Variable Transect Method Outperformed in Sampling Hymenopteran Flower Visitors in Brassica campestris L. var. toria Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. The Agroecosystem of Brassica campestris var. toria
2.2.1. Normal Cropping Period for Brassica campestris var. toria
2.2.2. Late Cropping Period for Brassica campestris var. toria
2.3. Sampling Methodology
2.3.1. Period of Observation and Field Data Collection
2.3.2. Methods and Sampling Design
Ground Bowl Traps (GBTs)
Elevated Bowl Traps (EBTs)
Observation Plots (OP)
Standardised Transect Walks (ST)
Variable Transect Walks (VT)
2.4. Statistical Analysis of Data
3. Results
Efficiency of All Sampling Methods
4. Discussion
4.1. Diversity of Floral Visitors Based on Sampling Methods
4.2. Comparative Efficiency of Transect and Non-Transect Sampling Methods
4.3. Influence of Trap Colour and Position on Sampling Efficiency
4.4. Implications for Future Research and Conservation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.; Altieri, M. Plant biodiversity enhances bees and other insect pollinators in agro-ecosystems: A review. Agron. Sustain. Dev 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Mcgregor, S.E. Insect Pollination of Cultivated Crop Plants; USDA/ARS Agriculture Handbook 496; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1976; p. 139.
- Tepedino, V.J. The importance of bees and other insect pollinators in maintaining floral species composition. Great Basin Nat. Mem. 1979, 3, 17. [Google Scholar]
- Free, J.B. Insect Pollination of Crops; Academic Press: London, UK, 1993; p. 684. [Google Scholar]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissiere, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Landaverde-González, P.; Quezada-Euán, J.J.G.; Theodorou, P.; Murray, T.E.; Husemann, M.; Ayala, R.; Moo-Valle, H.; Vandame, R.; Paxton, R.J. Sweat bees on hot chilies: Provision of pollination services by native bees in traditional slash-and-burn agriculture in the Yucatán Peninsula of tropical Mexico. J. Appl. Ecol. 2017, 54, 1814–1824. [Google Scholar] [CrossRef]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. The Assessment Report on Pollinators, Pollination and Food Production; IPBES Secretariat: Bonn, Germany, 2016; Available online: https://ipbes.net (accessed on 3 March 2025).
- Faegri, K.; Van Der Pijl, L. Principales of Pollination Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 256. [Google Scholar]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Pollination of Coffea canephora in relation to local and regional agroforestry management. Oecologia 2003, 134, 607–615. [Google Scholar] [CrossRef]
- Culley, T.M.; Weller, S.G.; Sakai, A.K. The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 2002, 17, 361–369. [Google Scholar] [CrossRef]
- Abrol, D.P. Pollination Biology: Biodiversity Conservation and Agricultural Production; Springer: Dordrecht, The Netherlands, 2012; p. 792. [Google Scholar]
- Stanley, J.; Sah, K.; Subbanna, A.R.N.S. How efficient is the Asian honey bee, Apis cerana in pollinating mustard, Brassica campestris var. toria? Pollination behavior, pollinator efficiency, pollinator requirements and impact of pollination. J. Agric. Res. 2017, 56, 439–451. [Google Scholar] [CrossRef]
- Sarma, A.K.; Chauhan, J.S. Pollinator diversity in Brassica crops and significance of pollinators in improving productivity: A review. J. Agric. Res. 2015, 1, 35–40. [Google Scholar]
- Taba, N.; Sharma, P.; Devadas, V.S.; Hazarika, G.N.; Monlai, S. Performance of Toria (Brassica campestris L.) Varieties under Namsai Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2101–2103. [Google Scholar]
- Williams, I.H. The pollination requirements of swede rape (Brassica napus L.) and of turnip rape (Brassica campestris L.). J. Agric. Sci. 1978, 91, 343–348. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Benefits of Insect Pollination in Brassicaceae: A Meta-Analysis of Self-Compatible and Self-Incompatible Crop Species. Agriculture 2022, 12, 446. [Google Scholar] [CrossRef]
- Brittain, C.; Kremen, C.; Klein, A.M. Biodiversity buffers pollination from changes in environmental conditions. Glob. Chang. Biol. 2013, 19, 540–547. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Garratt, M.P.D.; Powney, G.D.; Shaw, R.F.; Osborne, J.L.; Soroka, J.; Lindström, S.A.M.; Stanley, D.; Ouvrard, P.; Edwards, M.E.; et al. Meta-Analysis Reveals That Pollinator Functional Diversity and Abundance Enhance Crop Pollination and Yield. Nat. Commun. 2019, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Rader, R.; Howlett, B.G.; Cunningham, S.A.; Westcott, D.A.; Newstrom-Lloyd, L.E.; Walker, M.K.; Teulon, D.A.J.; Edwards, W. Alternative Pollinator Taxa Are Equally Efficient but Not as Effective as the Honeybee in a Mass Flowering Crop. J. Appl. Ecol. 2009, 46, 1080–1087. [Google Scholar] [CrossRef]
