) in protected crops against Nezara viridula (Pentatomidae): Less is more. Crop Prot. 2022, 161, 106069. [Google Scholar] [CrossRef]
- Balzan, M.V.; Wäckers, F.L. Flowers to selectively enhance the fitness of a host-feeding parasitoid: Adult feeding by Tuta absoluta and its parasitoid Necremnus artynes. Biol. Control 2013, 67, 21–31. [Google Scholar] [CrossRef]
- Arnó, J.; Oveja, M.F.; Gabarra, R. Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biol. Control 2018, 122, 41–50. [Google Scholar] [CrossRef]
- McIntosh, H.R.; Skillman, V.P.; Galindo, G.; Lee, J.C. Floral resources for Trissolcus japonicus, a parasitoid of Halyomorpha halys. Insects 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.T. Systematics, biology, and hosts of the Mymaridae and Mymarommatidae (Insecta: Hymenoptera): 1758–1984. Entomography 1986, 4, 185–243. [Google Scholar]
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest. Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Riley, D.; Sparks, A.; Srinivasan, R.; Kennedy, G.; Fonsah, G.; Scott, J.; Olson, S. Chapter 3—Thrips: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 49–71. ISBN 978-0-12-802441-6. [Google Scholar]
- Abenaim, L.; Bedini, S.; Greco, A.; Giannotti, P.; Conti, B. Predation capacity of the banded thrips Aeolothrips intermedius for the biological control of the onion thrips Thrips tabaci. Insects 2022, 13, 702. [Google Scholar] [CrossRef]
- Mouratidis, A.; De Lima, A.P.; Dicke, M.; Messelink, G.J. Predator-prey interactions and life history of Orius laevigatus and O. majusculus feeding on flower and leaf-inhabiting thrips. Biol. Control 2022, 172, 104954. [Google Scholar] [CrossRef]
- Venzon, M.; Janssen, A.; Sabelis, M.W. Prey Preference and reproductive success of the generalist predator Orius laevigatus. Oikos 2002, 97, 116–124. [Google Scholar] [CrossRef]
- Harwood, J.D.; Desneux, N.; Yoo, H.J.S.; Rowley, D.L.; Greenstone, M.H.; Obrycki, J.J.; O’neil, R.J. Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: A molecular approach. Mol. Ecol. 2007, 16, 4390–4400. [Google Scholar] [CrossRef]
- Arnó, J.; Roig, J.; Riudavets, J. Evaluation of Orius majusculus and O. laevigatus as predators of Bemisa tabaci and estimation of their prey preference. Biol. Control 2008, 44, 1–6. [Google Scholar] [CrossRef]
- Patrzich, R.; Klumpp, M. Vergleich Der phytophagen und räuberischen thripse (Thysanoptera) auf unterschiedlich bewirtschafteten weizenfeldern in Hessen/Comparison of phytophagous and predatory thrips (Thysanoptera) in winter wheat fields in Hesse with different systems of management. J. Plant Dis. Prot. 1991, 98, 464–470. [Google Scholar]
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.P.; Cierpisz, M. The effects of locality and host plant on the body size of Aeolothrips ιntermedius (Thysanoptera: Aeolothripidae) in the Southwest of Poland. Insects 2019, 10, 266. [Google Scholar] [CrossRef]
- Pumariño, L.; Alomar, O.; Lundgren, J.G. Effects of floral and extrafloral resource diversity on the fitness of an omnivorous bug, Orius insidiosus. Entomol. Exp. Appl. 2012, 145, 181–190. [Google Scholar] [CrossRef]
