Foliar Application of Melatonin Improves Photosynthesis and Secondary Metabolism in Chenopodium quinoa Willd. Seedlings Under High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Melatonin Treatments
2.2. Sample Collection and Preservation
2.3. Leaf Photosynthesis Measurement
2.4. Leaf Pigment Measurements
2.5. Leaf Total Polyphenol and Flavonoid Measurements
2.6. Polyphenol Oxidase (PPO) Measurement
2.7. Leaf Saponin Measurement
2.8. Statistical Analysis
3. Results
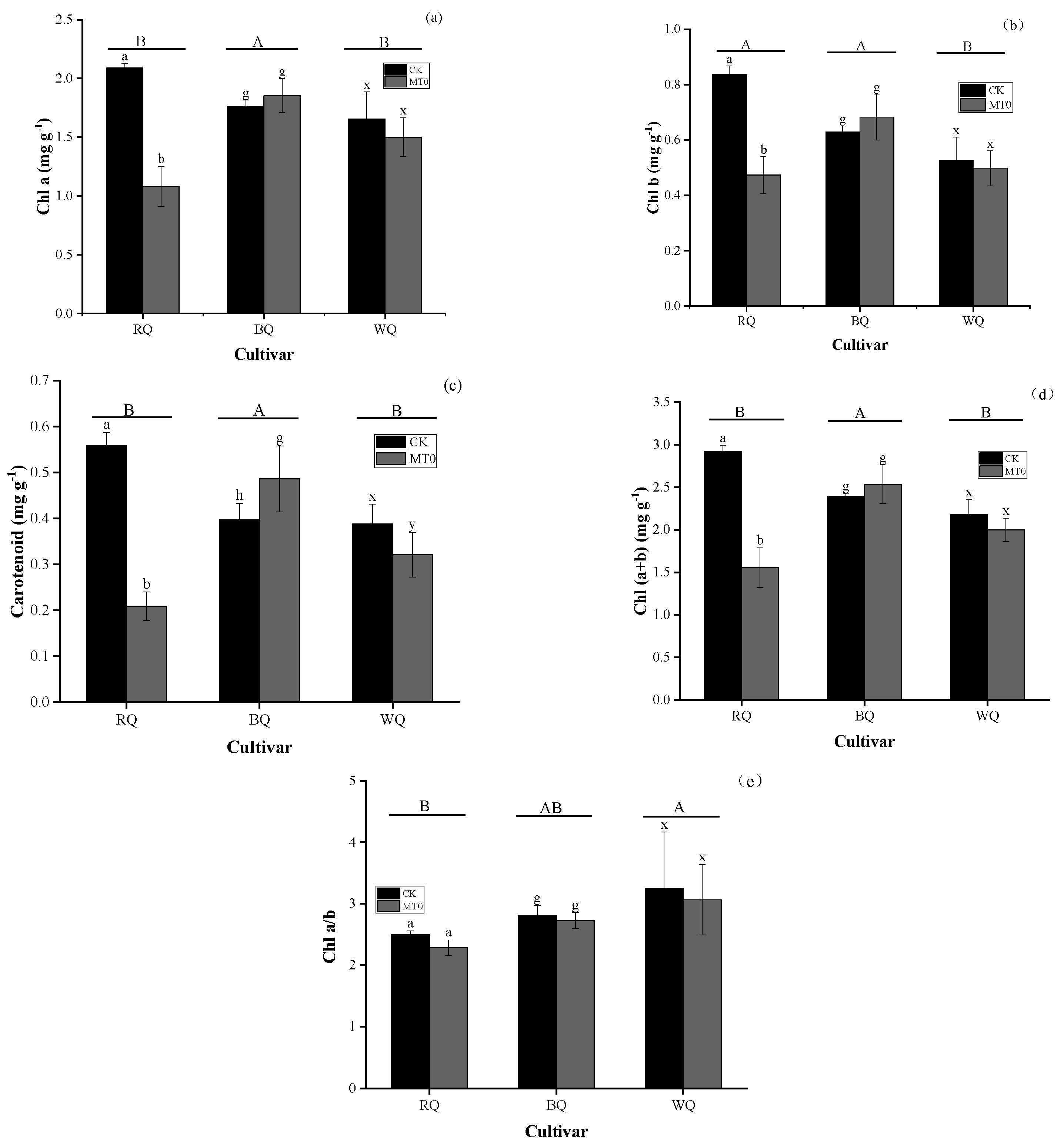
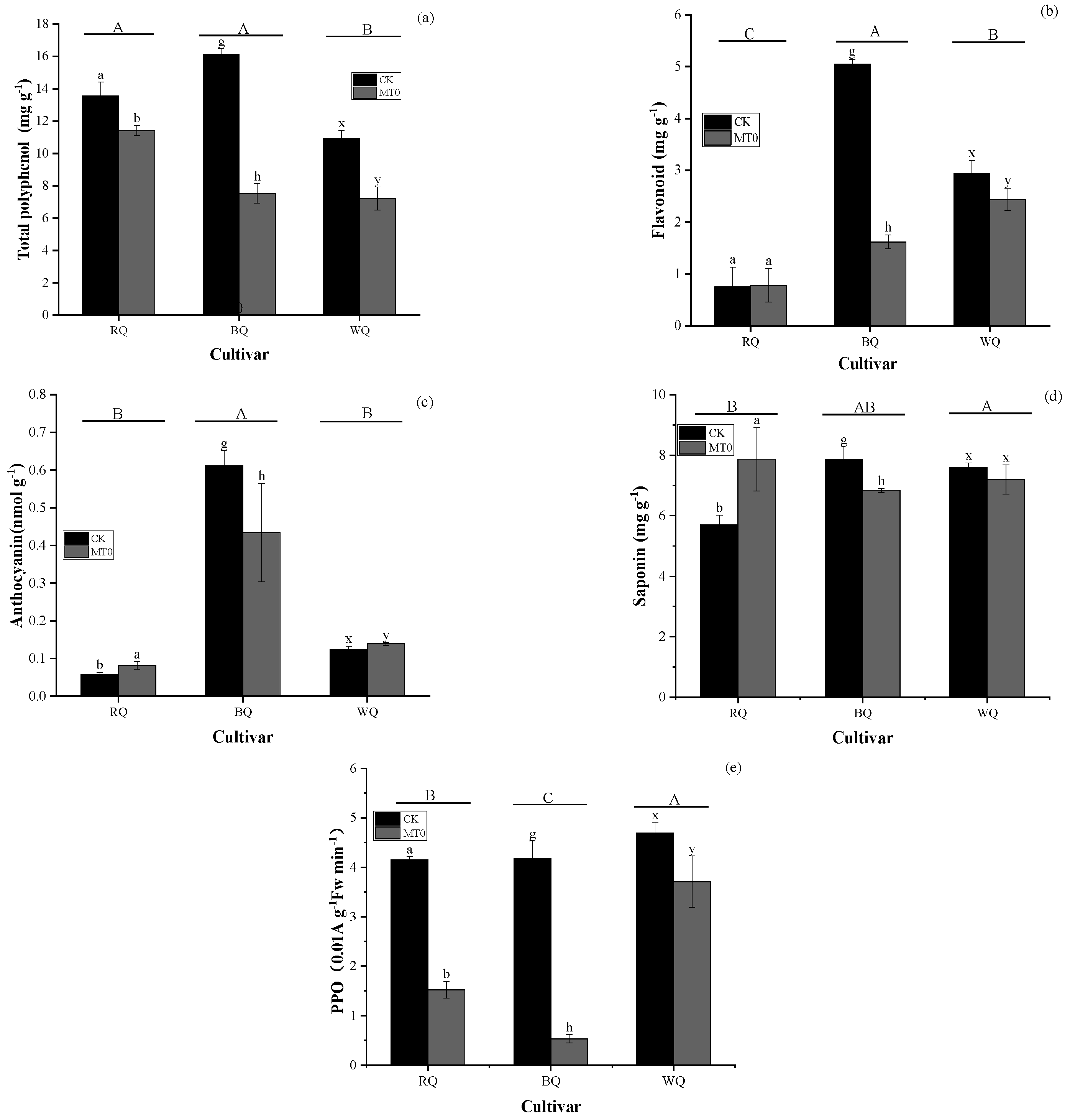
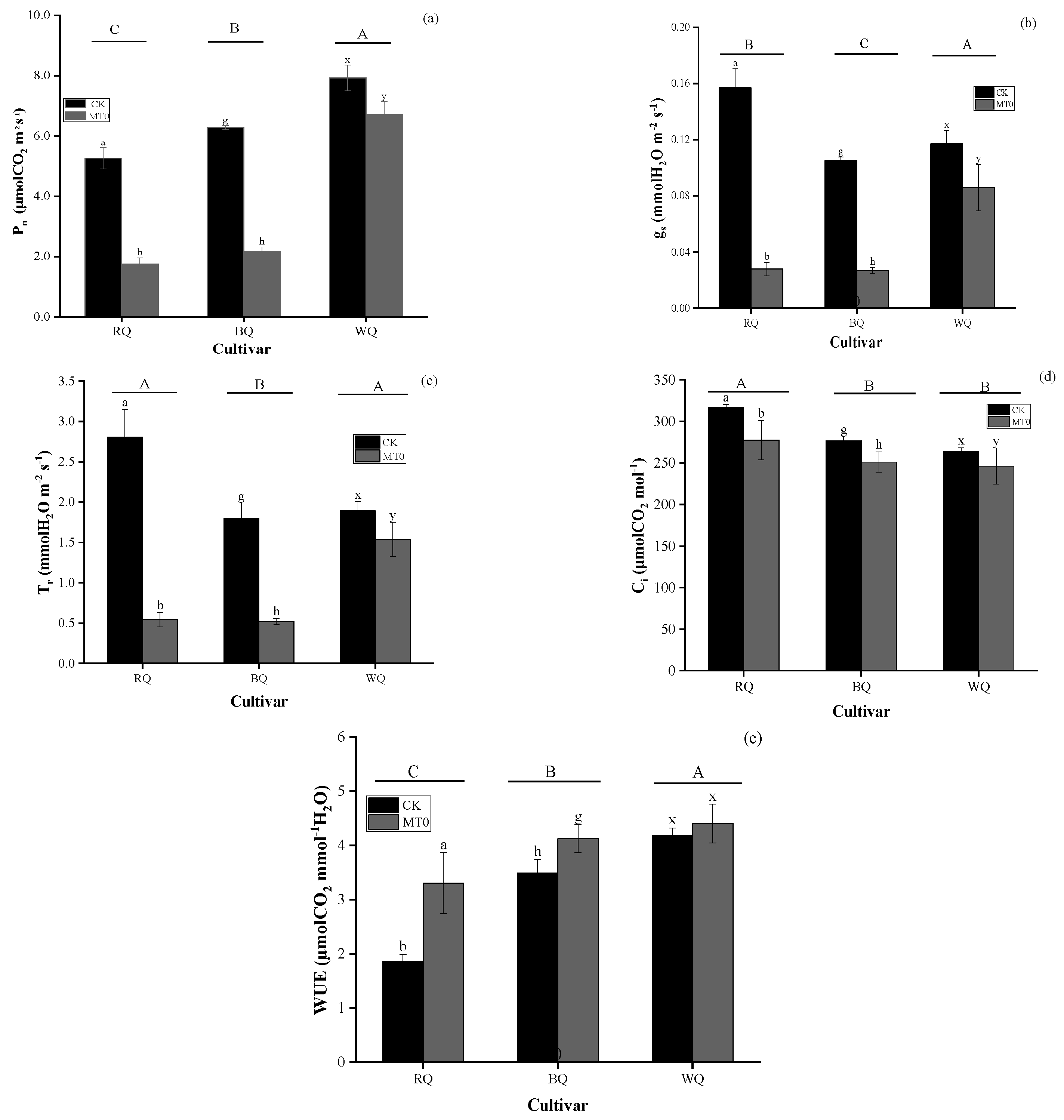
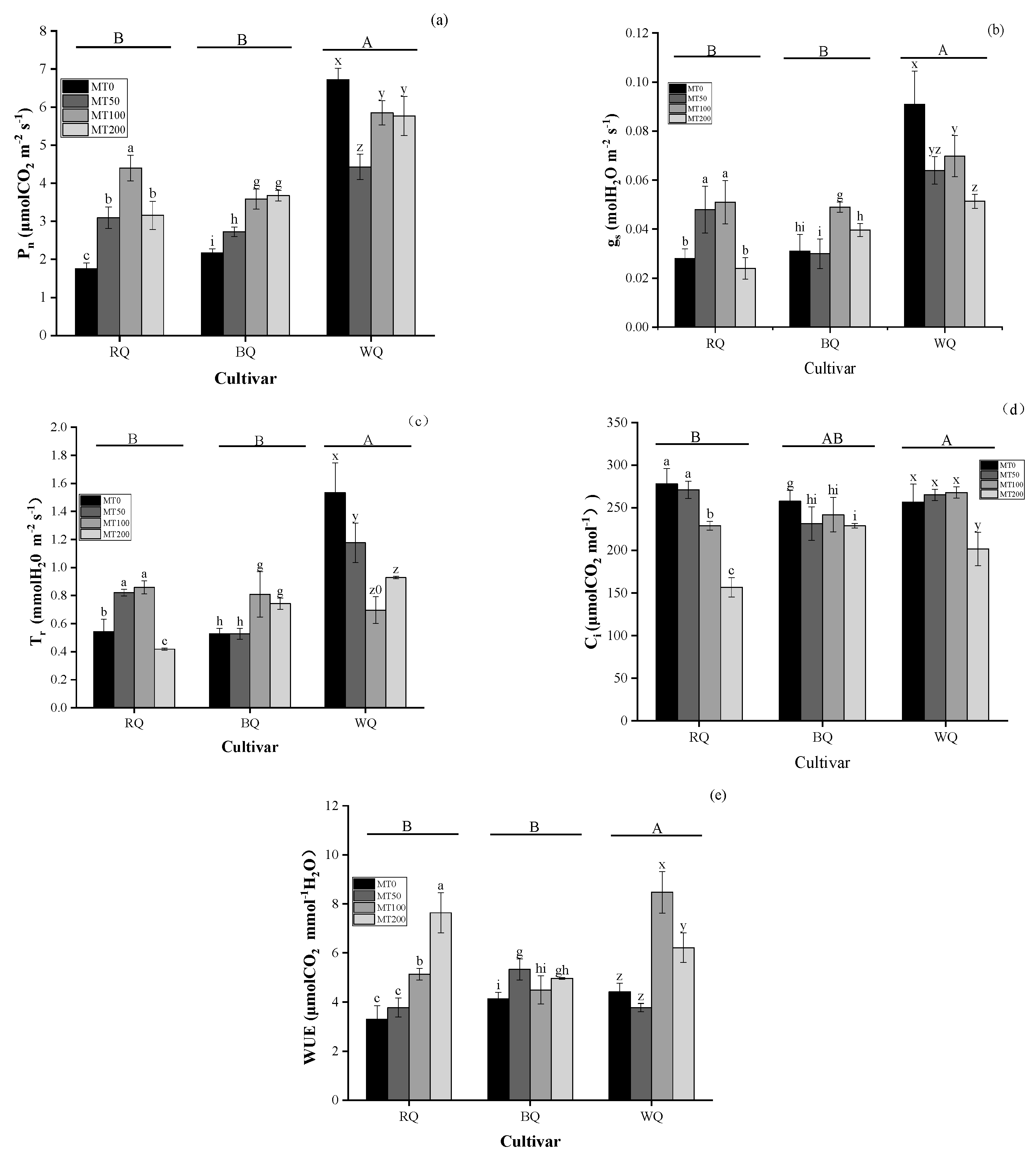
3.1. Impacts of Heat Stress and Cultivar on Metabolite and Gas Exchange Parameters
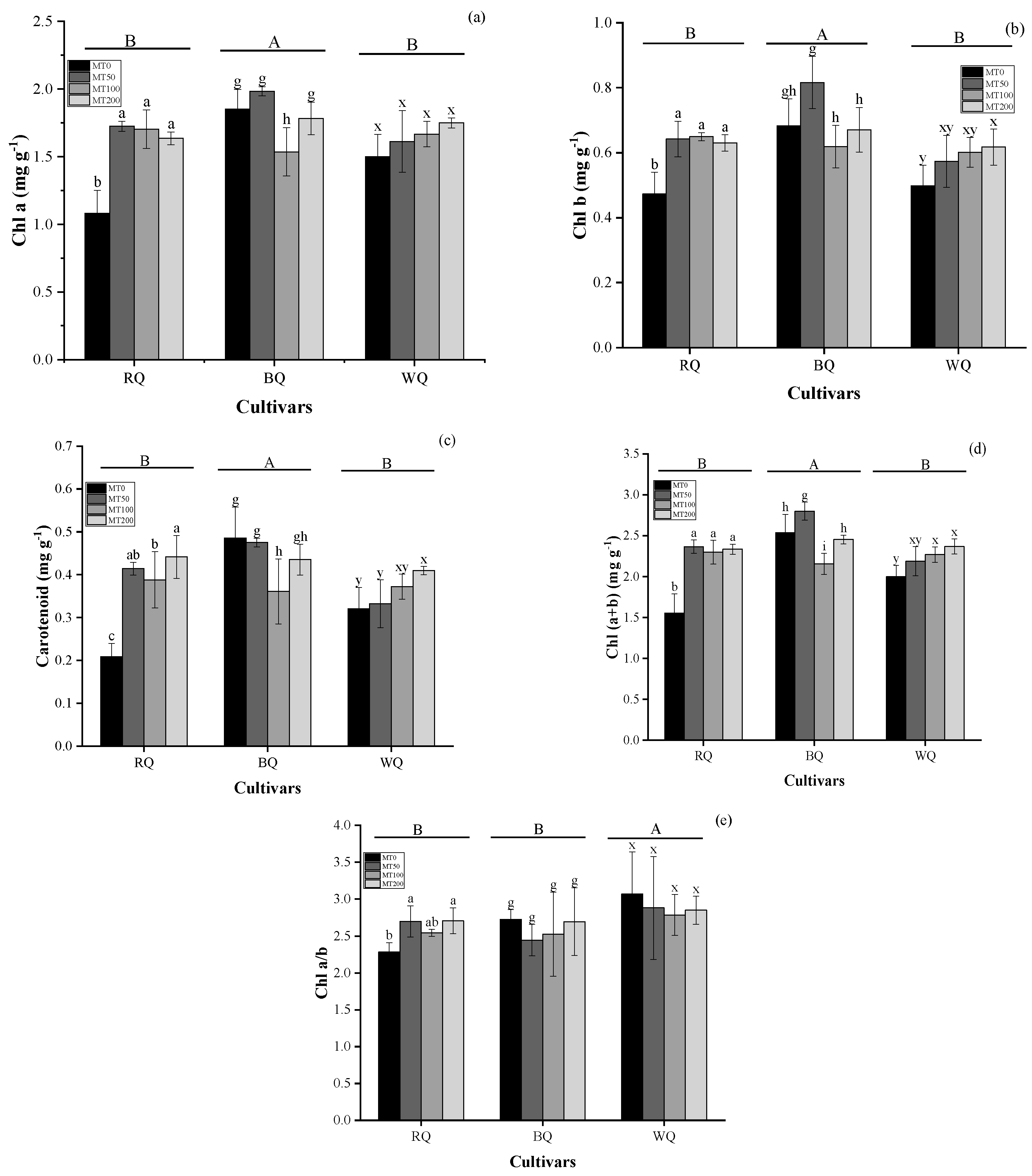
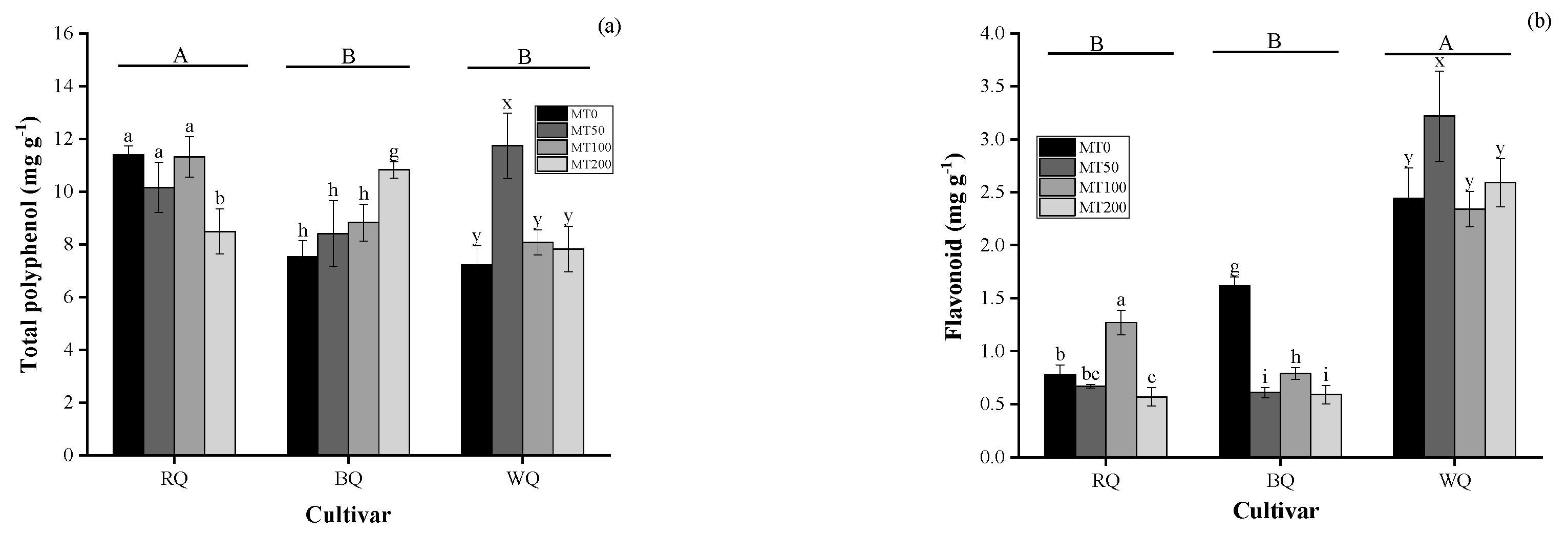
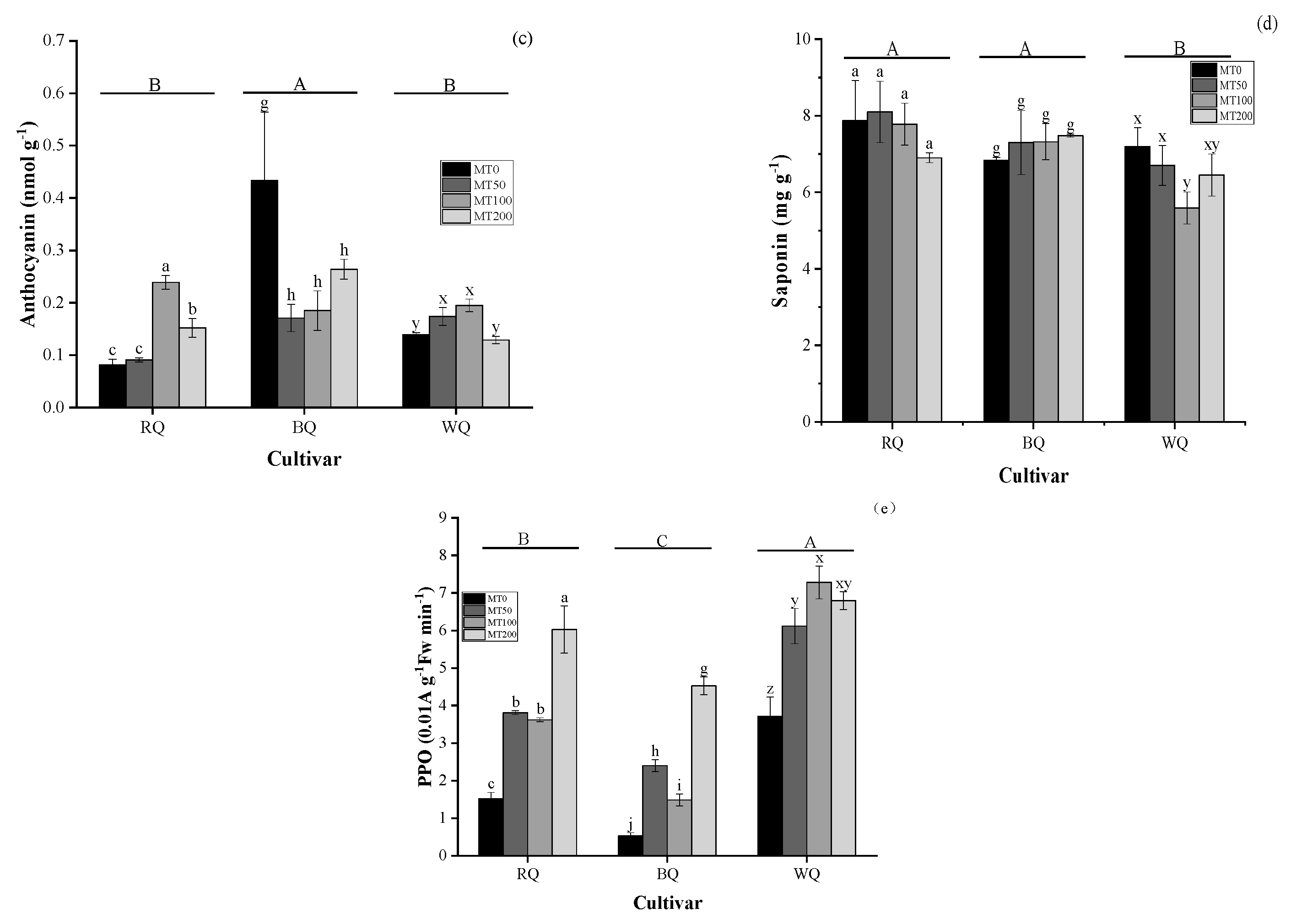
3.2. Impacts of Cultivar and MT on Metabolite and Gas Exchange Parameters in Plants Exposed to Heat Stress
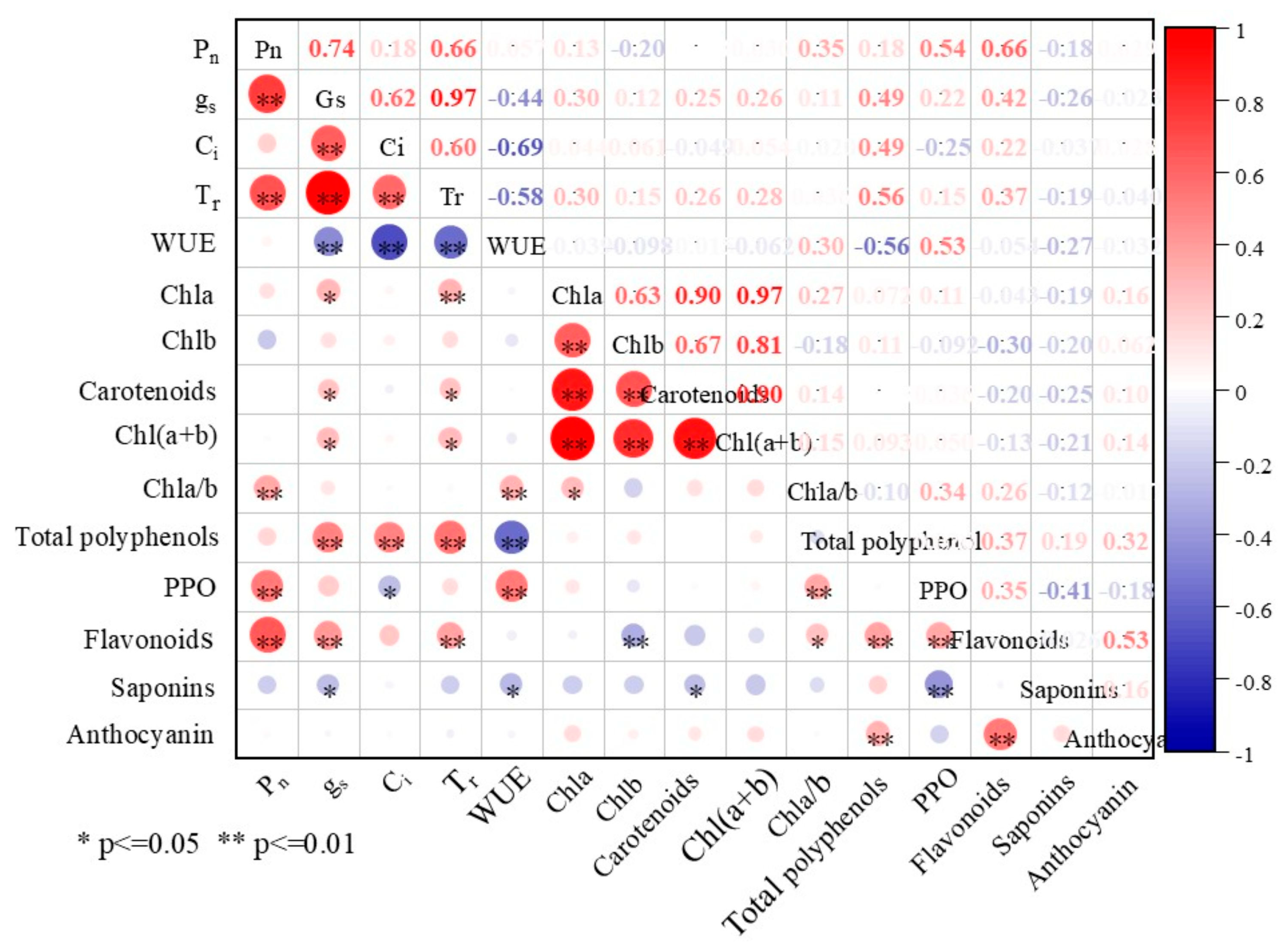
3.3. Correlation Analysis of the Photosynthetic Parameters and Some Biochemical Traits in Plants Exposed to High Temperatures
4. Discussion
4.1. Changes in Photosynthetic Efficiency Under Favorable Growth Conditions and Heat Stress Conditions
4.2. Metabolites and Photosynthesis in Plants Exposed to Heat Stress with MT Spraying
4.3. Correlation Analysis of Photosynthetic Parameters and Some Biochemical Traits of Quinoa Cultivars with MT and Exposure to High Temperatures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilcacundo, R.; Hernández, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R. Nutritional composition and bioactive components in quinoa (Chenopodium quinoa Willd.) greens: A review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Luna, A.; Pimentel Cortes, V.; Fuentes Carmonas, F.; Diaz-Sobac, R. Quinoa leaf as a nutritional alternative. Cienc. Inv. Agric. 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Swieca, M.; Sulkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts-In vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef]
