Abstract
Utilizing environmentally friendly techniques for pathogen control in agriculture is a sustainable and eco-friendly approach to managing crop diseases. These techniques leverage the natural environment and ecosystem dynamics to reduce pathogen pressure, minimize the use of chemical inputs, and promote long-term agricultural productivity. Key strategies include crop rotation, intercropping, and maintaining biodiversity, all of which disrupt pathogen life cycles and enhance soil health. Biological control, such as introducing natural antagonists like beneficial fungi or bacteria, suppresses pathogen populations while promoting plant resilience. Additionally, practices such as mulching, soil solarization, and water management optimize environmental conditions to limit the development and spread of pathogens. These techniques also contribute to integrated pest management by providing sustainable, cost-effective solutions that reduce chemical dependency and mitigate climate change and other environmental impacts. This review discusses the importance of utilizing environmentally friendly techniques, highlighting their advantages, practical challenges, and limitations in different agro-ecological settings, and their role in advancing sustainable agriculture.
1. Introduction
1.1. Importance of Plant Disease Management
Although plant disease management has always been important, it has become increasingly complex due to recent challenges related to climate change, the appearance of emerging pathogens, resistance to fungicides, the reduction of allowed registered pesticides, and the difficulties in developing new products for plant pathogen control [1,2]. Throughout history, plant disease management has evolved across four principal phases: (i) limited intervention in old agricultural fields; (ii) mechanical and agronomical suppression techniques (plowing, rotations); (iii) extensive use of pesticides; and (iv) integrated pest management (IPM) endeavoring to achieve ecological, economic, and social balance [3].
1.2. Overview of Environmental Techniques for Pathogen Control
Environmental techniques for pathogen control are crucial components of sustainable agriculture and environmental management, aiming to minimize the reliance on chemical pesticides while preserving ecosystem health. These techniques use a variety of strategies, from physical methods and biological control to cultural practices, to create unfavorable conditions for pathogens or improve host organism resilience (Figure 1). Each approach leverages natural principles to manage pathogens more effectively while reducing potential adverse impacts on soil, water, and non-target organisms.
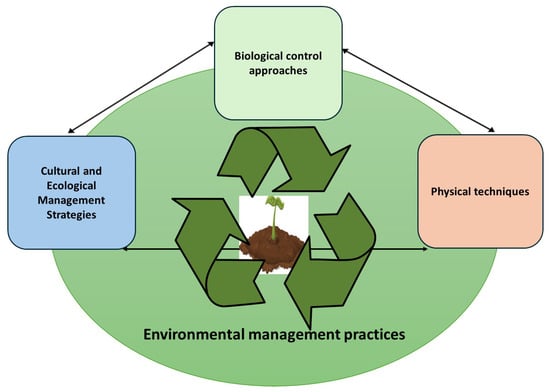
Figure 1.
Environmental management practices used for the control of plant pathogens.
One key group of environmental pathogen control techniques involves physical methods, which manipulate environmental conditions to disrupt pathogen life cycles. For example, soil solarization is a widely used technique that relies on covering the soil with transparent plastic sheeting, allowing solar radiation to heat the soil to temperatures lethal to many soil-borne pathogens. This process can effectively reduce pathogen loads without the use of synthetic chemicals [4].
Similarly, controlled irrigation practices can limit excess moisture, which often promotes fungal and bacterial pathogens. Adjusting irrigation schedules or using drip irrigation helps reduce humidity and soil moisture, creating an environment less conducive to pathogen proliferation [5].
Biological control methods are another foundational component of environmental pathogen management, using natural antagonists to suppress harmful organisms. Beneficial microorganisms such as Bacillus, Pseudomonas, Trichoderma, and Gliocladium species (spp.) can act as biocontrol agents against a wide range of plant pathogens. These organisms use multiple mechanisms, including competition, parasitism, antibiosis, and the induction of plant defense responses, to limit pathogen growth and infection. Biological control has gained considerable attention because of its environmentally friendly nature and ability to establish long-term pathogen suppression [6].
Cultural practices play an essential role in the management of pathogens by altering the conditions that support the survival and infection of a pathogen [7,8]. Crop rotation, for instance, is a long-standing practice that helps break pathogen life cycles by rotating crops with non-host plants, reducing pathogen populations in the soil over time. Cover cropping, another cultural practice, improves soil structure and supports beneficial microorganisms that compete with or inhibit pathogens. Additionally, modifying planting dates and practicing proper sanitation can prevent pathogen spread and reduces inoculum sources for the following season.
Another approach gaining interest is represented by the use of organic soil amendments, such as compost, animal manure, and plant residues [9]. These amendments serve as carbon sources for microbes, improving soil health and fertility while enhancing its microbial community, which competes with or directly inhibits pathogens. Organic matter additions have also been found to increase populations of beneficial microbes that produce enzymes and antimicrobial compounds, adding another layer of defense against soil-borne pathogens. Additionally, organic amendments can increase soil water-holding capacity and nutrient availability, helping plants better resist pathogens. Research has shown that the addition of specific composts can suppress soil-borne pathogens like Pythium and Fusarium and increase plant health by enhancing the soil microbiome’s diversity and resilience.
Genetic resistance through breeding is another environmentally sustainable method for pathogen control, through which resistant plant varieties are developed to naturally withstand certain pathogens. Breeding plants for disease resistance can significantly reduce disease incidence without relying on chemical inputs. However, pathogens can evolve and overcome these resistances, making it necessary to develop and rotate resistant crop varieties regularly.
IPM combines these techniques into a cohesive strategy, aiming for long-term pathogen control with a minimal environmental impact [10]. By using a combination of cultural practices, biological control, and physical barriers, IPM seeks to prevent pathogen establishment and spread while also monitoring pathogen populations to apply chemical interventions only as a last resort. IPM is especially valuable because it allows for flexible management strategies tailored to specific pathogen threats and environmental conditions, thereby optimizing pathogen control and reducing ecological impact.
In summary, environmentally friendly techniques for pathogen control leverage physical, biological, and cultural methods to limit pathogen proliferation while promoting a balanced ecosystem. These methods minimize the use of chemical inputs and contribute to sustainable agricultural practices. The continued innovation and integration of these approaches, along with advancements in plant breeding and microbial applications, hold promise for effective and environmentally responsible pathogen management.
1.3. Scope and Objectives of the Review
The review aims to provide a comprehensive analysis of non-chemical strategies for the sustainable and eco-friendly management of plant pathogens across agricultural and natural ecosystems. By examining physical techniques, biological control, soil health management, and integrated pest management, the study seeks to evaluate the effectiveness, benefits, and limitations of environmentally friendly techniques for controlling plant pathogens. While previous reviews have typically focused on individual environmental control methods or specific pathogen groups, this review uniquely synthesizes the synergistic interactions between multiple non-chemical approaches across diverse agricultural systems and environmental conditions. Unlike the existing literature, which often treats techniques in isolation, our comprehensive analysis systematically evaluates the combined effectiveness of environmentally friendly management strategies, providing novel insights into optimization pathways for sustainable pathogen control. This review distinctively addresses current knowledge gaps in understanding the mechanistic interactions between soil health, microbial communities, and pathogen suppression under varying climatic conditions, offering evidence-based recommendations for climate-resilient disease management. Ultimately, the study aims to serve as a valuable resource for researchers, agronomists, and policymakers by promoting sustainable pathogen management practices that minimize environmental impacts, support agricultural productivity, and reduce reliance on chemical pesticides.
2. Environmental Factors and Plant–Pathogen Interactions
2.1. Abiotic Factors
Pathogenic fungi, oomycetes, bacteria, phytoplasmas, viruses, and viroids present causal agents of plant diseases that provoke important qualitative and quantitative yield reductions. Notwithstanding the progress of science and technology, yield losses of crop production at a global level from various pests, reach about 30% of the total production today. The development of plant diseases caused by these pathogens is affected by different abiotic factors such as temperature, CO2, light intensity, relative humidity, precipitation patterns, soil moisture, soil composition, and the pH of the growing medium [11,12,13,14,15]. Temperature is a key factor since it impacts pathogen activation and disease development. Soil pH may influence plant susceptibility to plant diseases and the activity of microbial communities in a changed pH environment [14,16]. Unbalanced nutrition and contaminants importantly influence pathogen development and plant resilience [15]. All of these abiotic factors collectively influence the interaction between the plant pathogen and its host, which significantly shapes the dynamics of plant disease through the temporal dimension.
2.2. Biotic Factors
Biotic factors, including microbial communities and biodiversity, play a vital role in controlling plant pathogens by naturally suppressing diseases and promoting plant health. Microbial communities inhibit pathogens through competition, antimicrobial compound production, and parasitism, while also priming plant immune responses. Biodiversity enhances ecosystem resilience, reducing pathogen spread through the “dilution effect” and ensuring functional redundancy. Specific bacteria, such as Bacillus spp. and Pseudomonas spp., are known to inhibit pathogens like Fusarium spp. through competition and parasitism [17,18]. Specific interactions, such as mycoparasitism by Trichoderma spp. or predation by soil organisms, further reduce pathogen populations. Symbiotic organisms like mycorrhizal fungi and nitrogen-fixing bacteria [19] improve plant stress tolerance and indirectly lower susceptibility to pathogens. Microbial communities can trigger systemic resistance in plants, enhancing their ability to fend off diseases [18]. Despite challenges like environmental variability and pathogen adaptation, these biotic interactions are increasingly harnessed in agriculture through biocontrol agents (BCAs), microbiome engineering, and conservation practices. Practices like crop rotation and minimal tillage preserve microbial diversity, reducing disease pressure by disrupting pathogen life cycles [20]. In addition, healthy soils with rich microbial communities are less conducive to disease, as seen in suppressive soils where pathogens are present but do not cause significant disease [17]. Biotic factors offer a sustainable and effective approach to plant pathogen control, highlighting the importance of maintaining biodiversity and leveraging microbial communities for improved plant health. While the benefits of enhancing soil microbial diversity are clear, challenges remain, such as the unpredictability of certain plant growth-promoting rhizobacteria (PGPR) and the potential risks associated with using organic amendments that may harbor antibiotic resistance genes [20].
2.3. Impact of Climate Change on Plant Disease Dynamics
More recently, climate change has also had an important impact on plant pathogens and their interactions with plant hosts. Thus, climate change, through alterations in environmental factors, significantly shapes the dynamics of plant diseases by affecting plant pathogens, plant hosts, and their interactions. Labouyrie et al. [21] showed that climate change based on warming and altered humidity enhanced the presence of certain functional microorganism groups, such as plant pathogens, but reduced the abundance of others, such as beneficial arbuscular mycorrhizal fungi. Climate change may further promote the appearance of new pathogenic strains and the spread of plant pathogens into new geographic areas [22,23,24,25,26,27,28]. Climate change, especially temperature fluctuations, influences the pathogenesis of phytopathogenic fungi, whose life stages are intricately and closely linked to temperature [29,30]. Increased temperatures influence the emergence of certain heat-tolerant bacteria and raise the synthesis of their extracellular polysaccharides, as well as increased replication and altered transmission patterns of phytopathogenic viruses [31]. Changes in relative humidity patterns, such as increased precipitation or prolonged dry periods, can favor or reduce the development of plant pathogens [13,32,33]. Furthermore, extreme climate events like storms, hail, and floods can damage plants, facilitating pathogen entry and helping their spread via water and wind.
Climate change influences plant resilience and plants’ sanitary status, impacting their susceptibility to plant pathogens. Higher temperatures hasten the growth of bacteria and fungus, which increases the prevalence of disease [31]. As they multiply in warmer climates, heat-adapted bacteria such as Burkholderia glumea are becoming more dangerous pathogens. Plant physiology may be impacted by elevated CO2 concentrations, which may weaken their defensive mechanisms, but increased CO2 may also alter the efficacy of particular pathogens [31]. Numerous diseases that were previously limited to warmer locations are now reported in new areas due to the expansion of the geographical range of numerous pathogens caused by climate change [34,35]. Increasingly frequent extreme weather events may facilitate the spread of diseases and worsen their management [34]. The variations in the timing of plant growth and growing seasons may result in host plant and pathogen mismatches and increase or decrease the disease pressure. Moreover, alterations in biodiversity and the makeup of microbial communities resulting from climate change can impact the equilibrium of beneficial and detrimental organisms, thereby affecting overall disease dynamics. As climate change advances, understanding and addressing its effects on plant diseases will be essential for sustaining crop yields and assuring food security [31].
3. Plant Pathogens Control
3.1. Physical Techniques
Physical environmentally friendly techniques (Figure 2) play an important role in managing plant pathogens by utilizing non-chemical techniques that manipulate the environment to reduce or eliminate pathogen populations [36]. These methods are eco-friendly and sustainable, and they can be integrated with other management strategies for desired results [37].
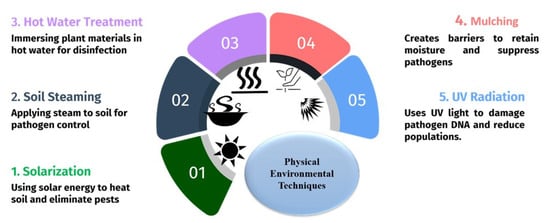
Figure 2.
Physical management practices used for the control of plant pathogens.
3.1.1. Solarization and Soil Heating
Solarization is an environmentally friendly technique in which solar energy is used to heat the soil to reduce soil-borne pathogens, weeds, and pests [38]. This method involves covering the soil with transparent polyethylene sheets during the hottest months, usually summer [39]. The transparent film allows short-wave solar radiation to penetrate the soil while preventing the escape of longer-wavelength radiations, creating a greenhouse effect. The trapped heat raises the temperature of the soil to a level that is lethal to many pathogens and pests such as bacteria, fungi, and nematodes [36]. The elevated temperatures directly damage pathogen cellular structures through protein denaturation, enzyme inactivation, and the disruption of cell membranes [40]. Solarization includes changes in soil microbial communities, often favoring thermotolerant beneficial microorganisms that can further suppress pathogens through competition and antagonism [41]. Recent studies have shown that solarization can increase the soil temperature to 40–55 °C at depths of up to 15 cm, depending on the climatic conditions [42,43]. For example, the effectiveness of solarization in reducing F. oxysporum f.sp. conglutinans on rocket shows a significant reduction in disease outbreaks when combined with organic amendments [44].
Typically conducted in the summer, this process raises soil temperatures to levels that destroy many harmful pathogens, nematodes, and weed seeds or seedlings. It is a safe method that leaves no toxic residues and can be applied at both small and large scales. Soil solarization enhances soil structure and increases nitrogen availability and other vital plant nutrients. Soil pests are effectively killed at temperatures exceeding 30–33 °C [45]. However, plant pathogens, weeds, and other soil-borne organisms vary in their sensitivity to heat. This method eradicates or reduces several soil-borne pathogens, such as Verticillium dahliae, F. oxysporum, Pythium ultimum, and Agrobacterium spp., Phytophthora cinnamomi, Sclerotium rolfsii, and Rhizoctonia solani [46,47]. Soil heating by other means such as steam treatment is also used to control pathogens [48]. Despite so many benefits in soil pathogen control, there are some limitations/disadvantages to using the soil solarization process effectively in many regions. Soil solarization efficacy varies dramatically between tropical, temperate, and arid regions; for example, temperate regions lack sufficient solar radiation or experience frequent cloud cover as compared to tropical regions [49,50]. The economic feasibility of soil solarization presents challenges for many farmers, particularly small-scale operations where initial investment in plastic sheets and the labor costs associated with proper installation may be prohibitive [51].
3.1.2. Soil Steaming
This technique involves using steam generated from burning fuel to transfer heat to the soil or substrate, raising it to pasteurization or sterilization temperatures. Steam at low pressure exceeds 100 °C, releasing significant energy as it condenses into water and effectively heating the soil with minimal moisture. The high-temperature steam penetrates soil particles, killing pathogens through the thermal denaturation of proteins and nucleic acids, the disruption of cell membranes, and the degradation of cytoplasm [52]. Unlike solarization, which creates a temperature gradient in the soil profile, steam treatment can achieve more uniform heating throughout the treated depth, providing more consistent pathogen control [53]. First introduced in Germany in 1888, it served as the primary technique for soil disinfection before the advent of soil fumigants. Pasteurization requires a higher temperature (100 °C). In areas where solarization is not useful for the purpose, soil steaming can be the alternative option [54]. According to Panth et al. [36], soil steaming at a temperature above 70 °C for 30 min can eradicate common soil-borne pathogens like Pythium and Verticillium. In agriculture, soil steaming is generally treated as a pasteurization method, with recommended temperatures of 71 °C for 30 min to eliminate most pathogenic fungi, bacteria, and nematodes, and 83 °C for 30 min to target resistant weed seeds. The use of a mobile steam applicator has also been practiced to improve strawberries yields in California and control root-knot nematode in cut flower production in Florida [55].
3.1.3. Hot Water Treatment and Thermal Inactivation
Hot water treatment is a widely used method for disinfecting plant materials such as bulbs, seeds, and cuttings [56]. This technique involves immersing plant materials in hot water at a specific temperature for a certain period, which inactivates pathogens without damaging plant tissues [57,58]. The mechanism relies on the differential thermal sensitivity between host tissue and pathogen cells. Most plant pathogens are inactivated at temperatures that can be tolerated by dormant plant tissues, creating a therapeutic window for treatment [59]. Heat causes protein denaturation, membrane disruption, and enzyme inactivation in pathogen cells, while dormant plant tissues can withstand these temperatures without significant damage to their cellular structures. The key to success is precise temperature control and timing to exploit this difference in thermal sensitivity [60]. Recent research has focused on optimizing temperature and exposure times to maximize pathogen control while minimizing damage to the host plant [61,62,63]. For instance, treating tomato seeds at 50 °C for 25 min or rice seeds at 60 °C for 2 min effectively reduced bacterial and fungal pathogens without affecting seed germination rates [57,64]. Thermal inactivation can also be applied to irrigation water, compost, and other substrates to reduce the pathogen load [65]. Studies have shown that heating irrigation water to 60 °C for 20 min significantly reduces the presence of Phytophthora species, a common water-borne pathogen in horticultural systems [2,66].
3.1.4. Mulching
By creating a physical barrier on the soil surface, mulching will further retain soil moisture and regulate temperature. Mulching controls plant pathogens through multiple mechanisms. Physically, it prevents soil splashing that would otherwise transport soil-borne pathogens to aerial plant parts during rainfall or irrigation [67]. The barrier also prevents direct contact between plant tissues and soil-borne pathogens. Biologically, mulches (especially organic types) foster diverse microbial communities that compete with or antagonize pathogens through antibiosis, hyper-parasitism, and induced systematic resistance in host plants [68,69]. Many organic mulches release antimicrobial compounds during decomposition that can directly inhibit pathogen growth. Modified soil temperature and moisture regimes under mulches often create conditions less favorable for pathogen development but more conducive to beneficial microbial growth [36]. This will significantly improve soil properties, support microbial diversity, and suppress plant pathogens, contributing to healthier crops. Biodegradable mulches, such as straw and wood chips, decompose over time, enriching the soil with organic matter, further fostering microbial activity, and improving the soil health status. Mulching also suppresses plant pathogens by reducing soil splashing and promoting beneficial microbes that compete with or inhibit harmful pathogens. The effectiveness of mulching varies with the type of mulch and site-specific conditions, making tailored applications crucial. By leveraging mulching as a sustainable practice, farmers can improve soil health, reduce disease incidence, and enhance agricultural productivity [70].
3.1.5. Ultraviolet (UV) Radiation Treatment
UV radiation, particularly in the UV-C spectrum (200–280 nm), damages pathogen DNA by inducing the formation of pyrimidine dimers, which disrupts cellular function and reproduction [71]. The controlled application of UV radiation can effectively reduce pathogen populations on plant surfaces, seeds, and postharvest products without chemical residues. Recent developments in UV technology have led to the creation of automated systems that can treat large volumes of plant material or growing spaces with precise UV dosages [72]. Studies have demonstrated that pulsed UV treatments can effectively control powdery mildew in greenhouse crops and extend the shelf life of postharvest fruits by inhibiting fungal decay organisms [73]. The main advantages of UV treatment include its non-residual nature, compatibility with organic production systems, and potential for automation in controlled environment agriculture.