- Junqueira, C.N.; Pereira, R.A.S.; da Silva, R.C.; Alves Cardoso Kobal, R.O.; Araújo, T.N.; Prato, A.; Pedrosa, J.; Martínez-Martínez, C.A.; Castrillon, K.P.; Felício, D.T.; et al. Do Apis and Non-Apis Bees Provide a Similar Contribution to Crop Production with Different Levels of Pollination Dependency? A Review Using Meta-Analysis. Ecol. Entomol. 2021, 47, 76–83. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-Bee Insects Are Important Contributors to Global Crop Pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef]
- Földesi, R.; Howlett, B.G.; Grass, I.; Batáry, P. Larger Pollinators Deposit More Pollen on Stigmas across Multiple Plant Species—A Meta-Analysis. J. Appl. Ecol. 2021, 58, 699–707. [Google Scholar] [CrossRef]
- Rader, R.; Howlett, B.G.; Cunningham, S.A.; Westcott, D.A.; Edwards, W. Spatial and temporal variation in pollinator effectiveness: Do unmanaged insects provide consistent pollination services to mass flowering crops? J. Appl. Ecol. 2012, 49, 126–134. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef]
- Winfree, R.; Fox, W.; Williams, J.; Reilly, N.M.; Cariveau, J.R.D.P. Abundance of common species, not species richness, drives delivery of a realworld ecosystem service. Ecol. Lett. 2015, 18, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Reilly, J.R.; Bartomeus, I.; Cariveau, D.P.; Williams, N.M.; Gibbs, J. Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 2018, 359, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Jauker, F.; Bondarenko, B.; Becker, H.C.; Steffan-Dewenter, I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric. For. Entomol. 2012, 14, 81–87. [Google Scholar] [CrossRef]
- Vaissiere, B.E.; Breno, M.F.; Barbara, G.H. Protocol to Detect and Assess Pollination Deficits in Crops: A Handbook for Its Use; FAO, UN: Rome, Italy, 2011; pp. 1–70. [Google Scholar]
- Wood, T.J.; Holland, J.M.; Goulson, D. Providing foraging resources for solitary bees on farmland: Current schemes for pollinators benefit a limited suite of species. J. Appl. Ecol. 2016, 54, 323–333. [Google Scholar] [CrossRef]
- Thompson, A.; Frenzel, M.; Schweiger, O.; Musche, M.; Groth, T.; Roberts, S.P.M.; Kuhlmann, M.; Knight, T.M. Pollinator sampling methods influence community patterns assessments by capturing species with different traits and at different abundances. Ecol. Indic. 2021, 132, 108284. [Google Scholar] [CrossRef]
- Westphal, C.; Bommarco, R.; Carre, G.; Lamborn, E.; Morison, N.; Petanidou, T.; Potts, S.G.; Roberts, S.P.M.; Szentgyo Rgyi, H.; Tscheulin, T.; et al. Measuring bee biodiversity in different European habitats and bio-geographical regions. Ecol. Monogr. 2008, 78, 653–671. [Google Scholar] [CrossRef]
- Potts, S.G.; Petanidou, T.; Roberts, S.; O’Toole, C.; Hulbert, A.; Willmer, P. Assessing pollinator biodiversity: Standardized methods for monitoring. J. Appl. Ecol. 2008, 45, 9–14. [Google Scholar]
- Potts, S.G.; Vulliamy, B.; Roberts, S.; O’toole, C.; Dafni, A.; Ne’eman, G.; Willmer, P. Role of nesting resources in organising diverse bee communities in a mediterranean landscape. Ecol. Entomol. 2005, 30, 78–85. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Oliver, T.H.; Breeze, T.D.; O’Connor, R.S.; Potts, S.G.; Roberts, S.P.M.; Garratt, M.P.D. Inventorying and monitoring crop pollinating bees: Evaluating the effectiveness of common sampling methods. Insects Conserv. Divers. 2022, 15, 299–311. [Google Scholar] [CrossRef]
- Sarma, A.K.; Deka, M.K.; Neog, B. Species richness of Hymenopteran flower visitors in Brassica campestris var. toria in Assam, India: A comparison of five sampling methods. AtaXE 2024, 70, 314–328. [Google Scholar] [CrossRef]
- Popic, T.J.; Davila, Y.C.; Wardle, G.M. Evaluation of common method for sampling invertebrate pollinator assemblages: Net samplings outperform Pan traps. PLoS ONE 2013, 8, e66665. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.W.; Hanula, J.L. Efficiency of Malaise traps and coloured pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Geroff, R.K.; Gibbs, J.; McCravy, K.W. Assessing bee (Hymenoptera: Apoidea) diversity of an Illinois restored tallgrass prairie: Methodology and conservation considerations. J. Insect Conserv. 2014, 18, 951–964. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Kunin, W.E. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Giovanetti, M.; Albertazzi, S.; Flaminio, S.; Ranalli, R.; Bortolotti, L.; Quaranta, M. Pollination in Agroecosystems: A Review of the Conceptual Framework with a View to Sound Monitoring. Land 2021, 10, 540. [Google Scholar] [CrossRef]
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.M.; Settele, J.; Szentgyörgyi, H.; Vaissière, B.E.; Vaitis, M.; et al. Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Leong, J.M.; Thorp, R.W. Colour-coded sampling: Pan trap colour preferences oligolectic and non-oligolectic bees associated with a vernal pool plant. Ecol. Entomol. 1999, 24, 329–335. [Google Scholar] [CrossRef]
- Cane, J.H.; Minckley, R.L.; Kervin, R.J. Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: Pitfalls of pan trapping. J. Kans. Entomol. Soc. 2000, 73, 225–231. Available online: http://www.jstor.org/stable/25085973 (accessed on 5 March 2025).
