Metagenomic Insights into How Understory Vegetation Enhances Soil Nitrogen Availability via Microbial Nitrogen Transformation in Poplar Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Soil Sampling and Basic Properties Determination
2.3. Soil Gross N Transformation
2.4. Soil Macrogenomic Analysis
2.5. Statistical Analyses
3. Results
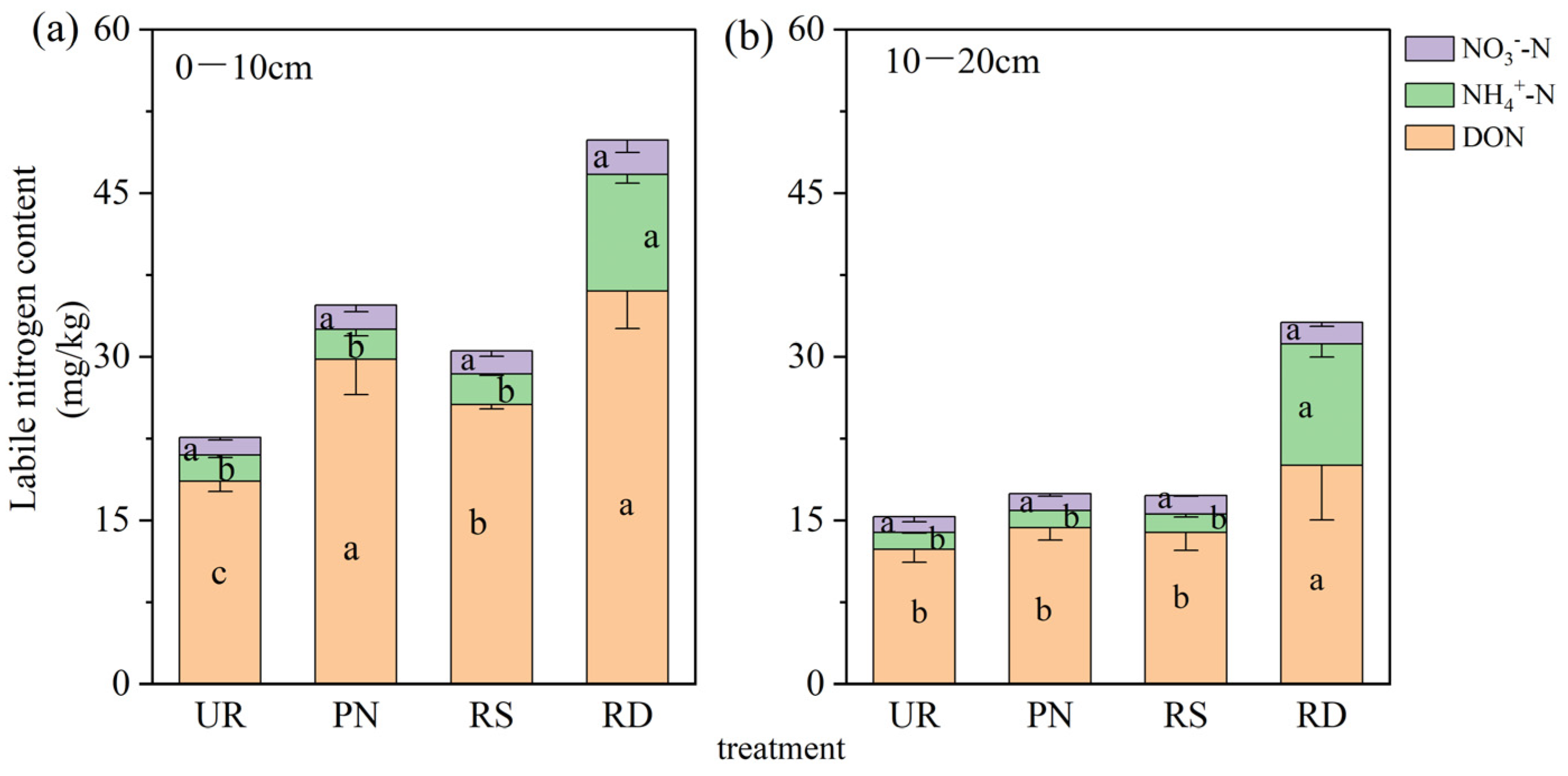
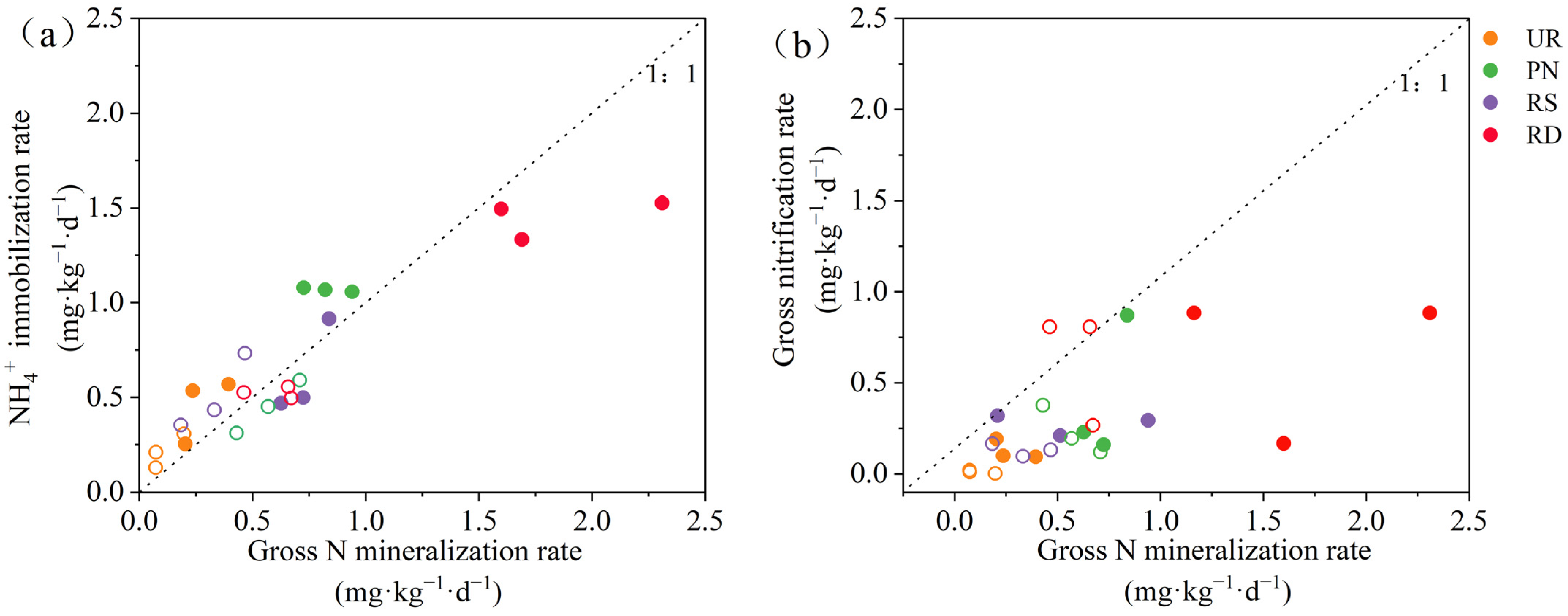
3.1. Soil N Transformation Under Different Understory Vegetation Treatments
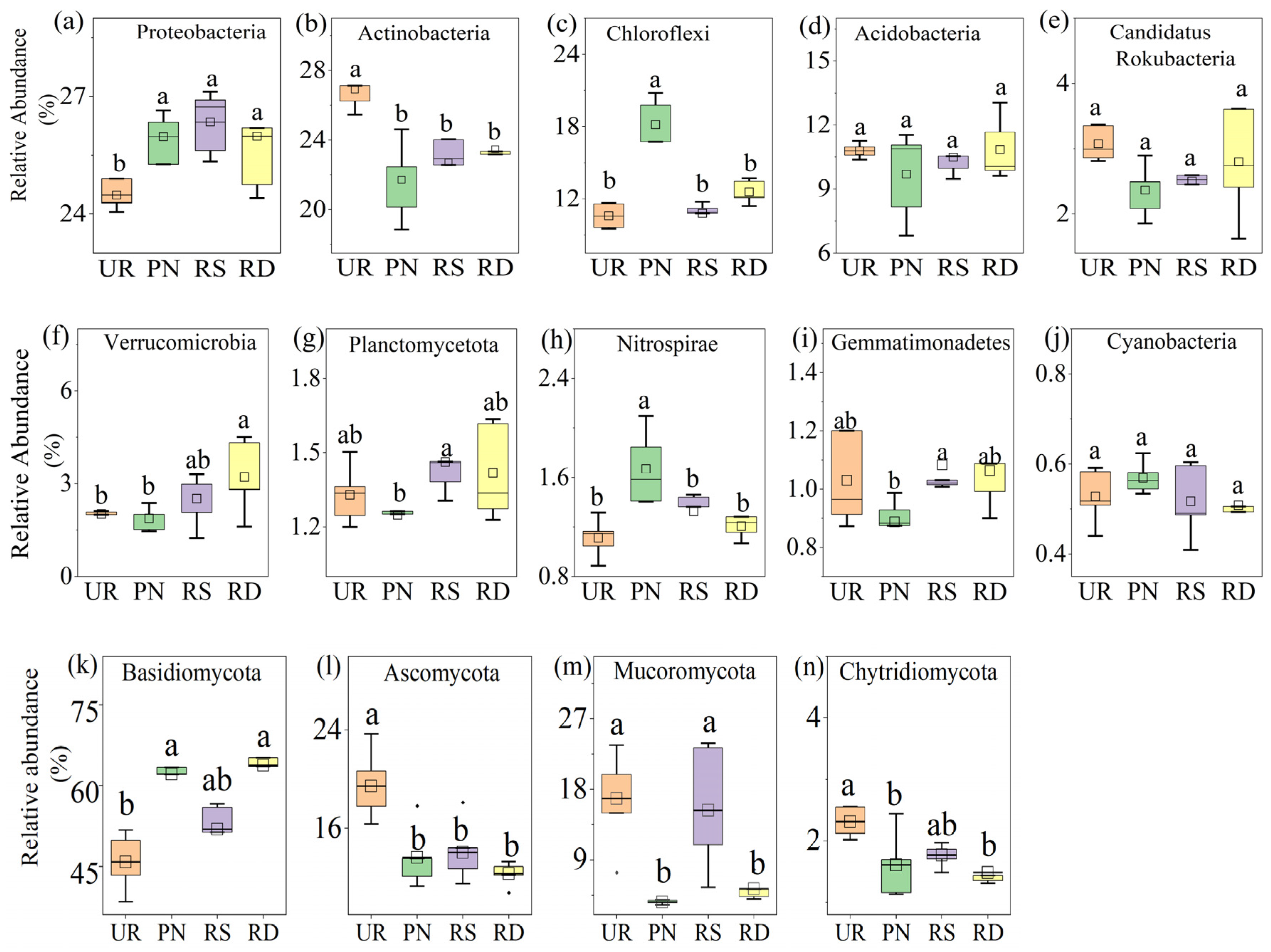
3.2. Soil Microbial Community Composition Under Different Understory Vegetation Treatments
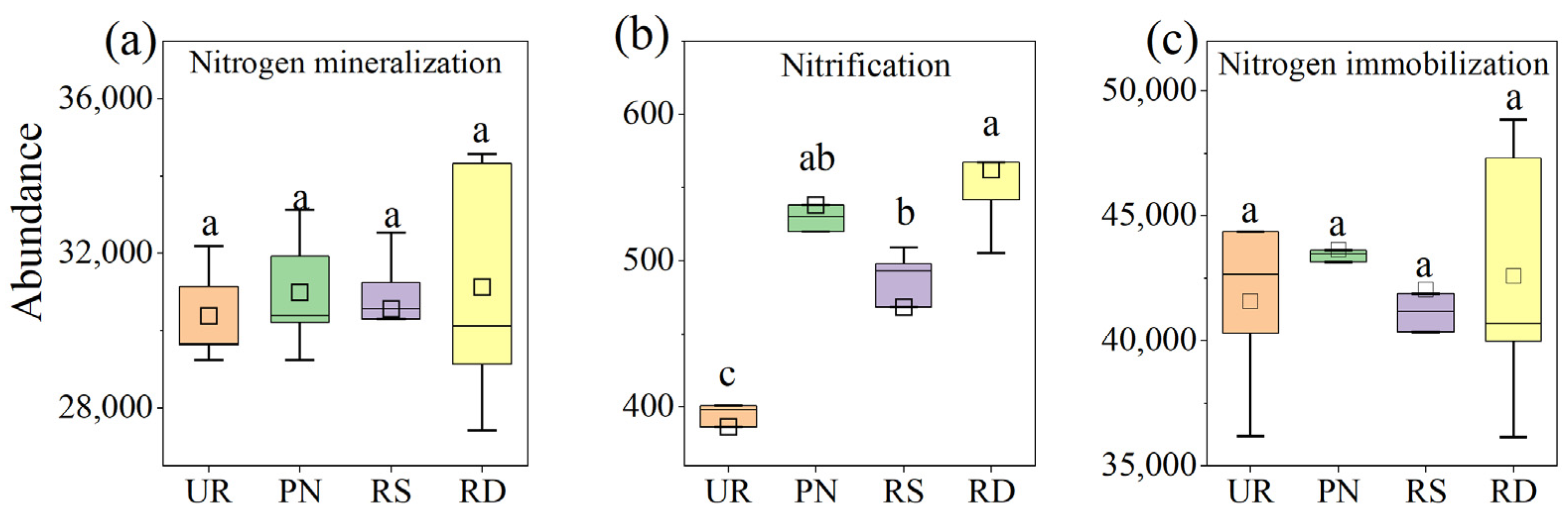
3.3. Functional Genes Related to N Transformation Under Different Understory Vegetation Treatments
3.4. Contribution Analysis of Microbial Composition, Functional Genes, and N Transformation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Sun, Y.J.; Saeed, S.; Zhang, B.; Luo, M. The difference of soil properties between pure and mixed Chinese fir (Cunninghamia lanceolata) plantations depends on tree species. Glob. Ecol. Conserv. 2020, 22, e01009. [Google Scholar] [CrossRef]
- Ghorbani, M.; Sohrabi, H.; Sadati, S.E.; Babaei, F. Productivity and dynamics of pure and mixed-species plantations of Populous deltoids Bartr. ex Marsh and Alnus subcordata C. A. Mey. For. Ecol. Manag. 2018, 409, 890–898. [Google Scholar] [CrossRef]
- Boulmane, M.; Oubrahim, H.; Halim, M.; Bakker, M.R.; Augusto, L. The potential of Eucalyptus plantations to restore degraded soils in semi-arid Morocco (NW Africa). Ann. For. Sci. 2017, 74, 5. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zhu, H.G.; Wen, Y.G.; Goodale, U.M.; Zhu, Y.L.; Yu, S.F.; Li, C.T.; Li, X.Q. Intensive management and declines in soil nutrients lead to serious exotic plant invasion in Eucalyptus plantations under successive short-rotation regimes. Land Degrad. Dev. 2020, 31, 297–310. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Wang, K. A hypothetical model that explains differing net effects of inorganic fertilization on biomass and/or abundance of soil biota. Theor. Ecol. 2015, 8, 505–512. [Google Scholar] [CrossRef]