3.1.6. Practical Challenges and Limitations of Physical Techniques
Despite their environmental benefits, physical techniques for plant pathogen control face several practical challenges and limitations (Figure 3).
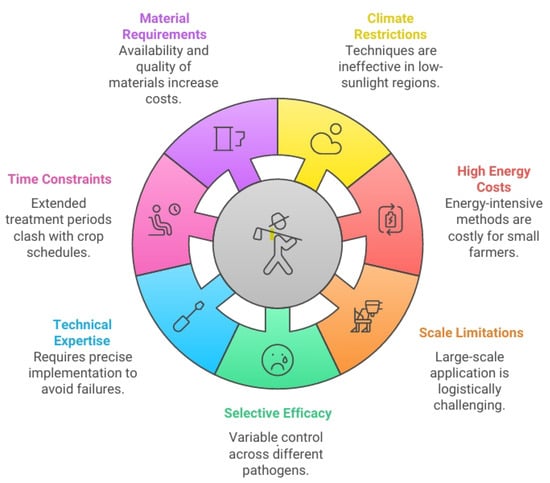
Figure 3.
Infographic showing the challenges and limitations of physical techniques in pathogen control.
a. Climate and geographic restrictions: Soil solarization’s effectiveness is highly dependent on local climate conditions, making it unsuitable for regions with limited solar radiation or shorter growing seasons. In temperate or cloudy regions, the soil may not reach the temperatures necessary for effective pathogen control [74].
b. Energy costs: Methods such as soil steaming and hot water treatment require significant energy inputs, making them economically challenging for small-scale farmers or in regions with high energy costs. The carbon footprint associated with these energy-intensive methods may partially offset their environmental benefits [75].
c. Scale limitations: Many physical techniques are more feasible at small to medium scales and become logistically challenging and costly for large-scale agricultural operations. For example, soil steaming in large field areas requires specialized equipment and significant resources [76].
d. Selective efficacy: Physical methods often provide variable control across different pathogen types. While some pathogens are highly susceptible to heat treatments, others, such as certain heat-resistant bacterial spores or deeply situated nematodes, may survive the treatment [77].
e. Technical expertise: The proper implementation of physical techniques often requires technical knowledge and precision. Incorrect application, for example, improper temperature or duration in hot water treatment, can either fail to control pathogens or damage the treated plant material [78].
f. Time constraints: Many physical techniques, particularly solarization, require extended treatment periods (4–6 weeks), which may not fit within tight crop rotation schedules or be economically viable for high-value cropping systems [79,80].
g. Material requirements: The quality and availability of materials, such as appropriate polythene films for solarization or suitable mulching materials, can limit implementation in certain regions or increase costs significantly [81,82].
3.1.7. Drawbacks or Potential Negative Impacts of Physical Techniques
Physical techniques that are generally considered environmentally friendly can still lead to some unintended negative impacts that are explained below (Figure 4).
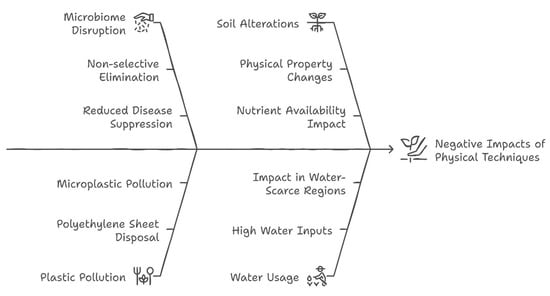
Figure 4.
Infographic showing negative impacts of physical techniques in pathogen control.
a. Microbiome disruption: Heat-based methods like solarization and steaming can eliminate beneficial soil microorganisms and pathogens, potentially disrupting soil ecological balance[36]. This non-selective elimination may reduce natural disease suppression and soil health in the long term [83].
b. Plastic pollution: Soil solarization typically relies on polyethylene sheets, which, if not properly disposed of or if fragmented during use, can contribute to microplastic pollution in agricultural soils. The environmental impact of plastic mulches remains a significant concern [84,85].
c. Recolonization vulnerability: sterilized or pasteurized soil creates an ecological vacuum that can be rapidly recolonized by pathogens if reintroduction occurs, sometimes leading to more severe disease outbreaks than in untreated soils that maintain competitive microbial communities [86].
d. Physical soil alterations: High-temperature treatments can alter soil physical and chemical properties, potentially affecting soil structure, organic matter content, and nutrient availability. These changes may positively or negatively impact subsequent crop growth [87,88].
e. Water usage: some physical techniques, particularly hot water treatment and certain mulching practices, require significant water inputs, which may be problematic in water-scarce regions [54].
f. Selection pressure: the repeated use of physical control methods can potentially select for heat-resistant pathogen strains over time, though this has been less documented than resistance to chemical control [89].
g. Phytotoxicity risks: the improper application of heat treatments can cause phytotoxicity for plants or seeds, potentially reducing germination rates, vigor, or yields [90].
3.2. Cultural and Ecological Management Techniques
3.2.1. Crop Rotation and Diversification
Crop rotation—defined as the sequential cultivation of different crops on the same field—is one of the oldest and most effective environmentally friendly strategies for managing plant diseases, especially those caused by soil-borne pathogens [91]. Its effectiveness is primarily attributed to the ability of rotations to interrupt pathogen life cycles by alternating host crops with non-hosts or less susceptible species, thereby reducing the accumulation of inoculum in the soil [92,93,94]. Numerous field studies have demonstrated the success of this approach. For example, Larkin et al. [95] showed that long-term rotation trials in potato systems incorporating Brassica break crops such as canola or rapeseed have consistently reduced the incidence of soil-borne diseases including Rhizoctonia canker, black scurf, and common scab by 20–40%, with corresponding yield increases. Further, the addition of cover crops, such as winter rye, enhances these effects by improving microbial activity and soil suppressiveness [95,96]. Furthermore, the incorporation of biofumigant crops like mustard can also directly suppress fungal pathogens through the release of allelopathic compounds [97].
The effectiveness of crop rotation in disease management is highly dependent on the biological characteristics of the target pathogen [98,99]. For instance, Shennan et al. [100] demonstrated that rotation is particularly successful against soil-borne pathogens with narrow host ranges and limited survival structures, such as Verticillium dahliae, with notable disease suppression observed when non-host crops like untreated lettuce were introduced into strawberry cropping systems. In contrast, rotation is far less effective against pathogens with broad host ranges or long-lived survival structures, such as Sclerotinia sclerotiorum, which can persist in the soil as sclerotia and evade disruption through rotational practices [36]. Similarly, foliar pathogens—particularly those dispersed over long distances by wind, like rust fungi—are typically unaffected by local crop rotations unless they rely on overwintering in crop residues or adjacent vegetation to complete their life cycles [101].
Comparative research across different agroecosystems reinforces the broad applicability of rotation as a disease management tool [102]. This is particularly evident in low-input and organic systems, where crop diversification—often implemented through rotation—is a cornerstone strategy for managing plant diseases in the absence of synthetic chemical controls. For example, in African smallholder systems, rotating maize with legumes or fallow has been associated with a lower incidence of root rots and parasitic nematodes, along with improved soil fertility [103,104]. In contrast, intensive systems such as the corn–soybean rotation dominant in the United States may offer limited pathogen suppression, as pathogens like Fusarium spp. and Pythium spp. can persist due to overlapping host susceptibility [105,106]. Furthermore, in European cereal systems, more diversified rotations, especially those exceeding two years, have been shown to reduce foliar disease severity and consequently reduce the need for fungicide applications [101]. Additionally, crop rotational diversity has been shown to enhance the disease-suppressive capacity of soil microbiomes by shaping bacterial community composition and increasing the abundance of functional groups that produce antifungal compounds [107,108]. Importantly, while overall microbial diversity may not always increase, it is the structure and functional potential of the microbial community that plays a more critical role in effective pathogen suppression [109,110].
Despite its strengths, crop rotation faces limitations. It is less applicable to perennial cropping systems where replanting occurs infrequently, although cover crops and rotation during replanting cycles can offer some control. For instance, fields previously infested with Fusarium oxysporum causing Panama disease in banana must be rotated out of bananas for many years to reduce pathogen pressure, a practice not always feasible due to land constraints or market demands [111,112,113,114]. Additionally, economic pressures in high-value agriculture may favor monoculture over more sustainable rotational practices [93].
Crop rotation and diversification are versatile, environmentally friendly tools that can significantly reduce the prevalence of soil-borne diseases and enhance soil health. However, their effectiveness is context-dependent, varying with pathogen ecology, crop choices, and management constraints. Integrating rotation with other disease management strategies enhances its reliability and long-term benefits. Crop rotation advantages and limitations are summarized in Table 1.

Table 1.
Summarized aspects of crop rotation.
3.2.2. Sanitation and Residue Management
Biofumigation
Biofumigation is a promising, environmentally friendly alternative for managing plant pathogens, particularly soil-borne diseases, by using biocidal compounds released from specific plant materials [121,122]. This method leverages the hydrolysis of glucosinolates found in Brassica species to produce isothiocyanates, which exhibit nematicidal and fungicidal properties [123,124,125,126,127]. Biofumigation has demonstrated significant efficacy against various soil-borne pathogens, such as F. oxysporum and R. solani, with suppression rates exceeding 60% in some cases [128]. Other studies showed that shredded plant materials from species such as Indian mustard, brown mustard, turnip (Brassica rapa), and radish (Raphanus sativus) release volatile sulfur compounds that effectively inhibit soil-borne pathogens affecting potatoes, including R. solani, Phytophthora spp., P. ultimum, and S. sclerotiorum [129]. For example, Indian mustard achieved an 80–100% inhibition of potato pathogens in Petri dishes at 25 °C, while black mustard (B. nigra) applied at 5% w/w reduced R. solani colonization by 75%, with effects lasting up to six months [130]. Other Brassica species, such as B. integrifolia and B. oleracea var. gongylodes, also showed strong inhibitory effects on Pythium and Fusarium species under similar conditions [131]. In addition to reducing dependence on synthetic pesticides [132], biofumigation can enhance soil fertility and stimulate beneficial microbial communities, such as Streptomyces [133], Actinobacteria [134], and arbuscular mycorrhiza fungi [135,136], contributing to overall soil health [137]. Nevertheless, studies on the effect that biofumigation may have on soil microbes show mixed results. While some reported increased microbial diversity [138], others noted declines [139], likely due to differences in materials, application rates, and conditions—highlighting the need for further research.
However, while biofumigation offers several advantages, it also faces notable challenges, such as seasonal limitations, as the availability of suitable plant biomass for biofumigation can be restricted during off-seasons [121]. In addition, the effectiveness of biofumigation can vary based on soil conditions, the plant species used, and the number of active compounds produced, as well as environmental factors, necessitating further research to optimize practices [121,137,140,141]. Furthermore, studies also suggested that the suppressive effect of biofumigant plants on pathogens is often temporary, gradually declining, rather than providing permanent control [131] and meanwhile inadvertently reducing populations of beneficial entomopathogenic nematodes [142]. Further research is required to understand how harmful soil organisms recover after biofumigation and how long its effects last. Moreover, under challenging conditions like drought, biofumigation alone may not enhance yields, highlighting the need to combine it with other sustainable practices for lasting disease control [143].
Despite its potential, the effectiveness of biofumigation can be inconsistent, and further research is needed to refine techniques and enhance its application in diverse agricultural contexts, including while other biofumigant sources are used. Plants belonging to the Liliaceae, Gramineae, Compositae, and Leguminosae families showed similar benefits [137,144].
Anaerobic Soil Disinfestation
Anaerobic soil disinfestation (ASD), also cited as reductive soil disinfestation, biological soil disinfestation, anaerobically mediated biological soil disinfestation, and soil reductive sterilization, presents both advantages and challenges in managing plant pathogens [145,146]. This method, which involves amending soil with easily decomposable organic matter, saturating it with water, and covering it to create anaerobic conditions for up to 3 weeks, has shown promise in suppressing soil-borne pathogens and pests [147,148,149].
However, its effectiveness can vary, based on environmental conditions and specific pathogen types. ASD has been shown to effectively suppress various soil-borne pathogens, including nematodes, bacteria, and fungi (i.e., Agrobacterium tumefaciens, R. solanacearum, Colletotrichum coccodes, F. oxysporum, Phytophthora capsici, S. sclerotiorum, and Pratylenchus penetrans), through the production of organic acids and volatile compounds under anaerobic conditions [150,151,152,153,154,155,156,157]. Ueki et al. (2018) [158] have demonstrated the efficient management of soil-borne pathogens through the activity of anaerobic bacteria such as Clostridium beijerinckii, which produce antifungal enzymes. Anaerobic soil disinfestation offers a sustainable, chemical-free alternative to conventional soil fumigation, making it well suited for organic agriculture and areas with strict pesticide regulations. This method can also be combined with other eco-friendly strategies, such as crop rotation, biological control agents, and organic amendments, for integrated disease management [155,159]. The process can lead to significant shifts in soil microbial communities, potentially enhancing soil health and disease suppression in subsequent cropping systems [155,160]. In this context, Hewavitharana et al. (2019) [161] described three microbial phases during ASD, marked by shifts in dominant microbial groups, as follows: in the first phase, aerobic and facultative organisms such as Bacillus, Pasteuria, Streptomyces, Pseudomonas, and Ascomycota fungi prevail. As oxygen depletes, the second phase sees a rise in facultative anaerobes from Firmicutes, Actinobacteria, Proteobacteria, and Zygomycota, with Bacillus spp. being strongly associated with lactic acid production. In the final phase, strict anaerobes like Clostridium spp. dominate, producing bioactive compounds that suppress pathogens [161]. In addition, ASD may lead to the recovery of several biocontrol agents (i.e., Bacillus spp., Trichoderma spp., Streptomyces spp., Coniothyrium minitans, and Chaetomium spp.) [153,156,162,163]. However, exploring the use of biocontrol agents to recolonize soils following ASD treatment is a promising strategy that warrants further research.
The type of carbon source applied in anaerobic soil disinfestation (ASD) significantly influences the structure of the soil microbial community [154,164,165]. When ethanol—a readily degradable carbon source—is used, it promotes the proliferation of Firmicutes species, while using alfalfa meal, a more resistant and complex carbon source, favors microbial groups from Firmicutes, Bacteroidetes, and Proteobacteria [166]. In addition, in a recent study, Duan et al. [167] emphasized the effectiveness of reductive soil disinfestation (RSD), especially when paired with targeted organic amendments, in controlling soil-borne pathogens and reducing high-risk antibiotic resistance genes (ARGs). This effect is largely mediated through shifts in soil microbial communities, particularly Actinobacteria, as well as changes in mobile genetic elements and soil total nitrogen. Supporting these findings, Chen et al. [168] also demonstrated that ASD significantly lowers the abundance of multiple ARGs. However, Chen et al. [169] reported that ASD can lead to a 4.1% increase in potential human pathogenic bacterial (HPB) species and a 23% rise in related genes. These shifts in bacterial community dynamics under ASD may enhance interactions between HPB and non-pathogenic microbes, potentially promoting the dissemination of resistance genes [169].
Despite its potential, the ASD technique meets challenges concerning its effectiveness, which can differ significantly based on soil type, carbon source, and environmental conditions, leading to inconsistent results [159,160]. Likewise, the complexity of implementing the ASD technique requires the careful management of soil moisture and temperature, as well as the selection of appropriate organic amendments, which can complicate its adoption in commercial settings [155,157]. Challenges may also involve optimizing conditions for anaerobiosis and ensuring consistent efficacy across different soil types [158]. While promising, the mechanisms behind the efficacy of ASD are not fully understood, necessitating further research to optimize its application and improve reliability [155,157]. In addition, the duality of ASD regarding soil ARG dynamics underscores the need for careful management practices in agricultural settings.
Intercropping and Mixed Cropping Systems
Intercropping—the simultaneous cultivation of two or more crop species in the same field—is a spatial diversification strategy with demonstrated potential to suppress both foliar and soil-borne plant pathogens [170]. Unlike crop rotation, which relies on temporal separation, intercropping modifies the cropping environment in real time by altering plant architecture, microclimate, and host spatial distribution [171]. This spatial heterogeneity is often unfavorable for pathogens and can significantly disrupt their transmission dynamics [172]. In this regard, a comprehensive review of over 200 studies found that disease incidence was reduced in approximately 73% of intercropping trials compared to monocultures, with the most consistent benefits observed for foliar fungal pathogens [172]. Several mechanisms highlight this protective effect. As inferred by Civitello et al. (2015) [173] and later by Chadfield et al. (2022) [174], host dilution reduces the density of susceptible plants, thereby limiting pathogen spread. Diverse canopies can hinder the wind and rain-splash dispersal of spores, while complex vegetation structures may alter humidity and temperature, reducing conditions favorable for infection [172,175]. In addition, Zhu et al. (2000) [175] demonstrated that planting mixtures of genetically diverse rice varieties significantly reduced the incidence of rice blast disease by 94%, primarily due to canopy-mediated spore interception and microclimate changes. Thus was highlighted the effectiveness of intercropping strategies in disease management. Moreover, plant–plant interactions at the physiological or biochemical level may contribute to disease suppression, including the induction of host defenses or allelopathic effects on pathogens in the rhizosphere [176,177]. Additionally, certain legume–cereal intercrops have been shown to enhance soil microbial diversity and suppressiveness, thereby further contributing to disease control [178,179]. Field studies confirmed that intercropping can be effective against a range of pathogens. In this regard, Wu et al. (2024) [180] and Zhang et al. (2019) [181] reported that cereal–legume intercropping systems often lead to reduced severity of foliar diseases such as powdery mildew and rust, largely due to canopy disruption and associated microclimatic effects. Similarly, in tropical smallholder systems, traditional combinations such as maize–bean or sorghum–pigeonpea have been shown to reduce leaf lesions and slow disease spread, likely by interrupting the continuous presence of susceptible hosts [172]. Soil-borne pathogens and nematodes also respond positively: in a meta-analyses based study, Chadfield et al. (2022) [174] showed average reductions of 55% in soil-borne disease and 40% in nematode damage across intercropped systems. Nonetheless, intercropping does not always yield positive outcomes; in approximately 27% of cases, the disease incidence remained unchanged or even increased—often due to incompatible crop combinations that result in dense, humid canopies conducive to foliar pathogen development [172,182].
Intercropping is particularly relevant for smallholder and organic systems, where it provides both agronomic and socio-economic benefits. For resource-limited farmers without access to fungicides, strategic intercrops can reduce disease pressure while offering yield stability through diversified outputs. In contrast, mechanized, large-scale agriculture has historically avoided intercropping due to equipment and market constraints. However, recent studies in high-input systems in Europe and North America are revisiting intercropping as a sustainable strategy. For instance, the strip-intercropping of oilseed rape with peas reduced Sclerotinia incidence in rape and rust in the legume, suggesting multi-pathogen control potential [183].
Despite its many advantages, intercropping also poses significant challenges. Managing multiple species with different agronomic needs requires the careful coordination of planting, harvesting, and input use, often increasing management complexity. Limitations in machinery, logistical hurdles associated with marketing diverse crops, and potential interspecies competition for light, water, or nutrients can further complicate implementation. From a plant health standpoint, there is also the risk of exacerbating disease pressure—particularly if both crops share susceptibility to the same pathogens or if intercrops serve as alternate hosts (e.g., grasses supporting rust life cycles). As such, successful intercropping relies on a deep understanding of crop–pathogen interactions and thoughtful system design.
Overall, when properly implemented, intercropping offers a robust, cost-effective strategy for managing multiple plant pathogens while enhancing agroecosystem resilience [184]. Its success is highly context-dependent but supported by a growing body of field-based evidence across diverse cropping systems. The summarized advantages and challenges of intercropping are presented in Table 2.

Table 2.
Summarized aspects of intercropping.