- Toler, T.R.; Evans, E.W.; Tepedino, V.J. Pan-trapping for Bee (Hymenoptera: Apiformes) in Utah’s West Desert: The importance of colour diversity. Pan-Pac Entomol. 2005, 81, 103–113. [Google Scholar]
- Roulston, T.H.; Smith, S.A.; Brewster, A.L. A comparison of Pan Trap and Intensive Net Sampling Techniques for documenting a bee (Hymenoptera: Apiformes) Fauna. J. Kans. Entomol. Soc. 2007, 80, 179–181. [Google Scholar] [CrossRef]
- Saunders, M.E.; Luck, G.W. Pan trap catches of pollinator insects vary with habitat. Aus. J. Entomol. 2013, 52, 106–113. [Google Scholar] [CrossRef]
- Lebuhn, G.; Droege, S.; Connor, E.F.; Gemmill-Herren, B.; Potts, S.G.; Minckley, R.L.; Griswold, T.; Jean, R.; Kula, E.; Roubik, E.W.; et al. Detecting insect pollinator declines on regional and global scales. Conserv. Biol. 2013, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.P.; Sharkov, A. Blue Pan Traps for collecting Stephanidae (Hymenoptera). J. Hymenopt. Res. 1997, 6, 422–423. [Google Scholar]
- Gollan, J.R.; Ashcroft, M.B.; Batley, M. Comparison of yellow and white pan traps in surveys of bee fauna in New South Wales, Australia (Hymenoptera: Apoidea: Anthophila). Aust. J. Entomol. 2011, 50, 174–178. [Google Scholar] [CrossRef]
- Prado, S.G.; Ngo, H.T.; Florez, J.A.; Collazo, J.A. Sampling bees in tropical forests and agroecosystems: A review. J. Insect Conserv. 2017, 21, 753–770. [Google Scholar] [CrossRef]
- Mayer, C.; Adler, L.; Armbruster, W.S.; Dafni, A.; Eardle, C.; Huan, S.Q.; Kevan, P.G.; Ollerton, J.; Packer, L.; Ssymank, A.; et al. Pollination ecology in the 21st century: Key questions for future research. J. Pollinat. Ecol. 2011, 3, 8–23. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Scheper, J.; Reemer, M.; van Kats, R.; Ozinga, W.A.; van der Linden, G.T.J.; Schaminée, J.H.J.; Siepel, H.; Kleijn, D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl. Acad. Sci. USA 2014, 111, 17552–17557. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Regional Meteorological Department, Kolkata. 2020. Available online: https://mausam.imd.gov.in/kolkata/ (accessed on 6 March 2025).
- Thakuria, C. Yield assessment of Indian mustard variety NRCHB101 with toria varieties TS 36 and TS 38 in Dibrugarh district of Assam. Pharma Innov. J. 2023, 12, 1502–1503. [Google Scholar]
