Biochar as a Stimulator of Zea mays Growth and Enzyme Activity in Soil Contaminated with Zinc, Copper, and Nickel
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Biochar Characteristics
2.2. Pot Experiment
2.3. Chemical, Physicochemical, and Biochemical Analyses
2.3.1. Soil
2.3.2. Plant
2.4. Calculations and Statistical Analysis
3. Results
3.1. The Effect of Heavy Metals and Biochar on the Chemical and Physicochemical Properties of Soil
3.2. The Effect of Heavy Metals and Biochar on Maize
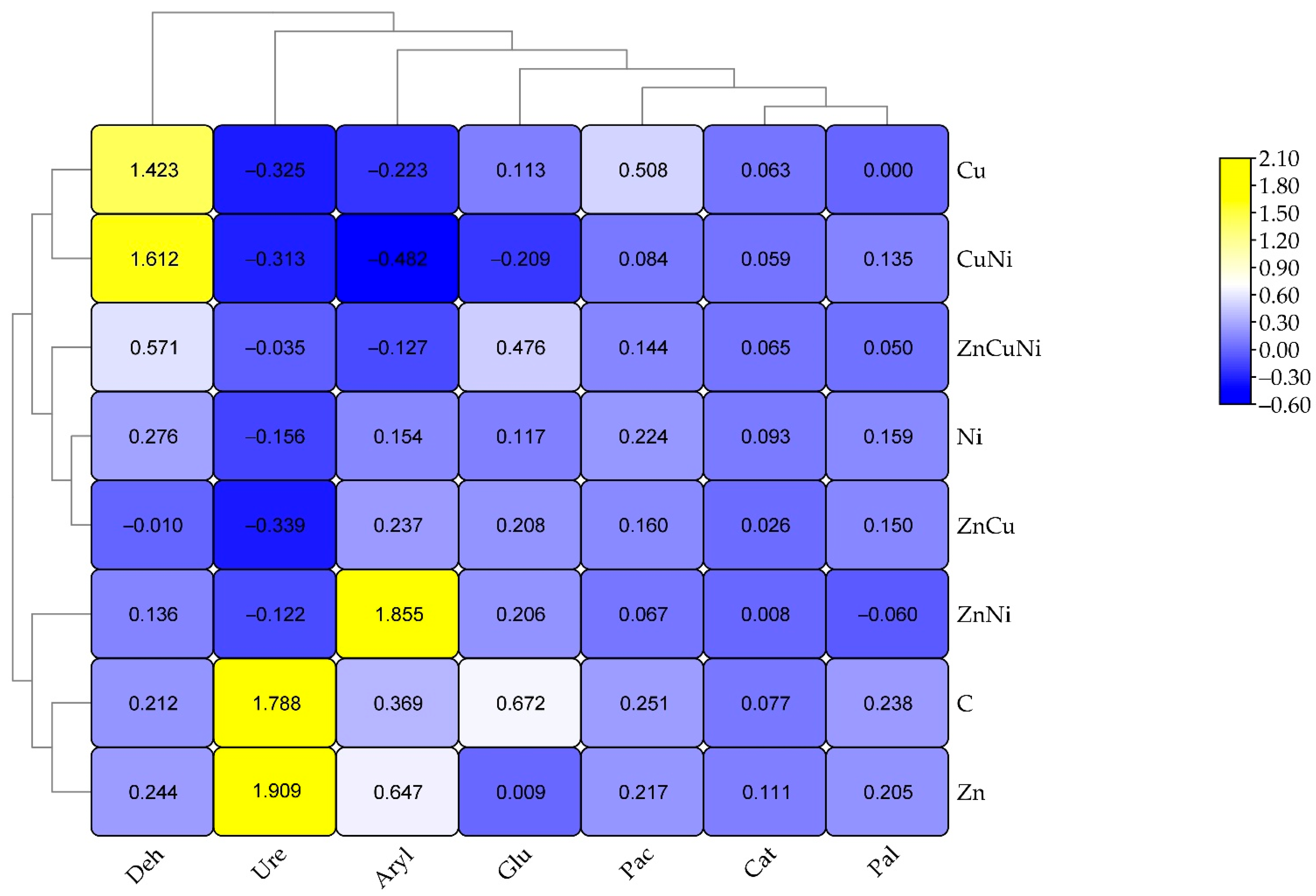
3.3. The Effect of Heavy Metals and Biochar on Soil Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar remediation of soil: Linking biochar production with function in heavy metal contaminated soils Review. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, I.; Usman, M.; Iqbal, R.; Rizwan, M.; Abdel-Hameed, U.K.; Haider, A.A.; Tariq, A. Biochar as a green sorbent for remediation of polluted soils and associated toxicity risks: A critical review. Separations 2023, 10, 197. [Google Scholar] [CrossRef]
- Rizwan, M.; Murtaza, G.; Zulfiqar, F.; Moosa, A.; Iqbal, R.; Ahmed, Z.; Irshad, S.; Khan, I.; Li, T.; Chen, J.; et al. Sustainable manufacture and application of biochar to improve soil properties and remediate soil contaminated with organic impurities: A systematic review. Front. Environ. Sci. 2023, 11, 1277240. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Latawiec, A.; Królczyk, J.; Bogacz, A.; Kawałko, D.; Bednik, M.; Dudek, M. Biochar improves maize growth but has a limited effect on soil properties: Evidence from a three-year field experiment. Sustainability 2021, 13, 3617. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Li, G.; Zhao, X.; Chen, H. Biochar amends saline soil and enhances maize growth: Three-year field experiment findings. Agronomy 2023, 13, 1111. [Google Scholar] [CrossRef]
- Choudhary, T.K.; Khan, K.S.; Hussain, Q.; Ashfaq, M. Nutrient availability to maize crop (Zea mays L.) in biochar amended alkaline subtropical Soil. J. Soil Sci. Plant Nutr. 2021, 21, 1293–1306. [Google Scholar] [CrossRef]
- Ahmad Bhat, S.; Kuriqi, A.; Dar, M.U.D.; Bhat, O.; Sammen, S.S.; Towfiqul Islam, A.R.M.; Elbeltagi, A.; Shah, O.; AI-Ansari, N.; Ali, R.; et al. Application of biochar for improving physical, chemical, and hydrological soil properties: A systematic review. Sustainability 2022, 14, 11104. [Google Scholar] [CrossRef]
- Haider, F.U.; Farooq, M.; Hussain, S.; Cheema, S.A.; Ain, N.U.; Virk, A.L.; Ejaz, M.; Janyshova, U.; Liqun, C. Biochar application for the remediation of trace metals in contaminated soils: Implications for stress tolerance and crop production; update. Ecotoxicol. Environ. Saf. 2022, 230, 113165. [Google Scholar] [CrossRef]
- Gyanwali, P.; Khanal, R.; Pokhrel, S.; Adhikari, K. Exploring the benefits of biochar: A review of production methods, characteristics, and applications in soil health and environment. Egypt. J. Soil Sci. 2024, 64, 855–884. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Kozioł, A.; Paliwoda, D.; Mikiciuk, G.; Benhadji, N. Biochar as a multi-action substance used to improve soil properties in horticultural and agricultural crops—A Review. Agriculture 2024, 14, 2165. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy metal pollution in the environment and its impact on health: Exploring green technology for remediation. Environ. Health Insights. 2023, 17, 11786302231201259. [Google Scholar] [CrossRef]
- Al-Trbany, A.; Elsayed, A.H.; El-Hakim, A.A.; Khorshed, M.A. Health risk from heavy metal contamination in maize. J. Angiother. 2024, 8, 1–12. [Google Scholar] [CrossRef]
- Atta, M.I.; Zehra, S.S.; Ali, H.; Ali, B.; Abbas, S.N.; Aimen, S.; Sarwar, S.; Ahmad, I.; Hussain, M.; Al-Ashkar, I.; et al. Assessing the effect of heavy metals on maize (Zea mays L.) growth and soil characteristics: Plants-implications for phytoremediation. PeerJ 2023, 11, e16067. [Google Scholar] [CrossRef] [PubMed]
- Elik, Ü.; Gül, Z. Accumulation potential of lead and cadmium metals in maize (Zea mays L.) and effects on physiological-morphological characteristics. Life 2025, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Futa, B.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P. Effect of natural aging of biochar on soil enzymatic activity and physicochemical properties in long-term field experiment. Agronomy 2020, 10, 449. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil enzyme activity response under the amendment of different types of biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Silva, A.M.; Mora-Motta, D.; Cherubin, M.R.; Ortiz–Morea, F.A. Soil enzyme responses to land use change in the tropical rainforest of the Colombian Amazon region. PLoS ONE 2021, 16, e0255669. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total. Environ. 2020, 1, 140446. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The Application of Biochar from Waste Biomass to Improve Soil Fertility and Soil Enzyme Activity and Increase Carbon Sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Polláková, N.; Hamar, J.; Šimanský, V.; Bartkowiak, A.; Lemanowicz, J. Secondary enrichment of soil by alkaline emissions: The specific form of anthropo-geogenic soil degradation near magnesite processing factories and possibilities of land management. Land Degrad. Dev. 2021, 32, 881–895. [Google Scholar] [CrossRef]