- Nguyen, H.; Herbohn, J.; Lamb, D.; Lamb, D.; Clendenning, J.; Meadows, J. A synthesis of the available evidence to guide the design of mixed-species forest plantings for smallholder and community forestry. Small Scale For. 2018, 17, 105–123. [Google Scholar] [CrossRef]
- Miller, K.; Aegerter, B.A.; Brenna, J. Relationship between soil properties and nitrogen mineralization in undisturbed soil cores from California agroecosystems. Commun. Soil Sci. Plant Anal. 2019, 50, 77–92. [Google Scholar] [CrossRef]
- Capek, P.; Choma, M.; Tahovská, K.; Kana, J.; Kopacek, J.; Santruckova, H. Coupling the resource stoichiometry and microbial biomass turnover to predict nutrient mineralization and immobilization in soil. Geoderma 2021, 385, 114884. [Google Scholar] [CrossRef]
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Deng, Y.; Cong, J.; Lu, H.; Sun, X.; Yang, C.; Yuan, T.; Li, D.; Zhou, J.; et al. Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Sci. Rep. 2015, 5, 7994. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kuang, Y.; Xie, X.; Peng, K.; Deng, Y.; Gan, Y.; Li, Q.; Zhang, Y. Insights into nitrogen biogeochemical cycling in mangrove wetland from genome-resolved metagenomic sequencing. J. Hydrol. 2024, 640, 131741. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Gibb, N.L.; Wakelin, S.A. The abundance of microbial functional genes in grassy woodlands is influenced more by soil nutrient enrichment than by recent weed invasion or livestock exclusion. Appl. Environ. Microbiol. 2010, 76, 5547–5555. [Google Scholar] [CrossRef]
- Yan, J.F.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.N.; Wu, J.H.; Fu, X.H.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Zhu, H.; Gong, L.; Luo, Y.; Tang, J.; Ding, Z.; Li, X. Effects of litter and root manipulations on soil bacterial and fungal community structure and function in a Schrenk’s spruce (Picea schrenkiana) forest. Front. Plant Sci. 2022, 13, 849483. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, A.; Zheng, Y.; Song, J.; Ru, J.; Zheng, M.; Hui, D.; Wan, S. Long-term litter removal rather than litter addition enhances ecosystem carbon sequestration in a temperate steppe. Funct. Ecol. 2021, 35, 2799–2807. [Google Scholar] [CrossRef]
