Putative Second-Site Mutations in the Barley Low Phytic Acid 1-1 (lpa 1-1) Genetic Background Further Reduce Seed Total Phosphorus
Abstract
1. Introduction
2. Materials and Methods
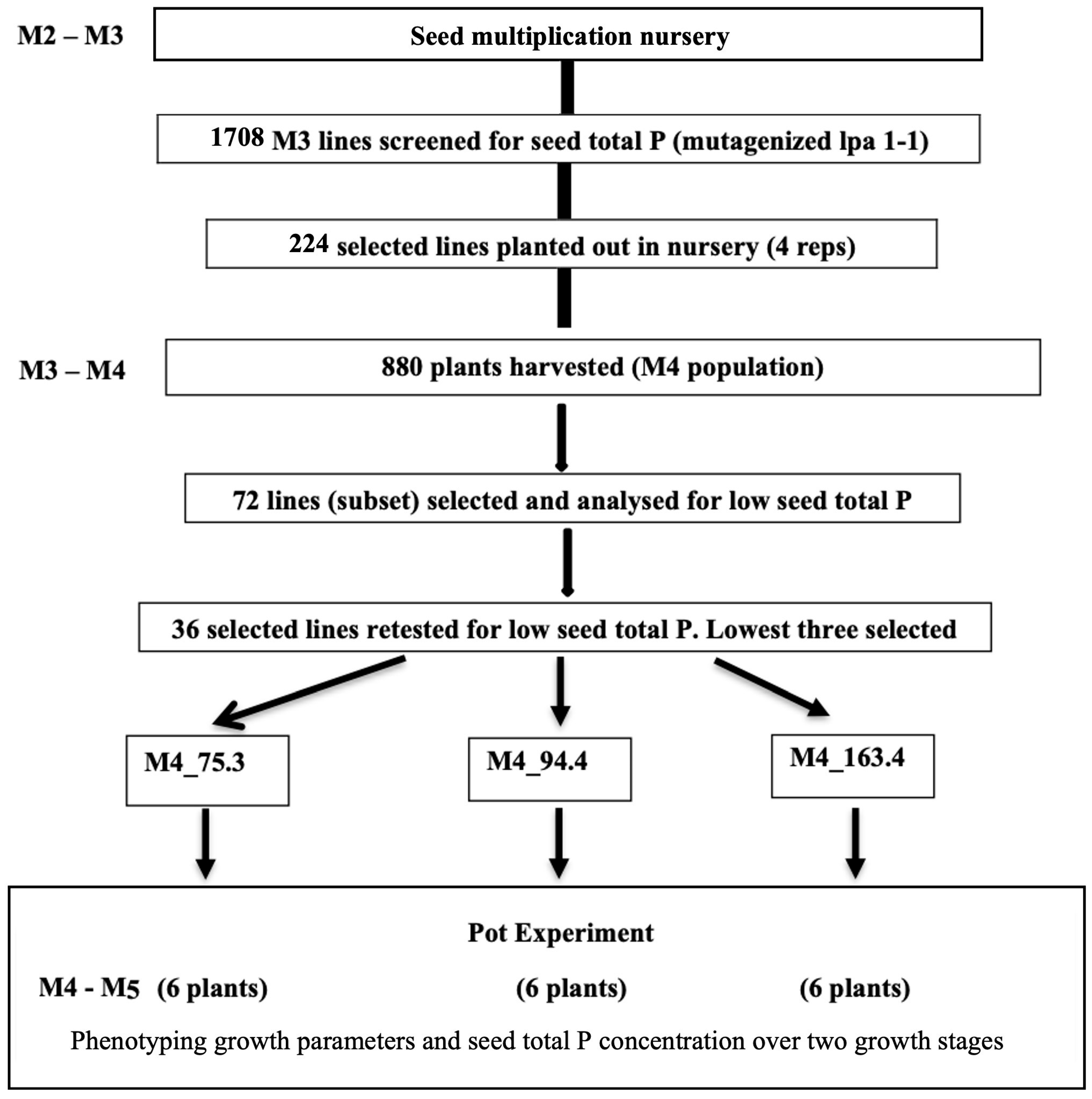
2.1. Overview of Screening Approach and Testing of Events of Interest
2.2. Experiment to Test P Uptake by Barley Mutants (M5)
2.3. TP and PA Analysis of Vegetative and Grain Tissue
2.4. Data Analysis
3. Results
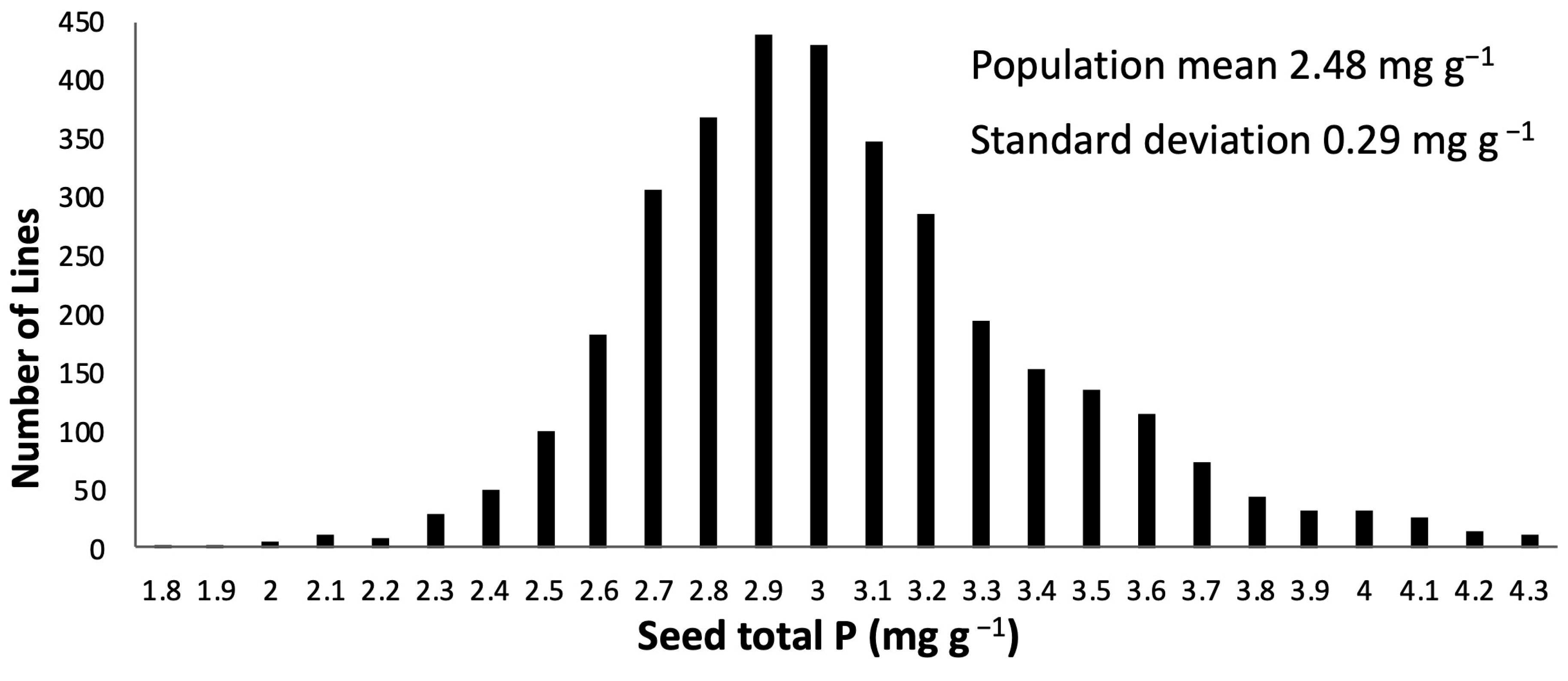
3.1. Selection for Low Seed TP in M3-M4 lpa 1-1 Barley
3.2. Seed TP, PA and Seed Weight of Three Putative Second-Site lpa Mutants in the M5 Generation
3.3. P Uptake and Utilization Efficiency in Three Novel lpa M4 Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Julia, C.C.; Rose, T.J.; Pariasca-Tanaka, J.; Jeong, K.; Matsuda, T.; Wissuwa, M. Phosphorus uptake commences at the earliest stages of seedling development in rice. J. Exp. Bot. 2018, 69, 233–5240. [Google Scholar] [CrossRef] [PubMed]
- Raboy, V.; Young, K.A.; Dorsch, J.A.; Cook, A. Genetics and breeding of seed phosphorus and phytic acid. J. Plant Physiol. 2001, 158, 489–497. [Google Scholar] [CrossRef]
- Raboy, V.; Cichy, K.; Peterson, K.; Reichman, S.; Sompong, U.; Srinives, P.; Saneoka, H. Barley (Hordeum vulgare L.) low phytic acid 1-1: An endosperm-specific, filial determinant of seed total phosphorus. J. Hered. 2014, 105, 656–665. [Google Scholar] [CrossRef]
- Tunney, H.; Breeuwsma, A.; Withers, P.J.A.; Ehlert, P.A.I. Phosphorus Fertilizer Strategies: Present and Future. In Phosphorus Loss from Soil to Water; Tunney, H., Carton, O.T., Brookes, P.C., Johnston, A.E., Eds.; CAB International: Wallingford, UK, 1997; pp. 177–203. [Google Scholar]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its efficient use in agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: Rethinking phosphorus management in the food chain. Environ. Sci. Technol. 2014, 48, 6523–6530. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Bennett, E.M. Reconsideration of the planetary boundary for phosphorus. Environ. Res. Lett. 2011, 6, 014009. [Google Scholar] [CrossRef]