- Hasnine, M.T.; Huda, M.E.; Khatun, R.; Saadat, A.H.M.; Ahasan, M.; Akter, S.; Uddin, M.F.; Monika, A.N.; Rahman, M.A.; Ohiduzzaman, M. Heavy Metal Contamination in Agricultural Soil at DEPZA, Bangladesh. Environ. Ecol. Res. 2017, 5, 510–516. [Google Scholar] [CrossRef]
- Obinnaa, I.B.; Ebere, E.C. Water pollution by heavy metal and organic pollutants: Brief review of sources, effects, and progress on remediation with aquatic plants. Anal. Meth. Environ. Chem. J. 2019, 2, 5–38. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Kumar, P. Heavy metals accumulation in crop plants: Sources, response mechanisms, stress tolerance and their effects. In Contaminants in Agriculture and Environment: Health Risks and Remediation; Kumar, V., Kumar, R., Singh, J., Kumar, P., Eds.; Agro Environ Media: Haridwar, India, 2019; Volume 1, pp. 38–57. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, C. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Intern. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Kuziemska, B.; Wysokinski, A.; Klej, P. The Content, uptake and bioaccumulation factor of copper and nickel in grass depending on zinc application and organic fertilization. Agriculture 2023, 13, 1676. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C.A. Role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Hambidge, M.; Cousins, R.J.; Costello, R.B. Zinc and health: Current status and future directions. J. Nutr. 2020, 130, 1437S–1446S. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Hippler, F.W.R.; Boaretto, R.M.; Quaggio, J.A.; Mattos Júnior, D. Copper in citrus production: Required but avoided. Citrus Res. Technol. 2017, 38, 99–106. [Google Scholar] [CrossRef]
- Pietrini, F.; Carnevale, M.; Beni, C.; Zacchini, M.; Gallucci, F.; Santangelo, E. Effect of different copper levels on growth and morpho-physiological parameters in giant reed (Arundo donax L.) in semi-hydroponic mesocosm experiment. Water 2019, 11, 1837. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, Y.F.; Zeb, A.; Hayat, K.; Alhoqail, W.A.; Soliman, M.H. Beneficial elements and their roles against soil pollution. In Beneficial Elements for Remediation of Heavy Metals in Polluted Soil; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–32. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; He, Z. The impact of heavy metal contamination on soil health. In Managing Soil Health for Sustainable Agriculture; Reicosky, D., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2018; Volume 2, pp. 1–36. [Google Scholar] [CrossRef]
- Ghazanfar, S.; Komal, A.; Waseem, A.; Hassan, W.; Iqbal, R.J.; Toor, S.; Nazar, S. Physiological effects of nickel contamination onplant growth. Nat. Volatiles Essent. Oils 2021, 8, 13457–13469. [Google Scholar]
- Akinola, S.A.; Babalola, O.O. The importance of adverse soil microbiomes in the light of omics: Implications for food safety Review. Plant Soil Environ. 2020, 66, 421–430. [Google Scholar] [CrossRef]
- Majewska, M.; Hanaka, A. Biochar in the bioremediation of metal-contaminated soils. Agronomy 2025, 15, 273. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Shi, L.; Li, G.; Pang, Z.; Liu, S.; Chen, Y.; Jia, B. Effects of biochar on soil chemical properties: A global meta-analysis of agricultural soil. Plant Soil Environ. 2022, 68, 272–289. [Google Scholar] [CrossRef]
- Haddad, S.A.; Mowrer, J.; Thapa, B. Biochar and compost from cotton residues inconsistently affect water use efficiency, nodulation, and growth of legumes under arid conditions. J. Environ. Manag. 2022, 307, 114558. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Singh, R.; Babu, J.N.; Kumar, R.; Srivastava, P.; Singh, P.; Raghubanshi, A.S. Multifaceted application of crop residue biochar as a tool for sustainable agriculture: An ecological perspective. Ecol. Eng. 2015, 77, 324–347. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Wewalwela, J.J.; Vithanage, M. Biochar for sustainable agriculture: Nutrient dynamics, soil enzymes, and crop growth. In Biochar from Biomass and Waste; Sik Ok, Y., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–224. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Indianraj, N. Influence of biochar on soil properties, soil health and crop productivity review. Int. J. Phytol. Res. 2023, 3, 4–9. [Google Scholar]
- Kumar Yadav, N.; Kumar, V.; Sharma, K.R.; Choudhary, R.S.; Butter, T.S.; Singh, G.; Kumar, M.; Kumar, R. Biochar and their impacts on soil properties and crop productivity: A review. J. Pharmacogn. Phytochem. 2018, 7, 49–54. [Google Scholar]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C:N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 182. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 26 May 2024).