3.3. Use of Resistant Cultivars
The deployment of disease-resistant cultivars is widely recognized as one of the most effective and environmentally friendly strategies for managing plant diseases [194]. By incorporating genetic resistance into crops, this approach enables plants to defend themselves against pathogens, minimizing the need for chemical controls and reducing yield losses [101,195]. Resistance may be qualitative (conferred via single major genes) or quantitative (involving multiple genes), with the former often providing complete resistance to specific pathogen races and the latter offering partial but more durable protection across diverse pathotypes [195,196]. The success of breeding programs in developing resistant varieties is well documented across both temperate and tropical regions [197,198]. For instance, the introduction of stem rust-resistant wheat lines has averted major epidemics in high-risk areas [199], while downy mildew-resistant pearl millet in India led to higher yields and resolved a long-standing disease challenge [200]. Similarly, in Africa, resistant cassava cultivars have successfully countered the Cassava Mosaic Virus, restoring productivity to severely affected regions [201]. Beyond conventional breeding, resistance has also been successfully deployed through horticultural techniques such as grafting, where susceptible scions are joined to resistant rootstocks—as in the case of watermelon grafted onto squash to manage F. oxysporum f. sp. niveum [202].
Host resistance plays a vital role in managing both soil-borne and foliar pathogens across a wide range of crops. For example, the development of lettuce cultivars resistant to Verticillium wilt [203], tomato lines resistant to Ralstonia [204] and Fusarium [205], and pea varieties resistant to Aphanomyces root rot [206] illustrates the effective application of genetic resistance to combat soil-borne diseases. In parallel, resistance to foliar pathogens such as rusts, blights, and mildews has been equally critical in protecting aboveground plant health [207]. Notably, Joobeur et al. [208] demonstrated that melon cultivars combining Fom-2 for Fusarium resistance and Pm-1 for powdery mildew resistance exemplify the potential of gene pyramiding to achieve broad-spectrum protection. These advances are consistently supported by multi-environment field trials, which showed that resistant genotypes contribute significantly to both disease suppression and yield stability [194,209].
Despite its proven benefits, host resistance is not without limitations—with one of the most significant being its long-term durability, particularly in the case of qualitative, single-gene resistance. As Brown [210] emphasized, pathogens with large, genetically diverse populations can rapidly adapt and overcome such resistance, resulting in the well-known ”boom-and-bust” cycles of disease control failure. A striking example comes from Leptosphaeria maculans in oilseed rape, where field populations overcame single-gene resistance within just three years; however, when combined with quantitative resistance, the effectiveness was maintained for a longer period [211]. This ongoing evolutionary arms race has rendered many formerly effective resistance genes ineffective over time, especially in cereal crops, where rust populations shift rapidly [212]. In response, breeding and management strategies such as gene pyramiding, cultivar rotation, and the use of varietal mixtures have been employed to extend resistance durability. For instance, wheat varietal mixtures containing diverse rust resistance genes have been shown to reduce epidemic severity and slow the emergence of virulent pathogen races [213]. Similarly, in Chinese rice systems, deploying mixtures of resistant and susceptible cultivars significantly reduced the blast incidence while preserving the long-term effectiveness of resistance genes [175].
While host resistance remains a fundamental component of plant disease management, several practical challenges continue to limit its widespread adoption. A major limitation is the specificity of resistance, which is typically effective against a single pathogen but offers no protection against others—for example, a tomato cultivar resistant to Fusarium wilt may still be vulnerable to bacterial spot [214]. Additionally, resistance traits can sometimes be linked to agronomic drawbacks, such as a lower yield or compromised fruit quality, though ongoing advances in breeding technologies are helping to overcome these limitations [215,216]. Another issue relates to unequal availability: minor and less-commercialized crops often receive limited breeding investment, and smallholder farmers may struggle to access resistant varieties due to affordability or distribution challenges. Looking ahead, transgenic and genome-edited resistance represent promising technological developments. However, their implementation remains restricted due to regulatory constraints and societal acceptance—particularly within organic agriculture. For instance, despite showing resistance to Huanglongbing, gene-edited citrus varieties have not yet reached commercial deployment due to regulatory delays [210].
Nevertheless, despite these limitations, host resistance continues to serve as a key element in plant disease management. Its value is particularly evident within integrated approaches, where it works synergistically with other strategies such as crop rotation, biological control, and targeted fungicide use. In many cropping systems, resistant cultivars form the first line of defense, providing farmers with a proactive and cost-effective tool to manage both endemic and emerging plant pathogens. The summarized advantages and limitations of resistant cultivar use are presented in Table 3.

Table 3.
Summarized aspects of the use of resistant cultivars.
3.4. Soilless Culture
Soilless culture systems—including hydroponics, nutrient film techniques, and inert substrates such as rockwool, coco coir, and perlite—have become an increasingly effective environmentally friendly strategy for managing plant diseases, particularly by circumventing soil-borne pathogens [248]. Originally developed to combat soil fatigue and persistent pathogen pressure in high-value crops, these systems enable cultivation in a controlled, soil-free environment where many traditional pathogens are unable to persist [249,250]. By replacing contaminated soils with sterile growing media and delivering nutrients through precisely regulated solutions, growers are able to disrupt pathogen life cycles that rely on soil as a reservoir. As a result, soilless systems have gained widespread adoption in the greenhouse cultivation of crops such as tomatoes, cucumbers, strawberries, and lettuce [251].
Their primary advantage lies in the effective exclusion of soil-borne pathogens, including Verticillium spp., Fusarium spp., and root-knot nematodes, which are otherwise difficult to manage in conventional systems [252]. For instance, strawberry growers using substrate-based systems report a marked reduction in Verticillium wilt incidence compared to field-grown production [249]. Similarly, greenhouse tomato cultivation in rockwool or coir minimizes the occurrence of Fusarium crown and root rot—previously a major concern necessitating routine soil fumigation [250]. These outcomes underscore the core biosecurity advantage of soilless systems: sterile, disposable media prevent the long-term persistence and re-emergence of soil-borne pathogens [253]. Despite their advantages, soilless culture systems are not entirely free from disease risks [254].
While these systems effectively eliminate many soil-borne pathogens, they introduce new vulnerabilities—particularly to waterborne diseases caused by oomycetes such as Pythium and Phytophthora. Calvo-Bado et al. [255] demonstrated that these pathogens can enter hydroponic systems through contaminated water sources or infected transplants and spread rapidly via recirculating nutrient solutions. In closed-loop systems, the infection of a single plant can lead to systemic outbreaks of root rot unless rigorous sanitation protocols are in place. Additionally, pathogens like F. oxysporum may be introduced via infected seedlings and can persist and disseminate within the system through spore dispersal or biofilm formation [256].
Nevertheless, one of the key advantages of soilless culture lies in its capacity for containment and control: systems can be disinfected between growing cycles, and waterborne pathogens can be managed through technologies such as UV irradiation or ozone sterilization of nutrient solutions—practices that are far more challenging to implement in traditional field soils [248]. From a systems-level perspective, soilless cultivation is particularly well suited to high-value horticultural crops grown in intensive production environments, such as European greenhouse complexes and peri-urban vegetable farming operations [257]. Its adoption has expanded in part as a response to soil degradation and contamination, especially in regions where traditional fumigants like methyl bromide have been phased out due to environmental concerns [249,258]. Soilless systems offer several compelling advantages: they effectively prevent soil-borne disease outbreaks [249], enable full sanitation and system reset between crop cycles [258], integrate seamlessly with controlled environment agriculture [248], and eliminate reliance on soil fumigants [256]. Moreover, these systems support uniform plant growth, which facilitates disease monitoring and agronomic management [251].
However, their implementation is not without challenges. The high initial investment, technical complexity, and need for specialized knowledge can pose barriers to entry, particularly for small-scale growers. In addition, the closed-loop nature of most systems increases the risk of rapid disease spread if contamination occurs, and foliar pathogens may still thrive if environmental controls—such as humidity and air circulation—are not carefully managed [259]. Finally, regulatory hurdles can arise for growers seeking organic certification, as hydroponically grown produce may not qualify under certain national or regional organic standards, thus limiting access to organic markets. Table 4 summarizes the principal advantages and challenges of the soilless technique.

Table 4.
Summarized aspects of soilless culture.
3.5. Biological Control Approaches
3.5.1. Microbial Inoculants as Biocontrol Agents
The exploration of novel microorganisms as BCAs has garnered increasing attention as a sustainable alternative to chemical pesticides in plant disease management. This approach aligns with the broader goals of environmentally friendly agriculture by leveraging naturally occurring microbial interactions to suppress plant pathogens.
Among the well-characterized bacterial genera, Bacillus, Streptomyces, and Lysobacter have shown considerable promise due to their ability exhibit a wide range of antagonistic activities against plant pathogens, including antibiosis, competition for nutrients and space, and the induction of plant systemic resistance [286,287]. Bacillus strains are identified as effective plant growth-promoting bacteria (PGPB) that serve as biocontrol agents against plant pathogens [288]. In particular, they are known for their prolific production of secondary metabolites such as lipopeptides, which not only inhibit pathogen growth but also enhance plant resilience through the induction of plant systemic resistance (PSR) [289]. Bacillus subtilis and Bacillus megaterium demonstrated significant suppression of Fusarium graminearum, a major wheat pathogen [290]. Some other bacterial strains, like Pseudomonas aeruginosa FG106, also induced PSR, enhancing their ability to fend off pathogens through the production of volatile compounds and biofilm formation [291]. Likewise, Streptomyces spp. have demonstrated strong antifungal activity against phytopathogens like Fusarium spp., largely attributed to their competitive behavior and production of antibiotics [292] Lysobacter enzymogenes has also emerged as an effective biocontrol agent, with studies highlighting its inhibitory effects on Alternaria solani and F. oxysporum, often outperforming conventional fungicides in controlled environments [293].
Similarly, beneficial fungi like Trichoderma spp. and mycorrhizal fungi have shown significant potential in suppressing root pathogens through the production of hydrolytic enzymes, mycoparasitism, and enhancement of plant stress tolerance [294]. In a comprehensive overview of biological control methods using beneficial microorganisms, Sarrocco [295] reviewed selected breakthroughs in biological control over the past 50 years, showcasing the role of Trichioderma isolates and mycoviruses that confer hypovirulence to plant pathogenic fungi. This innovative approach has shown promise in reducing the virulence of harmful fungi, thereby enhancing plant health and crop yields [296].
Arbuscular mycorrhizal fungi (AMF) also serve as biocontrol agents against plant-parasitic fungi and nematodes by competing for nutrients and space, altering rhizosphere interactions, and enhancing plant tolerance, thus providing an environmentally friendly alternative to synthetic chemical management methods [78]. For example, Bilgili, 2025, demonstrated the potential of AMF as biocontrol agents against plant pathogens. Species like Funneliformis mosseae, Rhizophagus intraradices, and Claroideoglomus etunicatum effectively reduced disease severity in pepper plants infected with Fusarium solani [297]. Similarly, Glomus fasciculatum, G. mosseae, and Acaulospora laevis reduced maize black bundle disease, with G. fasciculatum showing full suppression; they all improved plant growth, highlighting their dual role as biocontrol agents and growth promoters [298,299]. However, recent studies have emphasized the importance of strain specificity, environmental conditions, and microbial compatibility in determining the success of microbial inoculants in field applications [300]. While laboratory and greenhouse trials often report high efficacy, field performance can be inconsistent due to environmental fluctuations, microbial competition, and soil variability [286,301].
In parallel with these established genera, increasing efforts are being directed toward the identification and functional characterization of underexplored microbial species with biocontrol potential. For instance, Clonostachys rosea and Lysobacter capsici are currently under investigation for their activity against soil-borne pathogens, with preliminary studies yielding promising results [302]. Moreover, less conventional organisms such as Bdellovibrio bacteriovorus, a predatory bacterium that targets Gram-negative pathogens, and Paenibacillus spp., which are known to produce a range of antimicrobial and lytic enzymes, are gaining attention as potential sources of novel biopesticides [302]. These emerging taxa not only broaden the spectrum of biological control strategies but also offer opportunities to develop more targeted and ecologically compatible solutions.
Nevertheless, despite the growing body of evidence supporting the efficacy of microbial biocontrol agents, several challenges must be addressed to facilitate their widespread adoption. These include variability in field performance due to environmental influences, limitations in formulation stability and shelf-life, and regulatory complexities surrounding product registration and commercialization. Continued research into ecology, mechanisms of action, and application strategies of both established and emerging microbial taxa is, therefore, essential to fully realize their potential within integrated pest management systems. As the search for sustainable plant protection intensifies, microbial biocontrol remains a vibrant and evolving field with substantial implications for the future of crop health and environmental stewardship. The summarized aspects of BCAs application, including advantages and limitations, are presented in Table 5.

Table 5.
Summarized aspects of BCAs’ application.
3.5.2. Microbial Consortia and Synergistic Effects
Microbial consortia, involving the combination of different microbial species, have gained increasing attention as a strategy to enhance the effectiveness of biocontrol [295]. Recent studies indicated that multi-strain or multi-species formulations can provide broader-spectrum protection against plant pathogens while improving plant growth through synergistic plant–soil–microbe interactions [369]. For instance, the co-application of mycorrhizal fungi and rhizobacteria has been shown to promote plant vigor while simultaneously protecting against root pathogens [179,370,371]. Despite these benefits, developing and commercializing effective microbial consortia remains a challenge due to issues related to microbial compatibility, stability, and scalability. Ensuring that introduced microbes can establish and persist in the soil without being outcompeted by native microbial populations is a key concern that continues to be explored in ongoing research [372].
3.5.3. Organic Amendments and Soil Health in Disease Suppression
Organic amendments (OAs), such as composts, biochar, and green manures, represent another promising approach to microbiological disease control. These amendments contribute to soil health by enriching microbial diversity, enhancing nutrient cycling, and improving soil structure, which indirectly suppresses soil-borne pathogens [373]. Recent research highlights that organic amendments promote beneficial microbial communities that outcompete or inhibit plant pathogens, leading to long-term disease suppression. For example, fortified compost with Trichoderma sp. and biochar has been reported to enhance the abundance of disease-suppressive bacteria such as Pseudomonas and Bacillus spp. while simultaneously improving soil water retention, soil organic content, carbon sequestration, and soil microbial resilience [147,180,374]. Additionally, organic amendments can improve plant resilience against stressors by modulating rhizosphere microbiota and enhancing plant immune responses. Using water extracts derived from composts has also been shown to effectively suppress various soil-borne pathogens, suggesting that the suppressive effect is primarily biological, rather than chemical or physical [375].
However, one of the major limitations of organic amendment applications is their variable effectiveness, which is influenced by factors such as the soil type, the decomposition rate, and microbial community dynamics. In some cases, excessive organic matter inputs can lead to the proliferation of opportunistic pathogens or imbalances in soil microbial populations, potentially causing unintended negative effects on plant health [376]. The summarized aspects of OA application, including advantages and limitations, are presented in Table 6.

Table 6.
Summarized aspects of organic amendments application.
3.6. Challenges and Limitations of Microbiological Control
While microbiological control presents significant advantages over chemical methods, such as environmental safety, sustainability, and the potential for long-term disease suppression, it also faces considerable challenges. One of the primary drawbacks is the inconsistency in performance across different environmental conditions. Unlike chemical fungicides, which provide immediate and predictable effects, microbiological control often requires time to establish and may be influenced by biotic and abiotic interactions in the soil [415]. Furthermore, the regulatory approval process for microbial-based biocontrol agents remains complex and time-consuming, which can hinder their widespread adoption. Economic considerations, such as production costs, formulation stability, and application methods, also play a critical role in determining the feasibility of microbial-based approaches in large-scale agricultural systems [416,417].
3.7. Future Directions and Innovations
Despite these challenges, ongoing advances in microbial ecology, genomics, and biotechnology are continuously improving the effectiveness and applicability of microbiological control strategies. The integration of microbiome-based approaches with precision agriculture techniques, such as metagenomics and high-throughput sequencing, is providing valuable insights into how microbial communities interact with plant pathogens and how these interactions can be optimized for disease suppression [418]. Additionally, the use of bioinformatics tools and artificial intelligence in predicting microbial interactions and optimizing consortia formulations is opening new frontiers for the development of next-generation biocontrol strategies.
3.8. Conclusions
The microbiological control of plant pathogens offers a promising and environmentally friendly alternative to conventional disease management strategies. While microbial inoculants, organic amendments, and microbial consortia have demonstrated potential in controlling plant diseases, their effectiveness is influenced by a complex interplay of environmental, biological, and technical factors. Addressing the challenges associated with microbial-based disease control requires continued interdisciplinary research, technological advancements, and supportive regulatory frameworks to facilitate the transition toward sustainable and resilient agricultural systems.
4. Environmental Modification Techniques
This approach involves altering a plant physical and chemical environment to suppress or eliminate pathogens [419]. These strategies are often integrated with other management practices and aim to create unfavorable conditions for pathogen survival and development [36]. Adjusting factors such as pH, moisture, air circulation, and humidity can reduce plant disease incidence and severity without relying heavily on chemical treatments [11].
4.1. Soil Moisture Management
Soil moisture plays an important role in the development and spread of many soil-borne and foliar pathogens [420]. Excessive moisture can create conditions favourable to the growth of Oomycetes like Phytophthora and Pythium spp., which thrive in moist conditions, and poorly drained soils [421]. Managing soil moisture through proper irrigation practices, soil structure, and drainage system improvements can significantly reduce pathogen pressure [36]. Recent studies highlight the importance of drip and precision irrigation systems in reducing disease incidence; for example, drip irrigation reduced the spread of Phytophthora root rot in citrus orchards by keeping the soil surface drier which limited the pathogen’s ability to infect new roots [422,423]. Similarly, optimizing irrigation frequency and timing reduced the incidence of Fusarium wilt in greenhouse tomatoes by avoiding prolonged periods of soil saturation [424]. Improving soil structure through practices such as organic matter amendments and cover cropping can improve water infiltration and drainage, reducing the likelihood of waterlooging that promotes pathogen growth [425]. Cover crops like rye and clover improved soil porosity and reduced the incidence of Verticillium wilt in strawberries by preventing waterlogging and promoting a healthier soil environment [426].
4.2. Soil pH Adjustment
Soil pH influences the availability of nutrients and the activity of both pathogens and beneficial microbes [427]. Certain plant pathogens, such as Plasmodiophora brassicae (causal agent of clubroot disease in crucifers) and Pythium spp., thrive under acidic conditions [428]. Diseases like potato scab caused by S. scabies are more prevalent in alkaline soils. Adjusting soil pH to unfavorable levels for specific pathogens can be an effective disease management strategy [429]. Liming acidic soil to raise pH has been widely used to control clubroot disease in brassicas. Recent research by Geoffrey [430] found that applying lime to maintain a soil pH of 7.2 significantly reduced clubroot incidence in cabbage fields. Lowering soil pH using acidifying amendments like elemental sulfur or ammonium-based fertilizers can help control alkaline-soil pathogens [431]. Qi et al. [432] demonstrated that lowering soil pH below 5 reduced the severity of potato scab by limiting the growth of S. scabies. pH manipulation, integrating soil pH management with other practices, such as crop rotation and soil testing, can provide more sustainable and effective disease control [429].
4.3. Air Circulation and Humidity Control
Air circulation and humidity are critical factors in controlling foliar diseases, especially those caused by fungal pathogens such as B. cinerea (gray mold), Alternaria spp., and powdery mildew fungi [433]. High humidity and poor air circulation create a microclimate that promotes spore germination, infection, and the rapid spread of these pathogens [434]. Air circulation within plant canopies can be improved through pruning, appropriate plant spacing, and thinning [435]. For example, thinning apple trees and pruning lower branches improved airflow and reduced the incidence of apple scab (Venturia inaequalis) by 30% compared to unpruned controls [436]. Similarly, increased plant spacing in greenhouse cucumber production reduced the severity of downy mildew by lowering humidity levels within the canopy [437]. Humidity control in greenhouses and other enclosed environments is also essential for disease management. Techniques such as dehumidification, controlled irrigation, and ventilation can help maintain optimal humidity levels that discourage pathogen development [438]. For example, using dehumidifiers and controlled ventilation in a greenhouse reduced the incidence of B. cinerea in geraniums by 40% compared to conventional ventilation systems [439].