- Satheesh, N.; Fanta, S.W.; Yildiz, F. Review on structural, nutritional and anti-nutritional composition of Teff (Eragrostis tef) in comparison with Quinoa (Chenopodium quinoa Willd.). Cogent Food Agric. 2018, 4, 1546942. [Google Scholar]
- Yang, A.; Akhtar, S.; Amjad, M.; Iqbal, S.; Jacobsen, S. Growth and physiological responses of quinoa to drought and temperature stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Hinojosa, L.; Gonzalez, J.; Barrios-Masias, F.; Fuentes, F.; Murphy, K. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R.; et al. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef]
- Isobe, K.; Uziie, K.; Hitomi, S.; Furuya, U. Agronomic studies on quinoa (Chenopodium quinoa Willd.) cultivation in Japan. Jpn. J. Crop Sci. 2012, 81, 167–172. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Yan, H.; Ni, Y.; Akhlaq, M.; Zhou, J.; Xue, R. Effect of micro–spray on plant growth and chlorophyll fluorescence parameter of tomato under high temperature condition in a greenhouse. Sci. Hortic. 2022, 306, 111441. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, C.; Yan, H.; Li, J.; Ren, J.; Akhlaq, M.; Hameed, M.U.; Disasa, K.N. Physiological response of tomato and cucumber plants to micro-spray in high-temperature environment: A Scientific and effective means of alleviating crop heat stress. Agronomy 2023, 13, 2798. [Google Scholar] [CrossRef]
- Hou, M.; Ni, J.; Mao, H. Effects of airflow disturbance on the content of biochemical components and mechanical properties of cucumber seedling stems. Agriculture 2023, 13, 1125. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) cultivars grown under field conditions in southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef]