- Gawryluk, A.; Wyłupek, T.; Wolański, P. Assessment of Cu, Pb and Zn content in selected species of grasses and in the soil of the roadside embankment. Ecol. Evol. 2020, 10, 9841–9852. [Google Scholar] [CrossRef]
- Seeda, A.; Abou El-Nour, E.; Mervat, G.; Zaghloul, S. Interaction of copper, zinc, and their importance in plant physiology: Review, Acquisition and Transport. Middle East J. Appl. Sci. 2020, 10, 407–434. [Google Scholar] [CrossRef]
- Liščáková, P.; Nawaz, A.; Molnárová, M. Reciprocal effects of copper and zinc in plants. Int. J. Environ. Sci. Technol. 2022, 19, 9297–9312. [Google Scholar] [CrossRef]
- ISO 10390; Soil Quality–Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Carter, M.R. Soil Sampling and Methods of Analysis; Canadian Society of Soil Science; Lewis Publishers: London, UK, 1993. [Google Scholar]
- Klute, A. Methods of Soil Analysis; Agronomy Monograph 9; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Methods in Applied Soil Microbiology and Biochemistry; Academic London: London, UK, 1988; pp. 316–365. [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific value of Zea mays biomass derived from soil contaminated with chromium (VI) disrupting the soil’s biochemical properties. Energies 2023, 16, 3788. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. Available online: https://www.statistica.com (accessed on 23 April 2024).
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Nawab, J.; Ghani, J.; Khan, S.; Khan, M.A. Nutrient uptake and plant growthunder the influence of toxic elements. In Sustainable plant Nutritionunder Contaminated Environments; Springer: Cham, Switzerland, 2022; pp. 75–101. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Stoleru, V.; Gavrilescu, M. Analysis of Heavy Metal Impacts on Cereal Crop Growth and Development in Contaminated Soils. Agriculture 2023, 13, 1983. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of pollutants from wastewater by biochar: A review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Li, Z.; Niu, R.; Yu, J.; Yu, L.; Cao, D. Removal of cadmium from aqueous solution by magnetic biochar: Adsorption characteristics and mechanism. Environ. Sci. Pollut. Res. 2024, 31, 6543–6557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research status, trends, and mechanisms of biochar adsorption for wastewater treatment: A scientometric review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Saleem, A.; Ur Rahim, H.; Khan, U.; Irfan, M.; Akbar, W.A.; Akbar, Z.; Alatalo, J.M. Organic materials amendments can improve NPK availability and maize growth by reducing heavy metals stress in calcareous soil. Int. J. Environ. Sci. Technol. 2024, 21, 2533–2546. [Google Scholar] [CrossRef]