5. Integrated Environmentally Friendly Management
5.1. Combining Environmentally Friendly Techniques
Combining environmentally friendly techniques presents an appealing and sustainable solution, though it is not without inherent complexities and challenges [440,441]. Integrating various environmental methods for managing plant pathogens based on organic resource-mediated strategies like intercropping, crop rotation, and organic amendments enhances the resilience and sustainability of agroecosystems against plant pathogens [108]. Crop rotation interrupts the life cycles of soil-borne pathogens by incorporating a non-susceptible crop, leading to a decrease in pathogen populations. It helps in plant nutrition and the more effective management of plant diseases [442]. Intercropping improves plant diversity, inhibiting pathogen dissemination, and creating a more competitive environment for advantageous microbes [443]. Organic additives, such as compost and biochar, increase soil vitality and microbial diversity, thus providing unique benefits for long-term soil sustainability and contributing to improved disease resistance [444,445]. When combined, these methods encourage sustainable disease management by reducing dependence on chemical pesticides and fostering a balanced ecosystem that improves plant health and effectively manages plant pathogens.
Environmental management also enhances the success of genetic resistance and BCAs through synergistic mechanisms. In tomato cultivation, integrating tomato yellow leaf curl virus-resistant varieties with early planting and whitefly vector control measures yields far greater disease suppression than genetic resistance alone [396]. Soil health improvements through organic amendments further promote microbial diversity, enhancing the performance of biocontrol agents such as Trichoderma spp. against R. solani [446,447].
Nonetheless, several challenges impede the widespread adoption of integrated approaches. A major obstacle is the complexity of design and implementation. Effective integration requires extensive knowledge of pathogen biology, environmental interactions, and agronomic practices. For instance, while crop rotation is generally effective against soil-borne pathogens, it can inadvertently perpetuate diseases if alternative hosts are not properly excluded, as in the case of Fusarium spp. affecting both cotton and tomato [219]. Another challenge lies in the variability of environmental technique effectiveness, often dictated by climatic and edaphic conditions. Soil solarization, for example, requires sustained high temperatures to achieve adequate pathogen mortality, limiting its application in temperate regions [46]. Similarly, the success of biological control agents such as B. subtilis in suppressing bacterial diseases is influenced by soil moisture and temperature, leading to inconsistent field results [448]. The summarized aspects of the effectiveness of environmentally friendly techniques in controlling different types of plant pathogens are shown in Supplementary Table S1.
5.2. Integration with Chemical Control Methods
Combining environmental strategies with chemical control methods provides a comprehensive way to manage plant pathogens. Sustainable methods such as crop rotation, intercropping, and organic amendments can be integrated with the careful use of chemical fungicides or pesticides to lower pathogen pressure while decreasing chemical inputs. Moreover, genetic control techniques, like employing resistant plant varieties, enhance these strategies by offering natural disease resistance and minimizing the frequency of chemical applications [449,450]. This comprehensive strategy improves pathogen management while also fostering sustainable farming by protecting soil health, minimizing chemical resistance, and sustaining ecological equilibrium.
Moreover, integration plays a pivotal role in delaying the emergence of resistant pathogen strains. Chemical treatments applied in isolation, such as fungicides targeting B. cinerea, often select for resistant populations [451]. However, coupling environmental sanitation practices with chemical applications disrupts pathogen life cycles at multiple points. This strategy has proven effective, for example, against bacterial spot of tomato and pepper caused by Xanthomonas spp. [452]. Similarly, the use of reflective mulches and trap crops to limit aphid populations has significantly enhanced control of CMV, demonstrating how environmental interventions bolster chemical effectiveness [453]. Furthermore, when solarization is combined with minimal chemical applications, a synergistic effect often emerges, enabling effective disease suppression while reducing chemical residues in the environment [272].
Environmental methods may also be insufficient when facing viruses and phytoplasmas without complementary measures targeting insect vectors. For phytoplasma diseases like aster yellows, controlling the leafhopper vectors through habitat management must be supplemented with insecticide applications to achieve significant disease reduction [454]. Thus, while environmental interventions are critical, they often require integration with chemical or biological measures to be truly effective against complex pathogen systems. Economics poses a final significant barrier to integration. Integrated strategies generally demand higher upfront investment in knowledge acquisition, monitoring infrastructure, and sustainable inputs. This reality may discourage adoption by resource-limited farmers, who often prefer conventional pesticide-based systems for their immediate efficacy and lower initial costs [455].
Real-world applications further illustrate both the promise and limitations of integrated control. In viticulture, managing downy mildew (Plasmopara viticola) successfully combines resistant cultivars, canopy management for improved airflow, and minimal fungicide applications [456]. In rice, integrated management of bacterial blight (Xanthomonas oryzae pv. oryzae) emphasizes genetic resistance, field sanitation, and balanced fertilization, reserving bactericides for emergency intervention [457]. Similarly, controlling citrus Huanglongbing (‘Candidatus Liberibacter asiaticus’ integrates vector management, the rogueing of infected trees, and the deployment of tolerant rootstocks, highlighting the necessity of a multifaceted approach [458]. Ultimately, the integration of environmentally friendly and chemical disease management strategies represents a promising path towards sustainable agriculture. Future progress depends on advancing research into tailored, pathogen-specific protocols, supporting farmer education initiatives, and creating policies that encourage and subsidize integrated practices.
6. Quantitative and Comparative Contributions of Control Diseases Strategies
The review paper has presented a broad, qualitative evaluation of various environmentally friendly methods for plant pathogen control, across multiple pathosystems, providing some numerical data and comparative insights scattered across the sections and supporting a contextual and integrated use of the following key strategies:
(a) Host resistance and crop genetic diversity—top-tier effectiveness;
(b) Soil amendments, rotation, and organic inputs—medium to high (variable);
(c) Biological control—effective but climate-sensitive;
(d) Physical methods—useful for high-value crops or specific contexts;
(e) Integration of methods—highest overall performance.
Table 7 displays a synthesized summary based on the available data and references in the main text:

Table 7.
Summarized aspects of comparative contributions of control diseases strategies.
Different control strategies’ effectiveness can be quantitatively compared based on their ability to reduce disease incidence and severity as follows (Table 8). Physical techniques, biological control, and cultural and ecological management strategies are all effective methods for reducing plant diseases. However, their effectiveness can vary, depending on the specific crop, pathogen, and environmental conditions. Integrated disease management approaches, which combine multiple strategies, have been shown to be more effective than using a single strategy. By understanding the strengths and limitations of each method, farmers and agricultural practitioners can develop comprehensive disease management plans that optimize disease reduction while minimizing environmental impact.

Table 8.
Effectiveness of control strategies in the reduction in plant diseases.
7. Future Perspectives and Conclusions
Environmental techniques for managing plant pathogens represent a sustainable shift away from chemical-intensive agriculture, with strategies like soil solarization, hot water treatment, and steaming offering effective physical control. Cultural practices such as crop rotation, intercropping, and sanitation interrupt disease cycles and enhance agroecosystem resilience, while organic amendments—including compost, biochar, and green manures—improve soil health, promote beneficial microbes, and suppress pathogens through both direct and indirect mechanisms. Biological approaches, notably the application of antagonistic microorganisms, arbuscular mycorrhizal fungi, and plant growth-promoting rhizobacteria, have demonstrated multifaceted benefits in reducing pathogen loads and enhancing plant defense. Soilless cultivation techniques further contribute to plant health by minimizing soil-borne disease risks and improving environmental control in protected systems. Despite their proven potential, these techniques face limitations such as variability in effectiveness, challenges in standardization, and limited adoption due to labor or resource constraints. Looking ahead, emerging technologies like microbiome engineering and genome editing offer exciting possibilities to complement and amplify existing practices. Microbiome engineering can enable the design of tailored microbial consortia that improve disease suppression, nutrient cycling, and plant immunity—paving the way for precision biocontrol. Simultaneously, genetic modification and CRISPR-Cas technologies allow the development of crops with durable, multi-pathogen resistance traits that can be integrated with organic and microbial solutions for holistic plant protection.
To realize these advances, future research should focus on the following:
1. Optimizing synergies among environmental techniques—e.g., combining organic amendments with biocontrol agents or physical methods with crop diversification.
2. Advancing microbiome—based tools, supported by metagenomics and microbial ecology, to predict and manipulate soil suppressiveness.
3. Enhancing regulatory and risk assessment frameworks—for the use of engineered microbes and gene-edited crops in open-field conditions.
4. Conducting long-term field trials across diverse agroecological zones to validate the consistency and scalability of integrated strategies.
5. Bridging ecological knowledge with data science, enabling predictive modeling for disease outbreaks and microbiome responses to interventions.
In conclusion, the path to sustainable disease management lies in the integration of traditional ecological knowledge with cutting-edge biotechnological tools. A multidisciplinary and systems-based approach—uniting physical, biological, and cultural practices with innovations in genetic and microbial engineering—will be key to building resilient, productive, and environmentally responsible agricultural systems in the face of climate change and global food challenges.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15071551/s1, Table S1: Results of the effectiveness of environmentally friendly techniques in controlling different types of plant pathogens.
Author Contributions
Conceptualization, M.A.C., R.C., S.G., and S.M.; writing—original draft preparation, R.C., M.A.C., V.T., S.G., and S.M.; writing—review and editing, M.A.C., S.G., and S.M.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Acknowledgments
Sincere thanks are extended to A. Sosso (INRiM—Istituto Nazionale di Ricerca Metrologica, Turin, Italy) for their assistance with formatting the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, N.; Sundin, G.; Fuente, L.; Cubero, J.; Tatineni, S.; Brewer, M.; Zeng, Q.; Bock, C.; Cunniffe, N.; Wang, C.; et al. Key Challenges in Plant Pathology in the Next Decade. Phytopathology 2024, 114, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Senthilraja, N.; Gangwar, R.K.; Borad, C.K. Challenges and Opportunities in Plant Disease Management: A Brief Review. Madras Agric. J. 2024, 111, 1–3. [Google Scholar]
- He, D.-C.; Zhan, J.-S.; Xie, L.-H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Gill, H.K.; Aujla, I.S.; De Bellis, L.; Luvisi, A. The role of soil solarization in India: How an unnoticed practice could support pest control. Front. Plant Sci. 2017, 8, 1515. [Google Scholar] [CrossRef]
- Café-Filho, A.C.; Lopes, C.A.; Rossato, M. Management of Plant Disease Epidemics with Irrigation Practices. In Irrigation in Agroecosystems; Ondrašek, G., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 8. [Google Scholar] [CrossRef]
- Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae 2024, 10, 805. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D.L. Control of crop diseases through Integrated Crop Management to deliver climate-smart farming systems for low- and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Aytenew, M.; Wolancho, G. Effects of Organic Amendments on Soil Fertility and Environmental Quality: A Review. J. Plant Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Karlsson Green, K.; Stenberg, J.A.; Lankinen, Å. Making sense of Integrated Pest Management (IPM) in the light of evolution. Evol. Appl. 2020, 13, 1791–1805. [Google Scholar] [CrossRef]
- Gullino, M.; Pugliese, M.; Gilardi, G.; Garibaldi, A. Effect of increased CO2 and temperature on plant diseases: A critical appraisal of results obtained in studies carried out under controlled environment facilities. J. Plant Pathol. 2018, 100, 371–389. [Google Scholar] [CrossRef]
- Priya, P.; Patil, M.; Pandey, P.; Singh, A.; Babu, V.S.; Senthil-Kumar, M. Stress combinations and their interactions in plants database: A one-stop resource on combined stress responses in plants. Plant J. 2023, 116, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Narisawa, K.; Shimura, M.; Usuki, F.; Fukuhara, S.; Hashiba, T. Effects of Pathogen Density, Soil Moisture, and Soil pH on Biological Control of Clubroot in Chinese Cabbage by Heteroconium chaetospira. Plant Dis. 2005, 89, 285–290. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Stueven, M.; Pastrana, A.M.; Henry, P.M.; Dennehy, C.M.; Kirkpatrick, S.C.; Daugovish, O. The Effect of pH on Spore Germination, Growth, and Infection of Strawberry Roots by Fusarium oxysporum f. sp. fragariae, Cause of Fusarium wilt of Strawberry. Plant Dis. 2019, 103, 697–704. [Google Scholar] [CrossRef]
- Todorović, I.; Moënne-Loccoz, Y.; Raičević, V.; Jovičić-Petrović, J.; Muller, D. Microbial diversity in soils suppressive to Fusarium diseases. Front. Plant Sci. 2023, 14, 1228749. [Google Scholar] [CrossRef]
- Niekawa, E.T.G.; Simionato, A.S.; Barazetti, A.R.; Cano, B.G.; Emiliano, J.; Afonso, L.; de Lima Andreata, M.F.; Dealis, M.L.; Chryssafidis, A.L.; Andrade, G. Chapter 10—The microbial role in the control of phytopathogens—An alternative to agrochemicals. In Microbiome Stimulants for Crops; White, J., Kumar, A., Droby, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 159–177. [Google Scholar] [CrossRef]
- Yang, Y.; Singh, R.P.; Zhang, C.; You, X.; Li, Y. Chapter 23—Management of diversity and abundance of soil microorganisms to inhibit the occurrence of plant disease. In Microbial Essentialism; Pratap Singh, R., Manchanda, G., Sarsan, S., Kumar, A., Panosyan, H., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2024; pp. 519–559. [Google Scholar] [CrossRef]
- Kumar, A.P.; Murali, V. A Review on Soil and Phytomicrobiome for Plant Disease Management. Int. J. Environ. Clim. Change 2023, 13, 2890–2904. [Google Scholar]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Mikryukov, V.; Dulya, O.; Tedersoo, L.; Bahram, M.; et al. Patterns in soil microbial diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef]
- Matić, S.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emergence of leaf spot disease on leafy vegetable and ornamental crops caused by Paramyrothecium and Albifimbria species. Phytopathology 2019, 109, 1053–1061. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria leaf spot caused by Alternaria species: An emerging problem on ornamental plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Gullino, M.L.; Garibaldi, A.; Guarnaccia, V. Emerging leafy vegetable crop diseases caused by the Fusarium incarnatum-equiseti species complex. Phytopathol. Mediterr. 2020, 59, 303–317. [Google Scholar]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Trkulja, V.; Tomić, A.; Matić, S.; Trkulja, N.; Iličić, R.; Popović Milovanović, T. An Overview of the Emergence of Plant Pathogen ‘Candidatus Liberibacter solanacearum’ in Europe. Microorganisms 2023, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Caruso, A.G.; D’Errico, C.; Botto, C.S.; Noris, E.; Trkulja, V.; Panno, S.; Davino, S.; Moizio, M. Powdery mildew caused by Erysiphe corylacearum: An emerging problem on hazelnut in Italy. PLoS ONE 2024, 19, e0301941. [Google Scholar] [CrossRef]
- Tomic, A.; Trkulja, V.; Matic, S.; Trkulja, N.; Ilicic, R.; Scortichini, M.; Popovic Milovanovic, T. Net blotch (Pyrenophora teres Drechsler): An increasingly significant threat to barley production. Plant Prot. Sci. 2024, 60, 1–30. [Google Scholar] [CrossRef]
- Matić, S.; Cucu, M.A.; Garibaldi, A.; Gullino, M.L. Combined effect of CO2 and temperature on wheat powdery mildew development. Plant Pathol. J. 2018, 34, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Garibaldi, A.; Gullino, M.L. Combined and single effects of elevated CO2 and temperatures on rice bakanae disease under controlled conditions in phytotrons. Plant Pathol. 2021, 70, 815–826. [Google Scholar] [CrossRef]
- Kumar, D.; Mukhopadhyay, R. Climate change and plant pathogens: Understanding dynamics, risks and mitigation strategies. Plant Pathol. 2025, 74, 59–68. [Google Scholar] [CrossRef]
- Frías-De-León, M.; Brunner-Mendoza, C.; Reyes-Montes, M.; Duarte-Escalante, E. The Impact of Climate Change on Fungal Diseases; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Pagliarani, C.; Moine, A.; Chitarra, W.; Nerva, L.; Catoni, M.; Tavazza, R.; Matić, S.; Vallino, M.; Secchi, F.; Noris, E. The C4 protein of tomato yellow leaf curl Sardinia virus primes drought tolerance in tomato through morphological adjustments. Hortic. Res. 2022, 9, uhac164. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Kumar, A.; Mahanta, D.; Dange, M.M.; Trivedi, A.; Nandeha, N. Global Challenges Facing Plant Pathology: A Review on Multidisciplinary Approaches to Meet the Food Security. J. Sci. Res. Rep. 2024, 30, 884–892. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Deshi, S.; Wonang, D.; Dafur, B. Control of rots and spoilage of agricultural products: A review. Int. Lett. Nat. Sci. 2014, 18, 63–72. [Google Scholar]
- Lv, H.; Fang, Z.; Yang, L.; Zhang, Y.; Wang, Y. An update on the arsenal: Mining resistance genes for disease management of Brassica crops in the genomic era. Hortic. Res. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, K.; Maesaka, M.; Murase, J.; Asakawa, S. Solarization makes a great impact on the abundance and composition of microbial communities in soil. Soil Sci. Plant Nutr. 2015, 61, 641–652. [Google Scholar] [CrossRef]
- Gullino, M.L.; Garibaldi, A.; Gamliel, A.; Katan, J. Soil Disinfestation: From Soil Treatment to Soil and Plant Health. Plant Dis. 2022, 106, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.W.; Guo, H.; Claypool, J.T.; Marshall, M.N.; Perano, K.M.; Stapleton, J.J.; VanderGheynst, J.S. Managing compost stability and amendment to soil to enhance soil heating during soil solarization. Waste Manag. 2013, 33, 1090–1096. [Google Scholar] [CrossRef][Green Version]