- Hinds, J.; Barbercheck, M.E. Diversified floral provisioning enhances performance of the generalist predator, Orius insidiosus (Hemiptera: Anthocoridae). Biol. Control 2020, 149, 104313. [Google Scholar] [CrossRef]
- Huseynov, E.F. oglu Natural prey of the crab spider Runcinia Grammica (Araneae: Thomisidae) on Eryngium plants. Bull. Br. Arachnol. Soc. 2007, 14, 93–96. [Google Scholar] [CrossRef]
- Morse, D.H. Prey capture by the crab spider Misumena vatia (Clerck) (Thomisidae) on three common native flowers. Am. Midl. Nat. 1981, 105, 358–367. [Google Scholar] [CrossRef]
- Welti, E.A.R.; Putnam, S.; Joern, A. Crab spiders (Thomisidae) attract insect flower-visitors without UV signalling. Ecol. Entomol. 2016, 41, 611–617. [Google Scholar] [CrossRef]
- De Backer, L.; Caparros Megido, R.; Haubruge, É.; Verheggen, F. Macrolophus pygmaeus (Rambur) as an efficient predator of the tomato leafminer Tuta absoluta (Meyrick) in Europe. A Review. Biotechnol. Agron. Soc. Environ. 2014, 18, 536–543. [Google Scholar]
- Obrycki, J.J.; Kring, T.J. Predaceous Coccinellidae in biological control. Annu. Rev. Entomol. 1998, 43, 295–321. [Google Scholar] [CrossRef]
- Magro, A.; Hemptinne, J.L. The pool of Coccinellids (Coleoptera: Coccinellidae) to control Coccids (Homoptera: Coccoidea) in Portuguese citrus groves. Bol. San. Veg. Plagas 1999, 25, 311–320. [Google Scholar]
- Bogya, S. Spiders (Araneae) as Polyphagous Natural Enemies in Orchards. Ph.D. Thesis, Wangenigen University & Research, Wangenigen, The Netherlands, 1999. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I. Vegetational designs to enhance biological control of insect pests in agroecosystems. In Natural Enemies of Insect Pests in Neotropical Agroecosystems: Biological Control and Functional Biodiversity; Souza, B., Vázquez, L.L., Marucci, R.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–13. ISBN 978-3-030-24733-1. [Google Scholar]
- Silva-Neto, C.M.; Bergamini, L.L.; Elias, M.A.S.; Moreira, G.L.; Morais, J.M.; Bergamini, B.A.R.; Franceschinelli, E.V. High species richness of native pollinators in Brazilian tomato crops. Braz. J. Biol. 2016, 77, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Alvi, A.M.; Khan, K.A.; Rehmani, M.I.A.; Ansari, M.J.; Atta, S.; Ghramh, H.A.; Batool, T.; Tariq, M. Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum). Saudi J. Biol. Sci. 2018, 25, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, C.; Breeze, T.D.; Li, M.; Mashilingi, S.K.; Hua, J.; Zhang, W.; Zhang, X.; Zhang, S.; An, J. Bumblebee pollination enhances yield and flavor of tomato in Gobi Desert greenhouses. Agriculture 2022, 12, 795. [Google Scholar] [CrossRef]
- Roohanitaziani, R.; de Maagd, R.A.; Lammers, M.; Molthoff, J.; Meijer-Dekens, F.; van Kaauwen, M.P.W.; Finkers, R.; Tikunov, Y.; Visser, R.G.F.; Bovy, A.G. Exploration of a resequenced tomato core collection for phenotypic and genotypic variation in plant growth and fruit quality traits. Genes 2020, 11, 1278. [Google Scholar] [CrossRef]
| (%) | g/100 m2 | ||||
|---|---|---|---|---|---|
| Family | Species | WM | SM | WM | SM |