- Cong, M.; Hu, Y.; Sun, X.; Yan, H.; Yu, G.; Tang, G.; Chen, S.; Xu, W.; Jia, H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. [Google Scholar] [CrossRef]
- Mu, L.; Zhou, H.; Yang, K.; Wang, J.; Sun, S.; Lu, Z.; Wang, L.; Zhang, N.; Bao, L. Effect of biochar-based organic fertilizer on the growth of maize in cadmium-contaminated soil. Agriculture 2025, 15, 447. [Google Scholar] [CrossRef]
- Monga, Y.; Sharma, S.; Singh, S.; Gupta, A. Biochar, clay, zeolites, and microorganism-based methods for remediation of heavy metals. Curr. Green Chem. 2024, 11, 2–11. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, M.Z.; Cydzik-Kwiatkowska, A.; Blazejczyk, A.; Kumar, V.; Kintl, A.; Brtnicky, M. Effect of biochar on metal distribution and microbiome dynamic of a phytostabilized metalloid-contaminated soil following freeze–thaw cycles. Materials 2022, 15, 3801. [Google Scholar] [CrossRef] [PubMed]
- Nim, Y.S.; Wong, K.-B. The Maturation Pathway of Nickel Urease. Inorganics 2019, 7, 85. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- Conte, P.; Hanke, U.M.; Marsala, V.; Cimo, G.; Alonzo, G.; Glaser, B. Mechanisms of water interaction with pore systems of hydrochar and pyrochar from poplar forestry waste. J. Agric. Food Chem. 2014, 62, 4917–4923. [Google Scholar] [CrossRef]
- Nielsen, S.; Joseph, S.; Ye, J.; Chia, C.; Munroe, P.; van Zwieten, L.; Thomas, T. Crop-season and residual effects of sequentially applied mineral enhanced biochar and N fertiliser on crop yield, soil chemistry and microbial communities. Agric. Ecosyst. Environ. 2018, 255, 52–61. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, F.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: Effects on as soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef]