- Novák, V.; Hlaváčiková, H.; Novák, V.; Hlaváčiková, H. Soil temperature and heat transport in soils. In Applied Soil Hydrology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 303–318. [Google Scholar]
- Yates, S.; Ashworth, D.; Yates, M.; Luo, L. Active solarization as a nonchemical alternative to soil fumigation for controlling pests. Soil Sci. Soc. Am. J. 2011, 75, 9–16. [Google Scholar] [CrossRef]
- Gilardi, G.; Demarchi, S.; Gullino, M.L.; Garibaldi, A. Effect of Simulated Soil Solarization and Organic Amendments on Fusarium Wilt of Rocket and Basil Under Controlled Conditions. J. Phytopathol. 2014, 162, 557–566. [Google Scholar] [CrossRef]
- Elmore, C.; Stapleton, J.; Bell, C.; DeVay, J. Soil Solarization: A Non-Pesticidal Method for Controlling Diseases, Nematodes, and Weeds; University of California: Oakland, CA, USA, 1997. [Google Scholar]
- Stapleton, J.J.; DeVay, J.E. Soil solarization: A non-chemical approach for management of plant pathogens and pests. Crop Prot. 1986, 5, 190–198. [Google Scholar] [CrossRef]
- Kanaan, H.; Frenk, S.; Raviv, M.; Medina, S.; Minz, D. Long and short term effects of solarization on soil microbiome and agricultural production. Appl. Soil Ecol. 2018, 124, 54–61. [Google Scholar] [CrossRef]
- Castello, I.; D’emilio, A.; Raviv, M.; Vitale, A. Soil solarization as a sustainable solution to control tomato Pseudomonads infections in greenhouses. Agron. Sustain. Dev. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Dai, Y.; Senge, M.; Yoshiyama, K.; Zhang, P.; Zhang, F. Influencing factors, effects and development prospect of soil solarization. Rev. Agric. Sci. 2016, 4, 21–35. [Google Scholar] [CrossRef]
- Katan, J.; DeVay, J. Soil Solarization; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Gebreegziher, W.G. Agronomic use of solarization technology on soil fertility and pest management in dryland agriculture. Cogent Food Agric. 2024, 10, 2306692. [Google Scholar] [CrossRef]
- Ringselle, B.; De Cauwer, B.; Salonen, J.; Soukup, J. A Review of Non-Chemical Management of Couch Grass (Elymus repens). Agronomy 2020, 10, 1178. [Google Scholar] [CrossRef]
- Samtani, J.B.; Gilbert, C.; Weber, J.B.; Subbarao, K.V.; Goodhue, R.E.; Fennimore, S.A. Effect of Steam and Solarization Treatments on Pest Control, Strawberry Yield, and Economic Returns Relative to Methyl Bromide Fumigation. HortScience 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Luvisi, A.; Panattoni, A.; Materazzi, A. Heat treatments for sustainable control of soil viruses. Agron. Sustain. Dev. 2015, 35, 657–666. [Google Scholar] [CrossRef]
- Fennimore, S.A.; Martin, F.N.; Miller, T.C.; Broome, J.C.; Dorn, N.; Greene, I. Evaluation of a Mobile Steam Applicator for Soil Disinfestation in California Strawberry. HortScience 2014, 49, 1542–1549. [Google Scholar] [CrossRef]
- Phua, L.; Neo, S.; Khoo, G.; Yuk, H. Comparison of the efficacy of various sanitizers and hot water treatment in inactivating inoculated foodborne pathogens and natural microflora on mung bean sprouts. Food Control 2014, 42, 270–276. [Google Scholar] [CrossRef]
- Matić, S.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control 2014, 73, 59–67. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D. Hot water rinsing and brushing of fresh produce as an alternative to chemical treatment after harvest—The story behind the technology. Agronomy 2021, 11, 1653. [Google Scholar] [CrossRef]
- Forsberg, G.; Andersson, S.; Johnsson, L. Evaluation of hot, humid air seed treatment in thin layers and fluidized beds for seed pathogen sanitation/Bewertung der Saatgutbehandlung mit heißer, feuchter Luft und in einer Verwirbelungskammer zur Eliminierung von samenbürtigen Pathogenen. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 2002, 109, 357–370. [Google Scholar]
- Sultana, N.; Islam, M.; Noman, A.; Faruq, A.; Akter, N.; Islam, M. Standardization of Temperature and Duration for Hot Water Seed Treatment of Selected Vegetables. Agriculturists 2021, 19, 128–139. [Google Scholar]
- Mwando, N.; Ndlela, S.; Meyhöfer, R.; Subramanian, S.; Mohamed, S. Hot water treatment for post-harvest disinfestation of Bactrocera dorsalis (Diptera: Tephritidae) and its effect on cv. tommy atkins mango. Insects 2021, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.; Kang, D. Thermal and non-thermal treatment effects on Staphylococcus aureus biofilms formed at different temperatures and maturation periods. Food Res. Int. 2020, 137, 109432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hussain, I.; Azam, M.; Khan, M.; Akram, M.; Naveed, K.; Liu, H. Hot water treatment improves date drying and maintains phytochemicals and fruit quality characteristics of date palm (Phoenix dactylifera). Foods 2023, 12, 2405. [Google Scholar] [CrossRef]
- Berrios-Rodriguez, A.; Olanya, O.; Ukuku, D.; Niemira, B.; Orellana, L.; Mukhopadhyay, S.; Boyd, G. Inactivation of Listeria monocytogenes on post-harvest carrot and tomato by gamma radiation, sanitizer, biocontrol treatments and their combinations. LWT 2020, 118, 108805. [Google Scholar] [CrossRef]
- Spinks, A.; Dunstan, R.; Harrison, T.; Coombes, P.; Kuczera, G. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res. 2006, 40, 1326–1332. [Google Scholar] [CrossRef]
- Kantakhoo, J.; Imahori, Y. Antioxidative responses to pre-storage hot water treatment of red sweet pepper (Capsicum annuum L.) fruit during cold storage. Foods 2021, 10, 3031. [Google Scholar] [CrossRef]
- Haapala, T.; Palonen, P.; Korpela, A.; Ahokas, J. Feasibility of paper mulches in crop production—A review. Agric. Food Sci. 2014, 23, 60–79. [Google Scholar] [CrossRef]
- Kumar, V.; Abdul-Baki, A.; Anderson, J.D.; Mattoo, A.K. Cover Crop Residues Enhance Growth, Improve Yield, and Delay Leaf Senescence in Greenhouse-grown Tomatoes. HortScience 2005, 40, 1307–1311. [Google Scholar] [CrossRef]
- Fatima, T.; Teasdale, J.R.; Bunce, J.; Mattoo, A.K. Tomato response to legume cover crop and nitrogen: Differing enhancement patterns of fruit yield, photosynthesis and gene expression. Funct. Plant Biol. 2012, 39, 246–254. [Google Scholar] [CrossRef]
- Xu, D.; Ling, J.; Qiao, F.; Xi, P.; Zeng, Y.; Zhang, J.; Lan, C.; Jiang, Z.; Peng, A.; Li, P. Organic mulch can suppress litchi downy blight through modification of soil microbial community structure and functional potentials. BMC Microbiol. 2022, 22, 155. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Janisiewicz, W.; Takeda, F.; Glenn, D.; Camp, M.; Ii, W. Dark Period Following UV-C Treatment Enhances Killing of Botrytis cinerea Conidia and Controls Gray Mold of Strawberries. Phytopathology 2015, 106, 386–394. [Google Scholar] [CrossRef]
- Suthaparan, A.; Torre, S.; Stensvand, A.; Herrero, M.L.; Pettersen, R.; Gadoury, D.; Gislerød, H. Specific Light-Emitting Diodes Can Suppress Sporulation of Podosphaera pannosa on Greenhouse Roses. Plant Dis. 2010, 94, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J.; Dahlquist-Willard, R.; Achmon, Y.; Marshall, M.; VanderGheynst, J.; Simmons, C. Advances in Biosolarization Technology to Improve Soil Health and Organic Control of Soilborne Pests. In Proceedings of the 2016 Organic Agriculture Research Symposium, Asilomar, CA, USA, 20–23 January 2016. [Google Scholar]
- Fennimore, S.; Goodhue, R. Soil Disinfestation with Steam: A Review of Economics, Engineering, and Soil Pest Control in California Strawberry. Int. J. Fruit Sci. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Melander, B.; Liebman, M.; Davis, A.S.; Gallandt, E.R.; Bàrberi, P.; Moonen, A.C.; Rasmussen, J.; van der Weide, R.; Vidotto, F. Non-Chemical Weed Management. In Weed Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; Chapter 9; pp. 245–270. [Google Scholar] [CrossRef]
- Noble, R.; Roberts, S.J. Eradication of plant pathogens and nematodes during composting: A review. Plant Pathol. 2004, 53, 548–568. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, D.; Thakur, A.; Bhardwaj, S.; Angurana, R.; Katoch, V.; Kapoor, D. Success Story of Arbuscular Mycorrhizal Fungi as a Bio Protectant Against Major Plant Pathogens. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Springer Nature: Singapore, 2024; pp. 321–336. [Google Scholar]
- Tyagi, A.; Raj, H. Integration of soil solarization with bio-control agents for the management of stem rot of chrysanthemum. J. Pharmacogn. Phytochem. 2021, 10, 2468–2471. [Google Scholar] [CrossRef]
- Shavnam; Raj, H. Synergistic strategies for sustainable crop protection: Harnessing soil solarization and biofumigants to combat damping-off pathogens in Solanaceous vegetable crops. J. Plant Dis. Prot. 2024, 131, 2089–2098. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Löffler, P.; Eichhöfer, S.; David, J.; Muñoz, K.; Schaumann, G.E. Are agricultural plastic covers a source of plastic debris in soil? A first screening study. Soil 2022, 8, 31–47. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Mauromicale, G.; Monaco, A.L.; Longo, A.M.G. Improved efficiency of soil solarization for growth and yield of greenhouse tomatoes. Agron. Sustain. Dev. 2010, 30, 753–761. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Barman, M.; Bera, T.; De, M.; Chatterjee, D. Agriculture: Polymers in Crop Production Mulch and Fertilizer. In Encyclopedia of Polymer Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica Seed Meal Soil Amendments Transform the Rhizosphere Microbiome and Improve Apple Production Through Resistance to Pathogen Reinfestation. Phytopathology 2015, 105, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Streck, N.A.; Schneider, F.M.; Buriol, G.A. Effect of soil solarization on thermal regime of plastic greenhouse soil. Cienc. Rural 1994, 24, 229–233. [Google Scholar] [CrossRef]
- Salman, S.R.; Mettawee, S.A.G. Soil solar-warming with different types of mulch. Arab. Univ. J. Agric. Sci. 2005, 13, 877–889. [Google Scholar]
- Shlevin, E.; Mahrer, Y.; Katan, J. Effect of moisture on thermal inactivation of soilborne pathogens under structural solarization. Phytopathology 2004, 94, 132–137. [Google Scholar] [CrossRef]
- Kim, M.; Shim, C.; Lee, J.; Wangchuk, C. Hot Water Treatment as Seed Disinfection Techniques for Organic and Eco-Friendly Environmental Agricultural Crop Cultivation. Agriculture 2022, 12, 1081. [Google Scholar] [CrossRef]
- Shekhar, M.; Shivashankar, E.; Pandey, S.K. Crop Rotation and Intercropping Techniques, 1st ed.; ND Global Publication House: Sagar, India, 2024. [Google Scholar]
- Liu, C.; Plaza-Bonilla, D.; Coulter, J.A.; Kutcher, H.R.; Beckie, H.J.; Wang, L.; Gan, Y. Diversifying crop rotations enhances agroecosystem services and resilience. Adv. Agron. 2022, 173, 299–335. [Google Scholar] [CrossRef]
- Mihrete, T.B.; Mihretu, F.B. Crop Diversification for Ensuring Sustainable Agriculture, Risk Management and Food Security. Glob. Challenges 2025, 9, 2400267. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, H.; Hussain, N.; Nasim, W.; Mubeen, M.; Ahmad, S.; Hasanuzzaman, M. Fundamentals of crop rotation in agronomic management. In Agronomic Crops; Springer: Singapore, 2019; pp. 545–559. [Google Scholar]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef]
- Cook, R.J. Management of resident plant growth-promoting rhizobacteria with the cropping system: A review of experience in the US Pacific Northwest. Eur. J. Plant Pathol. 2007, 119, 255–264. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Hubbard, J.C.; Koike, S.T. Evaluation of broccoli residue incorporation into field soil for Verticillium wilt control in cauliflower. Plant Dis. 1999, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.; Kur, A.; Bueno, E.; von Wettberg, E. Defining and improving the rotational and intercropping value of a crop using a plant–soil feedbacks approach. Crop Sci. 2020, 60, 2195–2203. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Shennan, C.; Muramoto, J.; Baird, G.; Zavatta, M.; Toyama, L.; Mazzola, M.; Koike, S.T. Anaerobic soil disinfestation (ASD): A strategy for control of soil borne diseases in strawberry production. In Proceedings of the International Symposium on Innovation in Integrated and Organic Horticulture, Avignon, France, 8–12 June 2015; Volume 1137, pp. 113–120. [Google Scholar] [CrossRef]
- Tronsmo, A.M.; Collinge, D.B.; Djurle, A.; Munk, L.; Yuen, J.; Tronsmo, A. Plant Pathology and Plant Diseases; CAB International: Boston, MA, USA, 2020. [Google Scholar]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Rusinamhodzi, L. Crop Rotations and Residue Management in Conservation Agriculture. In Conservation Agriculture; Farooq, M., Siddique, K., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kheyrodin, H. Crop rotations for managing soil-borne plant diseases. Afr. J. Food Sci. Technol. 2011, 2, 1–9. [Google Scholar]
- Marburger, D.A.; Venkateshwaran, M.; Conley, S.P.; Esker, P.D.; Lauer, J.G.; Ané, J.M. Crop rotation and management effect on Fusarium spp. populations. Crop Sci. 2015, 55, 365–376. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Yang, X.B. Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis. 2000, 84, 94–99. [Google Scholar] [CrossRef]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Zhao, J.; Zhang, J.; Cai, Z.; Huang, X. High carbon resource diversity enhances the certainty of successful plant pathogen and disease control. New Phytol. 2023, 237, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Gilbert, J.A. Microbial diversity—Exploration of natural ecosystems and microbiomes. Curr. Opin. Genet. Dev. 2015, 35, 66–72. [Google Scholar] [CrossRef]
- De Corato, U. Soil microbiota manipulation and its role in suppressing soil-borne plant pathogens in organic farming systems under the light of microbiome-assisted strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Kema, G.H.; Drenth, A.; Dita, M.; Jansen, K.; Vellema, S.; Stoorvogel, J.J. Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 2021, 11, 628888. [Google Scholar] [CrossRef]
- Siamak, S.B.; Zheng, S. Banana Fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems. Hortic. Plant J. 2018, 4, 208–218. [Google Scholar] [CrossRef]
- Viljoen, A.; Ma, L.J.; Molina, A.B. CHAPTER 8: Fusarium wilt (panama disease) and monoculture in banana production: Resurgence of a century-old disease. In Emerging Plant Diseases and Global Food Security; The American Phytopathological Society: St. Paul, MN, USA, 2020; pp. 159–184. [Google Scholar]
- CABI. Fusarium oxysporum f.sp. Cubense (Panama disease of banana). In CABI Compendium; CABI: Wallingford, UK, 2022. [Google Scholar]
- Bekele, D.; Worku, W.; Mulatu, Z.; Admasu, A.; Shimeles, F.; Dobocha, D. Evaluation of Alternative Break Crops in Rotation with Bread Wheat (Triticum aestivum L.) in South-Eastern Ethiopia. J. Aquac. Livest. Prod. 2024, 5, 1–6. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crops Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Abdulkadir, K.N. Cropping Systems Diversification as an approach to Enhancing Crop Productivity. Preprints 2023. [Google Scholar]
- Roesch-McNally, G.E.; Arbuckle, J.G.; Tyndall, J.C. Barriers to implementing climate resilient agricultural strategies: The case of crop diversification in the US Corn Belt. Glob. Environ. Change 2018, 48, 206–215. [Google Scholar] [CrossRef]
- Larkin, R. Use of crop rotations, cover crops and green manures for disease suppression in potato cropping systems. Glob. J. Agric. Innov. Res. Dev. 2021, 8, 153–168. [Google Scholar] [CrossRef]
- Morris, E.; Fletcher, R.; Veresoglou, S. Effective methods of biofumigation: A meta-analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Prasad, P.; Kumar, J.; Pandey, S. Biofumigation: Success and prospects in soilborne plant disease management. JAPSA 2015, 1, 47–59. [Google Scholar]
- Angus, J.; Gardner, P.; Kirkegaard, J.; Desmarchelier, J. Biofumigation: Isothiocyanates released from Brassica roots inhibit growth of the take-all fungus. Plant Soil 1994, 162, 107–112. [Google Scholar] [CrossRef]
- Brown, P.; Morra, M. Control of soilborne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Yang, Y.J.; Li, S.Y.; Hu, G.W.; Liao, X.J.; Hu, X.S.; Zhang, Y. Research progress on degradation pathways and products of glucosinolates. Acta Bot.-Boreali-Occident. Sin. 2011, 31, 1490–1496. [Google Scholar]
- Hanschen, F.; Winkelmann, T. Biofumigation for fighting replant disease—A review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Wang, L.L.; Jiang, H.; Qiu, Y.J.; Dong, Y.Y.; Hamouda, H.I.; Balah, M.A.; Mao, X.Z. Biochemical characterization of a novel myrosinase Rmyr from Rahnella inusitata for high-level preparation of sulforaphene and sulforaphane. J. Agric. Food Chem. 2022, 70, 2303–2311. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Miao, Z.; Guo, R.; Zhao, Z. Biofumigation for management of soilborne plant diseases. Chin. J. Biol. Control 2006, 22, 296. [Google Scholar]
- Larkin, R.; Griffin, T. Control of soilborne diseases of potato using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Yulianti, T.; Sivasithamparam, K.; Turner, D.W. Saprophytic and pathogenic behaviour of R. solani AG2-1 (ZG-5) in a soil amended with Diplotaxis tenuifolia or Brassica nigra manures and incubated at different temperatures and soil water content. Plant Soil 2007, 294, 277–289. [Google Scholar] [CrossRef]
- Fan, C.; Liu, J.; Wu, Y.; Xiong, G.; He, Y. Screening of several plants suppressing soil borne plant fungi by biofumigation. J. Yunnan Agric. Univ. 2007, 22, 654–658. [Google Scholar] [CrossRef]
- Sihag, M.; Kumar, V.; Rana, M.; Srivastava, S.; Singh, S. Biofumigation: Prospects for control of soil borne plant diseases. J. Biopestic. 2022, 15, 136–149. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Wang, Y.; Lu, X.; Wang, P.; Su, Z.; Zhang, X.; Ma, P. Control efficacy of broccoli residues on cotton Verticillium wilt and its effect on soil bacterial community at different growth stages. Sci. Agric. Sin. 2019, 52, 4505–4517. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, Z.; Wang, G.; Ma, Y. Integration of Pseudomonas aeruginosa with biofumigation to control phytophthora blight of pepper. Jiangsu J. Agric. Sci. 2015, 31, 290–297. [Google Scholar] [CrossRef]
- Walker, B.A.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Soil microbial community dynamics during ryegrass green manuring and brassica biofumigation. Appl. Soil Ecol. 2022, 179, 104600. [Google Scholar] [CrossRef]
- Walker, B.A.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Ten years of green manuring and biofumigation alters soil characteristics and microbiota. Appl. Soil Ecol. 2023, 187, 104836. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Fang, W.; Li, Y.; Yan, D.; Cao, A.; Wang, Q. A review of biofumigation effects with plant materials. New Plant Prot. 2024, 1, e21. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, R.; Zhang, C.; Yao, X.; Yang, Z.; Li, S.; Liu, T.; Zheng, C.; Wang, X.; Xu, N. Changes in soil microbial diversity and control of Fusarium oxysporum in continuous cropping cucumber greenhouses following biofumigation. Emir. J. Food Agric. 2018, 30, 644–653. [Google Scholar] [CrossRef]
- Sennett, L.; Goyer, C.; Burton, D.; Zebarth, B.; Whitney, S. Chemical fumigation and biofumigation alter soil bacterial community diversity and composition. FEMS Microbiol. Ecol. 2022, 98, fiac026. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, J.; Kirkegaard, J. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J. Biofumigation potential of brassicas: II. Effect of environment and ontogeny on glucosinolate production and implications for screening. Plant Soil 1998, 201, 91–101. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Chen, D.; Zebarth, B.; Goyer, C.; Comeau, L.; Nahar, K.; Dixon, T. Effect of biofumigation on population densities of Pratylenchus spp. and Verticillium spp. and potato yield in Eastern Canada. Am. J. Potato Res. 2022, 99, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Zhang, D.; Fang, W.; Song, Z.; Ren, L.; Li, Q.; Li, W.; Wang, Q.; Yan, D.; Li, Y.; et al. Progresses and challenges in soil-borne disease prevention and control technology. Plant Prot. 2023, 49, 260–269. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Shinmura, A. Principle and effect of soil sterilization methods by reducing the redox potential of soil. PSJ Soilborne Dis. Workshop Rep. 2004, 22, 2–12, (In Japanese with English Summary). [Google Scholar]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. An assessment of the modulation of the population dynamics of pathogenic Fusarium oxysporum f. sp. lycopersici in the tomato rhizosphere by means of the application of Bacillus subtilis QST 713, Trichoderma sp. TW2 and two composts. Biol. Control 2020, 142, 104158. [Google Scholar] [CrossRef]