- Deka, B.C.; Parisa, D.; Singha, A.K.; Siangshai, R.; Massar, D.A. (Eds.) Impact of Technologies on Oilseeds Production in North Eastern Region; ICAR-Agricultural Technology Application Research Institute (ATARI): Zone-VII: Umiam, Meghalaya, India, 2018; p. 28. [Google Scholar]
- Belavadi, V.V.; Ganeshaiah, K.N. Insect Pollination Manual; Indian Council of Agricultural Research: New Delhi, India, 2013; pp. 1–44. [Google Scholar]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011; pp. 1–832. [Google Scholar]
- Shapiro, L.H.; Tepedino, V.J.; Minckley, R.L. Bowling for bees: Optimal sample number for “bee bowl” sampling transects. J. Insect Conserv. 2014, 18, 1105–1113. [Google Scholar] [CrossRef]
- Moreira, E.F.; Silva Santos, R.L.S.; Penna, U.L.; Coca, C.A.; Oliveira, F.F.; Blandina Felipe Viana, B.F. Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- Droege, S.; Tepedino, V.J.; LeBuhn, G.; Link, W.; Minckley, R.L.; Chen, Q.; Conrad, C. Spatial scale and sampling interval effects on abundance and species richness of native bees. Biol. Conserv. 2010, 143, 1068–1074. [Google Scholar]
- Rogers, S.R.; Tarpy, D.R.; Burrack, H.J. Bee Species Diversity Enhances Productivity and Stability in a Perennial Crop. PLoS ONE 2014, 9, e97307. [Google Scholar] [CrossRef]
- Devi, M.; Sharma, H.K.; Thakur, R.K.; Bhardwaj, S.K.; Rana, K.; Thakur, M.; Ram, B. Diversity of insect pollinators in reference to seed set of mustard (Brassica juncea L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2131–2144. [Google Scholar] [CrossRef]
- Berglund, H.L.; Milberg, P. Sampling of fower-visiting insects: Poor correspondence between the catches of colour pan−trap and sweep netting. Eur. J. Entomol. 2019, 116, 425–431. [Google Scholar] [CrossRef]
- Lezzeri, M.; Lozano, V.; Brundu, G.; Floris, I.; Pusceddu, M.; Quaranta, M.; Satta, A. Standardized transect walks outperform pan traps in assessing wild bee community in a Mediterranean protected area (Asinara National Park, Italy). Biodivers. Conserv. 2024, 33, 2329–2344. [Google Scholar] [CrossRef]
- Gibbs, J.; Joshi, N.K.; Wilson, J.K.; Rothwell, N.L.; Powers, K.; Haas, M.; Gut, L.; Biddinger, D.J.; Isaacs, R. Does passive sampling accurately reflect the bee (Apoidea:Anthophila) communities pollinating apple and sour cherry orchards? Environ. Entomol. 2017, 46, 579–588. [Google Scholar] [CrossRef] [PubMed]