- Min, K.; Zheng, T.; Zhu, X.; Bao, X.; Lynch, L.; Liang, C. Bacterial community structure and assembly dynamics hinge on plant litter quality. FEMS Microbiol. Ecol. 2023, 99, fiad118. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Gou, X.; Reich, P.B.; Qiu, L.; Shao, M.; Wei, G.; Wang, J.; Wei, X. Leguminous plants significantly increase soil nitrogen cycling across global climates and ecosystem types. Glob. Change Biol. 2023, 29, 4028–4043. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R.; Futuyma, D.J.; Shaffer, H.B.; Simberloff, D. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, H.; Gao, K.; Fang, Y.; Wang, K.; Zhu, T.; Zhu, J.; Cheng, Y.; Li, D. Plant species diversity enhances soil gross nitrogen transformations in a subtropical forest, southwest China. J. Appl. Ecol. 2023, 60, 1364–1375. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.; Liu, H.; Yang, X.; Zhang, J.; Tong, Z. Soil bacterial community structure and functions but not assembly processes are affected by the conversion from monospecific Cunninghamia lanceolata plantations to mixed plantations. Appl. Soil Ecol. 2023, 185, 104775. [Google Scholar] [CrossRef]
- Pereira, A.P.; Durrer, A.; Gumiere, T.; Gonçalves, J.L.; Robin, A.; Bouillet, J.P.; Wang, J.; Verma, J.P.; Singh, B.K.; Cardoso, E.J. Mixed Eucalyptus plantations induce changes in microbial communities and increase biological functions in the soil and litter layers. For. Ecol. Manage. 2019, 433, 332–342. [Google Scholar] [CrossRef]
- Maxwell, T.L.; Fanin, N.; Parker, W.C.; Bakker, M.R.; Belleau, A.; Meredieu, C.; Munson, A.D. Tree species identity drives nutrient use efficiency in young mixed-species plantations, at both high and low water availability. Funct. Ecol. 2022, 36, 2069–2083. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Fang, S.; Tian, Y. Tree species composition influenced microbial diversity and nitrogen availability in rhizosphere soil. Plant Soil Environ. 2015, 61, 438–443. [Google Scholar] [CrossRef]