- Eisa, S.; Eid, M.; Abd, E.; Hussin, S.; Abdel-Ati, A.; El-Bordeny, N.; Ali, S.; Al-Sayed, H.; Lotfy, M.; Masoud, A.; et al. ‘Chenopodium quinoa’ Willd. A new cash crop halophyte for saline regions of Egypt. Aust. J. Crop Sci. 2017, 11, 343–351. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.; Ghaffar, A.; Kausar, A.; Zeidi, M.; Kadambot, H.; Siddique, K.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Shang, Y.; Wang, X.; Du, G.; Lü, D. Preharvest application of melatonin induces anthocyanin accumulation and related gene upregulation in red pear (Pyrus ussuriensis). J. Integr. Agr. 2021, 20, 2126–2137. [Google Scholar] [CrossRef]
- Arnao, M.; Hernandez-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886. [Google Scholar] [CrossRef]
- Dey, S.; Biswas, A.; Deng, Y.; Birhanie, Z.; Wentao, C.; Li, D. Exogenous melatonin enhances low-temperature stress of jute seedlings through modulation of photosynthesis and antioxidant potential. Heliyon 2023, 9, e19125. [Google Scholar] [CrossRef]
- Karumannil, S.; Khan, T.; Kappachery, S.; Gururani, M. Impact of exogenous melatonin application on photosynthetic machinery under abiotic stress conditions. Plants 2023, 12, 2948. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, H.; Xu, X.; Yang, J.; Wang, G.; Wei, Z.; Xu, T.; Yin, J. Study on the method and mechanism of seedling picking for pepper (Capsicum annuum L.) plug seedlings. Agriculture 2024, 14, 11. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Song, J.; Zhang, R.; Cai, W. Does the daily light integral influence the sowing density of tomato plug seedlings in a controlled environment? Horticulturae 2024, 10, 730. [Google Scholar] [CrossRef]
- Liang, D.; Gao, F.; Ni, Z.; Lin, L.; Deng, Q.; Tang, Y.; Wang, X.; Luo, X.; Xia, H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules 2018, 23, 584. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ferrante, A.; Nafees, M.; Darras, A.; Nazir, M.M.; AlShaqhaa, M.A.; Elsaid, F.A. Melatonin and salicylic acid synergistically improve arsenic induced oxidative stress tolerance in ornamental sword lily. Sci. Hortic. 2023, 322, 112389. [Google Scholar] [CrossRef]