- Simiele, M.; Sferra, G.; Lebrun, M.; Renzone, G.; Bourgerie, S.; Scippa, G.S.; Morabito, D.; Scaloni, A.; Trupiano, D. In-depth study to decipher mechanisms underlying Arabidopsis thaliana tolerance to metal(loid) soil contamination in association with biochar and/or bacteria. Environ. Exp. Bot. 2021, 182, 104335. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of Biochar-Derived Sewage Sludge on Heavy Metal Adsorption and Immobilization in Soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, K.; McGrouther, K.; Che, L.; Hu, G.; Wang, Q.; Liu, X.; Shen, L.; Huang, H.; Ye, Z.; et al. Bioavailability of Cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. J. Soils Sediments 2017, 17, 751–762. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef]
- Rehman, A.; Arif, M.S.; Tufail, M.A.; Shahzad, S.M.; Farooq, T.H.; Ahmed, W.; Mehmood, T.; Farooq, M.R.; Javed, Z.; Shakoor, A. Biochar potential to relegate metal toxicity effects is more soil driven than plant system: A global meta-analysis. J. Clean. Prod. 2021, 316, 128276. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Y.; Wang, N.; Ma, F.; Yuan, Y. Effects of Ageing on Surface Properties of Biochar and Bioavailability of Heavy Metals in Soil. Agriculture 2024, 14, 1631. [Google Scholar] [CrossRef]
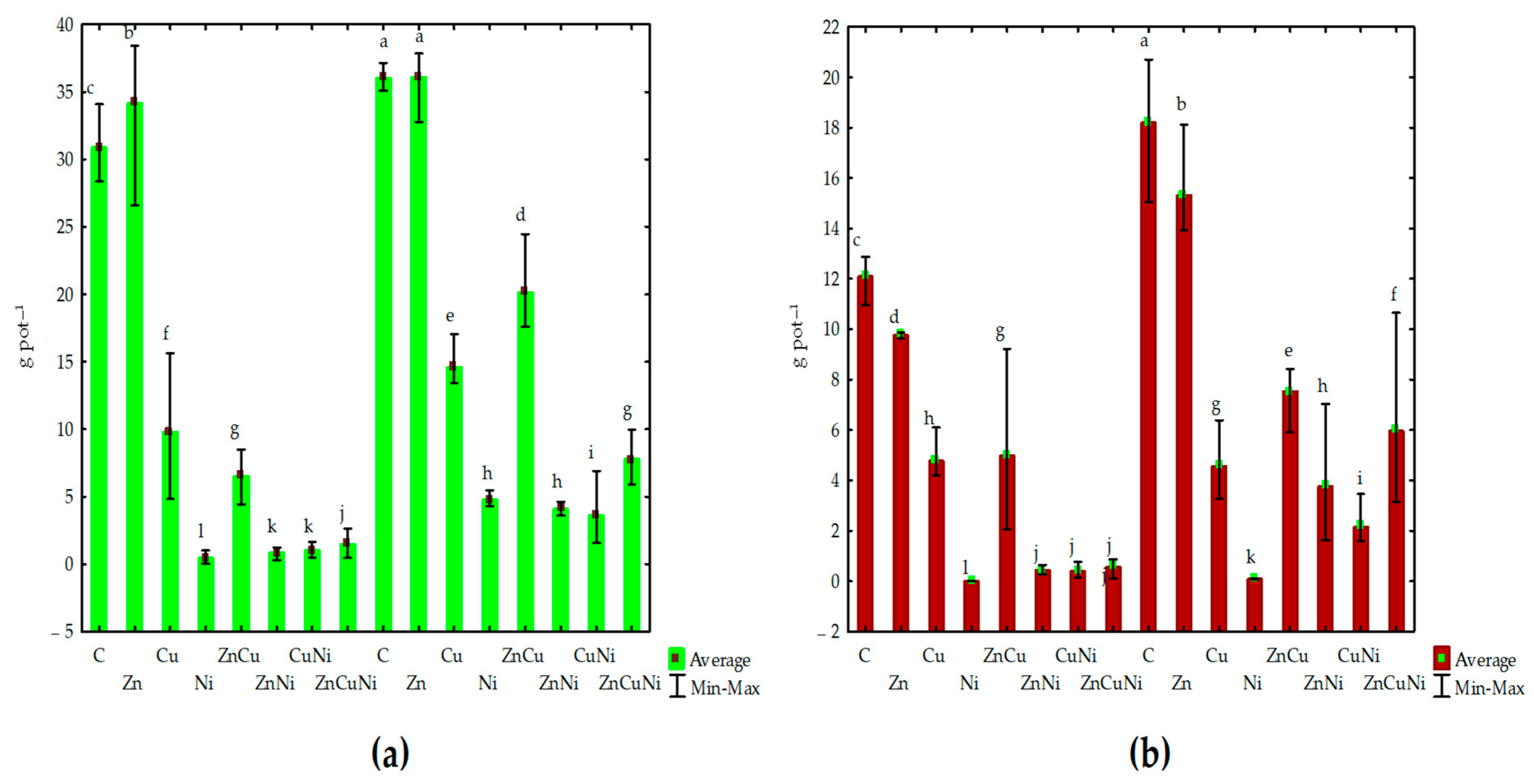
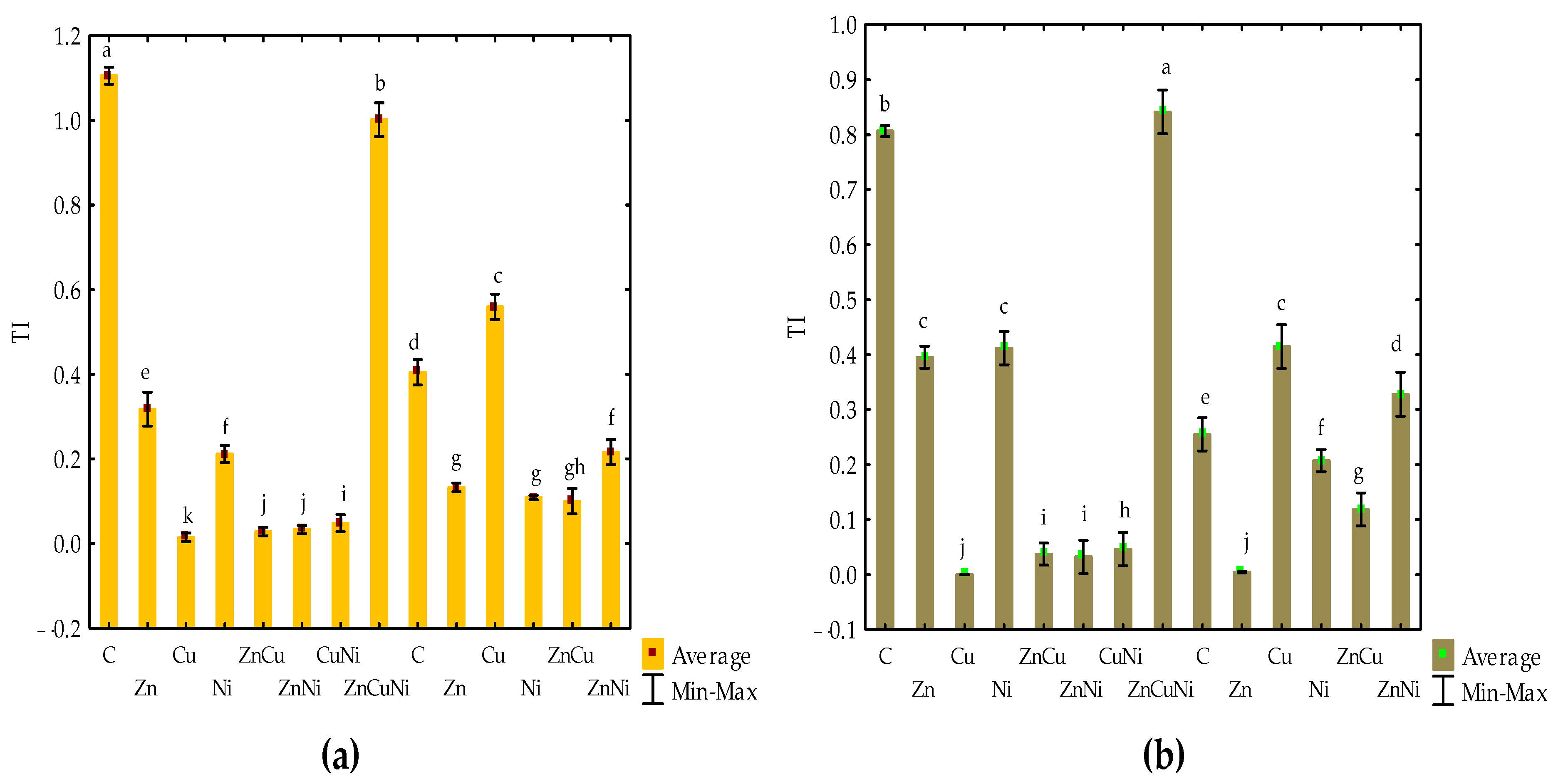
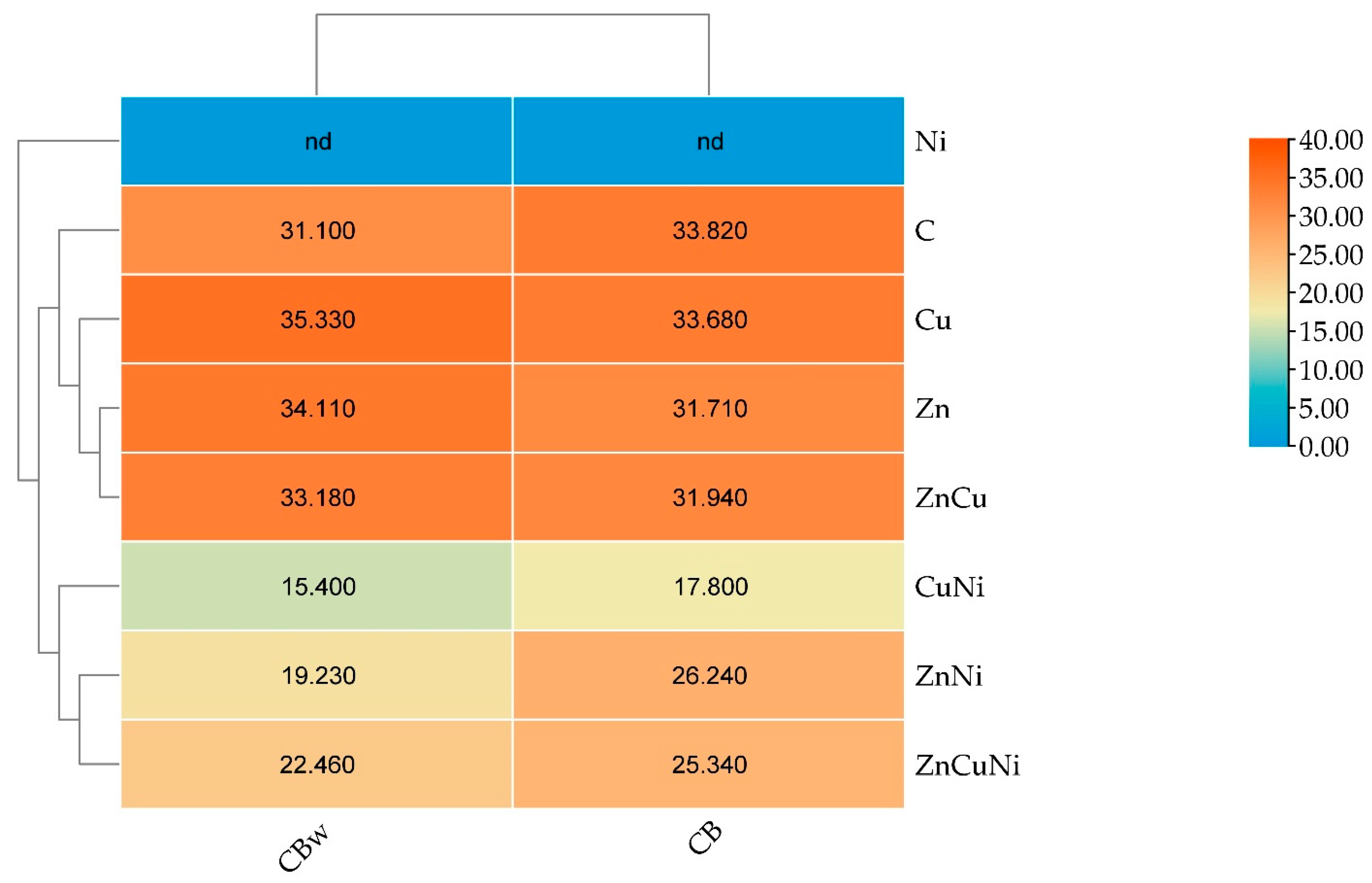
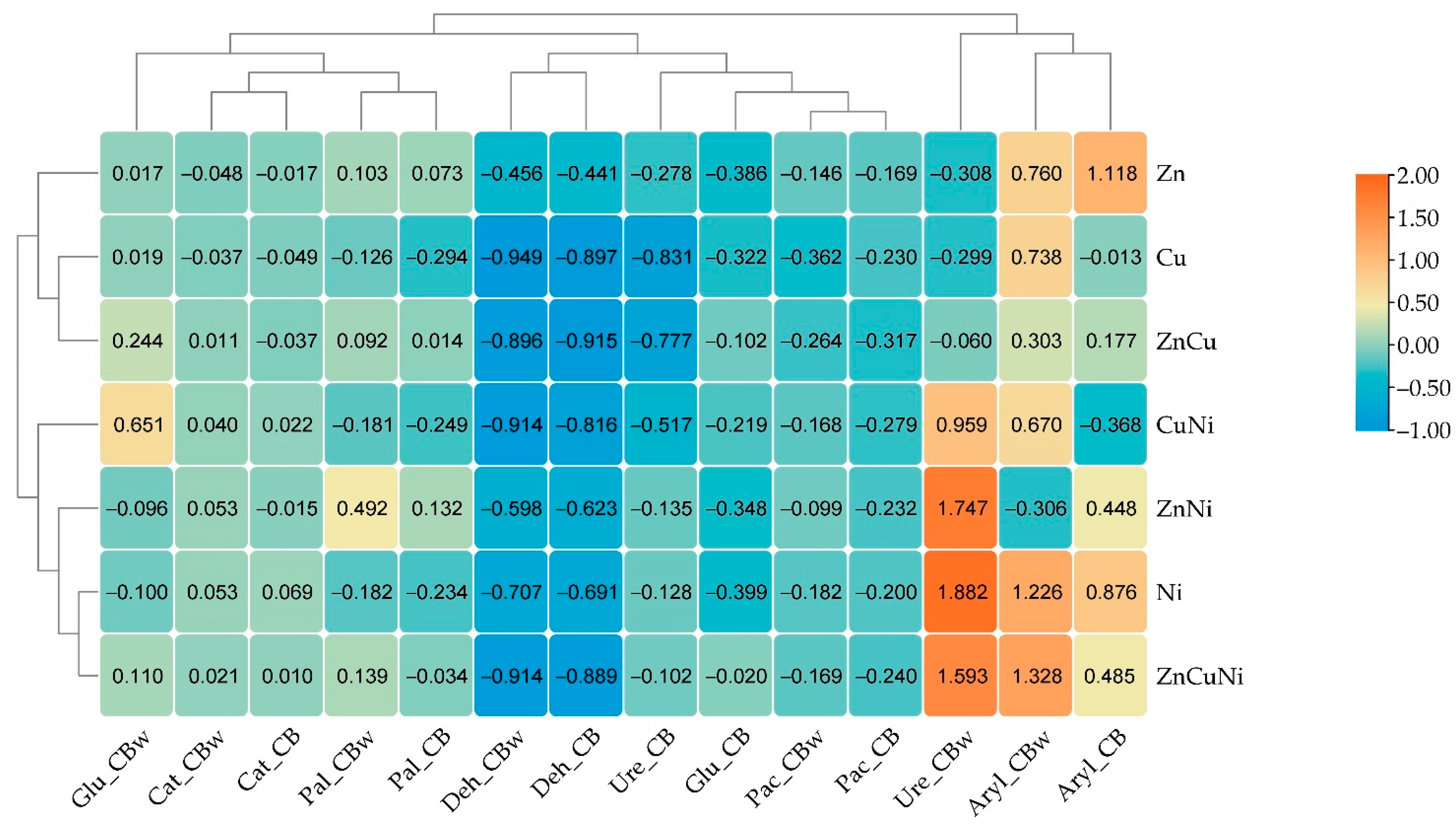
| Number | Treatment | Biochar Dose, g kg−1 Soil | Heavy Metal Dose, mg kg−1 d.m. |
|---|---|---|---|
| 1 | C | 0 | 0 |
| 2 | Zn | 420 | |
| 3 | Cu | 420 | |
| 4 | Ni | 420 | |
| 5 | ZnCu | 210 (Zn) + 210 (Cu) | |
| 6 | ZnNi | 210 (Zn) + 210 (Ni) | |
| 7 | CuNi | 210 (Cu) + 210 (Ni) | |
| 8 | ZnCuNi | 140 (Zn) + 140 (Cu) + 140 (Ni) | |
| 9 | C | 15 | 0 |
| 10 | Zn | 420 | |
| 11 | Cu | 420 | |
| 12 | Ni | 420 | |
| 13 | ZnCu | 210 (Zn) + 210 (Cu) | |
| 14 | ZnNi | 210 (Zn) + 210 (Ni) | |
| 15 | CuNi | 210 (Cu) + 210 (Ni) | |
| 16 | ZnCuNi | 140 (Zn) + 140 (Cu) + 140 (Ni) |
| Treatment | Heavy Metal Tested | CBw | CB |
|---|---|---|---|
| C | Zn | 22.762 i ± 1.020 | 11.579 j ± 1.011 |
| Cu | 3.974 k ± 0.080 | 2.472 k ± 0.052 | |
| Ni | 6.022 jk ± 0.093 | 2.806 k ± 0.067 | |
| Zn | Zn | 375.520 b ± 4.070 | 385.261 b ± 2.228 |
| Cu | Cu | 406.119 a ± 5.120 | 395.320 b ± 2.478 |
| Ni | Ni | 415.202 a ± 4.152 | 308.419 c ± 3.121 |
| ZnCu | Zn | 161.615 e ± 2.223 | 191.695 de ± 1.975 |
| Cu | 201.646 d ± 2.026 | 211.529 d ± 1.999 | |
| ZnNi | Zn | 194.430 de ± 1.268 | 188.732 de ± 1.853 |
| Ni | 195.699 de ± 1.732 | 194.927 cd ± 1.278 | |
| CuNi | Cu | 204.011 d ± 2.098 | 182.323 de ± 1.645 |
| Ni | 186.535 de ± 1.365 | 167.257 e ± 1.874 | |