- Nziguheba, G.; Zingore, S.; Kihara, J.; Merckx, R.; Njoroge, S.; Otinga, A.; Vandamme, E.; Vanlauwe, B. Phosphorus in smallholder farming systems of sub-Saharan Africa: Implications for agricultural intensification. Nutr. Cycl. Agroecosyst. 2016, 104, 321–340. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Sutton, M.A.; Reay, D.S.; Heal, K.V.; Hermann, L.; Kabbe, C.; Spears, B.M. Global actions for a sustainable phosphorus future. Nat. Food 2021, 2, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.J.; Pariasca-Tanaka, J.; Rose, M.T.; Fukuta, Y.; Wissuwa, M. Genotypic variation in grain phosphorus concentration; and opportunities to improve P-use efficiency in rice. Field Crops Res. 2010, 119, 154–160. [Google Scholar] [CrossRef]
- Shenoy, V.V.; Kalagudi, G.M. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef]
- Vandamme, E.; Wissuwa, M.; Rose, T.J.; Dieng, I.; Drame, K.N.; Fofana, M.; Senthilkumar, K.; Venuprasad, R.; Jallow, D.; Segda, Z.; et al. Genotypic variation in grain P loading across diverse rice growing environments and implications for field P balances. Front. Plant Sci. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Rothwell, S.A.; Doody, D.G.; Johnston, C.; Forber, K.J.; Cencic, O.; Rechberger, H.; Withers, P.J.A. Phosphorus stocks and flows in an intensive livestock dominated food system. Resour. Conserv. Recycl. 2020, 163, 105065. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef]
- Guttieri, M.; Bowen, D.; Dorsch, J.A.; Raboy, V.; Souza, E. Identification and characterization of a low phytic acid wheat. Crop Sci. 2004, 44, 418–424. [Google Scholar] [CrossRef]
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A potential cereal for producing healthy and functional foods. Food Qual. Saf. 2022, 6, fyac012. [Google Scholar] [CrossRef]
- Dorsch, J.A.; Cook, A.; Young, K.A.; Anderson, J.M.; Bauman, A.T.; Volkmann, C.J.; Murthy, P.P.; Raboy, V. Seed phosphorus and inositol phosphate phenotype of barley low phytic acid genotypes. Phytochemistry 2003, 62, 691–706. [Google Scholar] [CrossRef]
- Raboy, V.; Peterson, K.; Jackson, C.; Marshall, J.M.; Hu, G.; Saneoka, H.; Bregitzer, P. A substantial fraction of barley (Hordeum vulgare L.) low phytic acid mutations have little or no effect on yield across diverse production environments. Plants 2015, 4, 225–239. [Google Scholar] [CrossRef]