- Zrig, A.; Saleh, A.M.; Sheteiwy, M.S.; Hamouda, F.; Selim, S.; Abdel-Mawgoud, M.; Almuhayawi, M.S.; Okla, M.K.; Abbas, Z.K.; Al-Qahtani, W.H.; et al. Melatonin priming as a promising approach to improve biomass accumulation and the nutritional values of Chenopodium quinoa sprouts: A genotype-based study. Sci. Hortic. 2022, 301, 111088. [Google Scholar] [CrossRef]
- Austin, J.; Jeon, Y.; Cha, M.; Park, S.; Cho, Y. Effects of photoperiod, light intensity and electrical conductivity on the growth and yield of quinoa (Chenopodium quinoa Willd.) in a closed-type plant factory system. Korean J. Hortic. Sci. Technol. 2016, 34, 405–416. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED supplemental lighting improves quality of tomato seedlings by increasing biomass accumulation in a controlled environment. Agronomy 2024, 14, 1888. [Google Scholar] [CrossRef]
- Ahmad, I.; Cheng, Z.; Meng, H.; Liu, T.; Nan, W.; Khan, M.; Wasila, H.; Khan, A. Effect of intercropped garlic (Allium sativum) on chlorophyl contents, photosynthesis and antioxidant enzymes in pepper. Pak. J. Bot. 2013, 45, 1889–1896. [Google Scholar]
- Shanghai Institute of Plant Physiology, Chinese Academy of Sciences/Shanghai Society of Plant Physiology. A Modern Guide to Plant Physiology Experiments, 1st ed.; Science Press: Beijing, China, 1999; pp. 212–317. (In Chinese) [Google Scholar]
- Xu, C.; He, C.; Wang, Y.; Bi, Y.; Jiang, H. Effect of drought and heat stresses on photosynthesis, pigments, and xanthophyll cycle in alfalfa (Medicago sativa L.). Photosynthetica 2020, 58, 1226–1236. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Cai, X.; Wang, Q.; Dai, S. Heat-responsive photosynthetic and signaling pathways in plants: Insight from proteomics. Int. J. Mol. Sci. 2017, 18, 2191. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Y.Y.; Xing, D.K. The differential response of intracellular water metabolism derived from intrinsic electrophysiological information in Morus alba L. and Broussonetia papyrifera (L.) vent. subjected to water shortage. Horticulturae 2022, 8, 182. [Google Scholar] [CrossRef]