- McCravy, K.W.; Ruholl, J.D. Bee (Hymenoptera: Apoidea) diversity and sampling methodology in a Midwestern USA deciduous forest. Insects 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, S.M.; Samways, M.J. Optimising coloured pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Nuttman, C.V.; Otieno, M.; Kwapong, P.K.; Combey, R.; Willmer, P.; Potts, S.G. The utility of aerial pan-trapping for assessing insect pollinators across vertical strata. J. Kans. Entomol. Soc. 2011, 84, 260–270. [Google Scholar] [CrossRef]
- Grundel, R.; Frohnapple, K.J.; Jean, R.P.; Pavlovic, N.B. Effectiveness of bowl trapping and netting for inventory of a bee community. Environ. Entomol. 2011, 40, 374–380. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Ecologically selective coloured traps. Ecol. Entomol. 1984, 9, 35–41. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, H.; Felix, J.J.; Bianchi, A.; Jauker, F.; Luo, S.; Werf, W. Wild pollinators enhance oilseed rape yield in small-holder farming systems in China. BMC Ecol. 2017, 17, 6. [Google Scholar] [CrossRef]
- Ludewig, M.J.; Landaverde-González, P.; Götz, K.P.; Chmielewski, F.-M. Initial assessment to understand the effect of air temperature on bees as floral visitors in urban orchards. J. Insect Conserv. 2023, 27, 1013–1022. [Google Scholar] [CrossRef]
- Casiá-Ajché, Q.B.; Escobedo-Kenefic, N.; Escobar-González, D.; Cardona, E.; Mejía-Coroy, A.; Morales-Siná, J.; Enríquez, E.; Landaverde-González, P. Unveiling the effects of land use and intra-seasonal variation on bee and plant diversity and their ecological interactions in vegetation surrounding coffee plantations. Front. Bee Sci. 2024, 2, 1408854. [Google Scholar] [CrossRef]
- Tsang, T.P.; De Santis, A.A.; Armas-Quiñonez, G.; Ascher, J.S.; Ávila-Gómez, E.S.; Báldi, A.; Ballare, K.; Balzan, M.V.; Banaszak-Cibicka, W.; Bänsch, S.; et al. Land use change consistently reduces α-but not β-and γ-diversity of bees. Glob. Chang. Biol. 2025, 31, e70006. [Google Scholar] [CrossRef] [PubMed]
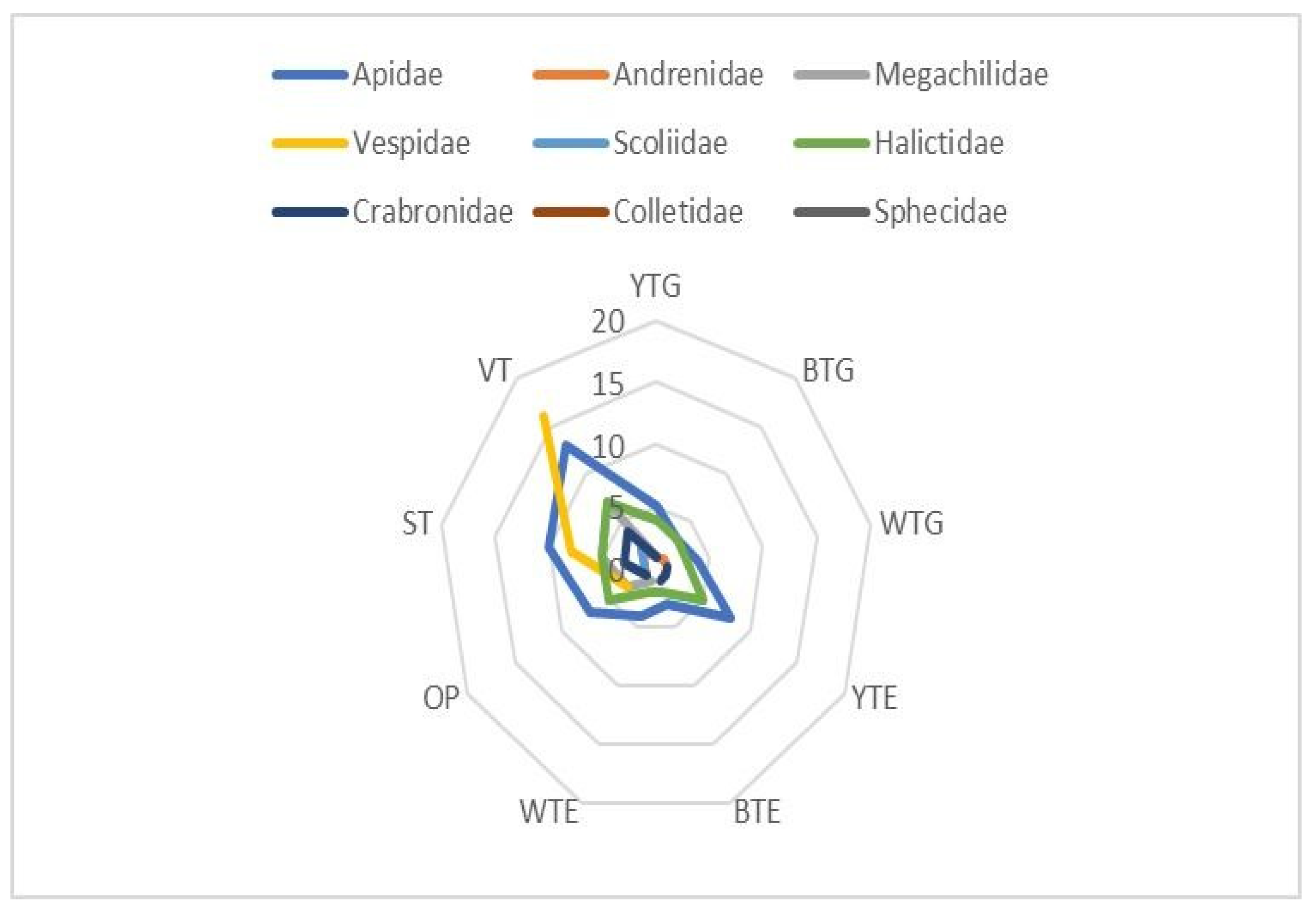
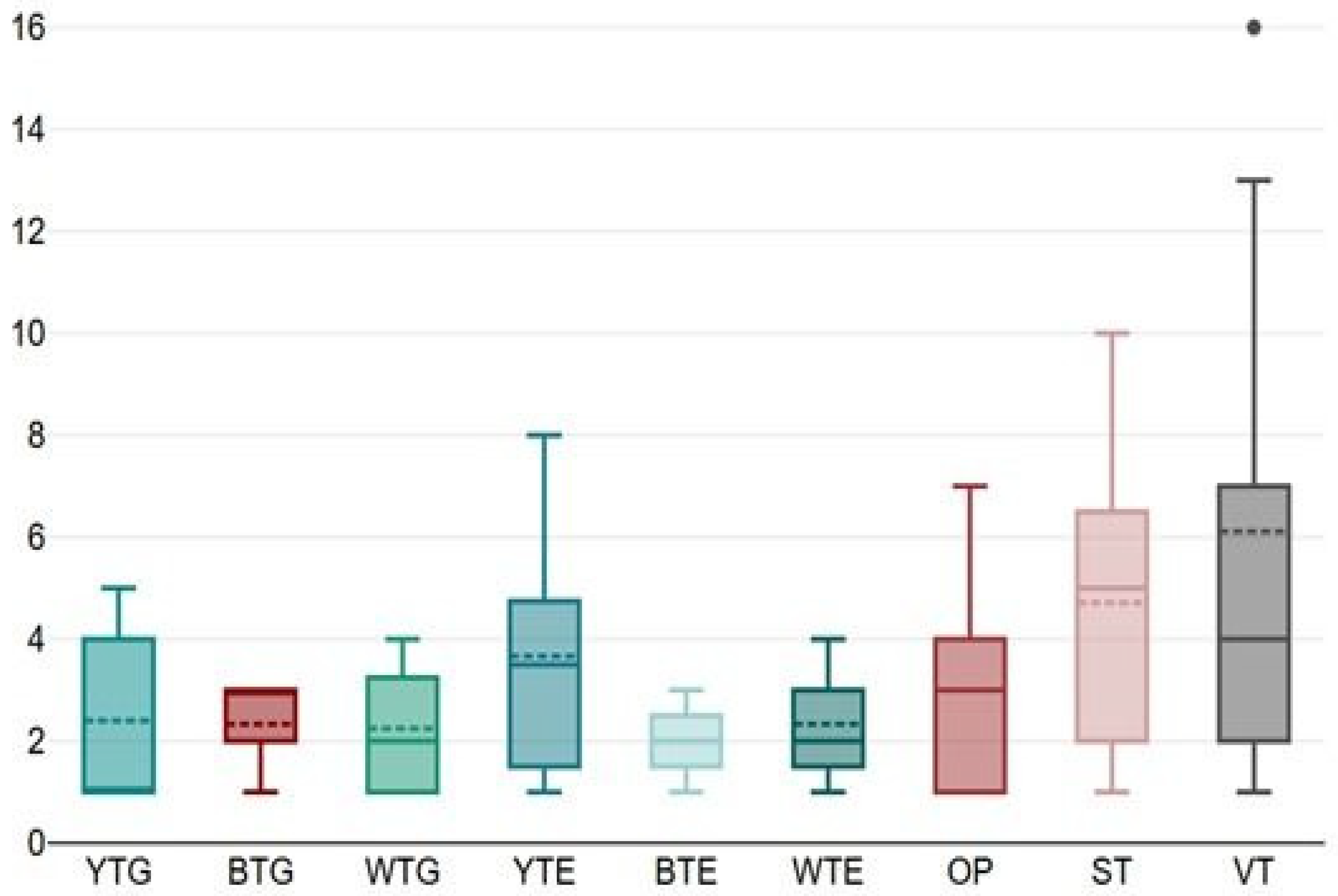

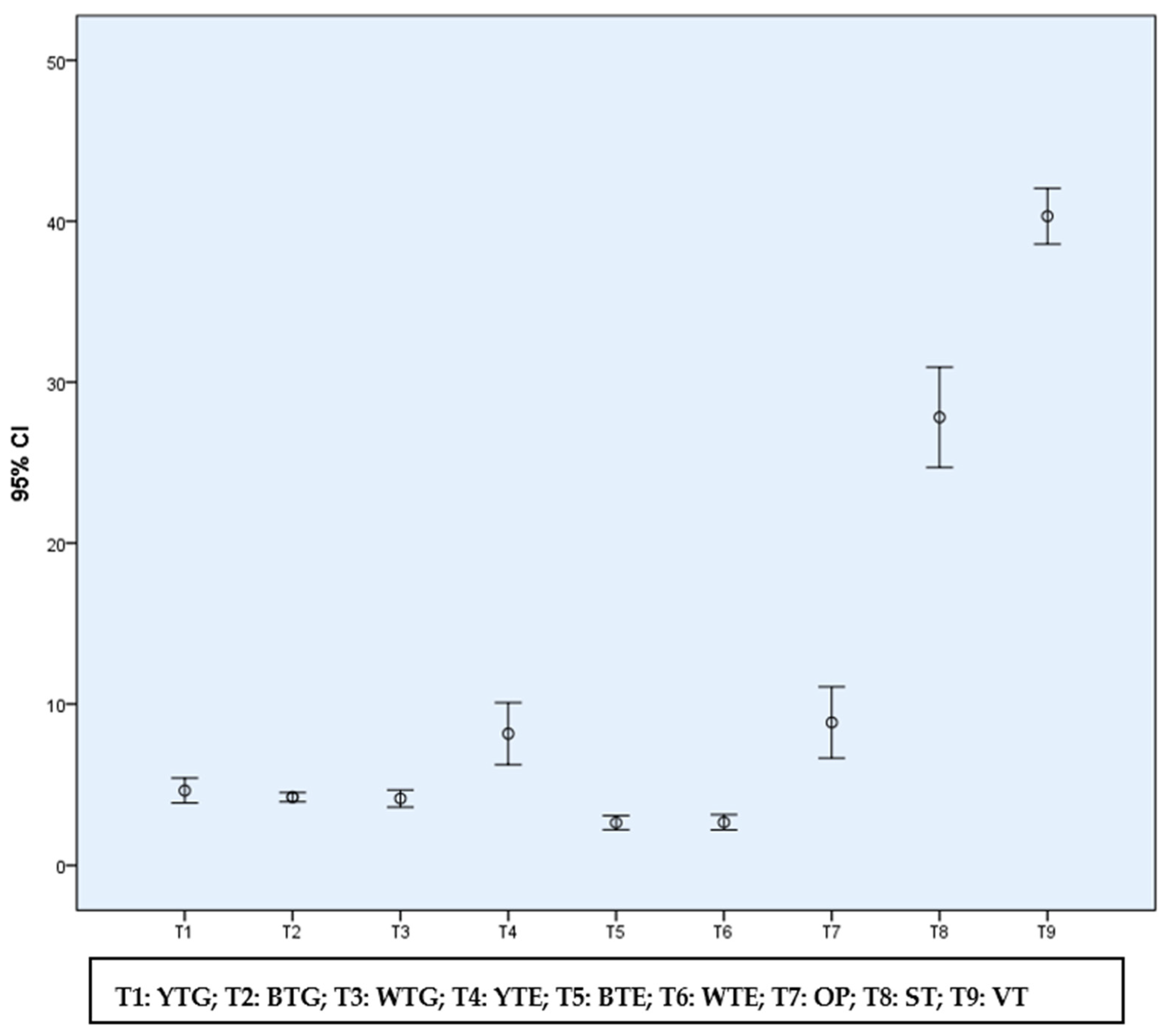