| ZnCuNi | Zn | 78.252 h ± 1.786 | 108.232 g ± 1.765 |
| Cu | 88.951 h ± 1.259 | 157.465 ef ± 1.899 | |
| Ni | 81.529 h ± 1.223 | 146.107 f ± 1.367 |
| Treatment | Corg | Ntotal | pHKCl | HAC | EBC | CEC | BCSR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | |
| C | 7.605 i ± 0.011 | 11.795 f ± 0.062 | 1.230 c ± 0.011 | 1.385 b ± 0.013 | 5.73 e ± 0.05 | 5.98 e ± 0.05 | 15.56 a ± 0.19 | 13.31 b ± 0.19 | 31.00 g ± 0.26 | 35.00 f ± 0.34 | 46.56 f ± 0.20 | 48.31 e ± 0.27 | 66.49 i ± 0.37 | 72.38 f ± 0.41 |
| Zn | 7.965 gh ± 0.010 | 13.085 c ± 0.054 | 1.325 c ± 0.010 | 1.350 bc ± 0.011 | 5.76 e ± 0.04 | 6.08 d ± 0.05 | 15.45 a ± 0.08 | 12.75 c ± 0.28 | 29.50 gh ± 0.23 | 27.00 h ± 0.31 | 44.95 g ± 0.25 | 39.75 k ± 0.31 | 65.63 i ± 0.29 | 67.79 h ± 0.32 |
| Cu | 7.345 k ± 0.013 | 12.220 d ± 0.047 | 1.305 d ± 0.013 | 1.420 a ± 0.012 | 5.97 de ± 0.05 | 6.16 c ± 0.02 | 9.19 i ± 0.19 | 12.75 c ± 0.26 | 65.00 a ± 0.50 | 28.00 h ± 0.28 | 74.19 a ± 0.39 | 40.75 j ± 0.33 | 87.62 a ± 0.39 | 68.22 h ± 0.29 |
| Ni | 7.115 l ± 0.023 | 13.785 ab ± 0.042 | 1.340 c ± 0.012 | 1.385 b ± 0.012 | 6.35 b ± 0.03 | 6.55 a ± 0.01 | 9.75 g ± 0.04 | 9.38 h ± 0.28 | 30.50 g ± 0.30 | 46.00 b ± 0.38 | 40.25 jk ± 0.35 | 55.38 c ± 0.41 | 75.75 e ± 0.36 | 82.99 b ± 0.38 |
| ZnCu | 7.765 h ± 0.033 | 13.845 a ± 0.045 | 1.195 f ± 0.012 | 1.275 e ± 0.011 | 5.95 de ± 0.02 | 6.10 cd ± 0.02 | 13.69 b ± 0.18 | 11.81 de ± 0.16 | 31.00 g ± 0.33 | 31.00 g ± 0.32 | 44.69 g ± 0.33 | 42.81 h ± 0.39 | 69.36 g ± 0.28 | 72.40 f ± 0.42 |
| ZnNi | 7.485 j ± 0.027 | 12.035 e ± 0.036 | 1.275 e ± 0.011 | 1.345 b ± 0.013 | 6.13 c ± 0.04 | 6.30 b ± 0.03 | 11.44 e ± 0.17 | 10.31 f ± 0.15 | 36.00 f ± 0.35 | 45.00 bc ± 0.29 | 47.44 f ± 0.39 | 55.31 c ± 0.42 | 75.86 e ± 0.43 | 81.31 b ± 0.44 |
| CuNi | 8.165 g ± 0.030 | 13.645 b ± 0.027 | 1.385 b ± 0.010 | 1.385 b ± 0.012 | 6.10 cd ± 0.03 | 6.30 b ± 0.01 | 12.19 d ± 0.18 | 11.81 ef ± 0.18 | 48.00 b ± 0.32 | 41.00 e ± 0.35 | 60.19 b ± 0.40 | 52.81 d ± 0.37 | 79.73 c ± 0.46 | 77.63 d ± 0.47 |
| ZnCuNi | 7.295 j ± 0.029 | 13.825 a ± 0.029 | 1.235 f ± 0.011 | 1.435 a ± 0.013 | 6.03 d ± 0.02 | 6.30 b ± 0.03 | 12.38 cd ± 0.28 | 10.31 f ± 0.09 | 44.00 c ± 0.25 | 33.00 fg ± 0.36 | 56.38 c ± 0.42 | 43.31 h ± 0.35 | 78.02 c ± 0.48 | 76.18 e ± 0.37 |
| Treatment | Heavy Metal Tested | AP | R | ||
|---|---|---|---|---|---|
| CBw | CB | CBw | CB | ||