- Xing, D.; Chen, L.; Wu, Y.; Zwiazek, J. Leaf physiological impedance and elasticity modulus in Orychophragmus violaceus seedlings subjected to repeated osmotic stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Wang, X.; Wang, L.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Nie, R.; Wei, X.; Jin, N.; Su, S. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 2024, 103, 675–687. [Google Scholar] [CrossRef]
- Peak, J.; Lee, Y.; Kim, Y.; Yoon, K. Antioxidant and antimicrobial activities of quinoa (Chenopodium quinoa Willd.) seeds cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Siddika1, M.R.; Rakib, M.A.; Zubair, M.A.; Islam, M.M.; Haque, M.S.; Al-Khayri, J.M. Regulatory mechanism of enhancing polyphenol oxidase activity in leaf of Basella alba induced by high temperature stress. Emir. J. Food Agric. 2015, 27, 82–93. [Google Scholar] [CrossRef]
- Qian, G.; Li, X.; Zhang, H.; Zhang, H.; Zhou, J.; Ma, X.; Sun, W.; Yang, W.; He, R.; Wahab, A.; et al. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa Willd.) grains of different colors. Food Chem. X 2023, 17, 100594. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, S.; Wellman, G.; Schmöckel, S. Saponins of quinoa: Structure, function and opportunities. In The Quinoa Genome; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 119–138. [Google Scholar]
- Mahmood, S.; Parveen, A.; Hussain, I.; Javed, S.; Iqbal, M. Possible involvement of secondary metabolites in the thermotolerance of maize seedlings. Int. J. Agric. Biol. 2014, 16, 1075–1082. [Google Scholar]
- Jahan, M.; Shu, S.; Wang, Y.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Melatonin alleviates heat induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Jahan, M.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Ei-Yazied, A.; Alabdallah, N.; Hikal, M.; Mohamed, M.; Ibrahim, M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-ZnO stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S. A comparative assessment of photosynthetic pigments and defense enzymes in ex vitro and in vitro propagated plants of banana (Musa spp.). Biocatal. Agric. Biotechnol. 