| Sl. No. | Family | Total No. of Species Sampled | No. of Species Sampled by Different Sampling Techniques | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| YTG (T1) | BTG (T2) | WTG (T3) | YTE (T4) | BTE (T5) | WTE (T6) | OP (T7) | ST (T8) | VT (T9) | |||
| 1 | Apidae | 15 | 5 (33.3) | 3 (20.0) | 4 (26.7) | 8 (53.3) | 3 (20.0) | 4 (26.7) | 7 (46.7) | 10 (66.7) | 13 (86.7) |
| 2 | Andrenidae | 02 | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | - | - | 1 (50.0) | 1 (50.0) | 2 (100.0) |
| 3 | Megachilidae | 08 | 1 (12.5) | - | - | 4 (50.0) | - | 1 (12.5) | 3 (37.5) | 5 (62.5) | 7 (87.5) |
| 4 | Vespidae | 18 | - | - | - | 3 (18.8) | - | - | 3 (18.8) | 8 (44.4) | 16 (88.9) |
| 5 | Scoliidae | 02 | - | - | - | - | - | - | - | 1 (50.0) | 2 (100.0) |
| 6 | Halictidae | 09 | 4 (44.4) | 3 (33.3) | 3 (33.3) | 5 (55.6) | 2 (22.2) | 2 (22.2) | 5 (55.6) | 5 (55.6) | 7 (77.8) |
| 7 | Crabronidae | 05 | 1 (20.0) | - | 1 (20.0) | 1 (20.0) | 1 (20.0) | - | 1 (20.0) | 3 (60.0) | 4 (80.0) |
| 8 | Colletidae | 01 | - | - | - | - | - | - | - | - | 1 (100.0) |
| 9 | Sphecidae | 04 | - | - | - | - | - | - | 1 (25.0) | - | 3 (75.0) |
| Total | 64 | 12 | 07 | 09 | 22 | 06 | 07 | 21 | 34 | 54 | |
| % species sampled | 18.8 | 10.9 | 14.1 | 34.4 | 9.4 | 10.9 | 32.8 | 53.1 | 84.4 | ||
| % deviation from mean (=19.1) | −37.2 | −63.4 | −52.9 | +15.2 | −68.6 | −63.4 | +9.9 | +78.0 | +182.7 | ||
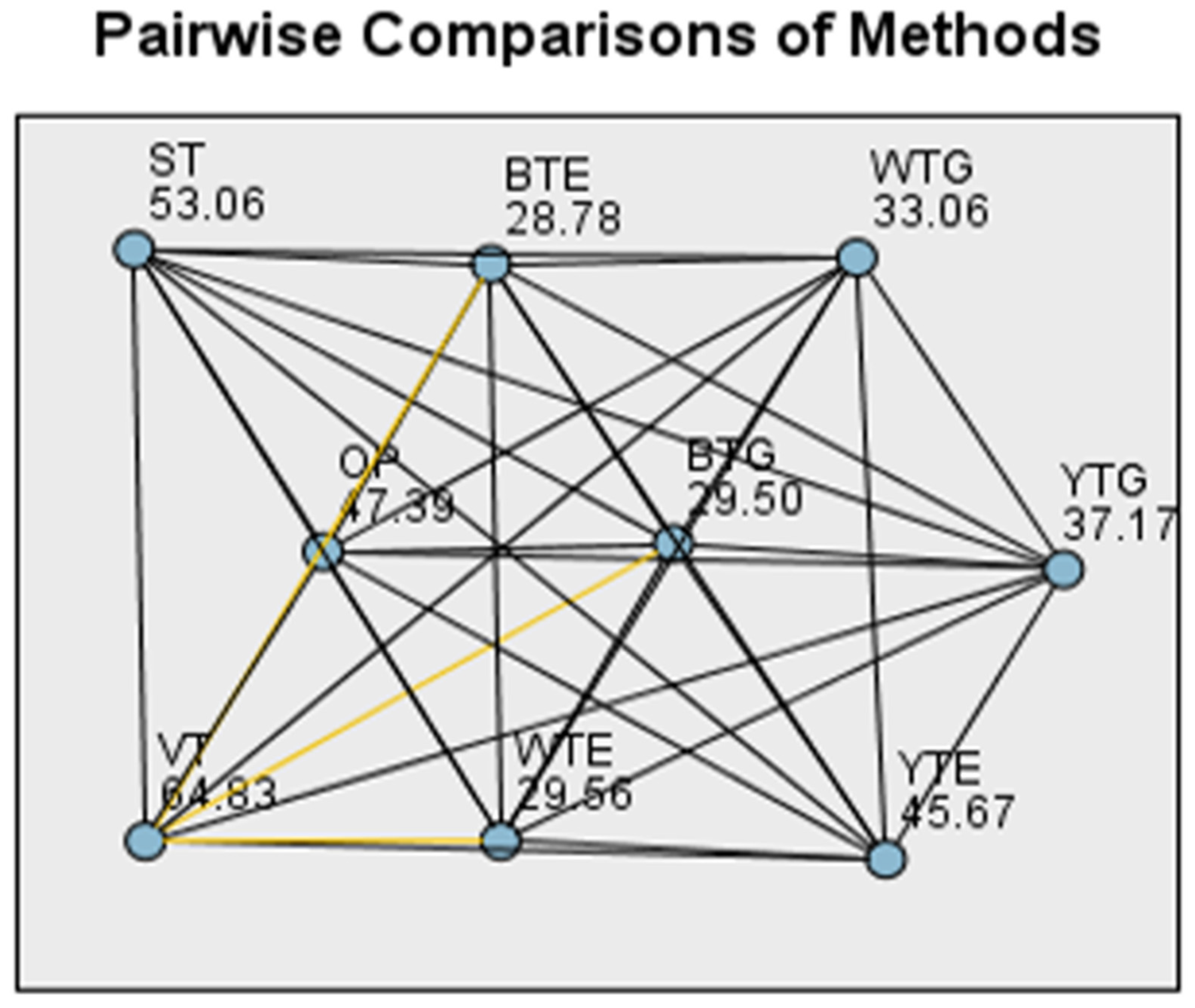
| Pair 1–Pair 2 | Test Statistic | Std. Error | Std. Test Statistic | Sig. | Adj. Sig. |
|---|---|---|---|---|---|
| BTG–VT | −35.333 | 10.617 | −3.328 | 0.001 | 0.031 |
| BTE–VT | −36.056 | 10.617 | −3.396 | 0.001 | 0.025 |
| WTE–VT | −35.278 | 10.617 | −3.323 | 0.001 | 0.032 |
| WTG–VT | −31.778 | 10.617 | −2.993 | 0.003 | 0.099 |
| YTG–VT | −27.667 | 10.617 | −2.606 | 0.009 | 0.330 |
| BTE–ST | −24.278 | 10.617 | −2.287 | 0.022 | 0.800 |
| WTE–ST | −23.500 | 10.617 | −2.213 | 0.027 | 0.967 |
| BTG–ST | −23.556 | 10.617 | −2.219 | 0.027 | 0.954 |
| WTG–ST | −20.000 | 10.617 | −1.884 | 0.060 | 1.000 |
| YTE–VT | −19.167 | 10.617 | −1.805 | 0.071 | 1.000 |
| BTE–OP | −18.611 | 10.617 | −1.753 | 0.080 | 1.000 |
| BTG–OP | −17.889 | 10.617 | −1.685 | 0.092 | 1.000 |
| WTE–OP | −17.833 | 10.617 | −1.680 | 0.093 | 1.000 |
| OP–VT | −17.444 | 10.617 | −1.643 | 0.100 | 1.000 |
| BTE–YTE | 16.889 | 10.617 | 1.591 | 0.112 | 1.000 |