- Pereira, A.P.; Araujo, A.S.; Santana, M.C.; Lima, A.Y.; Araujo, V.L.; Verma, J.P.; Cardoso, E.J. Enzymatic stoichiometry in tropical soil under pure and mixed plantations of Eucalyptus with N2-fixing trees. Sci. Agric. 2023, 80, e20210283. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Radoglou, K.; Derrien, D.; Spyroglou, G.; Angeli, N.; Tsioni, G.; Fotelli, M.N. Carbon sequestration and soil nitrogen enrichment in Robinia pseudoacacia L. post-mining restoration plantations. Front. For. Global Change 2023, 6, 1190026. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.; Chang, S.X.; Jiang, P.; Zhou, G.; Liu, J. Understory vegetation management affected greenhouse gas emissions and labile organic carbon pools in an intensively managed Chinese chestnut plantation. Plant Soil 2014, 376, 363–375. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Qin, G.Z.; Zhai, Z.; Zhou, S.C.; Tang, L.Z.; Tian, Y. Diverse understory vegetation alleviates nitrogen competition with crop trees in poplar plantations. Forests 2021, 12, 705. [Google Scholar] [CrossRef]
- Deng, J.J.; Fang, S.; Fang, X.M.; Kuang, Y.W.; Lin, F.; Liu, C. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z. Silviculture of poplar plantation in China: A review. Chin. J. Appl. Ecol. 2008, 19, 2308–2316. [Google Scholar] [CrossRef]
- Ge, X.M.; Tian, Y.; Tang, L.Z. Nutrient distribution indicated whole-tree harvesting as a possible factor restricting the sustainable productivity of a poplar plantation system in China. PLoS ONE 2015, 10, e0125303. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, S.; Tian, Y.; Song, H.; Dun, X. The response of understory plant diversity and nutrient accumulation to stand structure of poplar plantation. Chin. J. Ecol. 2014, 33, 1170–1177. [Google Scholar] [CrossRef]