| C | Zn | 7.106 h ± 0.071 | 6.433 h ± 0.048 | 6.418 g ± 1.020 | 6.831 g ± 0.078 |
| Cu | 2.507 h ± 0.023 | 2.231 h ± 0.031 | 2.745 g ± 1.020 | 6.790 g ± 0.055 | |
| Ni | 3.292 h ± 0.035 | 1.512 h ± 0.016 | 2.117 g ± 1.020 | 2.043 g ± 0.025 | |
| Zn | Zn | 338.325 c ± 4.571 | 314.242 c ± 1.386 | 419.970 b ± 3.769 | 510.949 a ± 4.039 |
| Cu | Cu | 313.369 cd ± 3.524 | 331.435 c ± 2.125 | 354.623 c ± 2.534 | 372.265 bc ± 1.904 |
| Ni | Ni | 408.228 b ± 2.989 | 440.135 a ± 3.187 | 465.322 ab ± 2.776 | 492.658 a ± 2.597 |
| ZnCu | Zn | 304.755 c ± 2.256 | 255.199 d ± 1.870 | 343.656 c ± 3.026 | 376.423 bc ± 2.034 |
| Cu | 111.981 ef ± 1.897 | 116.288 ef ± 1.098 | 326.657 c ± 2.732 | 303.389 cd ± 2.026 | |
| ZnNi | Zn | 252.661 d ± 1.798 | 191.788 de ± 1.788 | 309.218 cd ± 2.098 | 317.824 c ± 2.123 |
| Ni | 341.060 c ± 2.265 | 176.302 de ± 1.487 | 378.479 bc ± 2.111 | 332.689 c ± 2.059 | |
| CuNi | Cu | 146.696 e ± 1.567 | 112.115 ef ± 1.275 | 171.383 e ± 1.137 | 167.872 e ± 1.754 |
| Ni | 189.981 de ± 1.246 | 123.429 e ± 1.524 | 234.164 d ± 1.273 | 269.995 d ± 1.673 | |
| ZnCuNi | Zn | 111.603 ef ± 1.452 | 76.548 f ± 1.071 | 116.676 f ± 1.153 | 175.928 e ± 1.561 |
| Cu | 97.064 f ± 1.015 | 99.665 f ± 1.039 | 106.885 f ± 1.211 | 106.378 f ± 1.275 | |
| Ni | 136.206 e ± 1.125 | 45.348 g ± 1.011 | 194.887 e ± 1.327 | 177.301 e ± 1.325 | |
| Treatment | Dehydrogenases μmol TFF kg−1 d.m. h−1 | Catalase mol O2 kg−1 d.m. h−1 | Urease mmol N-NH4 kg−1 d.m. h−1 | Acid Phosphatase | Alkaline Phosphatase | β-Glucosidase | Arylsulfatase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol PNP kg−1 d.m. h−1 | ||||||||||||||
| CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | CBw | CB | |
| C | 3.040 b ± 0.027 | 3.684 a ± 0.007 | 0.378 e ± 0.007 | 0.407 bc ± 0.004 | 0.364 h ± 0.028 | 1.015 b ± 0.030 | 1.491 c ± 0.014 | 1.865 a ± 0.014 | 0.868 f ± 0.027 | 1.075 d ± 0.015 | 0.418 e ± 0.003 | 0.699 a ± 0.007 | 0.667 h ± 0.013 | 0.913 g ± 0.042 |
| Zn | 1.655 d ± 0.040 | 2.059 c ± 0.014 | 0.360 f ± 0.004 | 0.400 b ± 0.006 | 0.252 i ± 0.027 | 0.733 f ± 0.025 | 1.274 e ± 0.009 | 1.550 b ± 0.014 | 0.957 e ± 0.027 | 1.153 c ± 0.014 | 0.425 e ± 0.005 | 0.429 e ± 0.003 | 1.174 e ± 0.087 | 1.934 a ± 0.055 |
| Cu | 0.156 n ± 0.007 | 0.378 k ± 0.014 | 0.364 f ± 0.008 | 0.387 de ± 0.007 | 0.255 i ± 0.029 | 0.172 j ± 0.027 | 0.952 i ± 0.002 | 1.436 d ± 0.100 | 0.759 h ± 0.027 | 0.759 h ± 0.003 | 0.426 e ± 0.003 | 0.474 d ± 0.004 | 1.159 e ± 0.007 | 0.901 g ± 0.027 |