| BTG–YTE | −16.167 | 10.617 | −1.523 | 0.128 | 1.000 |
| WTE–YTE | 16.111 | 10.617 | 1.517 | 0.129 | 1.000 |
| YTG–ST | −15.889 | 10.617 | −1.497 | 0.135 | 1.000 |
| WTG–OP | −14.333 | 10.617 | −1.350 | 0.177 | 1.000 |
| WTG–YTE | −12.611 | 10.617 | −1.188 | 0.235 | 1.000 |
| ST–VT | −11.778 | 10.617 | −1.109 | 0.267 | 1.000 |
| YTG–OP | −10.222 | 10.617 | −0.963 | 0.336 | 1.000 |
| YTG–YTE | −8.500 | 10.617 | −0.801 | 0.423 | 1.000 |
| BTE–YTG | 8.389 | 10.617 | 0.790 | 0.429 | 1.000 |
| BTG–YTG | 7.667 | 10.617 | 0.722 | 0.470 | 1.000 |
| WTE–YTG | 7.611 | 10.617 | 0.717 | 0.473 | 1.000 |
| YTE–ST | −7.389 | 10.617 | −0.696 | 0.486 | 1.000 |
| OP–ST | −5.667 | 10.617 | −0.534 | 0.594 | 1.000 |
| BTE–WTG | 4.278 | 10.617 | 0.403 | 0.687 | 1.000 |
| WTG–YTG | 4.111 | 10.617 | 0.387 | 0.699 | 1.000 |
| BTG–WTG | −3.556 | 10.617 | −0.335 | 0.738 | 1.000 |
| WTE–WTG | 3.500 | 10.617 | 0.330 | 0.742 | 1.000 |
| YTE–OP | −1.722 | 10.617 | −0.162 | 0.871 | 1.000 |
| BTE–WTE | −0.778 | 10.617 | −0.073 | 0.942 | 1.000 |
| BTE–BTG | 0.722 | 10.617 | 0.068 | 0.946 | 1.000 |
| BTG–WTE | −0.056 | 10.617 | −0.005 | 0.996 | 1.000 |
| a | ||||||||
| Treatment (Sampling Method) | Normal Cropping Period (TS 36 Variety) | Late Cropping Period (TS 67 Variety) | ||||||
| Jajimukh | Panimirigaon | Kutuha | Mean | Jajimukh | Panimirigaon | Kutuha | Mean | |
| Treatment 1 (YTG) | 4.01 | 4.13 | 4.51 | 4.21 b | 4.81 | 4.28 | 4.81 | 4.63 c |
| Treatment 2 (BTG) | 3.49 | 3.73 | 3.93 | 3.72 c | 4.12 | 4.21 | 4.35 | 4.23 d |
| Treatment 3 (WTG) | 3.51 | 3.53 | 3.88 | 3.64 c | 4.07 | 3.97 | 4.38 | 4.14 d |
| Treatment 4 (YTE) | 7.65 | 7.88 | 9.03 | 8.19 a | 7.49 | 7.98 | 9.00 | 8.16 b |
| Treatment 5 (BTE) | 2.19 | 2.38 | 2.50 | 2.36 d | 2.50 | 2.59 | 2.84 | 2.64 e |
| Treatment 6 (WTE) | 2.19 | 2.46 | 2.61 | 2.42 d | 2.46 | 2.71 | 2.83 | 2.67 e |
| Treatment 7 (OP) | 7.76 | 8.11 | 9.20 | 8.35 a | 8.13 | 8.58 | 9.84 | 8.85 a |
| Site Mean | 4.40 c | 4.60 b | 5.09 a | 4.70 | 4.80 b | 4.90 b | 5.44 a | 5.05 |
| Deviation (%) from OM (4.88) | (−) 9.84 | (−) 5.74 | (+) 4.30 | (−) 3.69 | (−) 1.64 | (+) 0.41 | (+) 11.48 | (+) 3.28 |
| b | ||||||||
| Treatment (Sampling Method) | Jajimukh | Panimirigaon | Kutuha | Mean | ||||
| Treatment 1 (YTG) | 4.41 | 4.21 | 4.66 | 4.43 b | ||||