- Holmes, R.M.; Mcclelland, J.W.; Sigman, D.M.; Fry, B.; Peterson, B.J. Measuring 15N–NH4+ in marine, estuarine and fresh waters: An adaptation of the ammonia diffusion method for samples with low ammonium concentrations. Mar. Chem. 1998, 60, 235–243. [Google Scholar] [CrossRef]
- Mary, B.; Recous, S.; Robin, D. A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol. Biochem. 1998, 30, 1963–1979. [Google Scholar] [CrossRef]
- Matsushima, M.; Chang, S.X. Effects of understory removal, N fertilization, and litter layer removal on soil N cycling in a 13-year-old white spruce plantation infested with Canada bluejoint grass. Plant Soil 2007, 292, 243–258. [Google Scholar] [CrossRef]
- Trentini, C.P.; Villagra, M.; Pámies, D.G.; Laborde, V.B.; Bedano, J.C.; Campanello, P.I. Effect of nitrogen addition and litter removal on understory vegetation, soil mesofauna, and litter decomposition in loblolly pine plantations in subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Kopecky, M.; Hederová, L.; Macek, M.; Klinerová, T.; Wild, J. Forest plant indicator values for moisture reflect atmospheric vapour pressure deficit rather than soil water content. New Phytol. 2024, 244, 1801–1811. [Google Scholar] [CrossRef]
- Greiser, C.; Hederová, L.; Vico, G.; Wild, J.; Macek, M.; Kopecky, M. Higher soil moisture increases microclimate temperature buffering in temperate broadleaf forests. Sci. Total Environ. Agric. For. Meteorol. 2023, 345, 109828. [Google Scholar] [CrossRef]
- Ullah, S.; Liu, W.; Shah, J.A.; Shen, F.; Liao, Y.; Duan, H. Effects of canopy nitrogen addition and understory vegetation removal on nitrogen transformations in a subtropical forest. Forests 2024, 15, 962. [Google Scholar] [CrossRef]
- Gei, M.G.; Powers, J.S. Do legumes and non-legumes tree species affect soil properties in unmanaged forests and plantations in Costa Rican dry forests? Soil Biol. Biochem. 2013, 57, 264–272. [Google Scholar] [CrossRef]