- Gilardi, G.; Pugliese, M.; Gullino, M.; Garibaldi, A. Evaluation of different carbon sources for anaerobic soil disinfestation against Rhizoctonia solani on lettuce in controlled production systems. Phytopathol. Mediterr. 2020, 59, 77–96. [Google Scholar] [CrossRef]
- Lopes, E.A.; Canedo, E.J.; Gomes, V.A.; Vieira, B.S.; Parreira, D.F.; Neves, W.S. Anaerobic soil disinfestation for the management of soilborne pathogens: A review. Appl. Soil Ecol. 2022, 174, 104408. [Google Scholar] [CrossRef]
- Goud, J.; Termorshuizen, A.; Blok, W.; van Bruggen, A. Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis. 2004, 88, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, E.; Burelle, N.; Hong, J.; Butler, D.; Noling, J.; He, Z.; Booker, B.; Sances, F. Comparison of anaerobic soil disinfestation and drip-applied organic acids for raised-bed specialty crop production in Florida. Acta Hortic. 2014, 1044, 221–228. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Lamers, J.; Mazzola, M.; Rosskopf, E.; Kokalis-Burelle, N.; Momma, N.; Butler, D.; Kobara, Y. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions. Acta Hortic. 2014, 1044, 165–175. [Google Scholar] [CrossRef]
- Shrestha, U.; Augé, R.M.; Butler, D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Guo, H.; Di Gioia, F.; Zhao, X.; Ozores-Hampton, M.; Swisher, M.; Hong, J.; Rosskopf, E. Optimizing anaerobic soil disinfestation for fresh market tomato production: Nematode and weed control, yield, and fruit quality. Sci. Hortic. 2017, 218, 105–116. [Google Scholar] [CrossRef]
- Rosskopf, E.; Serrano-Pérez, P.; Hong, J.; Shrestha, U.; Rodríguez-Molina, M.; Martin, K.; Butler, D. Anaerobic soil disinfestation and soilborne pest management. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Springer: Berlin/Heidelberg, Germany, 2015; pp. 277–305. [Google Scholar]
- Shrestha, U.; Dee, M.E.; Ownley, B.H.; Butler, D.M. Anaerobic soil disinfestation reduces germination and affects colonization of Sclerotium rolfsii sclerotia. Phytopathology 2018, 108, 342–351. [Google Scholar] [CrossRef]
- Strauss, S.L.; Kluepfel, D.A. Anaerobic soil disinfestation: A chemical-independent approach to pre-plant control of plant pathogens. J. Integr. Agric. 2015, 14, 2309–2318. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef]
- Di Gioia, F.; Ozores-Hampton, M.; Hong, J.; Kokalis-Burelle, N.; Albano, J.; Zhao, X.; Rosskopf, E. The effects of anaerobic soil disinfestation on weed and nematode control, fruit yield, and quality of Florida fresh-market tomato. HortScience 2016, 51, 703–711. [Google Scholar] [CrossRef]
- Stremińska, M.A.; Runia, W.T.; Termorshuizen, A.J.; Feil, H.; Van Der Wurff, A.W.G. Anaerobic soil disinfestation in microcosms of two sandy soils. Commun. Agric. Appl. Biol. Sci. 2014, 79, 15–18. [Google Scholar]
- Hewavitharana, S.; Klarer, E.; Reed, A.; Leisso, R.; Poirier, B.; Honaas, L.; Mazzola, M. Temporal dynamics of the soil metabolome and microbiome during simulated anaerobic soil disinfestation. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef] [PubMed]
- Thaning, C.; Gerhardson, B. Reduced sclerotial soil-longevity by whole-crop amendment and plastic covering/Feldversuche zum Einfluss einer Bodenabdeckung mit Kunststoffplane und zur Einarbeitung von Grünmasse auf die Überdauerung von Sklerotien. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 2001, 143–151. [Google Scholar]
- Gholami, M.; Khakvar, R.; Niknam, G. Introduction of some new endophytic bacteria from Bacillus and Streptomyces genera as successful biocontrol agents against Sclerotium rolfsii. Arch. Phytopathol. Plant Prot. 2014, 47, 122–130. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Mazzola, M.; Muramoto, J.; Shennan, C. Anaerobic disinfestation induced changes to the soil microbiome, disease incidence and strawberry fruit yields in California field trials. Appl. Soil Ecol. 2018, 127, 74–86. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Wang, F.; Cai, Z. Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Appl. Microbiol. Biotechnol. 2016, 100, 5581–5593. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.; Wang, Y.; Liu, Z.; Cai, T.; Zhu, D.; Duan, G. Effects of reductive soil disinfestation on potential pathogens and antibiotic resistance genes in soil. J. Environ. Sci. 2025, 150, 373–384. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Xu, R.; Song, J.; Wei, X.; Liu, X.; Wang, Y. Effects of anaerobic soil disinfestation on antibiotics, human pathogenic bacteria, and their associated antibiotic resistance genes in soil. Appl. Soil Ecol. 2024, 195, 105266. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Yu, R.P.; Dresbøll, D.B.; Finckh, M.R.; Justes, E.; van der Werf, W.; Fletcher, A.; Carlsson, G.; Li, L. Intercropping: Ecosystem functioning and sustainable agriculture. Plant Soil 2025, 506, 1–6. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Keshavarz Afshar, R.; Liu, X.; Herbert, S.J. Understanding intercropping to improve agricultural resiliency and environmental sustainability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–256. [Google Scholar]
- Boudreau, M.A. Diseases in intercropping systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef]
- Chadfield, V.G.A.; Hartley, S.E.; Redeker, K.R. Associational resistance through intercropping reduces yield losses to soil-borne pests and diseases. New Phytol. 2022, 235, 2393–2405. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defence. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Cipollini, D.; Morris, K.; Gurusinghe, S.; Weston, L.A. Ecological realism and rigor in the study of plant-plant allelopathic interactions. Plant Soil 2023, 489, 1–39. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Zampieri, E.; Sillo, F.; Metelli, G.; Cucu, M.A.; Montesano, V.; Quagliata, G.; Philipp, L.; Brescia, F.; Conte, A.; Giovannini, L.; et al. Insights into the influence of intercropping and arbuscular mycorrhizal inoculation on two modern durum wheat cultivars and their associated microbiota. Biol. Fertil. Soils 2025, 61, 85–107. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zhai, Z. Soil organic carbon, carbon fractions, and microbial community under various organic amendments. Sci. Rep. 2024, 14, 25431. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Y.; Tang, L.; Zheng, Y.; Makowski, D.; Yu, Y.; Zhang, F.; van der Werf, W. Intercropping cereals with faba bean reduces plant disease incidence regardless of fertilizer input; a meta-analysis. Eur. J. Plant Pathol. 2019, 154, 931–942. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Alarcón-Segura, V.; Grass, I.; Breustedt, G.; Rohlfs, M.; Tscharntke, T. Strip intercropping of wheat and oilseed rape enhances biodiversity and biological pest control in a conventionally managed farm scenario. J. Appl. Ecol. 2022, 59, 1513–1523. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Sairam, M. Intercropping—A low input agricultural strategy for food and environmental security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.E.; Anderson, P.; Carlsson, G.; Friberg, H.; Larsson, M.C.; Wallenhammar, A.C.; Lundin, O. The potential of intercropping for multifunctional crop protection in oilseed rape (Brassica napus L.). Front. Agron. 2021, 3, 782686. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. The efficiency of a durum wheat-winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil 2010, 330, 19–35. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Z.G.; Bao, X.G.; Sun, J.H.; Yang, S.C.; Wang, P.; Li, L. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 2021, 4, 943–950. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, Y.; Li, X.; Qin, H.; Wang, J.; Zhang, S.; Li, X.F. Increased overyielding probability and yield stability from a 5-year cotton-based intercropping. Eur. J. Agron. 2024, 156, 127145. [Google Scholar] [CrossRef]
- Martin-Guay, M.O.; Paquette, A.; Dupras, J.; Rivest, D. The new green revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; van Der Werf, W. Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv. Agron. 2020, 160, 1–50. [Google Scholar] [CrossRef]
- Carolan, K.; Helps, J.; van den Berg, F.; Bain, R.; Paveley, N.; van den Bosch, F. Extending the durability of cultivar resistance by limiting epidemic growth rates. Proc. Biol. Sci. 2017, 284, 20170828. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Rutkoski, J. Advances and challenges in genomic selection for disease resistance. Annu. Rev. Phytopathol. 2016, 54, 79–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Lubberstedt, T.; Xu, M. The genetic and molecular basis of plant resistance to pathogens. J. Genet. Genom. 2013, 40, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ragimekula, N.; Varadarajula, N.N.; Mallapuram, S.P.; Gangimeni, G.; Reddy, R.K.; Kondreddy, H.R. Marker assisted selection in disease resistance breeding. J. Plant Breed. Genet. 2013, 1, 90–109. [Google Scholar]
- Pathania, A.; Rialch, N.; Sharma, P.N. Marker-assisted selection in disease resistance breeding: A boon to enhance agriculture production. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 187–213. [Google Scholar] [CrossRef]
- Tomar, S.S. Breeding Strategies for Stem Rust Resistance in Wheat. Ph.D. Dissertation, Cornell University, Ithaca, NY, USA, 2011. [Google Scholar]
- Thakur, R.P.; Rai, K.N.; Khairwal, I.S.; Mahala, R.S. Strategy for downy mildew resistance breeding in pearl millet in India. J. SAT Agric. Res. 2008, 6, 1–11. [Google Scholar]
- Legg, J.P.; Thresh, J.M. Cassava mosaic virus disease in East Africa: A dynamic disease in a changing environment. Virus Res. 2000, 71, 135–149. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Hayes, R.J.; Vallad, G.E.; Qin, Q.M.; Grube, R.C.; Subbarao, K.V. Variation for resistance to Verticillium wilt in lettuce (Lactuca sativa L.). Plant Dis. 2007, 91, 439–445. [Google Scholar] [CrossRef]
- Kim, S.G.; Hur, O.S.; Ro, N.Y.; Ko, H.C.; Rhee, J.H.; Sung, J.S.; Baek, H.J. Evaluation of resistance to Ralstonia solanacearum in tomato genetic resources at seedling stage. Plant Pathol. J. 2016, 32, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for resistance to Fusarium wilt of tomato: A review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mena, S.; Rubiales, D.; González, M. Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum. Plants 2024, 13, 2454. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; Lu, J.; Sánchez-Martín, J.; Wulff, B.B.H. Resistance that stacks up: Engineering rust and mildew disease control in the cereal crops wheat and barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef]
- Joobeur, T.; King, J.J.; Nolin, S.J.; Thomas, C.E.; Dean, R.A. The fusarium wilt resistance locus Fom-2 of melon contains a single resistance gene with complex features. Plant J. 2004, 39, 283–297. [Google Scholar] [CrossRef]
- de Vallavieille-Pope, C. Management of disease resistance diversity of cultivars of a species in single fields: Controlling epidemics. Comptes Rendus Biol. 2004, 327, 611–620. [Google Scholar] [CrossRef]
- Brown, J.K.M. Durable resistance of crops to disease: A Darwinian perspective. Annu. Rev. Phytopathol. 2015, 53, 513–539. [Google Scholar] [CrossRef]
- Brun, H.; Chèvre, A.M.; Fitt, B.D.L.; Powers, S.; Besnard, A.L.; Ermel, M.; Huteau, V.; Marquer, B.; Eber, F.; Renard, M.; et al. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010, 185, 285–299. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Singh, R.P.; Wagoire, W.W.; Payne, T.S. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 2000, 84, 203. [Google Scholar] [CrossRef]
- Mundt, C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Chen, X.; Zhou, J.M. From plant immunity to crop disease resistance. J. Genet. Genom. 2022, 49, 693–703. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Newman, T.E.; Thomas, W.J.W.; Batley, J.; Edwards, D. The complex relationship between disease resistance and yield in crops. Plant Biotechnol. J. 2024, 22, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hao, Z.; Ning, Y.; He, Z. Revisiting growth–defence trade-offs and breeding strategies in crops. Plant Biotechnol. J. 2024, 22, 1198–1205. [Google Scholar] [CrossRef]
- Khan, A.H.; Hassan, M.; Khan, M.N. Conventional plant breeding program for disease resistance. In Plant Disease Management Strategies for Sustainable Agriculture Through Traditional and Modern Approaches; Springer: Berlin/Heidelberg, Germany, 2020; pp. 27–51. [Google Scholar] [CrossRef]
- Trudgill, D.L. Resistance to and tolerance of plant parasitic nematodes in plants. Annu. Rev. Phytopathol. 1991, 29, 167–192. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Sakr, N. Durable genetic plant resistance: A key to sustainable pathogen management. Open Agric. J. 2023, 17, e187433152306220. [Google Scholar] [CrossRef]
- Stam, R.; McDonald, B.A. When resistance gene pyramids are not durable—The role of pathogen diversity. Mol. Plant Pathol. 2018, 19, 521. [Google Scholar] [CrossRef]
- Sharma, H.C.; Ortiz, R. Host plant resistance to insects: An eco-friendly approach for pest management and environment conservation. J. Environ. Biol. 2002, 23, 111–135. [Google Scholar]
- Paul, M.; Mahla, J.S.; Upadhyay, D.K.; Das, D.; Wankhade, M.; Kumar, M.; Lallawmkimi, M.C. Integration of Genetic Resistance Mechanisms in Sustainable Crop Breeding Programs-A Review. J. Adv. Biol. Biotechnol. 2025, 28, 193–211. [Google Scholar] [CrossRef]
- Boiteux, L.S.; de Noronha Fonseca, M.E.; Vieira, J.V.; de Cássia Pereira-Carvalho, R. Breeding for resistance to viral diseases. In Plant Breeding for Biotic Stress Resistance; Springer: Berlin/Heidelberg, Germany, 2012; pp. 57–79. [Google Scholar]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Walkey, D.G. Control through resistant cultivars. In Applied Plant Virology; Springer: Dordrecht, The Netherlands, 1991; pp. 244–269. [Google Scholar] [CrossRef]
- Ntui, V.O.; Tripathi, J.N.; Kariuki, S.M.; Tripathi, L. Cassava molecular genetics and genomics for enhanced resistance to diseases and pests. Mol. Plant Pathol. 2024, 25, e13402. [Google Scholar] [CrossRef]
- Yuvashree, B.; Johnson, I.; Karthikeyan, M.; Anandham, R. Downy mildew of millets—An overview. Plant Sci. Today 2024, 11. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sinha, S. Natural and engineered resistance: Implications for managing the cassava mosaic disease. In Geminivirus: Detection, Diagnosis and Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–548. [Google Scholar]
- Agrawal, M.K.; Fageria, M.S.; Dhaka, R.S. Breeding for multiple disease resistance in vegetables: A review. Agric. Rev. 2000, 21, 125–128. [Google Scholar]
- Stuthman, D.D.; Leonard, K.J.; Miller-Garvin, J. Breeding crops for durable resistance to disease. Adv. Agron. 2007, 95, 319–367. [Google Scholar] [CrossRef]
- Wiesner-Hanks, T.; Nelson, R. Multiple disease resistance in plants. Annu. Rev. Phytopathol. 2016, 54, 229–252. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.P.; Caixeta, E.T.; Moreira, K.F.; de Oliveira, A.C.B.; de Freitas, K.N.P.; Pereira, A.A.; Rosado, R.D.S.; Zambolim, L.; Cruz, C.D. Marker-assisted pyramiding of multiple disease resistance genes in coffee genotypes (Coffea arabica). Agronomy 2021, 11, 1763. [Google Scholar] [CrossRef]
- Kumar, A.; Jindal, S.K.; Dhaliwal, M.S.; Sharma, A.; Kaur, S.; Jain, S. Development of multiple disease resistant tomato lines through marker assisted breeding and their evaluation for horticultural traits. Indian Phytopathol. 2022, 75, 47–55. [Google Scholar] [CrossRef]
- Tiwari, S.; Tomar, R.S.; Chand, S.; Singh, N.K. Combining multiple rust resistance genes by phenotypic and marker assisted selection in wheat (Triticum aestivum L.). Indian J. Genet. Plant Breed. 2014, 74, 181–188. [Google Scholar] [CrossRef]
- Milczarek, D.; Plich, J.; Tatarowska, B.; Flis, B. Early selection of potato clones with resistance genes: The relationship between combined resistance and agronomical characteristics. Breed. Sci. 2017, 67, 416–420. [Google Scholar] [CrossRef][Green Version]
- Motsnyi, I.I.; Molodchenkova, O.O.; Nargan, T.P.; Nakonechnyy, M.Y.; Mishchenko, I.A.; Lyfenko, S.P.; Mishchenko, L.T. Impact of Alien Genes on Disease Resistance, Drought Tolerance, and Agronomic Traits in Winter Wheat Commercial Varieties. Open Agric. J. 2022, 16. [Google Scholar] [CrossRef]
- Wind, J.J. Balancing trait improvement with tradeoff side-effects using genome editing technology. In A Roadmap for Plant Genome Editing; Springer Nature: Cham, Switzerland, 2024; pp. 69–77. [Google Scholar]
- Avelino, J.; Ten Hoopen, G.M.; DeClerck, F.A. Ecological mechanisms for pest and disease control in coffee and cacao agroecosystems of the Neotropics. In Ecosystem Services from Agriculture and Agroforestry; Routledge: Londen, UK, 2012; pp. 91–117. [Google Scholar]
- Djian-Caporalino, C.; Palloix, A.; Fazari, A.; Marteu, N.; Barbary, A.; Abad, P.; Sage-Palloix, A.M.; Mateille, T.; Risso, S.; Lanza, R.; et al. Pyramiding, alternating or mixing: Comparative performances of deployment strategies of nematode resistance genes to promote plant resistance efficiency and durability. BMC Plant Biol. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Jomanga, K.E.; Lucas, S.S.; Mgenzi, A.R.; Gaudence, M.; Kiurugo, R.F.S.; Biseko, E. Review on broken-down resistance to diseases and its management; the hidden challenge in breeding and production of banana and plantains in developing countries. Int. J. Curr. Sci. Res. Rev. 2022, 5, 3939–3953. [Google Scholar] [CrossRef]
- Taylor, N.P.; Cunniffe, N.J. Modelling quantitative fungicide resistance and breakdown of resistant cultivars: Designing integrated disease management strategies for Septoria of winter wheat. PLoS Comput. Biol. 2023, 19, e1010969. [Google Scholar] [CrossRef]
- Akem, C.; Ceccarelli, S.; Erskine, W.; Lenné, J. Using genetic diversity for disease resistance in agricultural production. Outlook Agric. 2000, 29, 25–30. [Google Scholar] [CrossRef]
- Chakravarthy, A.K.; Jose Luis, E.V.; Onkara Naik, S.; Rajkumar, B. Economic and ecological values of resistant plants. In Experimental Techniques in Host-Plant Resistance; Springer: Singapore, 2019; pp. 253–263. [Google Scholar]
- Vanloqueren, G.; Baret, P.V. Why are ecological, low-input, multi-resistant wheat cultivars slow to develop commercially? A Belgian agricultural ‘lock-in’ case study. Ecol. Econ. 2008, 66, 436–446. [Google Scholar] [CrossRef]
- van Hove, L.; Gillund, F. Is it only the regulatory status? Broadening the debate on cisgenic plants. Environ. Sci. Eur. 2017, 29, 22. [Google Scholar] [CrossRef]
- van Hove, L.; Gillund, F. Is It Only the Regulatory Status? Broadening the Debate on Cisgenic Plants. In Cisgenic Crops: Safety, Legal and Social Issues; Chaurasia, A., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 269–288. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. An overview of soil and soilless cultivation techniques—Chances, challenges and the neglected question of sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Gullino, M.L.; Gilardi, G.; Garibaldi, A. Emerging soilborne diseases of horticultural crops and new trends in their management. Acta Hortic. 2010, 883, 37–47. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Bihari, C.; Ahamad, S.; Kumar, M.; Kumar, A.; Kamboj, A.D.; Singh, S.; Srivastava, V.; Gautam, P. Innovative Soilless Culture Techniques for Horticultural Crops: A Comprehensive Review. Int. J. Environ. Clim. Change 2023, 13, 4071–4084. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a Circular Economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Postma, J. The status of biological control of plant diseases in soilless cultivation. In Recent Developments in Management of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–146. [Google Scholar] [CrossRef]