2023, 51, 102799. [Google Scholar] [CrossRef]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Aparecido, L.M.T.; Woo, S.; Suazo, C.; Hultine, K.R.; Blonder, B. High water use in desert plants exposed to extreme heat. Ecol. Lett. 2020, 23, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Slot, M.; Garcia, M.N.; Winter, K. Temperature response of CO2 exchange in three tropical tree species. Funct. Plant Biolog. 2016, 43, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. Effects of exogenous melatonin on physiological response of cucumber seedlings under high temperature stress. Master’s Thesis, Northwest A&F University, Xianyang, China, 2010. [Google Scholar]
- Kaur, K.; Asthir, B. Regulation of polyphenol catabolism in amelioration of high-temperature stress vis-a-vis antioxidant defense system in wheat. Cereal Res. Commun. 2022, 50, 987–998. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous melatonin treatment induces disease resistance against botrytis cinerea on post-harvest grapes by activating defence. Foods 2022, 11, 2231. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biol. Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, H.; Wang, H.; Wang, C.; He, C.; Xu, M.; Zhang, W.; Zhang, W.; Zhang, Z.; Su, L. Melatonin improves saponin biosynthesis and primary root growth in Psammosilene tunicoides hairy roots through multiple hormonal signaling and transcriptional pathwanys. Ind. Crops Prod. 2023, 200, 116819. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar] [CrossRef]
- Mastebroek, H.D.; Limburg, H.; Gilles, T.; Marvin, H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd.). J. Sci. Food Agric. 2000, 80, 152–156. [Google Scholar] [CrossRef]

| Two-Way ANOVA of Cultivar Level and Temperature Level (p < 0.05) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TEM | Cultivar | TEM × Cultivar | ||||||||