- Sun, L.J.; Ataka, M.; Kominami, Y.; Yoshimura, K.; Kitayama, K. A constant microbial C/N ratio mediates the microbial nitrogen mineralization induced by root exudation among four co-existing canopy species. Rhizosphere 2021, 17, 100317. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Cong, J.; Wang, M.; Zhao, M.; Lu, H.; Xie, C.; Yang, C.; Yuan, T.; Li, D.; et al. Variations of soil microbial community structures beneath broadleaved forest trees in temperate and subtropical climate zones. Front. Microbiol. 2017, 8, 200. [Google Scholar] [CrossRef]
- Sánchez, L.F.; García, M.J.; Chacón, N. Nitrogen mineralization in soils under grasses and under trees in a protected Venezuelan savanna. Acta Oecol. 1997, 18, 27–37. [Google Scholar] [CrossRef]
- Kundu, K.; Bergmann, I.; Hahnke, S. Carbon source—A strong determinant of microbial community structure and performance of an anaerobic reactor. J. Biotechnol. 2013, 168, 616–624. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, X.; Xiang, W.; Chen, L.; Ouyang, S.; Lei, P. Vegetation restoration drives dynamics of soil nitrogen content and availability in the subtropics. Catena 2023, 220, 106720. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecol. Lett. 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes—Ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, X.; Wu, X.; Ding, M.; Duo, L. Lawn vegetation regulation changes the structure and function of soil bacterial community at airport. Chin. J. Ecol. 2023, 43, 5072–5083. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, Z.; Yang, J.; Zhao, Y.; Yang, Z.; Hu, Q. Effects of natural vegetative restoration on soil fungal and bacterial communities in bare patches of the southern Taihang Mountains. Ecol. Evol. 2019, 9, 10432–10441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, F.; Wang, Z.; Xiang, L.; Fu, Y.; Zhao, Z.; Kengara, F.O.; Mei, Z.; He, C.; Bian, Y.; et al. Soil properties and organochlorine compounds co-shape the microbial community structure: A case study of an obsolete site. Environ. Res. 2024, 240, 117589. [Google Scholar] [CrossRef] [PubMed]