| Treatment 2 (BTG) | 3.80 | 3.97 | 4.14 | 3.97 bc | ||||
| Treatment 3 (WTG) | 3.79 | 3.75 | 4.13 | 3.89 c | ||||
| Treatment 4 (YTE) | 7.57 | 7.93 | 9.02 | 8.17 a | ||||
| Treatment 5 (BTE) | 2.35 | 2.49 | 2.67 | 2.50 d | ||||
| Treatment 6 (WTE) | 2.33 | 2.59 | 2.72 | 2.55 d | ||||
| Treatment 7 (OP) | 7.95 | 8.35 | 9.52 | 8.61 a | ||||
| Mean | 4.60 | 4.75 | 5.27 | OM = 4.88 | ||||
| Deviation (%) from OM | (−) 5.74 | (−) 2.66 | (+) 7.99 | - | ||||
| a | |||||||
| Locations | Normal Cropping Period | Late Cropping Period | Pooled Mean (over Cropping Periods) | ||||
| T8 ST | T9 VT | Mean | T8 ST | T9 VT | Mean | ||
| Jajimukh | 24.75 | 38.00 | 31.38 c | 26.75 | 39.69 | 33.22 b | 32.30 c |
| Panimirigaon | 25.69 | 39.75 | 32.72 b | 27.69 | 40.19 | 33.94 b | 33.33 b |
| Kutuha | 27.43 | 42.56 | 35.00 a | 29.23 | 41.06 | 35.15 a | 35.08 a |
| Mean | 25.96 | 40.10 | 33.03 | 27.89 | 40.31 | 34.10 | 33.57 |
| b | |||||||
| Treatments | Normal Cropping Period | Late Cropping Period | Mean | ||||
| T8 (ST) | 25.96 | 27.89 | 26.93 | ||||
| T9 (VT) | 40.10 | 40.31 | 40.21 | ||||
| Mean | 33.03 | 34.10 | 33.57 | ||||
| t-test | 3.408 ** | 2.767 ** | 11.585 ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, A.K.; Neog, B.; Deka, M.K.; Carabet, A.; Stef, R. Variable Transect Method Outperformed in Sampling Hymenopteran Flower Visitors in Brassica campestris L. var. toria Ecosystem. Agronomy 2025, 15, 1281. https://doi.org/10.3390/agronomy15061281
Sarma AK, Neog B, Deka MK, Carabet A, Stef R. Variable Transect Method Outperformed in Sampling Hymenopteran Flower Visitors in Brassica campestris L. var. toria Ecosystem. Agronomy. 2025; 15(6):1281. https://doi.org/10.3390/agronomy15061281
Chicago/Turabian StyleSarma, Arup Kumar, Borsha Neog, Mukul Kumar Deka, Alin Carabet, and Ramona Stef. 2025. "Variable Transect Method Outperformed in Sampling Hymenopteran Flower Visitors in Brassica campestris L. var. toria Ecosystem" Agronomy 15, no. 6: 1281. https://doi.org/10.3390/agronomy15061281
APA StyleSarma, A. K., Neog, B., Deka, M. K., Carabet, A., & Stef, R. (2025). Variable Transect Method Outperformed in Sampling Hymenopteran Flower Visitors in Brassica campestris L. var. toria Ecosystem. Agronomy, 15(6), 1281. https://doi.org/10.3390/agronomy15061281