| Ni | 0.892 h ± 0.007 | 1.138 g ± 0.004 | 0.398 d ± 0.004 | 0.435 a ± 0.003 | 1.049 a ± 0.028 | 0.885 de ± 0.032 | 1.219 g ± 0.012 | 1.492 c ± 0.038 | 0.710 i ± 0.027 | 0.823 f ± 0.005 | 0.376 f ± 0.003 | 0.420 e ± 0.002 | 1.485 c ± 0.027 | 1.713 b ± 0.018 |
| ZnCu | 0.315 l ± 0.014 | 0.312 l ± 0.074 | 0.382 e ± 0.004 | 0.392 c ± 0.004 | 0.342 h ± 0.028 | 0.226 i ± 0.024 | 1.098 h ± 0.083 | 1.274 e ± 0.064 | 0.948 e ± 0.027 | 1.090 d ± 0.034 | 0.520 c ± 0.006 | 0.628 b ± 0.005 | 0.869 g ± 0.019 | 1.075 f ± 0.058 |
| ZnNi | 1.222 f ± 0.014 | 1.388 e ± 0.027 | 0.398 d ± 0.003 | 0.401 c ± 0.004 | 1.000 b ± 0.027 | 0.878 e ± 0.036 | 1.343 d ± 0.021 | 1.433 d ± 0.056 | 1.295 a ± 0.027 | 1.217 b ± 0.041 | 0.378 f ± 0.004 | 0.456 d ± 0.003 | 0.463 j ± 0.055 | 1.322 d ± 0.018 |
| CuNi | 0.260 m ± 0.054 | 0.679 i ± 0.043 | 0.393 d ± 0.005 | 0.416 b ± 0.006 | 0.713 f ± 0.029 | 0.490 g ± 0.040 | 1.241 f ± 0.060 | 1.345 d ± 0.025 | 0.711 i ± 0.027 | 0.807 fg ± 0.050 | 0.690 a ± 0.006 | 0.546 c ± 0.003 | 1.114 f ± 0.072 | 0.577 i ± 0.045 |
| ZnCuNi | 0.261 m ± 0.081 | 0.410 j ± 0.051 | 0.386 de ± 0.004 | 0.411 b ± 0.005 | 0.944 c ± 0.029 | 0.911 d ± 0.051 | 1.239 f ± 0.015 | 1.417 cd ± 0.024 | 0.989 d ± 0.027 | 1.038 d ± 0.023 | 0.464 d ± 0.004 | 0.685 ab ± 0.006 | 1.553 c ± 0.088 | 1.356 d ± 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Biochar as a Stimulator of Zea mays Growth and Enzyme Activity in Soil Contaminated with Zinc, Copper, and Nickel. Agronomy 2025, 15, 1543. https://doi.org/10.3390/agronomy15071543
Boros-Lajszner E, Wyszkowska J, Kucharski J. Biochar as a Stimulator of Zea mays Growth and Enzyme Activity in Soil Contaminated with Zinc, Copper, and Nickel. Agronomy. 2025; 15(7):1543. https://doi.org/10.3390/agronomy15071543
Chicago/Turabian StyleBoros-Lajszner, Edyta, Jadwiga Wyszkowska, and Jan Kucharski. 2025. "Biochar as a Stimulator of Zea mays Growth and Enzyme Activity in Soil Contaminated with Zinc, Copper, and Nickel" Agronomy 15, no. 7: 1543. https://doi.org/10.3390/agronomy15071543
APA StyleBoros-Lajszner, E., Wyszkowska, J., & Kucharski, J. (2025). Biochar as a Stimulator of Zea mays Growth and Enzyme Activity in Soil Contaminated with Zinc, Copper, and Nickel. Agronomy, 15(7), 1543. https://doi.org/10.3390/agronomy15071543