- Fraatz, M.A.; Naeve, S.; Hausherr, V.; Zorn, H.; Blank, L.M. A minimal growth medium for the basidiomycete Pleurotus sapidus for metabolic flux analysis. Fungal Biol. Biotechnol. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, P.; Zhang, W.; Yuan, Y.; Li, B.; Wang, J. Sampling and complementarity effects of plant diversity on resource use increases the invasion resistance of communities. PLoS ONE 2015, 11, 0150128. [Google Scholar] [CrossRef]
- Zhou, T.; Liang, G.P.; Reich, P.B.; Delgado, B.M.; Wang, C.K.; Zhou, Z.H. Promoting effect of plant diversity on soil microbial functionality is amplified over time. One Earth 2024, 7, 2139–2148. [Google Scholar] [CrossRef]
- Nie, S.Q.; Zhang, Z.F.; Mo, S.M.; Li, J.H.; He, S.; Kashif, M.; Liang, Z.W.; Shen, P.H.; Yan, B.; Jiang, C.J. Desulfobacterales stimulates nitrate reduction in the mangrove ecosystem of a subtropical gulf. Sci. Total Environ. 2021, 769, 144562. [Google Scholar] [CrossRef]
- Spasov, E.; Tsuji, J.M.; Hug, L.A.; Doxey, A.C.; Sauder, L.A.; Parker, W.J.; Neufeld, J.D. High functional diversity among Nitrospira populations that dominate rotating biological contactor microbial communities in a municipal wastewater treatment plant. ISME J. 2019, 14, 1857–1872. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, L.B.; Chakrabarti, S.; Cooper, J.; Choi, E.; Ganan, C.; Tolchinsky, B.; Triplett, E.W.; Daroub, S.H.; Martens, H.W. Nitrogen and phosphorous acquisition strategies drive coexistence patterns among archaeal lineages in soil. ISME J. 2023, 17, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, H.; Han, M.; Yang, L. Soil metagenomics reveals the effect of nitrogen on soil microbial communities and nitrogen-cycle functional genes in the rhizosphere of Panax ginseng. Front. Plant Sci. 2024, 15, 1411073. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Yang, F.; Ming, A.; Jia, H.; Zhou, B.; Xiong, J.; Lu, J. Mixture enhances microbial network complexity of soil carbon, nitrogen and phosphorus cycling in Eucalyptus plantations. For. Ecol. Manag. 2024, 553, 121632. [Google Scholar] [CrossRef]