- Bregitzer, P.; Raboy, V.; Obert, D.E.; Windes, J.M.; Whitmore, J.C. Registration of ‘Herald’ barley. Crop Sci. 2007, 47, 441. [Google Scholar] [CrossRef]
- Bregitzer, P.; Raboy, V.; Obert, D.E.; Windes, J.; Whitmore, J.C. Registration of ‘Clearwater’ low-phytate hulless spring barley. J. Plant Regist. 2008, 2, 1–4. [Google Scholar] [CrossRef]
- Roslinsky, V.; Eckstein, P.E.; Raboy, V.; Rossnagel, B.G.; Scoles, G.J. Molecular marker development and linkage analysis in three low phytic acid barley (Hordeum vulgare) mutant lines. Mol. Breed. 2007, 20, 323–330. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.; Broughton, S. A nonsense mutation in a putative sulphate transporter gene results in low phytic acid in barley. Funct. Integr. Genom. 2011, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2017, 541, 92–95. [Google Scholar] [CrossRef]
- Nilan, R.A.; Sideris, E.G.; Sander, C.; Konzak, C.F. Azide—A potent mutagen. Mutat. Res. 1973, 17, 142–144. [Google Scholar] [CrossRef]
- Agriculture and Horticulture Development Board. Nutrient Management Guide (RB209); Agriculture and Horticulture Development Board: Coventry, UK, 2019; Available online: https://ahdb.org.uk/nutrient-management-guide-rb209 (accessed on 19 May 2025).
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; Circular, California Agricultural Experiment Station: Santa Cruz, CA, USA, 1950; p. 347. [Google Scholar]
- Chen, P.S.; Toribara, T.Y.; Huber, W. Microdetermination of Phosphorus. Anal. Chem. 1956, 28, 1756–1758. [Google Scholar] [CrossRef]
- Richards, I. Review of AHDB-Funded Research on Phosphorus Management in Arable Crops; AHDB: Coventry, UK, 2019; Volume 93, pp. 1–21. [Google Scholar]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information; FAO: Rome, Italy, 2008; Volume 18. [Google Scholar] [CrossRef]
- R Core Team. RR: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: www.R-project.org/ (accessed on 3 May 2020).
- Rose, T.J.; Liu, L.; Wissuwa, M. Improving phosphorus efficiency in cereal crops: Is breeding for reduced grain phosphorus concentration part of the solution? Front. Plant Sci. 2013, 4, 444. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, A.; Bishi, S.K.; Akhila, P.; Latha, P.C.; Subrahmanyam, D.; Brajendra, P.; Anantha, M.S.; Ch, S.R.; Sakhare, A.S.; et al. Tolerance of Oryza sativa to low phosphate is associated with adaptive changes in root architecture and metabolic exudates. Plant Sci. 2025, 353, 112415. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.; Tan, Y.; Shu, Q.; Li, Y.; Liu, J.; Wang, J.; Shi, J.; Zou, X. Metabolite profiling reveals the metabolic features of the progenies resulting from the low phytic acid rice (Oryza sativa L.) mutant. J. Cereal Sci. 2021, 100, 103251. [Google Scholar] [CrossRef]
- Sacchi, G.A.; Nocito, F.F. Plant sulfate transporters in the low phytic acid network: Some educated guesses. Plants 2019, 8, 61. [Google Scholar] [CrossRef]
- Cominelli, E.; Pilu, R.; Sparvoli, F. Phytic acid and transporters: What can we learn from Low phytic acid mutants. Plants 2020, 9, 69. [Google Scholar] [CrossRef]
- Zhao, H.; Frank, T.; Tan, Y.; Zhou, C.; Jabnoune, M.; Arpat, A.B.; Cui, H.; Huang, J.; He, Z.; Poirier, Y.; et al. Disruption of OsSULTR3; 3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016, 211, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Kishor, D.S.; Lee, C.; Lee, D.; Venkatesh, J.; Seo, J.; Chin, J.H.; Jin, Z.; Hong, S.-K.; Ham, J.-K.; Koh, H.E. Novel allelic variant of Lpa1 gene associated with a significant reduction in seed phytic acid content in rice (Oryza sativa L.). PLoS ONE 2019, 14, e0209636. [Google Scholar] [CrossRef] [PubMed]
| Index | Calculation | Unit | Reference |
|---|---|---|---|
| Plant P | Shoot P Uptake = Shoot dry weight (g) × P concentration (mg/g) Root P Uptake = Root dry weight (g) × P concentration (mg/g) Grain P uptake = Grain dry weight (g) × P concentration (mg/g) Grain Yield = Grain dry weight (g) × 305,000 plants ha−1 Grain P offtake = 85% Grain dry weight (g) × P2O5 concentration | mg/g mg/g mg/g t/ha t/ha | [29] |
| Physiological Efficiency | g | [30] | |
| Agronomic Efficiency (AE) | mg | [30] | |
| Crop recovery | % | ||
| Yield Response (YR) | g | ||
| Phosphorus Harvest Index (PHI) | % | ||
| Yield P efficiency |
| Generation | n | Line Details | Seed Total P (mg g−1) | Seed Weight (mg) Mean ± s.e. | Single Seed P Content (μgP Seed−1) Mean ± s.e | ||
|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean ± s.e. | |||||