| Part | Trait | d.f. | F | p | d.f. | F | p | d.f. | F | p |
| Metabolite | Chl a | 1 | 42.333 | p < 0.001 | 2 | 7.590 | 0.03 | 2 | 37.065 | p < 0.001 |
| Chl b | 1 | 23.831 | p < 0.001 | 2 | 17.128 | p < 0.001 | 2 | 30.501 | p < 0.001 | |
| Carotenoid | 1 | 43.316 | p < 0.001 | 2 | 9.323 | 0.001 | 2 | 59.504 | p < 0.001 | |
| Chl (a + b) | 1 | 60.762 | p < 0.001 | 2 | 13.047 | p < 0.001 | 2 | 58.675 | p < 0.001 | |
| Chl a/b | 1 | 0.232 | 0.632 | 2 | 3.298 | 0.54 | 2 | 0.110 | 0.897 | |
| Total polyphenols | 1 | 492.445 | p < 0.001 | 2 | 92.488 | p < 0.001 | 2 | 79.721 | p < 0.001 | |
| Flavonoids | 1 | 165.502 | p < 0.001 | 2 | 233.437 | p < 0.001 | 2 | 113.377 | p < 0.001 | |
| Anthocyanin | 1 | 4.887 | 0.037 | 2 | 191.405 | p < 0.001 | 2 | 10.288 | 0.001 | |
| Saponin | 1 | 1.772 | 0.196 | 2 | 4.115 | 0.029 | 2 | 25.394 | p < 0.001 | |
| PPO | 1 | 547.419 | p < 0.001 | 2 | 114.264 | p < 0.001 | 2 | 56.858 | p < 0.001 | |
| Gas exchanges | Pn | 1 | 1413.01 | p < 0.001 | 2 | 889.563 | p < 0.001 | 2 | 120.800 | p < 0.001 |
| gs | 1 | 664.174 | p < 0.001 | 2 | 51.758 | p < 0.001 | 2 | 100.104 | p < 0.001 | |
| Tr | 1 | 619.071 | p < 0.001 | 2 | 47.159 | p < 0.001 | 2 | 111.985 | p < 0.001 | |
| Ci | 1 | 28.242 | p < 0.001 | 2 | 25.221 | p < 0.001 | 2 | 3.550 | 0.045 | |
| WUE | 1 | 43.277 | p < 0.001 | 2 | 76.739 | p < 0.001 | 2 | 9.495 | 0.001 | |
| Two-Way ANOVA of MT and Cultivar (p < 0.05) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MT | Cultivar | MT × Cultivar | ||||||||
| Part | Trait | d.f. | F | p | d.f. | F | p | d.f. | F | p |
| Metabolite | Chl a | 2 | 15.654 | p < 0.001 | 3 | 17.884 | p < 0.001 | 6 | 12.224 | p < 0.001 |
| Chl b | 2 | 10.869 | p < 0.001 | 3 | 22.390 | p < 0.001 | 6 | 5.575 | p < 0.001 | |
| Carotenoid | 2 | 12.712 | p < 0.001 | 3 | 22.105 | p < 0.001 | 6 | 13.790 | p < 0.001 | |
| Chl (a + b) | 2 | 25.975 | p < 0.001 | 3 | 33.621 | p < 0.001 | 6 | 17.763 | p < 0.001 | |
| Chl a/b | 2 | 0.328 | 0.805 | 3 | 5.037 | 0.01 | 6 | 1.171 | 0.337 | |