- Xu, H.; Chen, C.; Chen, W.; Pang, Z.; Zhang, G.; Zhang, W.; Kan, H. Metagenomics reveals soil nitrogen cycling after vegetation restoration: Influence of different vegetation restoration strategies. Appl. Soil Ecol. 2024, 204, 105695. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
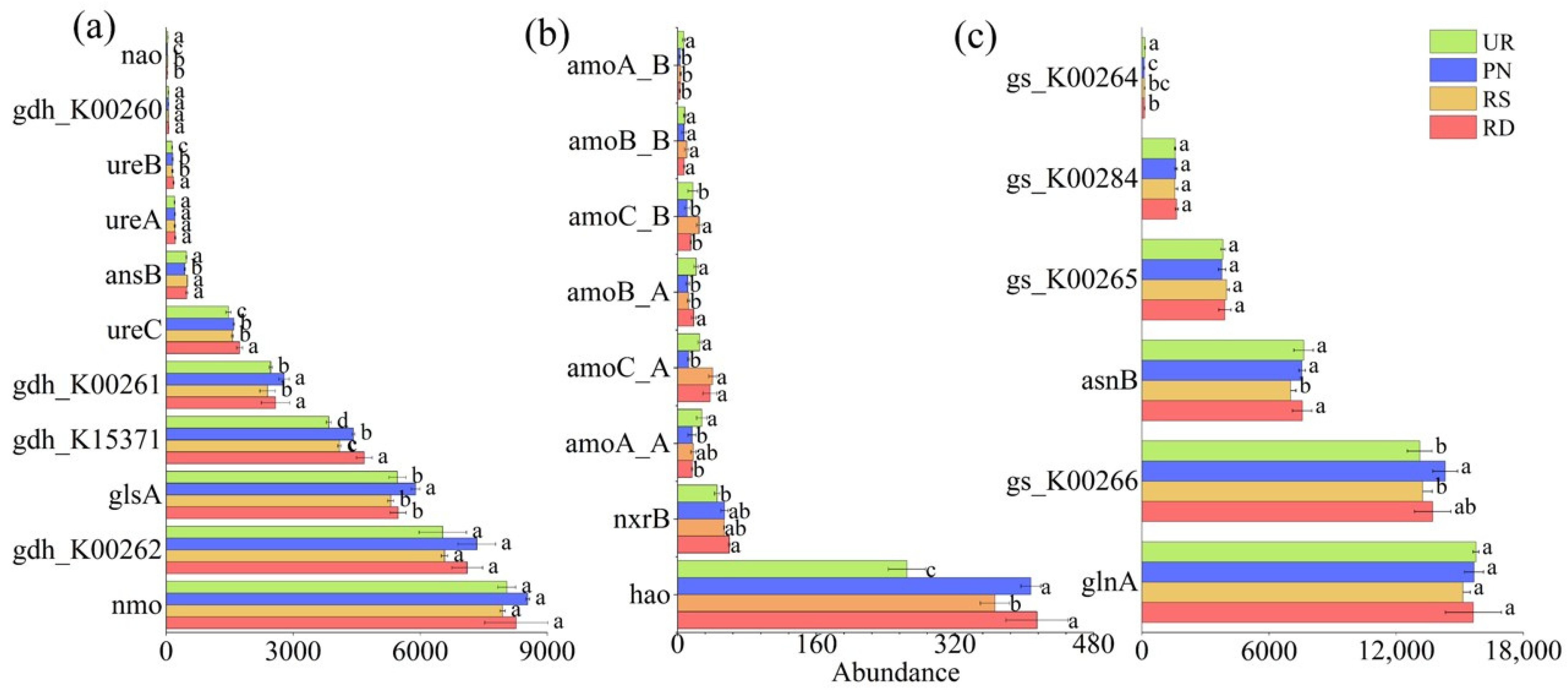
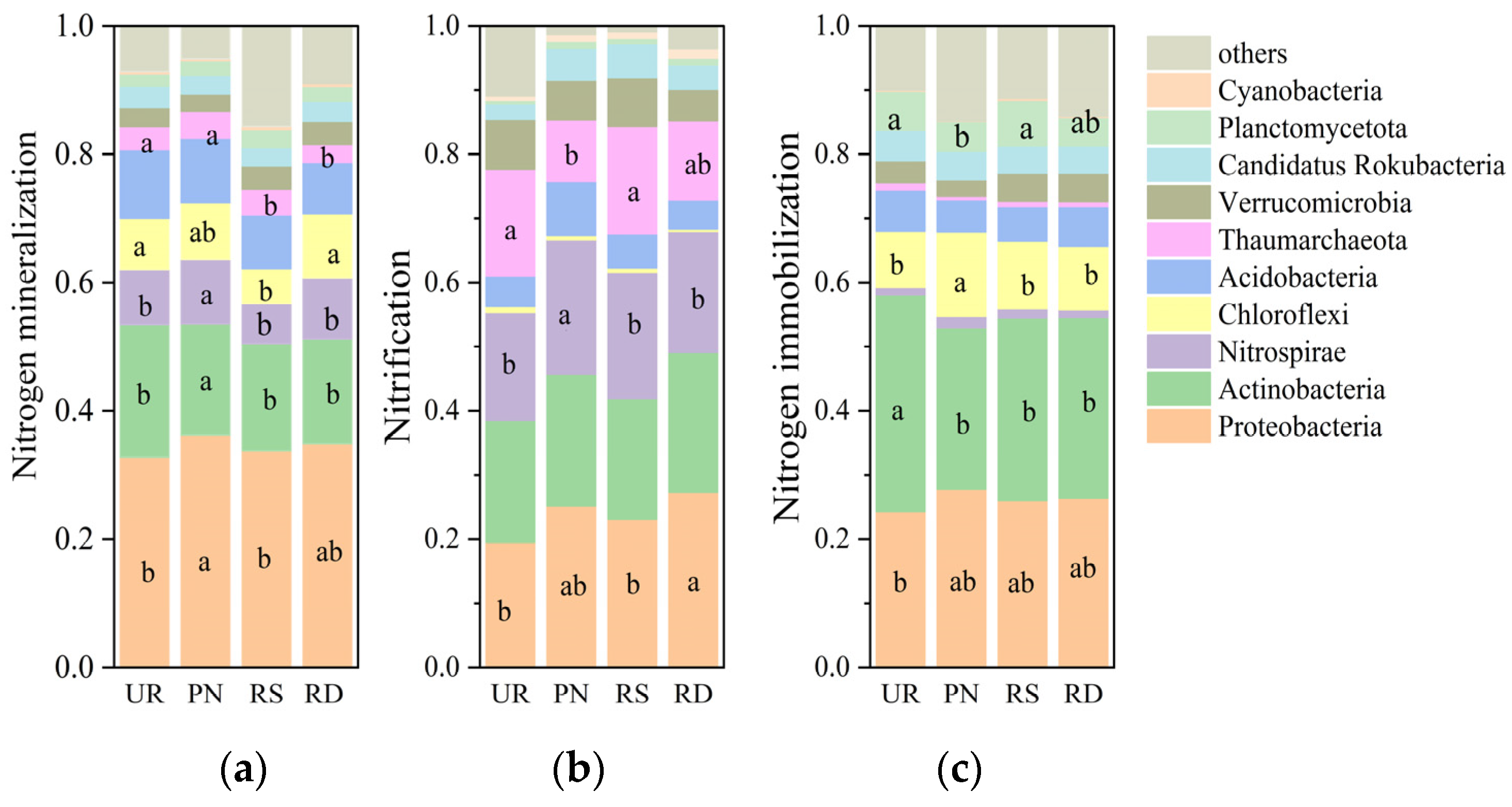
| Variable Y | x | Standardized Coefficient | Coefficient Standard Error | Adjusted R2 | p | Variance Inflation Factor |
|---|---|---|---|---|---|---|
| Gross N mineralization rate (mg·kg−1·d−1) | gdh_K15371 | 0.85 | 0.28 | 0.90 | <0.001 | 2.74 |
| gdh_K00261 | −0.57 | 0.14 | ||||
| ureB | 0.25 | 0.28 | ||||
| Gross nitrification rate (mg·kg−1·d−1) | amoA_B | 0.55 | 0.05 | 0.98 | <0.001 | 1.26 |
| hao | 0.49 | 0.05 | ||||
| amoC_B | −0.37 | 0.04 | ||||
| NH4+ immobilization rate (mg·kg−1·d−1) | gs_K00284 | 0.83 | 0.18 | 0.65 | <0.001 | 2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Li, T.; Ye, P.; Chen, Y.; Zhu, R.; Yan, R.; Yue, H.; Tian, Y. Metagenomic Insights into How Understory Vegetation Enhances Soil Nitrogen Availability via Microbial Nitrogen Transformation in Poplar Plantations. Agronomy 2025, 15, 1537. https://doi.org/10.3390/agronomy15071537
Jia W, Li T, Ye P, Chen Y, Zhu R, Yan R, Yue H, Tian Y. Metagenomic Insights into How Understory Vegetation Enhances Soil Nitrogen Availability via Microbial Nitrogen Transformation in Poplar Plantations. Agronomy. 2025; 15(7):1537. https://doi.org/10.3390/agronomy15071537
Chicago/Turabian StyleJia, Wenyu, Tong Li, Peilei Ye, Yuxin Chen, Ruoning Zhu, Ruixin Yan, Haoran Yue, and Ye Tian. 2025. "Metagenomic Insights into How Understory Vegetation Enhances Soil Nitrogen Availability via Microbial Nitrogen Transformation in Poplar Plantations" Agronomy 15, no. 7: 1537. https://doi.org/10.3390/agronomy15071537
APA StyleJia, W., Li, T., Ye, P., Chen, Y., Zhu, R., Yan, R., Yue, H., & Tian, Y. (2025). Metagenomic Insights into How Understory Vegetation Enhances Soil Nitrogen Availability via Microbial Nitrogen Transformation in Poplar Plantations. Agronomy, 15(7), 1537. https://doi.org/10.3390/agronomy15071537