| M3 | 1708 | All lines in initial TP screen | 1.9 | 4.2 | 2.84 ± 0.007 | 49 ± 0.152 | 139 ± 0.496 |
| M3 | 224 | Only selected lines | 1.2 | 3.6 | 2.50 ± 0.016 | 45 ± 0.4 | 112 ± 0.897 |
| Harrington | 3 | Control in M3 nursery | 4.1 ± 0.18 | 43 ± 1.24 | 176 ± 2.88 | ||
| lpa 1-1 | 3 | Control in M3 nursery | 3.2 ± 0.11 | 45 ± 1.32 | 143 ± 1.20 | ||
| M4 | 219 a | All lines screened for TP from field | 2.4 | 4.9 | 3.54 ± 0.029 | 38 ± 0.54 | 135 |
| M4 | 72 | Four plants of 18 selected M4 lines | 1.2 | 3.6 | 3.13 ± 0.040 | 39 ± 1.05 | 121 ± 3.6 |
| M5 (+P) | 9 | Three reps of lines 75.3, 94.4 and 163.4 with no P | 2.8 | 3.2 | 3.02 ± 0.130 | 43 ± 1.99 | |
| M5 (-P) | 9 | Three reps of lines 75.3, 94.4 and 163.4 with added P | 2.6 | 3.4 | 3.04 ± 0.219 | 47 ± 2.20 | |
| M5 (both treatments) | 18 | All M5 lines tested | 2.6 | 3.4 | 3.03 ± 0.175 | 45 ± 1.57 | |
| Nutrient P | Genotype | Seed Components (±SD) | ||
|---|---|---|---|---|
| Seed Total P (mg g−1) | Phytic Acid (%) | Single Seed Weight (mg) | ||
| No added P | 94.4 | 3.0 ± 0.10 a | 0.18 ± 0.01 a | 51 ± 3.22 ab |
| 163.4 | 3.3 ± 0.14 a | 0.15 ± 0.01 a | 40 ± 3.54 cd | |
| 75.3 | 3.1 ± 0.06 a | 0.15 ± 0.04 a | 52 ± 1.41 ab | |
| Harrington | 4.2 ± 0.21 c | 0.49 ± 0.13 b | 43 ± 1.53 cd | |
| LPA 1-1 | 3.7 ± 0.10 b | 0.25 ± 0.04 a | 45 ± 1.00 cd | |
| Added P | 94.4 | 2.9 ± 0.12 a | 0.17 ± 0.021 a | 45 ± 1.41 cd |
| 163.4 | 3.2 ± 0.06 a | 0.16 ± 0.04 a | 47 ± 2.12 bc | |
| 75.3 | 3.0 ± 0.06 a | 0.18 ± 0.01 a | 39 ± 2.65 d | |
| Harrington | 4.4 ± 0.20 c | 0.49 ± 0.09 b | 54 ± 1.73 a | |
| LPA 1-1 | 3.7 ± 0.06 b | 0.22 ± 0.01 a | 51 ± 0.58 ab | |
| Source of Variation | df | p Value and Significance | ||
| Genotype | 3 | 5.02 × 10−15 *** | 2.86 × 10−7 *** | 0.0006 *** |
| Nutrient P | 1 | 0.897 ns | 0.944 ns | 0.0957 ns |
| Interaction | 3 | 0.481 ns | 0.948 ns | 3.13 × 10−7 *** |
| Nutrient P Level (mg/L) | Genotype | Grain Yield (g) | Phosphorus Harvest Index (PHI) (%) | Harvest Index (HI) (g) | Yield/P Uptake |
|---|---|---|---|---|---|
| No added P | 94.4 | 12.73 ± 0.57 ab | 82 ± 0.05 a | 0.38 ± 0.02 a | 270 ± 0.01 ab |
| 163.4 | 10.20 ± 1.77 a | 80 ± 0.02 a | 0.35 ± 0.03 a | 270 ± 0.04 ab | |
| 75.3 | 9.40 ± 0.57 a | 80 ± 0.02 a | 0.36 ± 0.03 a | 270 ± 0.02 ab | |