- Vallance, J.; Déniel, F.; Floch, G.; Guérin-Dubrana, L.; Blancard, D.; Rey, P. Pathogenic and beneficial microorganisms in soilless cultures. Agron. Sustain. Dev. 2011, 31, 191–203. [Google Scholar] [CrossRef]
- Calvo-Bado, L.A.; Petch, G.; Parsons, N.R.; Morgan, J.A.W.; Pettitt, T.R.; Whipps, J.M. Microbial community responses associated with the development of oomycete plant pathogens on tomato roots in soilless growing systems. J. Appl. Microbiol. 2006, 100, 1194–1207. [Google Scholar] [CrossRef]
- Schnitzler, W. Pest and disease management of soilless culture. Acta Hortic. 2004, 648, 191–203. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- van Os, E.A.; Postma, J.; Pettitt, T.; Wohanka, W. Microbial optimisation in soilless cultivation, a replacement for methyl bromide. Acta Hortic. 2004, 635, 47–58. [Google Scholar] [CrossRef]
- Minuto, A.; Gullino, M.L.; Garibaldi, A. Disinfection of nutrient solution in closed soilless systems: Results in Italy. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, San Diego, CA, USA, 3–6 November 2003; pp. 37-1–37-4. [Google Scholar]
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. Agric. Eng. Int. Cigr J. Sci. Res. Dev. 2003, 1, 80–86. [Google Scholar]
- Khan, P.; Bora, L.C.; Borah, P.K.; Bora, P.; Talukdar, K. Efficacy of microbial consortia against bacterial wilt caused by Ralstonia solanacearum in hydroponically grown lettuce plant. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3046–3055. [Google Scholar] [CrossRef]
- Arumugam, T.; Sandeep, G.; Maheswari, M.U. Soilless farming of vegetable crops: An overview. Pharma Innov. J. 2021, 10, 773–785. [Google Scholar]
- Rajesh, E.; Basheer, S.; Baskar, K. Hydroponics soilless smart farming in improving productivity of crop using Intelligent Smart Systems. In Proceedings of the 2023 3rd International Conference on Innovative Practices in Technology and Management (ICIPTM), Uttar Pradesh, India, 22–24 February 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Wohanka, W. Root zone microbiology of soilless cropping systems. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–194. [Google Scholar]
- Feng, W.; Nukaya, A.; Satou, M.; Fukuta, N.; Ishiguro, Y.; Suga, H.; Kageyama, K. Use of LAMP detection to identify potential contamination sources of plant-pathogenic Pythium species in hydroponic culture systems of tomato and eustoma. Plant Dis. 2018, 102, 1357–1364. [Google Scholar] [CrossRef]
- Van Os, E.A. Disease Management IN Soilless Culture Systems. Acta Hortic. 2010, 385–393. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, Á. Optimization of nutrition in soilless systems: A review. Adv. Bot. Res. 2010, 53, 193–245. [Google Scholar] [CrossRef]
- Postma, J.; Os, E.V.; Bonants, P.J.M. Pathogen Detection and Management Strategies in Soilless Plant Growing Systems; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Zheng, Y. Advances in Understanding Plant Root Behaviour and Rootzone Management in Soilless Culture Systems; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 23–44. [Google Scholar] [CrossRef]
- Ally, N.M.; Neetoo, H.; Ranghoo-Sanmukhiya, V.M.; Coutinho, T.A. Greenhouse-grown tomatoes: Microbial diseases and their control methods: A review. Int. J. Phytopathol. 2023, 12, 99–127. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Gullino, M.L. Effect of potassium silicate and electrical conductivity in reducing powdery mildew of hydroponically grown tomato. Phytopathol. Mediterr. 2011, 50, 192–202. [Google Scholar]
- Gamliel, A. Soil and substrate health. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–383. [Google Scholar] [CrossRef]
- McGovern, R.J.; McSorley, R. Physical methods of soil sterilization for disease management including soil solarization. In Environmentally Safe Approaches to Crop Disease Control; CRC Press: Boca Raton, FL, USA, 2018; pp. 283–314. [Google Scholar]
- Cuervo, B.; Flórez, W.J.; González, V.J. Aspects to consider for optimizing a substrate culture system with drainage recycling. Agron. Colomb. 2012, 30, 379–387. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Pandey, K. Nutrient Management Strategies for Water and Nutrient Saving in Substrate Soilless Culture Under Protected Cultivation. In Artificial Intelligence and Smart Agriculture: Technology and Applications; Springer Nature: Singapore, 2024; pp. 369–386. [Google Scholar] [CrossRef]
- Voogt, W.; Bar-Yosef, B. Water and nutrient management and crops response to nutrient solution recycling in soilless growing systems in greenhouses. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–507. [Google Scholar]
- Guy, O.; Dai, N.; Cohen, S.; Bustan, A. Winter Strawberries Production in Greenhouse Soilless Culture under an Arid Climate–Cultivars, Phenology, Physiology, and Consequent Practices. In Recent Studies on Strawberries; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Jensen, M.H. Controlled Environment agriculture in deserts, tropics and temperate regions—A World Review. In Proceedings of the International Symposium on Design and Environmental Control of Tropical and Subtropical Greenhouses, Taichung, Taiwan, 15–18 April 2001; Volume 578, pp. 19–25. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M. Controlled environment ecosystem: A plant growth system to combat climate change through soilless culture. Crop Des. 2024, 3, 100044. [Google Scholar] [CrossRef]
- Wilkinson, A.; Gerlach, C.; Karlsson, M.; Penn, H. Controlled environment agriculture and containerized food production in northern North America. J. Agric. Food Syst. Community Dev. 2021, 10, 127–142. [Google Scholar] [CrossRef]
- Conner, D.S.; Christy, R.D. Consumer preferences for organic standards: Guiding demand-expansion strategies for organic food. J. Food Distrib. Res. 2002, 33, 46–51. [Google Scholar]
- Fahlevi, M.; Dandi, M.; Matroji, F.J.; Asetya, D.R. How Do Consumer Awareness, Health Consciousness, and Environmental Concern Drive the Willingness to Buy Hydroponic Vegetables? Proc. Iop Conf. Ser. Earth Environ. Sci. 2024, 1324, 012131. [Google Scholar] [CrossRef]
- Martini, M.; Fedi, A.; Murphy, B.; Dean, M.; Loera, B. More than organic: Consumer expectations of sustainability and quality. Evidences from a qualitative study in Italy. J. Food Prod. Mark. 2024, 30, 1–15. [Google Scholar] [CrossRef]
- Spendrup, S.; Bergstrand, K.J.; Thörning, R.; Hultberg, M. Consumer attitudes towards hydroponic cultivation of vegetables–Specifically exploring the impact of the fertilisation strategy (using mineral origin or food waste as fertilisers). Food Qual. Prefer. 2024, 113, 105085. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pool, J.A.; Calderón-Pérez, B.; Ruiz-Medrano, R.; Ortiz-Castro, R.; Xoconostle-Cazares, B. Bacillus strains as effective biocontrol agents against phytopathogenic bacteria and promoters of plant growth. Microb. Ecol. 2024, 87, 76. [Google Scholar] [CrossRef]
- Pellegrini, M.; Djebaili, R.; Pagnani, G.; Spera, D.M.; Del Gallo, M. Plant growth-promoting bacterial consortia render biological control of plant pathogens: A review. In Microorganisms for Sustainability; Springer Nature: Singapore, 2023; pp. 57–74. [Google Scholar]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting bacterial genera as biocontrol agents: Mechanisms, interactions and applications in sustainable agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Petkova, M.; Marcheva, M.; Petrova, A.L.; Slavova, V.; Shilev, S. Plant growth-promoting and biocontrol characteristics of four Bacillus strains and evaluation of their effects on wheat (Tr. aestivum L.). Int. J. Plant Biol. 2024, 16, 1. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant growth-promoting activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato and taro pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Ursan, M.; Boiu-Sicuia, O.A.; Voaides, C.; Stan, V.; Bubueanu, C.; Cornea, C.P. The potential of new Streptomyces isolates as biocontrol agents against Fusarium spp. In Proceedings of the “Agriculture for Life, Life for Agriculture” Conference Proceedings, Bucharest, Romania, 7–9 June 2018; Volume 1, pp. 594–600. [Google Scholar]
- Alharbi, A.A. Efficacy of the bacterium Lysobacter enzymogenes strain ch3B10 as a new biocontrol agent on the pathogenic fungi Alternaria solani and Fusarium oxysporum under laboratory conditions. Egypt. J. Biol. Pest Control 2022, 32, 70. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Sarrocco, S. Biological disease control by beneficial (micro) organisms: Selected breakthroughs in the past 50 years. Phytopathology 2023, 113, 732–740. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Ruan, S.; Nzabanita, C.; Wang, Y.; Guo, L. A mycovirus VIGS vector confers hypovirulence to a plant pathogenic fungus to control wheat FHB. Adv. Sci. 2023, 10, e2302606. [Google Scholar] [CrossRef]
- Bilgili, A. The effectiveness of arbuscular mycorrhizal fungal species (Funneliformis mosseae, Rhizophagus intraradices, and Claroideoglomus etunicatum) in the biocontrol of root and crown rot pathogens, Fusarium solani and Fusarium mixture in pepper. PeerJ 2025, 13, e18438. [Google Scholar] [CrossRef] [PubMed]
- Ravensberg, W.J. Registration of microbial pest control agents and products and other related regulations. In A Roadmap to the Successful Development and Commercialization of Microbial Pest Control Products for Control of Arthropods; Springer: Dordrecht, The Netherlands, 2011; pp. 171–233. [Google Scholar]
- Veerabhadraswamy, A.L.; Garampalli, R.H. Effect of arbuscular mycorrhizal fungi in the management of black bundle disease of maize caused by Cephalosporium acremonium. Sci. Res. Report. 2011, 1, 96–100. [Google Scholar]
- Zampieri, E.; Cucu, M.A.; Franchi, E.; Fusini, D.; Pietrini, I.; Centritto, M.; Balestrini, R. Characterization of Different Soil Bacterial Strains and Assessment of Their Impact on the Growth of Triticum turgidum spp. durum and Lens culinaris spp. culinaris. Curr. Microbiol. 2025, 82, 199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Khan, M.H.; Yuan, Z.; Hussain, S.; Cao, H.; Liu, Y. Response of soil microbiome structure and its network profiles to four soil amendments in monocropping strawberry greenhouse. PLoS ONE 2021, 16, e0245180. [Google Scholar] [CrossRef] [PubMed]
- Tomada, S. Underexplored Microbial Species in the Pipeline for the Development of Biopesticides. In Microbial Biocontrol Agents: Developing Effective Biopesticides; CABI: Wallingford, UK, 2022; pp. 202–225. [Google Scholar]
- Haritha, D.; Faiz Ahmed, M.; Bala, S.; Choudhury, D. Eco-friendly plant based on botanical pesticides. Plant Arch. 2021, 21, 2197–2204. [Google Scholar] [CrossRef]
- Lone, S.A.; Malik, A.; Padaria, J.C. Applications of Bacillus thuringiensis for Prevention of Environmental Deterioration. In Environmental Deterioration and Human Health; Springer: Dordrecht, Tthe Netherlands, 2014; pp. 73–95. [Google Scholar]
- Gašparovski, J. Effect of microbial biocontrol agents for plant diseases on soil microbiome. Biljn. Lek. 2021, 49, 170–177. [Google Scholar] [CrossRef]
- Patil, P.; Behera, S.K.; Raghu, S.; Annamalai, M. Biological control of insect pests in vegetable crops: An Eco-friendly approach. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1358–1373. [Google Scholar] [CrossRef]
- Vlaiculescu, A.; Varrone, C. Sustainable and eco-friendly alternatives to reduce the use of pesticides. In Pesticides in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–364. [Google Scholar]
- Hannusch, D.J.; Boland, G.J. Interactions of air temperature, relative humidity and biological control agents on grey mold of bean. Eur. J. Plant Pathol. 1996, 102, 133–142. [Google Scholar] [CrossRef]
- Fedele, G.; González-Domínguez, E.; Rossi, V. Influence of environment on the biocontrol of Botrytis cinerea: A systematic literature review. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–82. [Google Scholar]
- Bode, R.F.; Cervantez, O. Weather patterns determine success rates of two biocontrol agents on Cytisus scoparius in the USA. Entomol. Exp. Appl. 2024, 172, 1024–1032. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A. Climate change and resilience of biological control agents. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–93. [Google Scholar]
- Magan, N. Importance of ecological windows for efficacy of biocontrol agents. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–14. [Google Scholar]
- Cray, J.A.; Connor, M.C.; Stevenson, A.; Houghton, J.D.R.; Rangel, D.E.N.; Cooke, L.R.; Hallsworth, J.E. Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb. Biotechnol. 2016, 9, 330–354. [Google Scholar] [CrossRef]
- Xu, X.M.; Jeger, M.J. Combined use of two biocontrol agents with different biocontrol mechanisms most likely results in less than expected efficacy in controlling foliar pathogens under fluctuating conditions: A modeling study. Phytopathology 2013, 103, 108–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimopoulou, A.; Theologidis, I.; Varympopi, A.; Papafotis, D.; Mermigka, G.; Tzima, A.; Panopoulos, N.J.; Skandalis, N. Shifting perspectives of translational research in bio-bactericides: Reviewing the Bacillus amyloliquefaciens paradigm. Biology 2021, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Elango, S.; Shahni, Y.S.; Padamini, R.; Hazarika, S.; Wongamthing, R.; Oraon, S.; Panigrahi, C.K.; Kumar, A.; Thangaraj, R. Harnessing microbial antagonists for biological control of plant pathogens: A global perspective. Microbiol. Res. J. Int. 2024, 34, 1–17. [Google Scholar] [CrossRef]
- Gerbore, J.; Benhamou, N.; Vallance, J.; Le Floch, G.; Grizard, D.; Regnault-Roger, C.; Rey, P. Biological control of plant pathogens: Advantages and limitations seen through the case study of Pythium oligandrum. Environ. Sci. Pollut. Res. Int. 2014, 21, 4847–4860. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, P. Mechanisms of action of bacterial biological control agents. In Biological Management of Diseases of Crops; Springer: Dordrecht, The Netherlands, 2013; pp. 295–429. [Google Scholar]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Montoya-Martínez, A.C.; Ruiz, V.V.; Chávez-Luzanía, R.A.; Villa-Rodríguez, E.D.; Villalobos, S.d.L.S. Biological control agents for mitigating plant diseases. In New Insights, Trends, and Challenges in the Development and Applications of Microbial Inoculants in Agriculture; Elsevier: Amsterdam, The Netherlands, 2024; pp. 27–35. [Google Scholar]
- Dan Jensen, F.; Lumsden, R.D. Biological control of soilborne pathogens. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 319–337. [Google Scholar]
- Iftikhar, Y.; Sajid, A.; Shakeel, Q.; Ahmad, Z.; Ul Haq, Z. Biological antagonism: A safe and sustainable way to manage plant diseases. In Sustainability in Plant and Crop Protection; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–109. [Google Scholar]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Angelopoulou, D.J.; Naska, E.J.; Paplomatas, E.J.; Tjamos, S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014, 63, 1062–1069. [Google Scholar] [CrossRef]
- Alderley, C.L.; Greenrod, S.T.E.; Friman, V.P. Plant pathogenic bacterium can rapidly evolve tolerance to an antimicrobial plant allelochemical. Evol. Appl. 2022, 15, 735–750. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Clough, S.E.; Elphinstone, J.G.; Friman, V.P. Plant pathogenic bacterium Ralstonia solanacearum can rapidly evolve tolerance to antimicrobials produced by Pseudomonas biocontrol bacteria. J. Evol. Biol. 2024, 37, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I.; García-Arenal, F. Tolerance to plant pathogens: Theory and experimental evidence. Int. J. Mol. Sci. 2018, 19, 810. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Matić, S.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Influence of different biological control agents and compost on total and nitrification-driven microbial communities at rhizosphere and soil level in a lettuce - Fusarium oxysporum f. sp. lactucae pathosystem. J. Appl. Microbiol. 2019, 126, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Ferrocino, I.; Gullino, M.L. Effects of biocontrol agents and compost against the Phytophthora capsici of zucchini and their impact on the rhizosphere microbiota. Appl. Soil Ecol. 2020, 154, 103659. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukherjee, A.; Ganguli, S.; Chakraborti, A.; Roy, S.; Choudhury, S.S.; Subramaniyan, V.; Kumarasamy, V.; Sayed, A.A.; El-Demerdash, F.M.; et al. Marvels of Bacilli in soil amendment for plant-growth promotion toward sustainable development having futuristic socio-economic implications. Front. Microbiol. 2023, 14, 1293302. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bharat, N.; Thakur, A. Role of Plant Growth-Promoting Rhizobacteria (PGPR) and Bio-Control Agents (BCAs) in Crop Production. Int. J. Econ. Plants 2020, 7, 144–150. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Gaur, A.; Bhatti, L.; Khatak, S. Interaction between Plants and Microorganisms: Microbial Biological Control Agents against Plant Pathogens. Res. J. Biotechnol. 2023, 18, 134–144. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Diyarza-Sandoval, N.A.; Guevara-Avendaño, E.; Meza-Contreras, J.J.; Reverchon, F. Plant growth-promoting microorganisms from native plants: An untapped resource of biocontrol and biofertilizer agents. In Biocontrol Agents for Improved Agriculture; Academic Press: Cambridge, MA, USA, 2024; pp. 29–66. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef]
- Joshi, M.D.; Srivastava, A.K.; Ashaq, M.; Jaggi, S.; Gupta, P.; Hasan, W.; Gupta, S. Biocontrol Agents and Plant Protection. Uttar Pradesh J. Zool. 2024, 45, 109–131. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Bacillus thuringiensis based biopesticides for integrated crop management. In Biopesticides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–6. [Google Scholar]
- Tiwari, A.K. Advances in biological control strategies for sustainable pest management. Uttar Pradesh J. Zool. 2024, 45, 214–232. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Tjamos, S.E.; Antoniou, P.P. Biological management of plant diseases: Highlights on research and application. J. Plant Pathol. 2010, S17–S21. [Google Scholar]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Gusain, Y.S.; Kumar, V.; Sharma, A.K. Disease management through biological control agents: An eco-friendly and cost effective approach for sustainable agriculture-A Review. Agric. Rev. 2015, 36, 37. [Google Scholar] [CrossRef]
- Wong, C.K.F. Bioencapsulation of biocontrol agents as a management strategy for plant pathogens. In Microorganisms for Sustainability; Springer Nature: Singapore, 2023; pp. 339–358. [Google Scholar]
- Slininger, P.J.; Schisler, D.A. Biological control agents for suppression of post-harvest diseases of potatoes: Strategies on discovery and development. In Fungicides for Plant and Animal Diseases; InTech: London, UK, 2012; pp. 141–166. [Google Scholar] [CrossRef]
- Lange, L. Microbes and Microbial Products in Plant Protection. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 1992; pp. 252–270. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar]
- Gómez-Ramos, M.D.M.; Nannou, C.; Martínez Bueno, M.J.; Goday, A.; Murcia-Morales, M.; Ferrer, C.; Fernández-Alba, A.R. Pesticide residues evaluation of organic crops. A critical appraisal. Food Chem. X 2020, 5, 100079. [Google Scholar] [CrossRef]
- Walker, K.; Mendelsohn, M.; Matten, S.; Alphin, M.; Ave, D. The Role of Microbial Bt Products in U.S. Crop Protection. J. New Seeds 2003, 5, 31–51. [Google Scholar] [CrossRef]