| Total polyphenols | 2 | 24.150 | p < 0.001 | 3 | 7.999 | p < 0.001 | 6 | 26.185 | p < 0.001 | |
| Flavonoids | 2 | 10.374 | p < 0.001 | 3 | 649.204 | p < 0.001 | 6 | 31.719 | p < 0.001 | |
| Anthocyanin | 2 | 9.085 | p < 0.001 | 3 | 50.873 | p < 0.001 | 6 | 25.795 | p < 0.001 | |
| Saponin | 2 | 2.662 | p < 0.001 | 3 | 21.401 | 0.058 | 6 | 4.709 | p < 0.001 | |
| PPO | 2 | 352.764 | p < 0.001 | 3 | 663.052 | p < 0.001 | 6 | 30.043 | p < 0.001 | |
| Gas exchanges | Pn | 2 | 54.819 | p < 0.001 | 3 | 525.684 | p < 0.001 | 6 | 46.833 | p < 0.001 |
| gs | 2 | 17.545 | p < 0.001 | 3 | 133.390 | p < 0.001 | 6 | 18.592 | p < 0.001 | |
| Tr | 2 | 15.181 | p < 0.001 | 3 | 214.785 | p < 0.001 | 6 | 78.451 | p < 0.001 | |
| Ci | 2 | 68.464 | p < 0.001 | 3 | 4.848 | 0.012 | 6 | 17.124 | p < 0.001 | |
| WUE | 2 | 85.541 | p < 0.001 | 3 | 22.398 | p < 0.001 | 6 | 41.527 | p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, J.; Xing, D.; Wu, Y. Foliar Application of Melatonin Improves Photosynthesis and Secondary Metabolism in Chenopodium quinoa Willd. Seedlings Under High-Temperature Stress. Agronomy 2025, 15, 1556. https://doi.org/10.3390/agronomy15071556
Li M, Li J, Xing D, Wu Y. Foliar Application of Melatonin Improves Photosynthesis and Secondary Metabolism in Chenopodium quinoa Willd. Seedlings Under High-Temperature Stress. Agronomy. 2025; 15(7):1556. https://doi.org/10.3390/agronomy15071556
Chicago/Turabian StyleLi, Meiqing, Jinyang Li, Deke Xing, and Yanyou Wu. 2025. "Foliar Application of Melatonin Improves Photosynthesis and Secondary Metabolism in Chenopodium quinoa Willd. Seedlings Under High-Temperature Stress" Agronomy 15, no. 7: 1556. https://doi.org/10.3390/agronomy15071556
APA StyleLi, M., Li, J., Xing, D., & Wu, Y. (2025). Foliar Application of Melatonin Improves Photosynthesis and Secondary Metabolism in Chenopodium quinoa Willd. Seedlings Under High-Temperature Stress. Agronomy, 15(7), 1556. https://doi.org/10.3390/agronomy15071556