| Apiaceae | Anethum graveolens L. | 26 | 6 | ||
| Coriandrum sativum L. | 18 | 32 | |||
| Brassicaceae | Eruca vesicaria (L.) Cav. | 23 | 26 | 9 | 14 |
| Fabaceae | Pisum sativum L. | 7 | 368 | ||
| Lathyrus sativus L. | 12 | 12 | 301 | 361 | |
| Polygonaceae | Fagopyrum esculentum Moench | 33 | 214 | ||
| Boraginaceae | Phacelia tanacetifolia Benth. | 29 | 31 | ||
| Poaceae | Triticum aestivum L. | 14 | 150 | ||
| TOTAL | 100 | 100 | 866 | 620 | |
| Plant Species | Bee Genus | Treatment |
|---|---|---|
| Ammi majus | Andrena, Hylaeus, Lasioglossum, Nomiapis | CC |
| Anethum graveolens | Andrena, Hylaeus | WM |
| Centauria sp. | Halictus | CC |
| Coriandrum sativum | Andrena, Ceratina, Nomiapis | WM |
| Ecballium elaterium | Lasioglossum, Ceratina | SM |
| Eruca vesicaria | Amegilla, Andrena, Ceratina, Eucera, Halictus, Lasioglossum | SM |
| Fagopyrum esculentum | Andrena, Lasioglossum | SM |
| Lathyrus sativus | Eucera, Megachile, Osmia | SM |
| Phacelia tanacetifolia | Ceratina | SM |
| Rapistrum rugosum | Andrena, Eucera, Lasioglossum, Nomada | CT |
| Solanum lycopersicum | Andrena, Lasioglossum, Nomiapis | WM |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| 2021 | 2022 | |||||
| Yield Parameter | WM | SM | C | WM | SM | C |
| Weight (g/fruit) | 74.9 ± 0.7 a | 76.8 ± 0.9 a | 63.4 ± 0.6 b | 79.3 ± 1.2 A | 76.2 ± 2.2 A | 57.3 ± 1.3 B |
| BRIX | 5.2 ± 0.04 a | 5.2 ± 0.02 a | 5.2 ± 0.07 a | 5.2 ± 0.1 B | 5.3 ± 0.1 B | 6.1 ± 0.1 A |
| pH | 4.4 ± 0.03 a | 4.5 ± 0.03 a | 4.5 ± 0.04 a | 4.5 ± 0.02 A | 4.5 ± 0.02 A | 4.5 ± 0.03 A |
| L | 27.8 ± 0.1 b | 28.0 ± 0.3 b | 29.6 ± 0.4 a | 28.3 ± 0.3 A | 28.1 ± 0.3 A | 28.1 ± 0.6 A |
| a/b | 2.5 ± 0.01 a | 2.5 ± 0.04 a | 2.4 ± 0.05 a | 2.5 ± 0.04 A | 2.5 ± 0.03 A | 2.3 ± 0.03 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kati, V.; Stathakis, T.; Economou, L.; Mylonas, P.; Barda, M.; Angelioudakis, T.; Parlapani, A.B.; Tsamis, I.; Karamaouna, F. Processing Tomato Crop Benefits from Flowering Plants in Field Margins That Support Pollinators and Natural Enemies. Agronomy 2025, 15, 1558. https://doi.org/10.3390/agronomy15071558
Kati V, Stathakis T, Economou L, Mylonas P, Barda M, Angelioudakis T, Parlapani AB, Tsamis I, Karamaouna F. Processing Tomato Crop Benefits from Flowering Plants in Field Margins That Support Pollinators and Natural Enemies. Agronomy. 2025; 15(7):1558. https://doi.org/10.3390/agronomy15071558
Chicago/Turabian StyleKati, Vaya, Theodoros Stathakis, Leonidas Economou, Philippos Mylonas, Myrto Barda, Theodoros Angelioudakis, Athanasia Bratidou Parlapani, Ilias Tsamis, and Filitsa Karamaouna. 2025. "Processing Tomato Crop Benefits from Flowering Plants in Field Margins That Support Pollinators and Natural Enemies" Agronomy 15, no. 7: 1558. https://doi.org/10.3390/agronomy15071558
APA StyleKati, V., Stathakis, T., Economou, L., Mylonas, P., Barda, M., Angelioudakis, T., Parlapani, A. B., Tsamis, I., & Karamaouna, F. (2025). Processing Tomato Crop Benefits from Flowering Plants in Field Margins That Support Pollinators and Natural Enemies. Agronomy, 15(7), 1558. https://doi.org/10.3390/agronomy15071558