| Harrington | 12.25 ± 1.91 ab | 82 ± 0.05 a | 0.35 ± 0.07 a | 200 ± 0.02 c | |
| lpa 1-1 | 11.75 ± 1.34 ab | 81 ± 0.03 a | 0.39 ± 0.02 a | 220 ± 0.01 bc | |
| Added P | 94.4 | 15.65 ± 0.78 b | 83 ± 0.04 a | 0.37 ± 0.06 a | 290 ± 0.03 a |
| 163.4 | 15.30 ± 0.00 b | 81 ± 0.03 a | 0.40 ± 0.03 a | 260 ± 0.02 ab | |
| 75.3 | 14.75 ± 1.77 b | 81 ± 0.04 a | 0.38 ± 0.03 a | 270 ± 0.02 ab | |
| Harrington | 13.85 ± 0.35 ab | 84 ± 0.01 a | 0.38 ± 0.01 a | 200 ± 0.01 c | |
| lpa 1-1 | 15.23 ± 1.10 b | 86 ± 0.04 a | 0.42 ± 0.04 a | 230 ± 0.01 abc | |
| Source of Variation | df | p Value and Significance | |||
| Genotype | 4 | 0.104 ns | 0.582 ns | 0.505 ns | 1.02 × 10−5 *** |
| Nutrient P | 1 | 6.2 × 10−6 *** | 0.189 ns | 0.138 ns | 0.432 ns |
| Interaction | 4 | 0.193 ns | 0.958 ns | 0.793 ns | 0.852 ns |
| Genotype | Yield Response (g) | Physiological Efficiency (PE) (g g−1) | Agronomic Efficiency (AE) (mg) | Crop P Recovery (%) |
|---|---|---|---|---|
| 94.4 | 2.92 | 0.36 | 19.47 | 5.47 |
| 163.4 | 5.1 | 0.26 | 34.00 | 13.07 |
| 75.3 | 5.35 | 0.36 | 35.67 | 10.00 |
| Harrington | 1.6 | 0.16 | 10.67 | 6.60 |
| lpa 1-1 | 3.48 | 0.24 | 23.20 | 9.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agesa, B.L.; Raboy, V.; Withers, P.J.A.; Steele, K.A. Putative Second-Site Mutations in the Barley Low Phytic Acid 1-1 (lpa 1-1) Genetic Background Further Reduce Seed Total Phosphorus. Agronomy 2025, 15, 1550. https://doi.org/10.3390/agronomy15071550
Agesa BL, Raboy V, Withers PJA, Steele KA. Putative Second-Site Mutations in the Barley Low Phytic Acid 1-1 (lpa 1-1) Genetic Background Further Reduce Seed Total Phosphorus. Agronomy. 2025; 15(7):1550. https://doi.org/10.3390/agronomy15071550
Chicago/Turabian StyleAgesa, Beverly L., Victor Raboy, Paul J. A. Withers, and Katherine A. Steele. 2025. "Putative Second-Site Mutations in the Barley Low Phytic Acid 1-1 (lpa 1-1) Genetic Background Further Reduce Seed Total Phosphorus" Agronomy 15, no. 7: 1550. https://doi.org/10.3390/agronomy15071550
APA StyleAgesa, B. L., Raboy, V., Withers, P. J. A., & Steele, K. A. (2025). Putative Second-Site Mutations in the Barley Low Phytic Acid 1-1 (lpa 1-1) Genetic Background Further Reduce Seed Total Phosphorus. Agronomy, 15(7), 1550. https://doi.org/10.3390/agronomy15071550