- Lovell-Read, F.A.; Parnell, S.; Cunniffe, N.J.; Thompson, R.N. Using ’sentinel’ plants to improve early detection of invasive plant pathogens. PLoS Comput. Biol. 2023, 19, e1010884. [Google Scholar] [CrossRef]
- Pesti, R.; Kontra, L.; Paul, K.; Vass, I.; Csorba, T.; Havelda, Z.; Várallyay, É. Differential gene expression and physiological changes during acute or persistent plant virus interactions may contribute to viral symptom differences. PLoS ONE 2019, 14, e0216618. [Google Scholar] [CrossRef]
- Ward, M. Action against pest spread—The case for retrospective analysis with a focus on timing. Food Secur. 2016, 8, 77–81. [Google Scholar] [CrossRef]
- Marchand, P. The regulatory obstacles to bio control agents from Directive (EC) No 128/2009. Regsci 2024, 12. [Google Scholar] [CrossRef]
- Kiewnick, S. Practicalities of developing and registering microbial biologicalcontrol agents. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2007, 11. [Google Scholar] [CrossRef]
- Stewart, A. Commercial biocontrol—Reality or fantasy? Australas. Plant Pathol. 2001, 30, 127–131. [Google Scholar] [CrossRef]
- Strauch, O.; Strasser, H.; Hauschild, R.; Ehlers, R.U. Proposals for bacterial and fungal biocontrol agents. In Regulation of Biological Control Agents; Springer: Dordrecht, The Netherlands, 2011; pp. 267–288. [Google Scholar] [CrossRef]
- Das, M.M.; Abdulhameed, S. Agro-processing residues for the production of fungal bio-control agents. In Applied Environmental Science and Engineering for a Sustainable Future; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–126. [Google Scholar]
- Hao, Y.; Pan, X.; You, J.; Li, G.; Xu, M.; Rao, Z. Microbial production of branched chain amino acids: Advances and perspectives. Bioresour. Technol. 2024, 397, 130502. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Haridas, M.; Leza, H.; Abdulhameed, S. Customization of Bioreactor Technology for On-Site Production of Biocontrol Agents; Academic Press: Cambridge, MA, USA, 2022; pp. 23–43. [Google Scholar] [CrossRef]
- Ramanujam, B.; Prasad, R.D.; Sriram, S.; Rangeswaran, R. Mass production, formulation, quality control and delivery of Trichoderma for plant disease management. J. Plant Prot. Sci. 2010, 2, 1–8. [Google Scholar]
- Fravel, D.R.; Rhodes, D.J.; Larkin, R.P. Production and commercialization of biocontrol products. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 365–376. [Google Scholar]
- García-Bayona, L.; Comstock, L. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Jackson, M.A.; Behle, R.W.; Kobori, N.N.; Júnior, Í.D. Improved shelf life of dried Beauveria bassiana blastospores using convective drying and active packaging processes. Appl. Microbiol. Biotechnol. 2016, 100, 8359–8370. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Fulwider, V.M.; Duong, A.; Duffy, M.; Cafmeyer, J.; Ducceschi, S.; Hinton, C. Assessment of long-term stability of encapsulated agricultural biologicals in lipid-coated alginate beads. ACS Agric. Sci. Technol. 2023, 3, 389–398. [Google Scholar] [CrossRef]
- Llorens, E.; Agustí-Brisach, C. Biocontrol of plant diseases by means of antagonist microorganisms, biostimulants and induced resistance as alternatives to chemicals. Plants 2022, 11, 3521. [Google Scholar] [CrossRef]
- Fravel, D.R.; Keinath, A.P. Biocontrol of soilborne plant pathogens with fungi. In The Rhizosphere and Plant Growth; Springer: Dordrecht, The Netherlands, 1991; pp. 237–243. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, W. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, R.K.S.; Nirmalkar, V.K.; Dayasagar; Singh, Y. Ecological niches of Bacillus species and their multifaceted mechanisms in combatting plant pathogens. J. Sci. Res. Rep. 2024, 30, 514–525. [Google Scholar] [CrossRef]
- Fedele, G.; Bove, F.; González-Domínguez, E.; Rossi, V. A generic model accounting for the interactions among pathogens, host plants, biocontrol agents, and the environment, with parametrization for Botrytis cinerea on grapevines. Agronomy 2020, 10, 222. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. ECOLOGY. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Srivastava, R.; Khalid, A.; Singh, U.S.; Sharma, A.K. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control 2010, 53, 24–31. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world - bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C.; Graber, E.R. Biochar effects on the abundance, activity and diversity of the soil biota. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 327–389. [Google Scholar]
- El-Masry, M.; Khalil, A.; Hassouna, M.; Ibrahim, H. In situ and in vivo suppressiveeffect of agricultural composts and their water extracts on some phytopathogenic fungi. World J. Microbiol. Biotechnol. 2002, 18, 551–558. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Omokaro, G.O.; Osarhiemen, I.O.; Idama, V.; Airueghian, E.O.; West, S.T.; Igbigbi, F.E.; Nnake, D.C.; Obolokor, E.; Ahmed, A.; Omoshie, V.O. The role of organic amendments and their impact on soil restoration: A review. Asian J. Environ. Ecol. 2024, 23, 41–52. [Google Scholar] [CrossRef]
- Singh, S.; Devi, N.B.; Divakaran, D.; Kumar, A.; Singh, S.; Tyagi, G.; Nath, A.; Kumar, A. Soil fertility management: Role of organic amendments and bio-fertilizers: A review. Int. J. Res. Agron. 2024, 7, 766–772. [Google Scholar] [CrossRef]
- Lazarovits, G. Management of soil-borne plant pathogens with organic soil amendments: A disease control strategy salvaged from the past. Can. J. Plant Pathol. 2001, 23, 1–7. [Google Scholar] [CrossRef]
- Bellini, A.; Ferrocino, I.; Cucu, M.A.; Pugliese, M.; Garibaldi, A.; Gullino, M.L. A Compost Treatment Acts as a Suppressive Agent in Phytophthora capsici–Cucurbita pepo Pathosystem by Modifying the Rhizosphere Microbiota. Front. Plant Sci. 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, A.O.; Babalola, O.O.; Venturi, V.; Ayilara, M.S.; Adeleke, B.S.; Amoo, A.E.; Sobowale, A.A.; Fadiji, A.E.; Glick, B.R. Plant disease management: Leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 2021, 12, 700507. [Google Scholar] [CrossRef]
- Andreo-Jimenez, B.; Schilder, M.T.; Nijhuis, E.H.; Te Beest, D.E.; Bloem, J.; Visser, J.H.M.; van Os, G.; Brolsma, K.; de Boer, W.; Postma, J. Chitin- and keratin-rich soil amendments suppress Rhizoctonia solani disease via changes to the soil microbial community. Appl. Environ. Microbiol. 2021, 87, e00318-21. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. NPJ Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao Salles, J. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Yao, H. Biochar amendment stimulates utilization of plant-derived carbon by soil bacteria in an intercropping system. Front. Microbiol. 2019, 10, 1361. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–379. [Google Scholar]
- Hussain, M.I.; Khan, Z.I.; Akhter, P.; Al-Hemaid, F.M.; Al-Hashimi, A.; Elshikh, M.S.; Ahmad, K.; Yang, H.H. Potential of organic amendments for heavy metal contamination in soil–coriander system: Environmental fate and associated ecological risk. Sustainability 2022, 14, 11374. [Google Scholar] [CrossRef]
- Obriot, F.; Stauffer, M.; Goubard Delaunay, Y.; Cheviron, N.; Peres, G.; Eden, M.; Revallier, A.; Vieublé, L.; Houot, S. Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agric. Ecosyst. Environ. 2016, 232, 165–178. [Google Scholar] [CrossRef]
- Rosa, D.; Petruccelli, V.; Iacobbi, M.C.; Brasili, E.; Badiali, C.; Pasqua, G.; Di Palma, L. Functionalized biochar from waste as a slow-release nutrient source: Application on tomato plants. Heliyon 2024, 10, e29455. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Neeraj, A.; Siddiqui, M.A.; Singh, V. Nutrient management technologies and the role of organic matrix-based slow-release biofertilizers for agricultural sustainability: A review. Agric. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Neethu, C.B.; Vardhanan, Y.S. Development of slow-release fertilizer from animal origin wastes: Sustainable organic agriculural perspective. Curr. Agric. Res. J. 2023, 11, 69–77. [Google Scholar] [CrossRef]
- Noble, R. Risks and benefits of soil amendment with composts in relation to plant pathogens. Australas. Plant Pathol. 2011, 40, 157–167. [Google Scholar] [CrossRef]
- Dey, A.; Srivastava, P.C.; Pachauri, S.P.; Shukla, A.K. Release kinetics of some nutrients from a sandy loam soil treated with different organic amendments. Commun. Soil Sci. Plant Anal. 2019, 50, 2718–2732. [Google Scholar] [CrossRef]
- Rosskopf, E.; Gioia, F.; Hong, J.; Pisani, C.; Kokalis-Burelle, N. Organic amendments for pathogen and nematode control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, N.; Gutiérrez-Barranquero, J.; Vicente, A.; Cazorla, F. Enhancing soil quality and plant health through suppressive organic amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic amendments modulate soil Microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef]
- Pugliese, M.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. Organic amendments and soil suppressiveness: Results with vegetable and ornamental crops. In Soil Biology; Springer International Publishing: Cham, Switzerland, 2015; pp. 495–509. [Google Scholar]
- Sloot, M.; Maerowitz-McMahan, S.; Postma, J.; Limpens, J.; De Deyn, G.B. Soil-borne disease suppressiveness after short and long term application of fermented, composted or fresh organic amendment treatments in arable soils. Appl. Soil Ecol. 2024, 195, 105268. [Google Scholar] [CrossRef]
- Senesi, N.; Plaza, C. Role of Humification Processes in Recycling Organic Wastes of Various Nature and Sources as Soil Amendments. Clean-Soil Air Water 2007, 35, 26–41. [Google Scholar] [CrossRef]
- Calleja-Cervantes, M.E.; Fernández-González, A.J.; Irigoyen, I.; Fernández-López, M.; Aparicio-Tejo, P.M.; Menéndez, S. Thirteen years of continued application of composted organic wastes in a vineyard modify soil quality characteristics. Soil Biol. Biochem. 2015, 90, 241–254. [Google Scholar] [CrossRef]
- Brichi, L.; Fernandes, J.V.M.; Silva, B.M.; Vizú, J.d.F.; Junior, J.N.G.; Cherubin, M.R. Organic residues and their impact on soil health, crop production and sustainable agriculture: A review including bibliographic analysis. Soil Use Manag. 2023, 39, 686–706. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, G.; Rashid, M.S.; Zhang, J.; Lin, H.; Shu, Y. Effects of BC on metal uptake by crops (availability) and the vertical migration behavior in soil: A 3-year field experiments of crop rotation. Chemosphere 2024, 350, 141075. [Google Scholar] [CrossRef] [PubMed]
- Devi, G. Impact of organic amendments on Entomopathogenic nematodes: An overview. Int. J. Plant Soil Sci. 2024, 36, 461–467. [Google Scholar] [CrossRef]
- Reddy, P.P. Crop residue management and organic amendments. In Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Singapore, 2017; pp. 29–41. [Google Scholar]
- Usman, A.; Siddiqui, M.A. Integrated management of phytonematodes by the application of organic amendment and ploughing under field conditions. Arch. Phytopathol. Plant Prot. 2012, 45, 2437–2444. [Google Scholar] [CrossRef]
- Bollen, G.J.; Volker, D.; Wijnen, A.P. Inactivation of soil-borne plant pathogens during small-scale composting of crop residues. Neth. J. Plant Pathol. 1989, 95, 19–30. [Google Scholar] [CrossRef]
- Manga, M.; Muoghalu, C.C.; Acheng, P.O. Inactivation of faecal pathogens during faecal sludge composting: A systematic review. Environ. Technol. Rev. 2023, 12, 150–174. [Google Scholar] [CrossRef]
- Logo, A.; Boppré, B.; Fuchs, J.; Maurhofer, M.; Oberhänsli, T.; Thürig, B.; Widmer, F.; Flury, P.; Mayerhofer, J. Comprehensive analysis of 37 composts: Microbial indicators for soilborne disease suppression in three plant-pathogen systems. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zaccardelli, M.; De Nicola, F.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant Nutr. 2013, 13, 730–742. [Google Scholar] [CrossRef]
- Bezanson, G.S.; Ells, T.C.; Prange, R.K. Effect of composting on microbial contamination and quality of fresh fruits and vegetables—A mini-review. Acta Hortic. 2014, 1018, 631–638. [Google Scholar] [CrossRef]
- Churchill, D.B.; Alderman, S.C.; Mueller-Warrant, G.W.; Elliott, L.F.; Bilsland, D.M. Survival of weed seeds and seed pathogen propagates in composted grass seed straw. Appl. Eng. Agric. 1995, 12, 57–63. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Grebus, M.E. Status of biological control of plant diseases with composts. Compost Sci. Util. 1994, 2, 6–12. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Dunseith, P. Compost for management of weed seeds, pathogen, and early blight on brassicas in organic farmer fields. Agroecol. Sustain. Food Syst. 2015, 39, 3–18. [Google Scholar] [CrossRef]
- Vinnerås, B.; Agostini, F.; Jönsson, H. Sanitation by composting. In Microbes at Work; Springer: Berlin/Heidelberg, Germany, 2010; pp. 171–191. [Google Scholar]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease suppressive soils: New insights from the soil microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Aioub, A.A.A.; Ghosh, S.; AL-Farga, A.; Khan, A.N.; Bibi, R.; Elwakeel, A.M.; Nawaz, A.; Sherif, N.T.; Elmasry, S.A.; Ammar, E.E. Back to the origins: Biopesticides as promising alternatives to conventional agrochemicals. Eur. J. Plant Pathol. 2024, 169, 697–713. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Cernava, T.; Abdelfattah, A.; Gokul, J.; Korsten, L.; Berg, G. Microbiome approaches provide the key to biologically control postharvest pathogens and storability of fruits and vegetables. FEMS Microbiol. Ecol. 2020, 96. [Google Scholar] [CrossRef]
- Rivas-García, T.; González-Estrada, R.; Chiquito-Contreras, R.; Reyes-Pérez, J.; González-Salas, U.; Hernández-Montiel, L.; Murillo-Amador, B. Biocontrol of phytopathogens under aquaponics systems. Water 2020, 12, 2061. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Charkowski, A.; Sharma, K.; Parker, M.; Secor, G.; Elphinstone, J. Bacterial Diseases of Potato. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Puig-Sirera, A.; Provenzano, G.; González-Altozano, P.; Intrigliolo, D.; Rallo, G. Irrigation water saving strategies in Citrus orchards: Analysis of the combined effects of timing and severity of soil water deficit. Agric. Water Manag. 2021, 248, 106773. [Google Scholar] [CrossRef]
- Imbernón-Mulero, A.; Maestre-Valero, J.; Martínez-Alvarez, V.; García-García, F.; Jódar-Conesa, F.; Gallego-Elvira, B. Evaluation of an autonomous smart system for optimal management of fertigation with variable sources of irrigation water. Front. Plant Sci. 2023, 14, 1149956. [Google Scholar] [CrossRef]
- Maurya, S.; Dubey, S.; Kumari, R.; Verma, R. Management tactics for fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici (Sacc.): A review. Management 2019, 4, 1–7. [Google Scholar]
- Kulkarni, S. Sustainable Soil Conservation and Management: Principles, Issues, and Strategies. In Field Practices for Wastewater Use in Agriculture; Apple Academic Press: Cambridge, MA, USA, 2020; pp. 133–178. [Google Scholar]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Probiotic Bacteria, Anaerobic Soil Disinfestation, and Mustard Cover Crop Biofumigation Suppress Soilborne Disease and Increase Yield of Strawberry in a Perennial Organic Production System. Plant Dis. 2023, 107, 2490–2499. [Google Scholar] [CrossRef]
- Msimbira, L.; Smith, D. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Growth stimulation of two legumes (Vicia faba and Pisum sativum) using phosphate-solubilizing bacteria inoculation. Front. Microbiol. 2023, 14, 1212702. [Google Scholar] [CrossRef] [PubMed]
- Sommermann, L.; Babin, D.; Behr, J.; Chowdhury, S.; Sandmann, M.; Windisch, S.; Grosch, R. Long-term fertilization strategy impacts Rhizoctonia solani–microbe interactions in soil and rhizosphere and defense responses in lettuce. Microorganisms 2022, 10, 1717. [Google Scholar] [CrossRef]
- Geoffrey, R. Pests and pathogens. Veg. Brassicas Relat. Crucif. 2024, 40, 295. [Google Scholar]
- Pahalvi, H.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A. Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers; Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar]
- Qi, J.; Fu, D.; Wang, X.; Zhang, F.; Ma, C. The effect of alfalfa cultivation on improving physicochemical properties of soil microorganisms community structure of grey desert soil. Sci. Rep. 2023, 13, 13747. [Google Scholar] [CrossRef]
- Chai, A.; Yuan, L.; Li, X.; Li, L.; Shi, Y.; Xie, X.; Li, B. Effect of temperature and humidity on dynamics and transmission of Pseudomonas amygdali pv. lachrymans aerosols. Front. Plant Sci. 2023, 14, 1087496. [Google Scholar] [CrossRef]
- Gachomo, E.; Jimenez-Lopez, J.; Kayodé, A.; Baba-Moussa, L.; Kotchoni, S. Control of Major Diseases in Horticulture. In Fungicides for Plant and Animal Diseases; IntechOpen: London, UK, 2012. [Google Scholar]
- Kuroyanagi, T.; Yoshikoshi, H.; Kinoshita, T.; Kawashima, H. Use of air circulation to reduce wet leaves under high humidity conditions. Environ. Control Biol. 2014, 51, 215–220. [Google Scholar] [CrossRef]
- Autio, W.; Cooley, D. Summer pruning of apple: Impacts on disease management. Adv. Hortic. Sci. 2011, 25, 199–204. [Google Scholar]
- Daunde, A.; Baghele, R.; Khandare, V. Management of prevalent diseases of cucumber (Cucumis sativus) through integrated approach. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3022–3028. [Google Scholar] [CrossRef]
- Lecoq, L.; Flick, D.; Laguerre, O. Study of the water evaporation rate on stainless steel plate in controlled conditions. Int. J. Therm. Sci. 2017, 111, 450–462. [Google Scholar] [CrossRef]
- Baptista, F.; Bailey, B.; Meneses, J. Development of a warning system for controlling Botrytis cinerea in unheated tomato greenhouses. In Proceedings of the International Symposium on High Technology for Greenhouse Systems: GreenSys2009, Québec, QC, Canada, 14–19 June 2009; pp. 1263–1269. [Google Scholar]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Leoni, C.; Rossing, W.; van Bruggen, A.H.C. CHAPTER 4.2: Crop Rotation. In Plant Diseases and Their Management in Organic Agriculture; IPM, The American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 127–140. [Google Scholar] [CrossRef]
- Zhu, S.; Morel, J.B. Molecular mechanisms underlying microbial disease control in intercropping. Mol. Plant. Microbe. Interact. 2019, 32, 20–24. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Wang, C. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Rajput, P.; Choudhary, R.; Yadav, S.; Yadav, S.K.; Rajput, V.D.; Minkina, T.; Ercisli, S.; Matić, S. Multifaceted Characteristics of Biochar and Its Implementation in Environmental Management in a Sustainable Way. Environ. Qual. Manag. 2024, 34, e22305. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species–opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Murray-Watson, R.E.; Cunniffe, N.J. How the epidemiology of disease-resistant and disease-tolerant varieties affects grower behaviour. J. R. Soc. Interface 2022, 19, 20220517. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Hooks, C.R.; Fereres, A. Protecting crops from non-persistently aphid-transmitted viruses: A review on the use of barrier plants as a management tool. Virus Res. 2006, 120, 1–16. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Trivellone, V.; Krüger, K. The biology and ecology of Leafhopper transmission of phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria—II; Springer: Singapore, 2019; pp. 27–51. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Niño-liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef]
- Katan, J. Solar heating (solarization) of soil for control of soilborne pests. Annu. Rev. Phytopathol. 1981, 19, 211–236. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Antón-Domínguez, B.I.; López-Moral, A.; Romero-Salguero, F.J.; Trapero, A.; Trapero, C.; Agustí-Brisach, C. Bioprotection of Olive Trees Against Verticillium Wilt by Pomegranate and Carob Extracts. Plant Dis. 2024, 108, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).